Neuroprotective Effects of an Edible Pigment Brilliant Blue FCF against Behavioral Abnormity in MCAO Rats
Abstract
:1. Introduction
2. Results
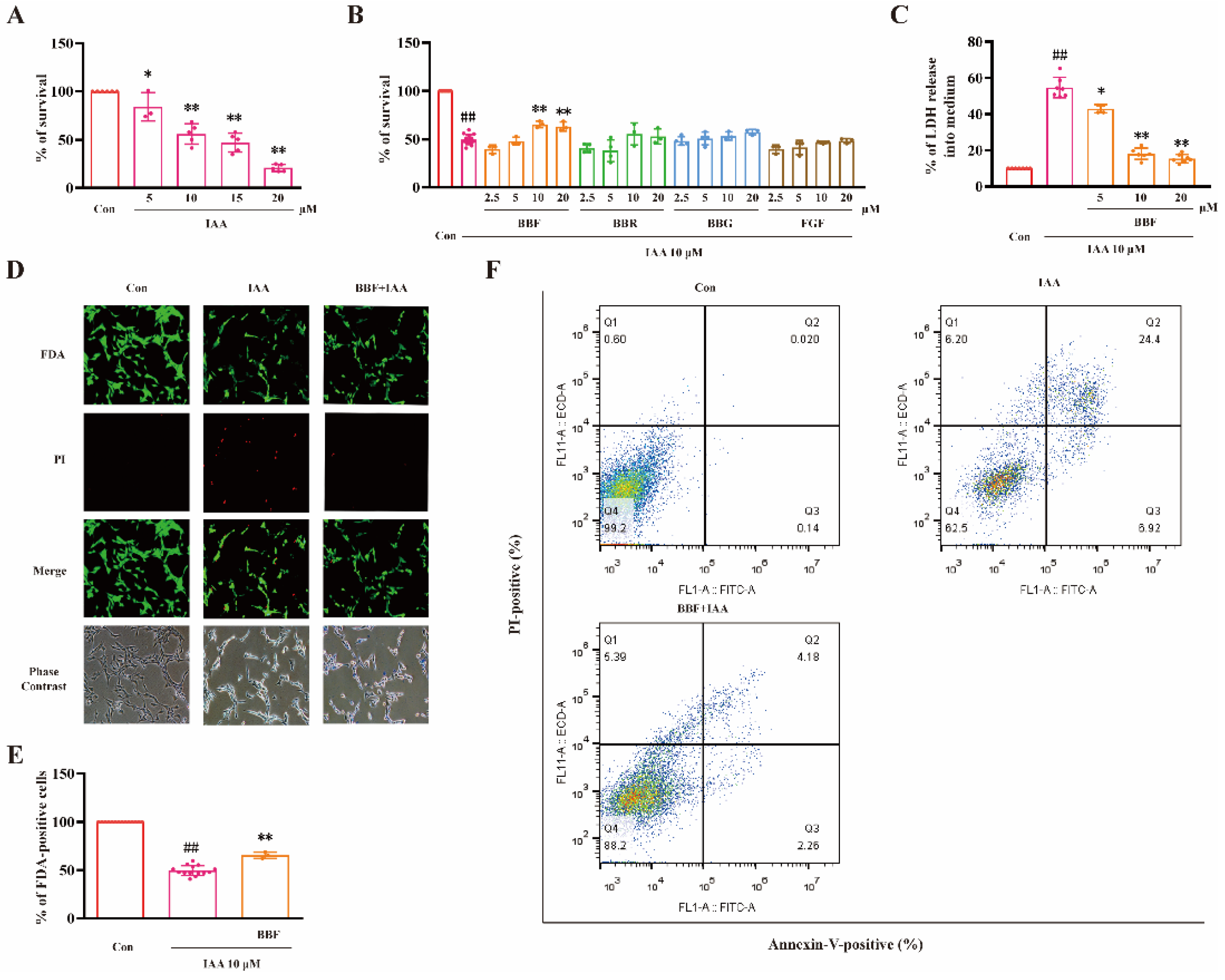
2.1. BBF but Not BBF Derivatives Prevents Chemical Hypoxia-Induced Cell Death in HT22 Cells
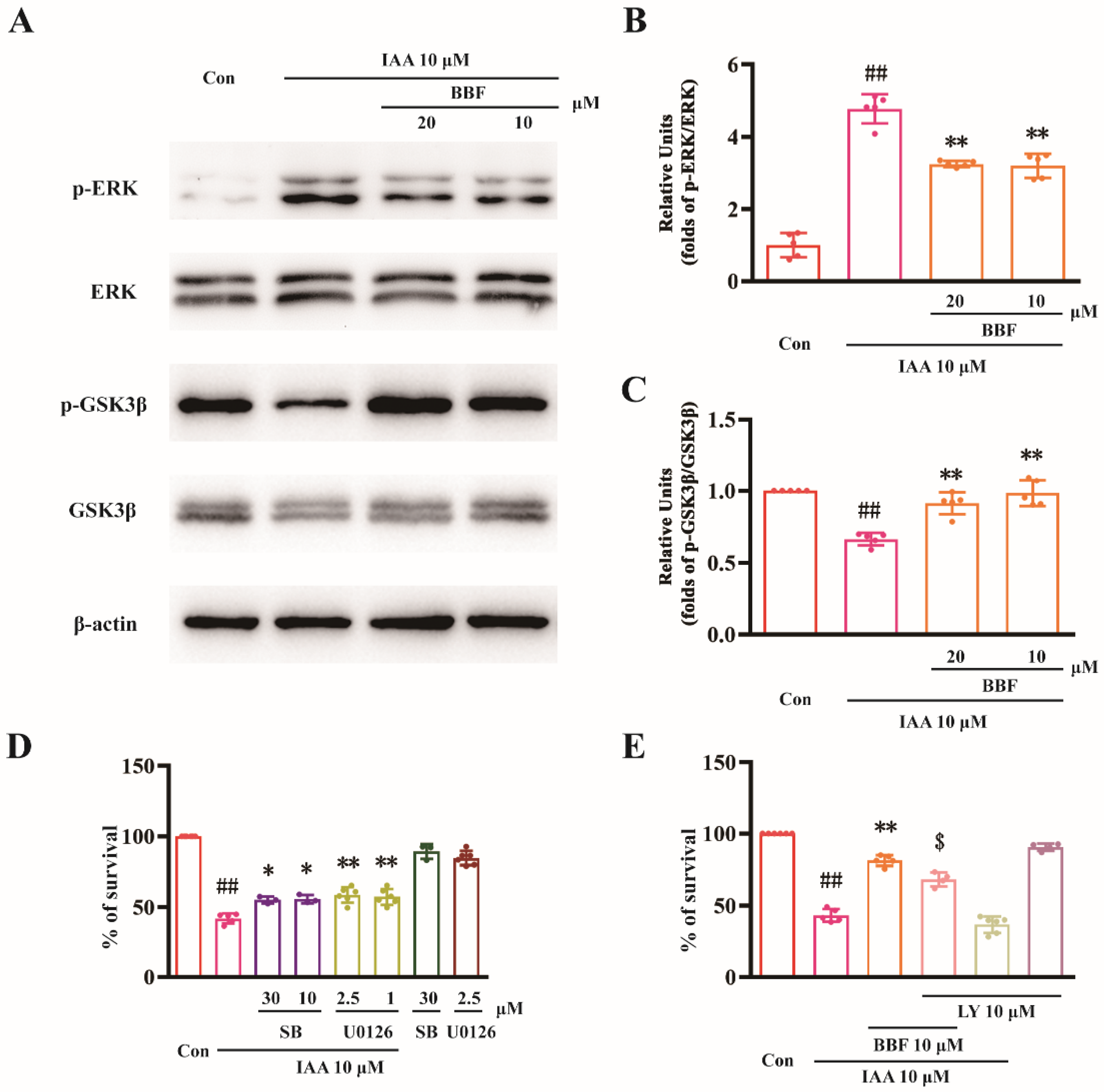
2.2. BBF Produces Anti-Chemical Hypoxia Protective Effects via the Inhibition of ERK and GSK3β Concurrently in HT22 Cells
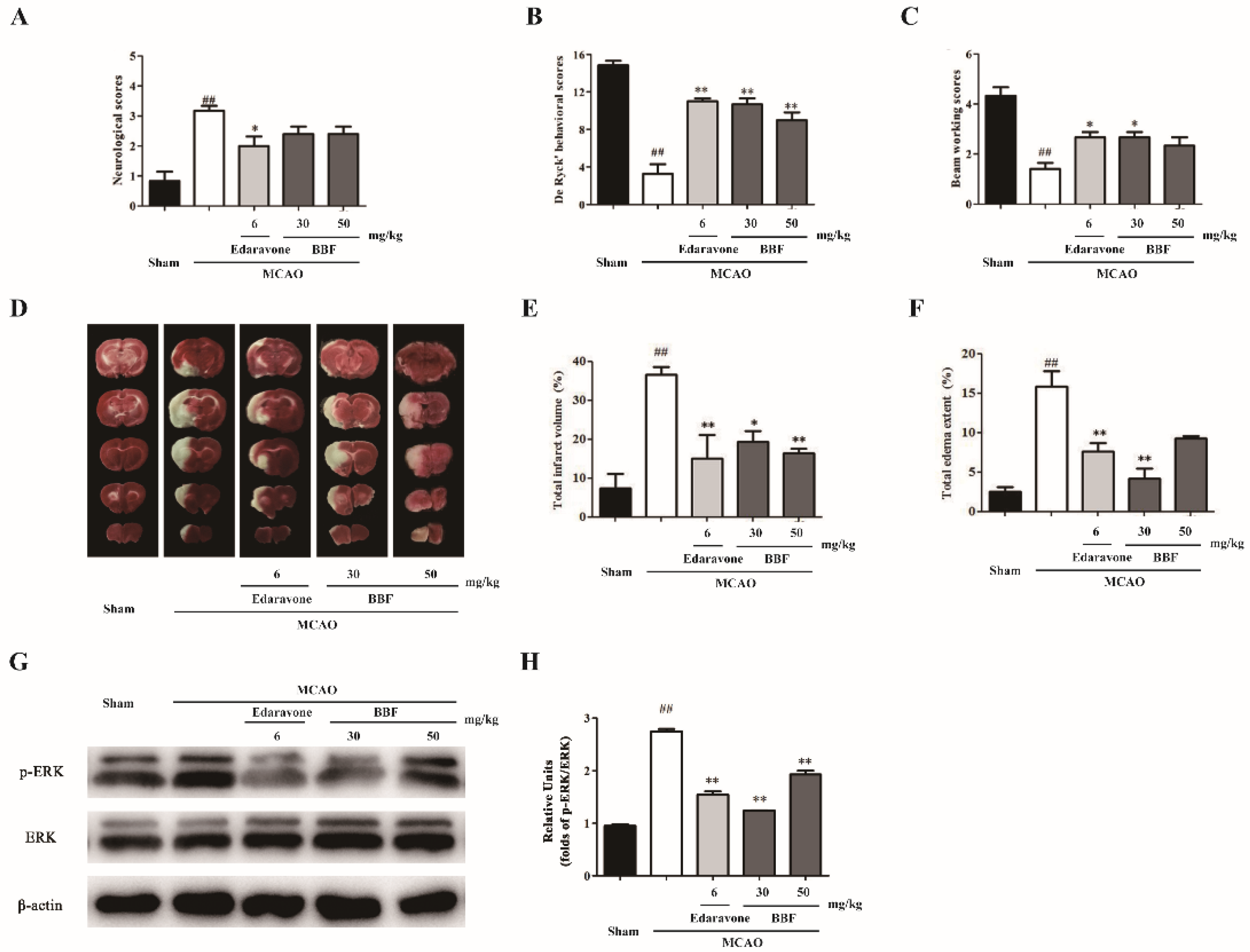
2.3. BBF Prevents Neurological and Behavioral Abnormity, Brain Infarct and ERK Activation in MCAO Rats
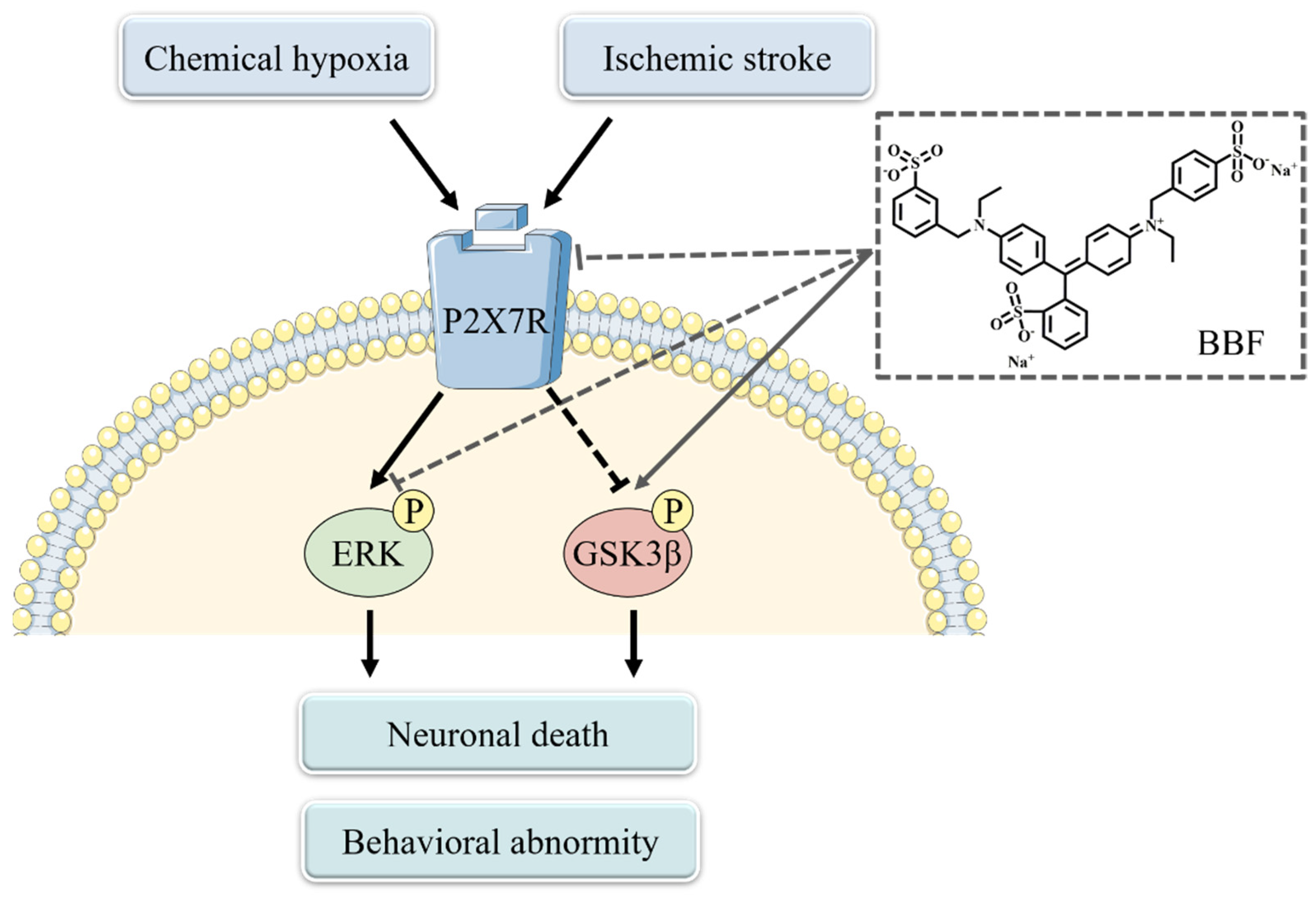
3. Discussion
4. Materials and Methods
4.1. HT22 Cells Culture and Treatments
4.2. Measurement of Cell Viability
4.3. Measurement of LDH Release
4.4. FDA/PI Double Staining Assay
4.5. Western Blotting Analysis
4.6. Animals and Surgery
4.7. Neurological Tests
4.8. Behavioral Tests
4.9. TTC Staining
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Caplan, L.R. Clinical Stroke Syndromes. Front. Neurol. Neurosci. 2016, 40, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Shanan, N.; GhasemiGharagoz, A.; Abdel-Kader, R.; Breitinger, H.G. The effect of Pyrroloquinoline quinone and Resveratrol on the Survival and Regeneration of Cerebellar Granular Neurons. Neurosci. Lett. 2019, 694, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Huang, C.; Chu, H.; Tang, Y.; Yang, X. Peptide5 Attenuates rtPA Related Brain Microvascular Endothelial Cells Reperfusion Injury via the Wnt/β-Catenin Signalling Pathway. Curr. Neurovasc. Res. 2021, 18, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kenna, J.E.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. Assessment of recombinant tissue plasminogen activator (rtPA) toxicity in cultured neural cells and subsequent treatment with poly-arginine peptide R18D. Neurochem. Res. 2020, 45, 1215–1229. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, G.; Kushwah, A.S.; Surampalli, G.; Singh, T.G.; Gupta, S. Arbutin protects brain against middle cerebral artery occlusion-reperfusion (MCAo/R) injury. Biochem. Biophys. Res. Commun. 2021, 577, 52–57. [Google Scholar] [CrossRef]
- Yang, L.; You, J.; Li, J.; Wang, Y.; Chan, Z. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611. [Google Scholar] [CrossRef]
- Yang, M.; Feng, Y.; Yan, S.; Wu, Z.; Xiao, X.; Sang, J.; Ye, S.; Liu, F.; Cui, W. Evans Blue Might Produce Pathologically Activated Neuroprotective Effects via the Inhibition of the P2X4R/p38 Signaling Pathway. Cell. Mol. Neurobiol. 2021, 41, 293–307. [Google Scholar] [CrossRef]
- Jiang, T.; Cheng, H.; Su, J.; Wang, X.; Wang, Q.; Chu, J.; Li, Q. Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathway. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2020, 62, 104715. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, A.I. Oxidative Stress in Ischemia/Reperfusion Injuries following Acute Ischemic Stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef]
- Zhang, D.; Han, S.; Wang, S.; Luo, Y.; Zhao, L.; Li, J. cPKCγ-mediated down-regulation of UCHL1 alleviates ischaemic neuronal injuries by decreasing autophagy via ERK-mTOR pathway. J. Cell. Mol. Med. 2017, 21, 3641–3657. [Google Scholar] [CrossRef]
- Duan, J.; Cui, J.; Yang, Z.; Guo, C.; Cao, J.; Xi, M.; Weng, Y.; Yin, Y.; Wang, Y.; Wei, G.; et al. Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3β/Nrf2 signaling. J. Neuroinflamm. 2019, 16, 24. [Google Scholar] [CrossRef]
- Shin, M.; Franks, C.E.; Hsu, K.L. Isoform-selective activity-based profiling of ERK signaling. Chem. Sci. 2018, 9, 2419–2431. [Google Scholar] [CrossRef]
- Lin, L.; Jadoon, S.S.; Liu, S.Z.; Zhang, R.Y.; Li, F.; Zhang, M.Y.; Ai-Hua, T.; You, Q.Y.; Wang, P. Tanshinone IIA Ameliorates Spatial Learning and Memory Deficits by Inhibiting the Activity of ERK and GSK-3β. J. Geriatr. Psychiatry Neurol. 2019, 32, 152–163. [Google Scholar] [CrossRef]
- Ting, L.L.; Lu, H.T.; Yen, S.F.; Ngo, T.H.; Tu, F.Y.; Tsai, I.S.; Tsai, Y.H.; Chang, F.Y.; Li, X.J.; Li, S.; et al. Expression of AHI1 Rescues Amyloidogenic Pathology in Alzheimer’s Disease Model Cells. Mol. Neurobiol. 2019, 56, 7572–7582. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef]
- Khlghatyan, J.; Evstratova, A.; Bozoyan, L.; Chamberland, S.; Chatterjee, D.; Marakhovskaia, A.; Soares Silva, T.; Toth, K.; Mongrain, V.; Beaulieu, J.M. Fxr1 regulates sleep and synaptic homeostasis. EMBO J. 2020, 39, e103864. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Z.Z.; Zhang, J.Y.; Zhang, Y.; Won, S.; Sun, J.; Yu, S.P.; Li, J.; Wei, L. GSK-3β Inhibition Induced Neuroprotection, Regeneration, and Functional Recovery After Intracerebral Hemorrhagic Stroke. Cell Transplant. 2017, 26, 395–407. [Google Scholar] [CrossRef]
- Crocetti, L.; Guerrini, G.; Giovannoni, M.P.; Melani, F.; Lamanna, S.; Di Cesare Mannelli, L.; Lucarini, E.; Ghelardini, C.; Wang, J.; Dahl, G. New Panx-1 Blockers: Synthesis, Biological Evaluation and Molecular Dynamic Studies. Int. J. Mol. Sci. 2022, 23, 4827. [Google Scholar] [CrossRef]
- Shakkour, Z.; Issa, H.; Ismail, H.; Ashekyan, O.; Habashy, K.J.; Nasrallah, L.; Jourdi, H.; Hamade, E.; Mondello, S.; Sabra, M.; et al. Drug Repurposing: Promises of Edaravone Target Drug in Traumatic Brain Injury. Curr. Med. Chem. 2021, 28, 2369–2391. [Google Scholar] [CrossRef]
- Jana, M.K.; Cappai, R.; Ciccotosto, G.D. Oligomeric Amyloid-β Toxicity Can Be Inhibited by Blocking Its Cellular Binding in Cortical Neuronal Cultures with Addition of the Triphenylmethane Dye Brilliant Blue G. ACS Chem. Neurosci. 2016, 7, 1141–1147. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, J.; Lin, J.; Jin, L.; Yu, R.; Mak, S.; Hu, S.; Sun, H.; Wu, X.; Zhang, Z.; et al. Indirubin-3-Oxime Prevents H2O2-Induced Neuronal Apoptosis via Concurrently Inhibiting GSK3β and the ERK Pathway. Cell. Mol. Neurobiol. 2017, 37, 655–664. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Wang, A.; Gao, Z.; Gao, X.; Chen, H.; Zhou, J.; Zhao, X.; Wang, Y. Safety and efficacy of Edaravone Dexborneol versus edaravone for patients with acute ischaemic stroke: A phase II, multicentre, randomised, double-blind, multiple-dose, active-controlled clinical trial. Stroke Vasc. Neurol. 2019, 4, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, F.; Huang, C.; Shentu, J.; Wang, M.; Sun, C.; Chen, L.; Yan, S.; Fang, F.; Wang, Y.; et al. 5-Hydroxycyclopenicillone Inhibits β-Amyloid Oligomerization and Produces Anti-β-Amyloid Neuroprotective Effects In Vitro. Molecules 2017, 22, 1651. [Google Scholar] [CrossRef]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a Marine Carotenoid, Reverses Scopolamine-Induced Cognitive Impairments in Mice and Inhibits Acetylcholinesterase in Vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, Y.; Lv, P.; Teng, Z.; Dong, Y.; Qi, Q.; Liu, Z. Edaravone attenuates oxidative stress induced by chronic cerebral hypoperfusion injury: Role of ERK/Nrf2/HO-1 signaling pathway. Neurol. Res. 2018, 40, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Huang, F.; Jiang, W.; Wang, W.; Xiang, J. Brilliant blue G attenuates neuro-inflammation via regulating MAPKs and NF-κB signaling pathways in lipopolysaccharide-induced BV2 microglia cells. Exp. Ther. Med. 2020, 20, 116. [Google Scholar] [CrossRef]
- Spadaro, A.; Rao, M.; Lorenti, M.; Romano, M.R.; Augello, A.; Eandi, C.M.; Platania, C.B.M.; Drago, F.; Bucolo, C. New Brilliant Blue G Derivative as Pharmacological Tool in Retinal Surgery. Front. Pharmacol. 2020, 11, 708. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Jian, Y.; Ma, Y.; Yan, W.; Chen, X.; Xu, H.; Li, L. Resveratrol induces apoptosis in human melanoma cell through negatively regulating Erk/PKM2/Bcl-2 axis. OncoTargets Ther. 2018, 11, 8995–9006. [Google Scholar] [CrossRef]
- Akter, R.; Zhyvoloup, A.; Zheng, B.; Bhatia, S.R.; Raleigh, D.P. The triphenylmethane dye brilliant blue G is only moderately effective at inhibiting amyloid formation by human amylin or at disaggregating amylin amyloid fibrils, but interferes with amyloid assays; Implications for inhibitor design. PLoS ONE 2019, 14, e0219130. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.W.; Cha, M.J.; Yoon, N.; Lee, C.Y.; Lee, J.; Seo, H.H.; Shin, S.; Choi, J.W.; Lee, S.; et al. TAK-733 inhibits inflammatory neointimal formation by suppressing proliferation, migration, and inflammation in vitro and in vivo. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Kuo, C.T.; Chen, Y.L.; Hsu, W.T.; How, S.C.; Cheng, Y.H.; Hsueh, S.S.; Liu, H.S.; Lin, T.H.; Wu, J.W.; Wang, S.S. Investigating the effects of erythrosine B on amyloid fibril formation derived from lysozyme. Int. J. Biol. Macromol. 2017, 98, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.; Wang, H.; Nan, F.; Liu, Y.; Zhang, M. Naofucong Ameliorates High Glucose Induced Hippocampal Neuron Injury Through Suppressing P2X7/NLRP1/Caspase-1 Pathway. Front. Pharmacol. 2021, 12, 647116. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Tong, Z. Formaldehyde-Crosslinked Nontoxic Aβ Monomers to Form Toxic Aβ Dimers and Aggregates: Pathogenicity and Therapeutic Perspectives. ChemMedChem 2021, 16, 3376–3390. [Google Scholar] [CrossRef]
- Merinas-Amo, R.; Martínez-Jurado, M.; Jurado-Güeto, S.; Alonso-Moraga, Á.; Merinas-Amo, T. Biological Effects of Food Coloring in In Vivo and In Vitro Model Systems. Foods 2019, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Hu, J.; Liu, X.; Deng, X. Increased expression of the P2X7 receptor in temporal lobe epilepsy: Animal models and clinical evidence. Mol. Med. Rep. 2019, 19, 5433–5439. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharm. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef]
- Ye, J.; Shang, H.; Du, H.; Cao, Y.; Hua, L.; Zhu, F.; Liu, W.; Wang, Y.; Chen, S.; Qiu, Z.; et al. An Optimal Animal Model of Ischemic Stroke Established by Digital Subtraction Angiography-Guided Autologous Thrombi in Cynomolgus Monkeys. Front. Neurol. 2022, 13, 864954. [Google Scholar] [CrossRef]
- Tanioka, M.; Park, W.K.; Park, J.; Lee, J.E.; Lee, B.H. Lipid Emulsion Improves Functional Recovery in an Animal Model of Stroke. Int. J. Mol. Sci. 2020, 21, 7373. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, M.-J.; Wang, Z.-Y.; Han, Q.-Q.; Wu, H.-Y.; Mao, X.-F.; Wang, Y.-X. Involvement of d-amino acid oxidase in cerebral ischaemia induced by transient occlusion of the middle cerebral artery in mice. Br. J. Pharm. 2019, 176, 3336–3349. [Google Scholar] [CrossRef]
- Feustel, P.J.; Ingvar, M.C.; Severinghaus, J.W. Cerebral oxygen availability and blood flow during middle cerebral artery occlusion: Effects of pentobarbital. Stroke 1981, 12, 858–863. [Google Scholar] [CrossRef]
- Belinga, V.F.; Wu, G.-J.; Yan, F.-L.; Limbenga, E.A. Splenectomy following MCAO inhibits the TLR4-NF-κB signaling pathway and protects the brain from neurodegeneration in rats. J. Neuroimmunol. 2016, 293, 105–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, P.; Deng, Y.; Ma, Z.; Guo, H.; Guo, H.; Hou, Y.; Wang, S.; Zou, W.; Sun, Y.; et al. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J. Neuroinflamm. 2018, 15, 206. [Google Scholar] [CrossRef]
- Tang, H.; Gamdzyk, M.; Huang, L.; Gao, L.; Lenahan, C.; Kang, R.; Tang, J.; Xia, Y.; Zhang, J.H. Delayed recanalization after MCAO ameliorates ischemic stroke by inhibiting apoptosis via HGF/c-Met/STAT3/Bcl-2 pathway in rats. Exp. Neurol. 2020, 330, 113359. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, J.; Xiao, X.; Zhang, D.; Feng, Y.; Wu, Z.; Mao, Y.; Mou, C.; Xie, Y.; Chen, X.; Liu, H.; et al. Neuroprotective Effects of an Edible Pigment Brilliant Blue FCF against Behavioral Abnormity in MCAO Rats. Pharmaceuticals 2022, 15, 1018. https://doi.org/10.3390/ph15081018
Le J, Xiao X, Zhang D, Feng Y, Wu Z, Mao Y, Mou C, Xie Y, Chen X, Liu H, et al. Neuroprotective Effects of an Edible Pigment Brilliant Blue FCF against Behavioral Abnormity in MCAO Rats. Pharmaceuticals. 2022; 15(8):1018. https://doi.org/10.3390/ph15081018
Chicago/Turabian StyleLe, Jingyang, Xiao Xiao, Difan Zhang, Yi Feng, Zhuoying Wu, Yuechun Mao, Chenye Mou, Yanfei Xie, Xiaowei Chen, Hao Liu, and et al. 2022. "Neuroprotective Effects of an Edible Pigment Brilliant Blue FCF against Behavioral Abnormity in MCAO Rats" Pharmaceuticals 15, no. 8: 1018. https://doi.org/10.3390/ph15081018
APA StyleLe, J., Xiao, X., Zhang, D., Feng, Y., Wu, Z., Mao, Y., Mou, C., Xie, Y., Chen, X., Liu, H., & Cui, W. (2022). Neuroprotective Effects of an Edible Pigment Brilliant Blue FCF against Behavioral Abnormity in MCAO Rats. Pharmaceuticals, 15(8), 1018. https://doi.org/10.3390/ph15081018






