Royal Jelly Components Encapsulation in a Controlled Release System—Skin Functionality, and Biochemical Activity for Skin Applications
Abstract
:1. Introduction
2. Results
2.1. Characterization of RJDS
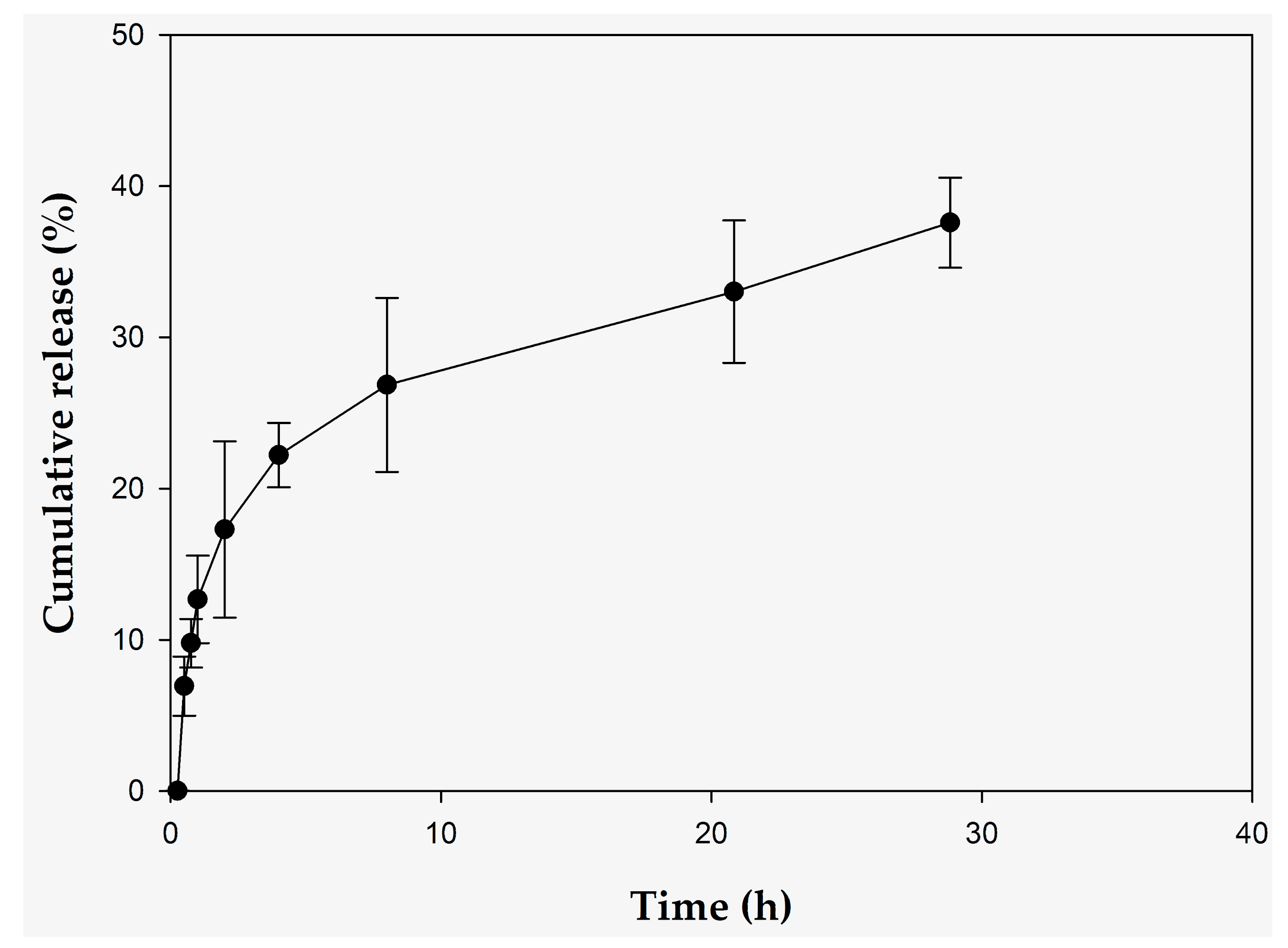
2.2. In Vitro Controlled Release Studies
2.3. In Cellulo Studies
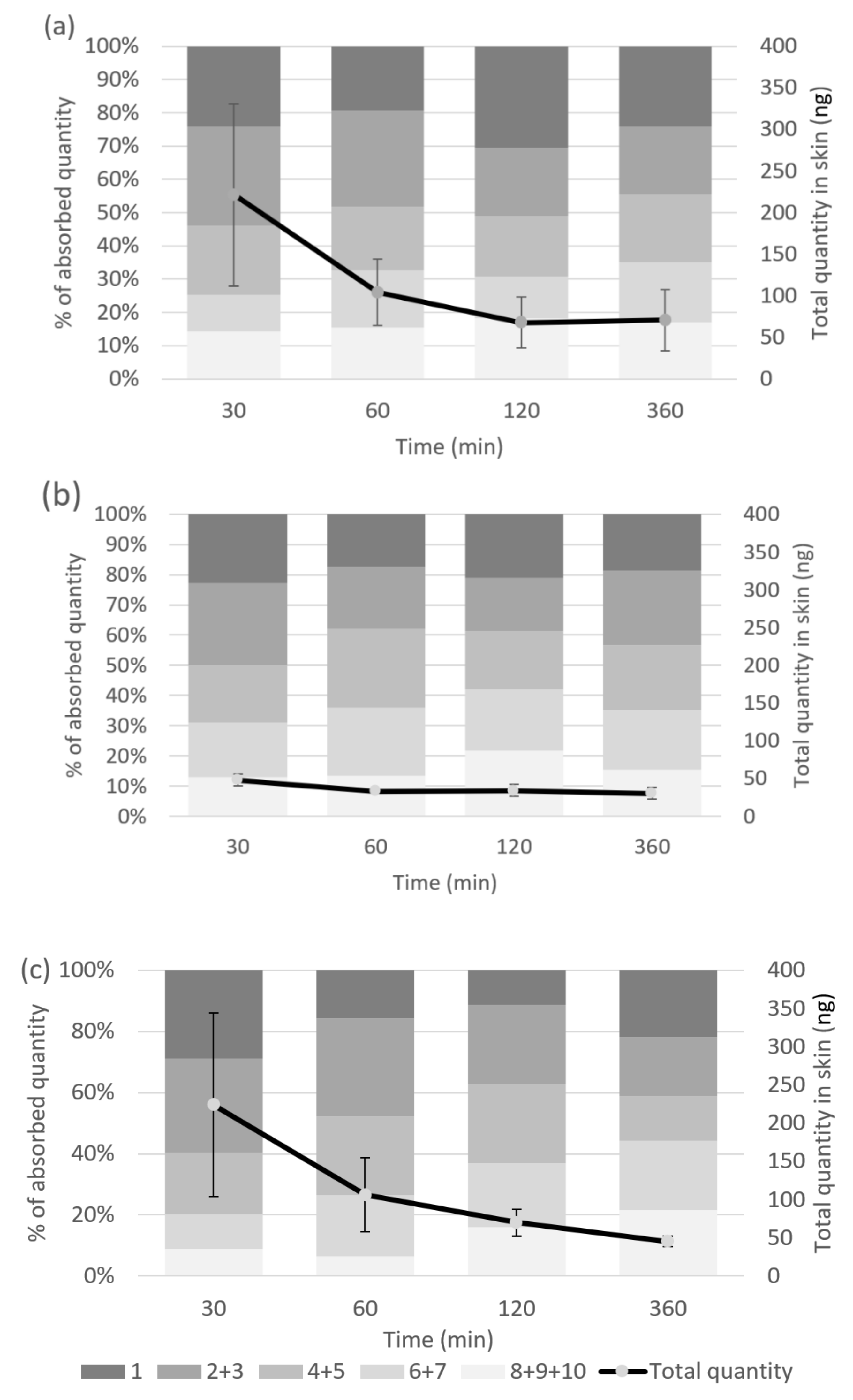
2.4. Tape Stripping
3. Discussion
4. Materials and Methods
4.1. Royal Jelly Encapsulation in A Liposome-Cyclodextrin Delivery System (RJDS)
Physicochemical Characterization of RJDS
4.2. Total Phenolic Content (TPC)
4.3. 10-Hydroxydecenoic Acid (10-HDA)
4.4. Stability of RJDS
4.5. In Vitro Controlled Release Study
4.6. Human Skin Cell Culture
4.7. MTT Cell Viability Assay
4.8. Gene Expression Analysis
4.9. Tape Stripping
4.10. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Spanidi, E.; Karapetsas, A.; Voulgaridou, G.P.; Letsiou, S.; Aligiannis, N.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Mourtzinos, I.; Pappa, A.; et al. A new controlled release system for propolis polyphenols and its biochemical activity for skin applications. Plants 2021, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.; Dosler, S.; Mericli, A.H. Chemical composition and antimicrobial activity of Verbascum caesareum. Chem. Nat. Compd. 2016, 52, 125–126. [Google Scholar] [CrossRef]
- Pavel, C.I.; Mărghitaş, L.A.; Bobiş, O.; Dezmirean, D.S.; Şapcaliu, A.; Radoi, I.; Mădaş, M.N. Biological Activities of Royal Jelly-Review. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 108–118. [Google Scholar]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New Findings on Biological Actions and Clinical Applications of Royal Jelly: A Review. J. Diet. Suppl. 2018, 15, 757–775. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. More than royal food-Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Virgiliou, C.; Kanelis, D.; Pina, A.; Gika, H.; Tananaki, C.; Zotou, A.; Theodoridis, G. A targeted approach for studying the effect of sugar bee feeding on the metabolic profile of Royal Jelly. J. Chromatogr. 2019, 1616, 460783. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; Almeida-Muradian, L.B. De Quality and standardisation of Royal Jelly. J. ApiProduct ApiMedical Sci. 2009, 1, 16–21. [Google Scholar] [CrossRef]
- Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar]
- Vucevic, D.; Melliou, E.; Vasilijic, S.; Gasic, S.; Ivanovski, P.; Chinou, I.; Colic, M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Laho, M.; Koh, L.; Mojžišov, A.; Majt, J.; Klaudiny, J. 10-HDA, a Major Fatty Acid of Royal Jelly, Exhibits pH Dependent Growth-Inhibitory Activity Against Different Strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef] [Green Version]
- Kawano, Y.; Makino, K.; Jinnin, M.; Sawamura, S.; Shimada, S.; Fukushima, S.; Ihn, H. Royal jelly regulates the proliferation of human dermal microvascular endothelial cells through the down-regulation of a photoaging-related microRNA. Drug Discov. Ther. 2019, 13, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Bouamama, S.; Merzouk, H.; Latrech, H.; Charif, N.; Bouamama, A. Royal jelly alleviates the detrimental effects of aging on immune functions by enhancing the in vitro cellular proliferation, cytokines, and nitric oxide release in aged human PBMCS. J. Food Biochem. 2021, 45, e13619. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, X.; Li, C.; Qian, H. Anti-senescence effect and molecular mechanism of the major royal jelly proteins on human embryonic lung fibroblast (HFL-I) cell line. J. Zhejiang Univ. SCIENCE B 2018, 19, 960–972. [Google Scholar] [CrossRef]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-Y.; De-Sheng, Y.; Wei-Zhang; Wang, J.-M.; Li, C.-Y.; Hui-Ye; Lei, K.-F.; Chen, X.-F.; Shen, N.-H.; Jin, L.-Q.; et al. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Fujii, A.; Kobayashi, S.; Kuboyama, N.; Furukawa, Y.; Kaneko, Y.; Ishihama, S.; Yamamoto, H.; Toyoyuki, T. Augmentation of Wound Healing by Royal Jelly (RJ) in Streptozotocin-Diabetic Rats. Jpn. J. Pharmacol. 1990, 53, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dynek, J.N.; Chan, S.M.; Liu, J.; Zha, J.; Fairbrother, W.J.; Vucic, D. Microphthalmia-Associated Transcription Factor Is a Critical Transcriptional Regulator of Melanoma Inhibitor of Apoptosis in Melanomas. Cancer Res. 2008, 2, 3124–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, S.Y. Changes in protein components and storage stability of Royal Jelly under various conditions. Food Chem. 1995, 54, 195–200. [Google Scholar] [CrossRef]
- Mendoza-Reséndez, R.; Gomez-Trevino, A.; Barriga-Castro, E.D.; Nunez, N.O.; Luna, C. Synthesis of Antibacterial Silver-based Nanodisks and Dendritic Structures Mediated by Royal Jelly. RSC Adv. 2014, 4, 1650–1658. [Google Scholar] [CrossRef] [Green Version]
- Complexes, D.C.; Saokham, P.; Muankaew, C.; Jansook, P. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Stella, V.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Eastburn, S.D.; Tao, B.Y. Applications of Modified Cyclodextrins. Biotechnol. Adv. 1994, 12, 325–339. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrin Inclusion Complexes. In Cyclodext. Technol; Springer: Dordrecht, The Netherlands, 1988; pp. 79–185. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Amin, M.; Kouhbanani, J.; Varma, R.S.; Maro, F.; Sarani, M. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Lin, E.Y.; Chen, Y.S.; Li, Y.S.; Chen, S.R.; Lee, C.H.; Huang, M.H.; Chuang, H.M.; Harn, H.J.; Yang, H.H.; Lin, S.Z.; et al. Liposome consolidated with cyclodextrin provides prolonged drug retention resulting in increased drug bioavailability in brain. Int. J. Mol. Sci. 2020, 21, 4408. [Google Scholar] [CrossRef]
- Yakavets, I.; Lassalle, H.; Scheglmann, D.; Wiehe, A. Temoporfin-in-Cyclodextrin-in-Liposome—A New Approach for Anticancer Drug Delivery: The Optimization of Composition. Nanomaterials 2018, 8, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldim, I.; Oliveira, A.M.; Souto, E.B.; Oliveira, W.P. Cyclodextrins-in-Liposomes: A Promising Delivery System for Lippia sidoides and Syzygium aromaticum Essential Oils. Life 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.C.; Flores, M.; Fernandez, R.J.R.; Vivas-mejia, P.E.; Barletta, G.L. A Fresh Look at the Potential of Cyclodextrins for Improving the Delivery of siRNA Encapsulated in Liposome Nanocarriers. ACS Omega 2022, 7, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, S.Y.; Dinarvand, R.; Esmaeili, M.; Mahjub, R.; Toliyat, T. Controlled-release drug delivery system based on fluocinolone acetonide—Cyclodextrin inclusion complex incorporated in multivesicular liposomes. Pharm. Dev. Technol. 2015, 20, 775–781. [Google Scholar] [CrossRef]
- Papagiannaros, A.; Dimas, K. Doxorubicin—PAMAM dendrimer complex attached to liposomes: Cytotoxic studies against human cancer cell lines. Int. J. Pharm. 2005, 302, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C. Advanced Drug Delivery Nanosystems: Perspectives and Regulatory Issues; Springer: Cham, Switzerland, 2015; pp. 195–198. [Google Scholar] [CrossRef]
- Spannhoff, A.; Kim, Y.K.; Raynal, N.J.; Gharibyan, V.; Su, M.; Zhou, Y.; Li, J.; Castellano, S.; Sbardella, G.; Issa, J.J.; et al. Scientific report might facilitate caste switching in bees. EMBO Rep. 2011, 12, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Bucekova, M.; Sojka, M.; Valachova, I.; Ma, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef] [Green Version]
- Antinelli, J.-F.; Zeggane, S.; Davico, R.; Rognone, C.; Faucon, J.; Lizzani, L. Evaluation of (E)-10-hydroxydec-2-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Li, J.; Feng, M.; Zhang, L.; Zhang, Z.; Pan, Y. Proteomics analysis of major royal jelly protein changes under different storage conditions. J. Proteome Res 2008, 7, 3339–3353. [Google Scholar] [CrossRef]
- Katsnelson, A. Cosmetics: Molecular beauty. Nature 2015, 526, 5–6. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, Y.; Cheng, Q.; Zhang, Q.; Fang, L.; Zheng, J. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget 2018, 9, 5480–5491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.M.; Hwang, E.; Lee, G.K.; Han, S.-M.; Cho, Y.; Kim, S.Y. Royal Jelly Protects Against Ultraviolet B—Induced Photoaging in Human Skin Fibroblasts via Enhancing Collagen Production 1 1. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Nitoiu, D.; Brennan-Crispi, D.; Addya, S.; Riobo, N.A.; Kelsell, D.P.; Mahoney, M.G. Cell Cycle- and Cancer-Associated Gene Networks Activated by Dsg2: Evidence of Cystatin A Deregulation and a Potential Role in Cell-Cell Adhesion. PLoS ONE 2015, 10, e0120091. [Google Scholar] [CrossRef] [Green Version]
- Van Itallie, C.M.; Tietgens, A.J.; Anderson, J.M. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol. Biol. Cell 2017, 28, 524–534. [Google Scholar] [CrossRef]
- Na, J.; Lee, K.; Na, W.; Shin, J.; Lee, M.; Yune, T.Y.; Lee, H.K.; Jung, H.; Kim, W.S.; Ju, B. Histone H3K27 Demethylase JMJD3 in Cooperation with NF- k B Regulates Keratinocyte Wound Healing. J. Investig. Dermatol. 2016, 136, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Odorisio, T. Epigenetic Control of Skin Re-Epithelialization: The NF-kB/JMJD3 Connection. J. Investig. Dermatol. 2016, 136, 738–740. [Google Scholar] [CrossRef] [Green Version]
- Westergren-Thorsson, G.U.; Bagher, M.A.; Andersson-Sjöland, A.N.; Thiman, L.E.N.A.; Löfdahl, C.L.Ö.; Hallgren, O.S.; Bjermer, L.E.I.F.; Larsson-Callerfelt, A.N.N.A.A. Original Article VEGF Synthesis is Induced by Prostacyclin and TGF-β in Distal Lung fi Broblasts from COPD Patients and Control Subjects: Implications for Pulmonary Vascular Remodelling. Respirology 2017, 23, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Jang, S.; Chung, J.H.; Kwon, O.; Jo, S.J. Inhibition of class I HDACs preserves hair follicle inductivity in postnatal dermal cells. Sci. Rep. 2021, 11, 24056. [Google Scholar] [CrossRef]
- Cabanel, M.; Pinheiro, T.; Cury, M.; Cheikh, E.L.; Carneiro, K. The epigenome as a putative target for skin repair: The HDAC inhibitor Trichostatin A modulates myeloid progenitor plasticity and behavior and improves wound healing. J. Transl. Med. 2019, 17, 247. [Google Scholar] [CrossRef] [Green Version]
- Detienne, G.; De Haes, W.; Ernst, U.R.; Schoofs, L.; Temmerman, L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 2014, 60, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, Y.; Fujita, Y.; Maruyama, H.; Araki, Y.; Ichihara, K.; Sato, A. Lifespan-Extending Effects of Royal Jelly and Its Related Substances on the Nematode Caenorhabditis elegans. PloS ONE 2011, 6, e23527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Lim, J.; Woo, K.; Kim, K. Piperonylic acid stimulates keratinocyte growth and survival by activating epidermal growth factor receptor (EGFR). Sci. Rep. 2018, 8, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Cui, L.; Xu, Y.; Guan, K. A Case of Anaphylaxis Caused by Major Royal Jelly Protein 3 of Royal Jelly and Its Cross-Reactivity with Honeycomb. J. Asthma Allergy 2021, 22, 1555–1557. [Google Scholar] [CrossRef]
- Rosmilah, M.; Shahnaz, M.; Patel, G.; Lock, J.; Rahman, D.; Masita, A.N.A. Characterization of major allergens of royal jelly Apis mellifera. Trop. Biomed. 2008, 25, 243–251. [Google Scholar]
- Nakashima, C.; Otsuka, A.; Seidel, J.A.; Kabashima, K. The effect of oral royal jelly administration on skin barrier function: A double-blind randomized placebo-controlled tria. Eur. J. Dermatol. 2018, 28, 563–564. [Google Scholar] [CrossRef]
- Okumura, N.; Ito, T.; Degawa, T.; Moriyama, M. Royal Jelly Protects against Epidermal Stress through Upregulation of the NQO1 Expression. Int. J. Mol. Sci. 2021, 22, 12973. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Syed, S.; Marshall, K.; Hosseinidoust, Z. Liposomal Nanovesicles for Efficient Encapsulation of Staphylococcal Antibiotics. ACS Omega 2019, 4, 10866–10876. [Google Scholar] [CrossRef]
- Gardikis, K.; Hatziantoniou, S.; Signorelli, M.; Pusceddu, M.; Micha-Screttas, M.; Schiraldi, A.; Demetzos, C.; Fessas, D. Thermodynamic and structural characterization of Liposomal-Locked in-Dendrimers as drug carriers. Colloids Surf. B Biointerfaces 2010, 81, 11–19. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Caparica-Santos, C.; Marcucci, M.C. Quantitative determination of trans- 10-Hydroxy-2-Decenoic Acid (10-HDA) in Brazilian royal jelly and commercial products containing royal jelly. Apic. Res. 2007, 46, 149–153. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letsiou, S.; Kalliampakou, K.; Gardikis, K.; Mantecon, L.; Infante, C.; Chatzikonstantinou, M.; Labrou, N.E.; Flemetakis, E. Skin protective effects of Nannochloropsis gaditana extract on H2O2-stressed human dermal fibroblasts. Front. Mar. Sci. 2017, 4, 221. [Google Scholar] [CrossRef] [Green Version]
- Ramakers, C.; Ruijter, J.M.; Lekanne Deprez, R.H.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.; Fluhr, J.W. The tape stripping procedure—Evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef] [PubMed]

| Time | Parameters | RJDS TPC (μg GA/mL) ± SD | RJL TPC (μg GA/mL) ± SD | ||||
|---|---|---|---|---|---|---|---|
| t (0) | 354 a | ± | 15 | 150 | ± | 15 | |
| 24th week | 25 °C | 306 c | ± | 16 | 135 | ± | 126 |
| 6 °C | 308 b | ± | 8 | 147 | ± | 11 | |
| 38 °C | 193 a–d | ± | 34 | 29 | ± | 101 | |
| UV | 282 d | ± | 17 | 100.5 | ± | 62 | |
| Time | Parameters | RJDS (ppm) ± SD | Pure Royal Jelly (ppm) ± SD | RJL (ppm) ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t (0) | 1142 a, b, e, f | ± | 107 | 1198 a | ± | 49 | 317 | ± | 14 | |
| 24th week | 25°C | 96 e–l | ± | 115 | 615 d, p | ± | 127 | N.D. | ||
| 6°C | 962 c, f–i | ± | 148 | 893 c, j, m, p, o | ± | 18 | N.D. | |||
| 38°C | 816 g, k, n, o | ± | 102 | 550 p, q | ± | 35 | N.D. | |||
| UV | 949 b, h, l, m, n | ± | 64 | 447 d, q | ± | 16 | N.D. | |||
| System | Z-Average Diameter (nm) ± SD | PI ± SD | ζ-Pot (mV) ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RJDS day 0 | 301 a | ± | 33 | 0.399 d | ± | 0.044 | −17 | ± | 4.0 |
| RJDS 6 °C week 4 | 308 b | ± | 45 | 0.251 a | ± | 0.065 | −19 | ± | 5.2 |
| RJDS 25 °C week 4 | 449 | ± | 101 | 0.404 e | ± | 0.063 | −20 | ± | 3.9 |
| RJDS 38 °C week 4 | 605 a, b | ± | 88 | 0.710 a–e | ± | 0.105 | −18 | ± | 3.8 |
| RJDS 6 °C week 8 | 428 | ± | 95 | 0.341 c | ± | 0.115 | −19 | ± | 4.2 |
| RJDS 6 °C week 24 | 508 | ± | 99 | 0.299 s b | ± | 0.112 | −25 | ± | 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanidi, E.; Athanasopoulou, S.; Liakopoulou, A.; Chaidou, A.; Hatziantoniou, S.; Gardikis, K. Royal Jelly Components Encapsulation in a Controlled Release System—Skin Functionality, and Biochemical Activity for Skin Applications. Pharmaceuticals 2022, 15, 907. https://doi.org/10.3390/ph15080907
Spanidi E, Athanasopoulou S, Liakopoulou A, Chaidou A, Hatziantoniou S, Gardikis K. Royal Jelly Components Encapsulation in a Controlled Release System—Skin Functionality, and Biochemical Activity for Skin Applications. Pharmaceuticals. 2022; 15(8):907. https://doi.org/10.3390/ph15080907
Chicago/Turabian StyleSpanidi, Eleni, Sophia Athanasopoulou, Angeliki Liakopoulou, Angeliki Chaidou, Sophia Hatziantoniou, and Konstantinos Gardikis. 2022. "Royal Jelly Components Encapsulation in a Controlled Release System—Skin Functionality, and Biochemical Activity for Skin Applications" Pharmaceuticals 15, no. 8: 907. https://doi.org/10.3390/ph15080907
APA StyleSpanidi, E., Athanasopoulou, S., Liakopoulou, A., Chaidou, A., Hatziantoniou, S., & Gardikis, K. (2022). Royal Jelly Components Encapsulation in a Controlled Release System—Skin Functionality, and Biochemical Activity for Skin Applications. Pharmaceuticals, 15(8), 907. https://doi.org/10.3390/ph15080907








