A Combined Chronic Low-Dose Soluble Epoxide Hydrolase and Acetylcholinesterase Pharmacological Inhibition Promotes Memory Reinstatement in Alzheimer’s Disease Mice Models
Abstract
:1. Introduction
2. Results
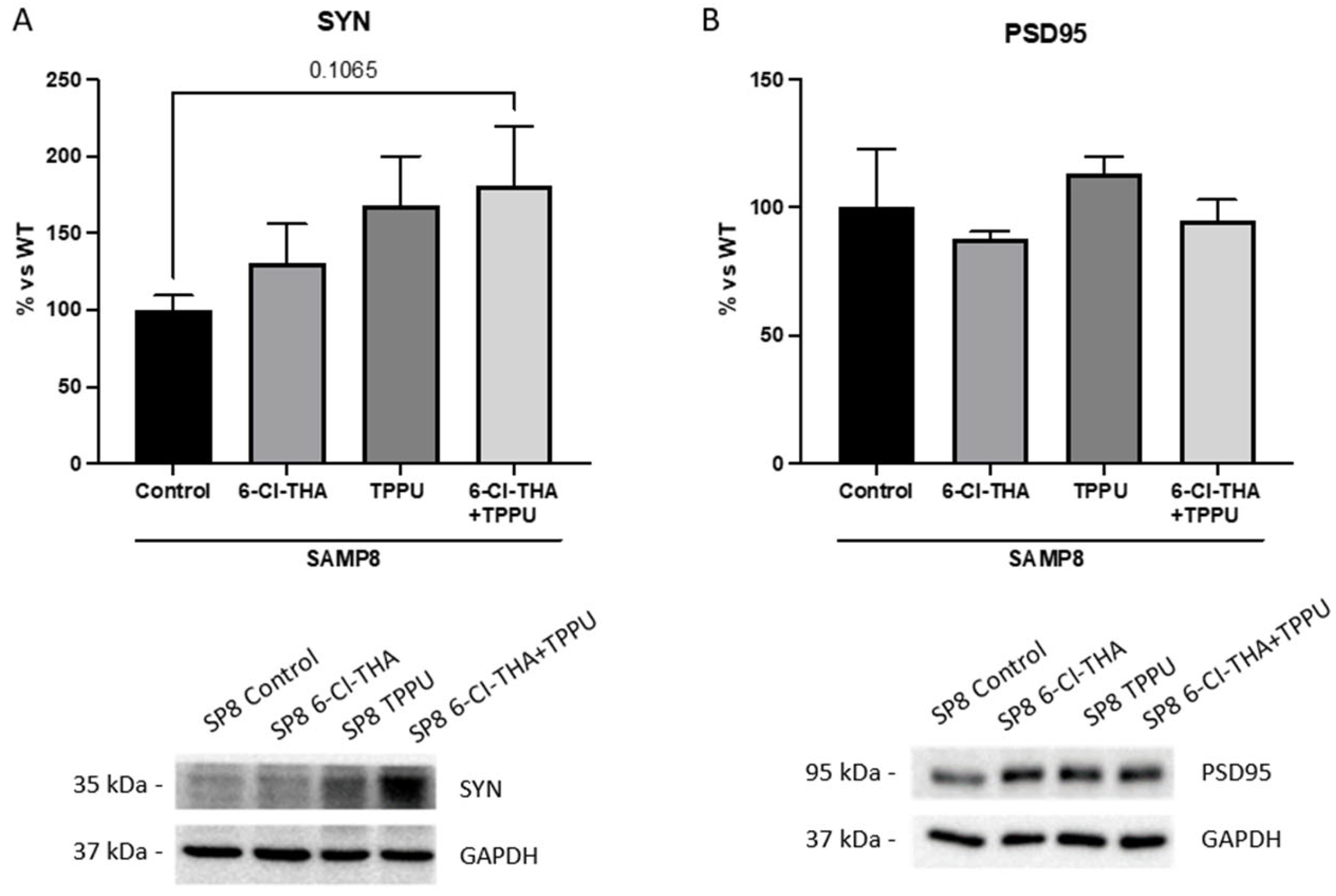
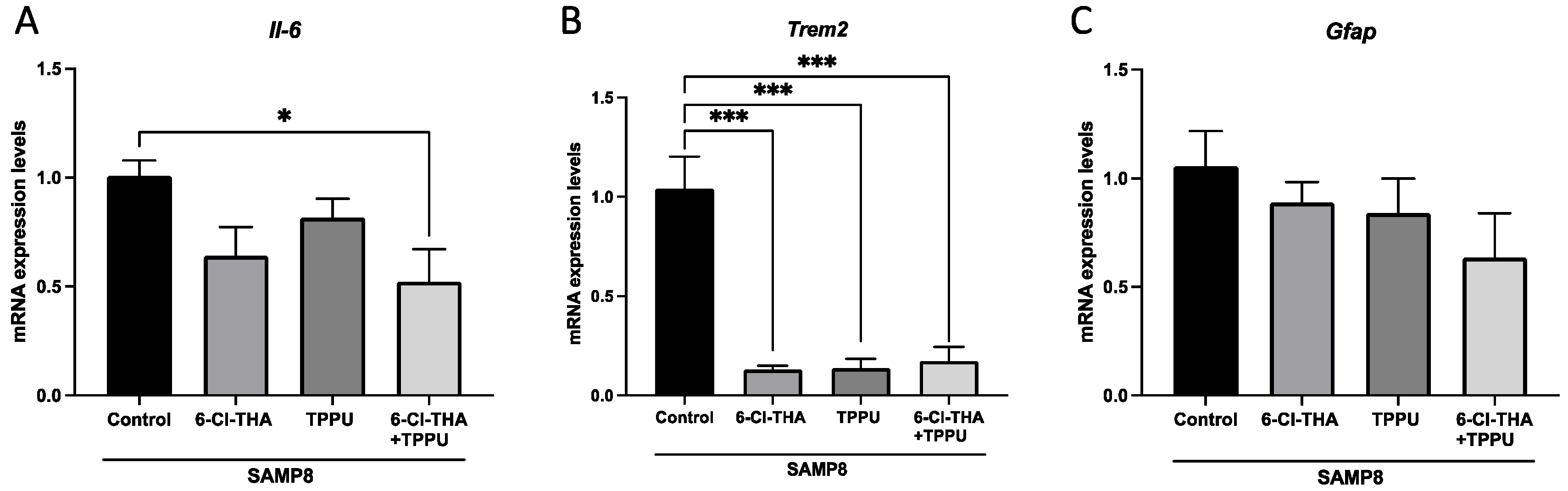
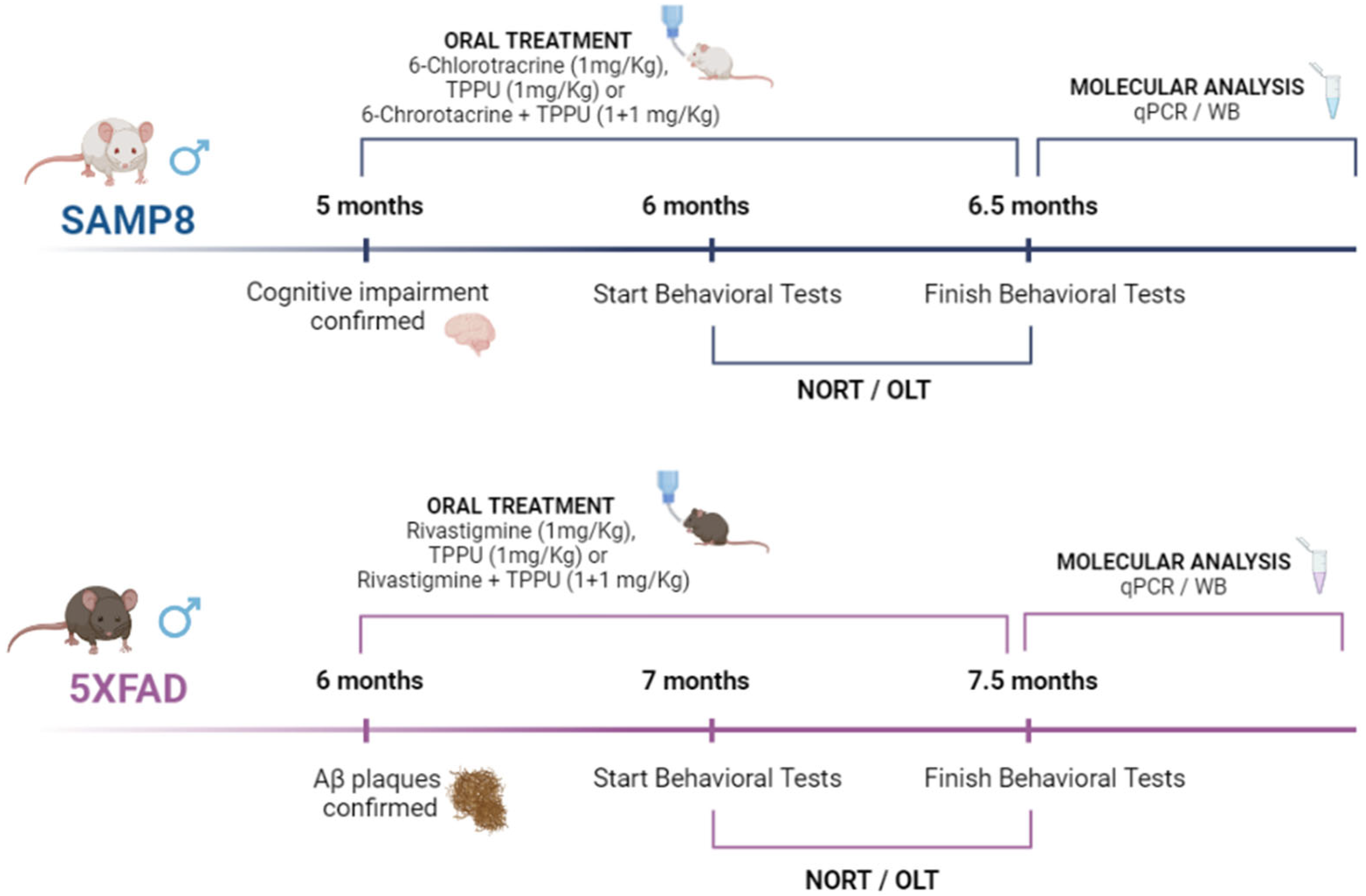
2.1. TPPU and 6-Cl-THA Chronic Low-Dose Co-Treatment Rescued SAMP8 Cognitive Decline, Ameliorating Neuroinflammation Markers
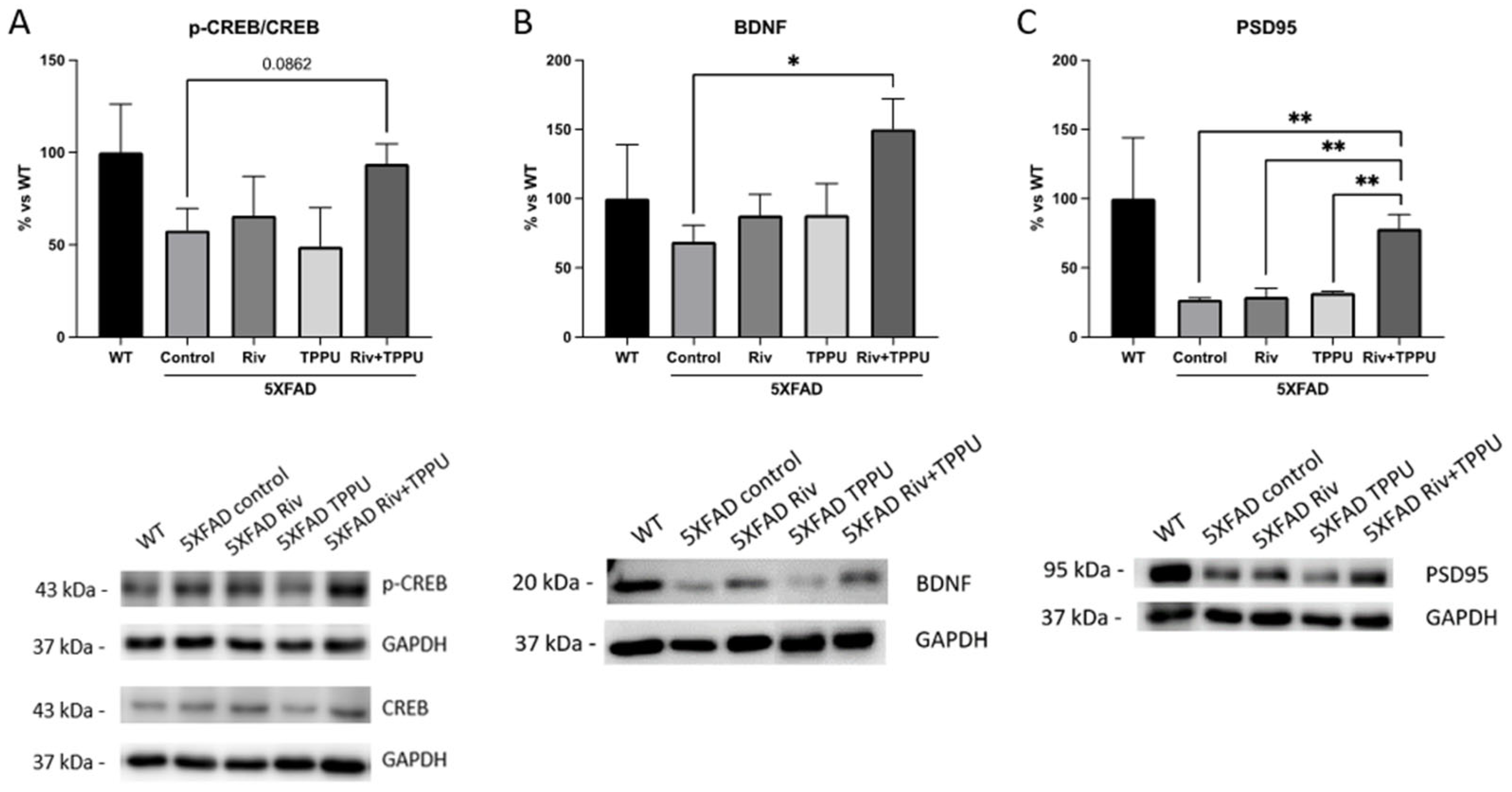
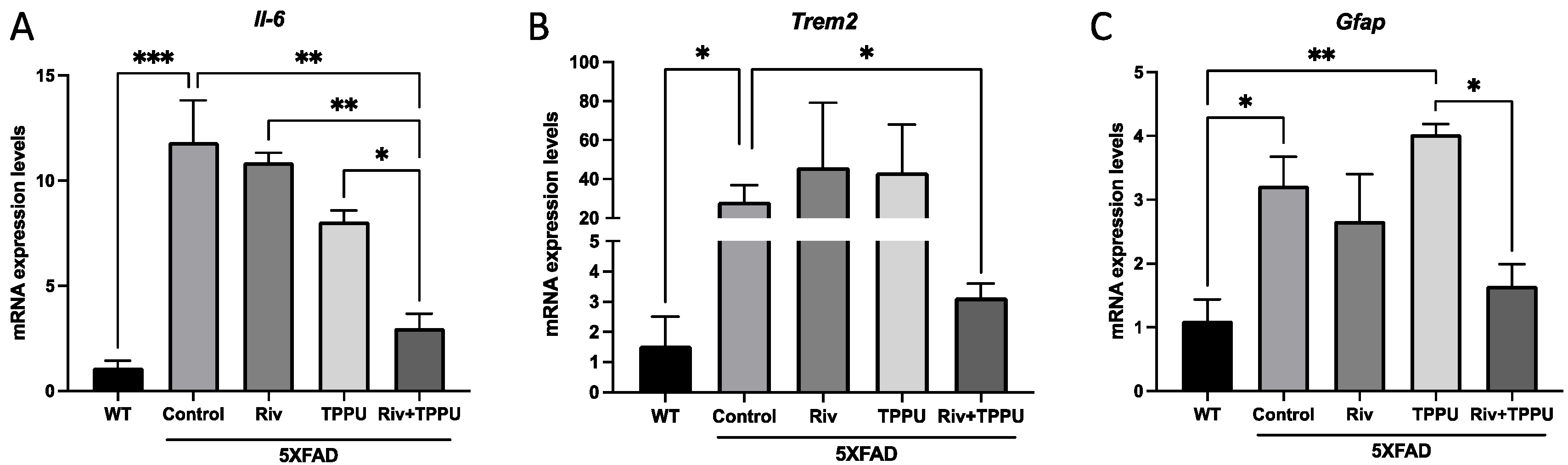
2.2. TPPU and Riv Chronic Low-Dose Co-Treatment Prevented 5XFAD Cognitive Decline, Improving Synaptic Markers and Ameliorating Neuroinflammation
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Behavioral Tests
4.3. Immunodetection Experiments by Western Blotting
4.4. RNA Extraction and Gene Expression Determination by qPCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finch, C.E. Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proc. Natl. Acad. Sci. USA 2010, 107, 1718–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palop, J.J.; Chin, J.; Mucke, L. A network dysfunction perspective on neurodegenerative diseases. Nature 2006, 443, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef]
- Iqbal, K.; Grundke-Iqbal, I. Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimer’s Dement. 2010, 6, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline. Alzheimer’s Dement. 2020, 6, e12050. [Google Scholar]
- Vasic, V.; Barth, K.; Schmidt, M.H.H. Neurodegeneration and neuro-regeneration—Alzheimer’s disease and stem cell therapy. Int. J. Mol. Sci. 2019, 20, 4272. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Camps, P.; Formosa, X.; Galdeano, C.; Munoz-Torrero, D.; Ramírez, L.; Gómez, E.; Isambert, N.; Lavilla, R.; Badia, A.; Clos, M.V. Pyrano [3, 2-c] quinoline−6-chlorotacrine hybrids as a novel family of acetylcholinesterase-and β-amyloid-directed anti-Alzheimer compounds. J. Med. Chem. 2009, 52, 5365–5379. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Codony, S.; Pujol, E.; Yang, J.; Leiva, R.; Escolano, C.; Puigoriol-Illamola, D.; Companys-Alemany, J.; Corpas, R.; Sanfeliu, C.; et al. Pharmacological Inhibition of Soluble Epoxide Hydrolase as a New Therapy for Alzheimer’s Disease. Neurotherapeutics 2020, 17, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Marowsky, A.; Burgener, J.; Falck, J.R.; Fritschy, J.-M.; Arand, M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience 2009, 163, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A. The role of soluble epoxide hydrolase and its inhibitors in depression. Brain Behav. Immun.-Health 2021, 16, 100325. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Hashimoto, K. Soluble Epoxide Hydrolase as a Therapeutic Target for Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 4951. [Google Scholar] [CrossRef]
- Pallàs, M.; Vázquez, S.; Sanfeliu, C.; Galdeano, C.; Griñán-Ferré, C. Soluble epoxide hydrolase inhibition to face neuroinflammation in Parkinson’s disease: A new therapeutic strategy. Biomolecules 2020, 10, 703. [Google Scholar] [CrossRef]
- Lee, H.-T.; Lee, K.-I.; Chen, C.-H.; Lee, T.-S. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflammation 2019, 16, 267. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.-P.; Zhang, X.-Y.; Morisseau, C.; Hwang, S.H.; Zhang, Z.-J.; Hammock, B.D.; Ma, X.-C. Discovery of soluble epoxide hydrolase inhibitors from chemical synthesis and natural products. J. Med. Chem. 2020, 64, 184–215. [Google Scholar] [CrossRef]
- Codony, S.; Pont, C.; Griñán-Ferré, C.; Di Pede-Mattatelli, A.; Calvó-Tusell, C.; Feixas, F.; Osuna, S.; Jarné-Ferrer, J.; Naldi, M.; Bartolini, M. Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2022, 65, 4909–4925. [Google Scholar] [CrossRef]
- Imig, J.D. Eicosanoid blood vessel regulation in physiological and pathological states. Clin. Sci. 2020, 134, 2707–2727. [Google Scholar] [CrossRef]
- Moreno, S.; Perno, C.F.; Mallon, P.W.; Behrens, G.; Corbeau, P.; Routy, J.; Darcis, G. Two-drug vs. three-drug combinations for HIV-1: Do we have enough data to make the switch? HIV Med. 2019, 20, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, T.E.; Morisseau, C.; Liu, J.-Y.; Inceoglu, B.; Jones, P.D.; Sanborn, J.R.; Hammock, B.D. 1-Aryl-3-(1-acylpiperidin-4-yl) urea inhibitors of human and murine soluble epoxide hydrolase: Structure− activity relationships, pharmacokinetics, and reduction of inflammatory pain. J. Med. Chem. 2010, 53, 7067–7075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregor, V.E.; Emmerling, M.R.; Lee, C.; Moore, C.J. The synthesis and in vitro acetylcholinesterase and butyrylcholinesterase inhibitory activity of tacrine (Cognex ®) derivaties. Bioorg. Med. Chem. Lett. 1992, 2, 861–864. [Google Scholar] [CrossRef]
- Matsui, N.; Takahashi, K.; Takeichi, M.; Kuroshita, T.; Noguchi, K.; Yamazaki, K.; Tagashira, H.; Tsutsui, K.; Okada, H.; Kido, Y. Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res. 2009, 1305, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Farr, S.A.; Kumar, V.B.; Armbrecht, H.J. The SAMP8 Mouse: A Model to Develop Therapeutic Interventions for Alzheimers Disease. Curr. Pharm. Des. 2012, 18, 1123–1130. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Hong, I.; Kang, T.; Yoo, Y.; Park, R.; Lee, J.; Lee, S.; Kim, J.; Song, B.; Kim, S.-Y.; Moon, M. Quantitative proteomic analysis of the hippocampus in the 5XFAD mouse model at early stages of Alzheimer’s disease pathology. J. Alzheimer’s Dis. 2013, 36, 321–334. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Dong, Y.; Pu, K.; Duan, W.; Chen, H.; Chen, L.; Wang, Y. Involvement of Akt/CREB signaling pathways in the protective effect of EPA against interleukin-1β-induced cytotoxicity and BDNF down-regulation in cultured rat hippocampal neurons. BMC Neurosci. 2018, 19, 52. [Google Scholar] [CrossRef]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, l6217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sodium oligomannate: First approval. Drugs 2020, 80, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Aducanumab: European agency rejects Alzheimer’s drug over efficacy and safety concerns. BMJ 2021, 375, n3127. [Google Scholar] [CrossRef] [PubMed]
- Pirolla, N.F.F.; Batista, V.S.; Dias Viegas, F.P.; Gontijo, V.S.; McCarthy, C.R.; Viegas, C.; Nascimento-Júnior, N.M. Alzheimer’s disease: Related targets, synthesis of available drugs, bioactive compounds under development and promising results obtained from multi-target approaches. Curr. Drug Targets 2021, 22, 505–538. [Google Scholar] [CrossRef]
- Sampietro, A.; Pérez-Areales, F.J.; Martínez, P.; Arce, E.M.; Galdeano, C.; Muñoz-Torrero, D. Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis. Pharmaceuticals 2022, 15, 545. [Google Scholar] [CrossRef]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. [Google Scholar] [CrossRef]
- Ghosh, A.; Comerota, M.M.; Wan, D.; Chen, F.; Propson, N.E.; Hwang, S.H.; Hammock, B.D.; Zheng, H. An epoxide hydrolase inhibitor reduces neuroinflammation in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2020, 12, eabb1206. [Google Scholar] [CrossRef]
- Chen, W.; Wang, M.; Zhu, M.; Xiong, W.; Qin, X.; Zhu, X. 14, 15-Epoxyeicosatrienoic Acid Alleviates Pathology in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2020, 40, 8188–8203. [Google Scholar] [CrossRef]
- Sun, C.-P.; Zhang, X.-Y.; Zhou, J.-J.; Huo, X.-K.; Yu, Z.-L.; Morisseau, C.; Hammock, B.D.; Ma, X.-C. Inhibition of sEH via stabilizing the level of EETs alleviated Alzheimer’s disease through GSK3β signaling pathway. Food Chem. Toxicol. 2021, 156, 112516. [Google Scholar] [CrossRef]
- Misik, J.; Nepovimova, E.; Pejchal, J.; Kassa, J.; Korabecny, J.; Soukup, O. Cholinesterase inhibitor 6-chlorotacrine-In vivo toxicological profile and behavioural effects. Curr. Alzheimer Res. 2018, 15, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Pallas, M.; Camins, A.; Smith, M.A.; Perry, G.; Lee, H.; Casadesus, G. From aging to Alzheimer’s disease: Unveiling “the switch” with the senescence-accelerated mouse model (SAMP8). J. Alzheimer’s Dis. 2008, 15, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Akiguchi, I.; Pallàs, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.-Y.; Chen, T.-K.; Zhou, J.-T.; He, S.-Y.; Yang, H.-Y.; Chen, Y.; Qu, W.; Feng, F.; Sun, H.-P. Dual GSK-3β/AChE inhibitors as a new strategy for multitargeting anti-Alzheimer’s disease drug discovery. ACS Med. Chem. Lett. 2018, 9, 171–176. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarne-Ferrer, J.; Griñán-Ferré, C.; Bellver-Sanchis, A.; Vázquez, S.; Muñoz-Torrero, D.; Pallàs, M. A Combined Chronic Low-Dose Soluble Epoxide Hydrolase and Acetylcholinesterase Pharmacological Inhibition Promotes Memory Reinstatement in Alzheimer’s Disease Mice Models. Pharmaceuticals 2022, 15, 908. https://doi.org/10.3390/ph15080908
Jarne-Ferrer J, Griñán-Ferré C, Bellver-Sanchis A, Vázquez S, Muñoz-Torrero D, Pallàs M. A Combined Chronic Low-Dose Soluble Epoxide Hydrolase and Acetylcholinesterase Pharmacological Inhibition Promotes Memory Reinstatement in Alzheimer’s Disease Mice Models. Pharmaceuticals. 2022; 15(8):908. https://doi.org/10.3390/ph15080908
Chicago/Turabian StyleJarne-Ferrer, Júlia, Christian Griñán-Ferré, Aina Bellver-Sanchis, Santiago Vázquez, Diego Muñoz-Torrero, and Mercè Pallàs. 2022. "A Combined Chronic Low-Dose Soluble Epoxide Hydrolase and Acetylcholinesterase Pharmacological Inhibition Promotes Memory Reinstatement in Alzheimer’s Disease Mice Models" Pharmaceuticals 15, no. 8: 908. https://doi.org/10.3390/ph15080908
APA StyleJarne-Ferrer, J., Griñán-Ferré, C., Bellver-Sanchis, A., Vázquez, S., Muñoz-Torrero, D., & Pallàs, M. (2022). A Combined Chronic Low-Dose Soluble Epoxide Hydrolase and Acetylcholinesterase Pharmacological Inhibition Promotes Memory Reinstatement in Alzheimer’s Disease Mice Models. Pharmaceuticals, 15(8), 908. https://doi.org/10.3390/ph15080908









