From Automated Synthesis to In Vivo Application in Multiple Types of Cancer—Clinical Results with [68Ga]Ga-DATA5m.SA.FAPi
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Radiochemistry
2.2. Clinical Safety
2.3. In-Human Tracer Uptake and Biodistribution
3. Discussion
4. Materials and Methods
4.1. Radiochemistry
4.2. Clinical
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sollini, M.; Kirienko, M.; Gelardi, F.; Fiz, F.; Gozzi, N.; Chiti, A. State-of-the-art of FAPI-PET imaging: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4396–4414. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Younis, M.H.; Zhang, Y.; Cai, W.; Lan, X. Clinical summary of fibroblast activation protein inhibitor-based radiopharmaceuticals: Cancer and beyond. Eur. J. Nucl Med. Mol. Imaging 2022, 49, 2844–2868. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, L.; Airò Farulla, L.S.; Demirci, E.; Clerici, I.; Omodeo Salè, E.; Ceci, F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines 2022, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Argalia, G.; Zanoni, L.; Fanti, S.; Ambrosini, V. New PET Radiotracers for the Imaging of Neuroendocrine Neoplasms. Curr. Treat. Options Oncol. 2022, 23, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Te Beek, E.T.; Burggraaf, J.; Teunissen, J.J.M.; Vriens, D. Clinical pharmacology of radiotheranostics in oncology. Clin. Pharmacol. Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Giesel, F.L.; Kratochwil, C.; Serfling, S.E. Radioligands Targeting Fibroblast Activation Protein (FAP). Cancers 2021, 13, 5744. [Google Scholar] [CrossRef]
- Kamali Zonouzi, S.; Pezeshki, P.S.; Razi, S.; Rezaei, N. Cancer-associated fibroblasts in colorectal cancer. Clin. Transl. Oncol. 2022, 24, 757–769. [Google Scholar] [CrossRef]
- Aertgeerts, K.; Levin, I.; Shi, L.; Snell, G.P.; Jennings, A.; Prasad, G.S.; Zhang, Y.; Kraus, M.L.; Salakian, S.; Sridhar, V.; et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J. Biol. Chem. 2005, 280, 19441–19444. [Google Scholar] [CrossRef]
- Yadav, D.; Ballal, S.; Yadav, M.P.; Tripathi, M.; Roesch, F.; Bal, C. Evaluation of [68Ga]Ga-DATA-TOC for imaging of neuroendocrine tumours: Comparison with [68Ga]Ga-DOTA-NOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 860–869. [Google Scholar] [CrossRef]
- Puré, E. The road to integrative cancer therapies: Emergence of a tumor-associated fibroblast protease as a potential therapeutic target in cancer. Expert Opin. Ther. Targets 2009, 13, 967–973. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Zi, F.; He, J.; He, D.; Li, Y.; Yang, L.; Cai, Z. Fibroblast activation protein α in tumor microenvironment: Recent progression and implications (review). Mol. Med. Rep. 2015, 11, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.M.T.; Yao, T.-W.; Chowdhury, S.; Nadvi, N.A.; Osborne, B.; Church, W.B.; McCaughan, G.W.; Gorrell, M.D. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010, 277, 1126–1144. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom.-Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Meng, T.; Shang, Q.; Pang, Y.; Chen, H. Uncommon Metastases From Occult Breast Cancer Revealed by 18F-FDG and 68Ga-FAPI PET/CT. Clin. Nucl. Med. 2022, 47, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhao, L.; Shang, Q.; Meng, T.; Zhao, L.; Feng, L.; Wang, S.; Guo, P.; Wu, X.; Lin, Q.; et al. Positron emission tomography and computed tomography with [68Ga]Ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1322–1337. [Google Scholar] [CrossRef]
- Shang, Q.; Zhao, L.; Pang, Y.; Meng, T.; Chen, H. Differentiation of Reactive Lymph Nodes and Tumor Metastatic Lymph Nodes With 68Ga-FAPI PET/CT in a Patient With Squamous Cell Lung Cancer. Clin. Nucl. Med. 2022, 47, 458–461. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef]
- Can, C.; Gündoğan, C.; Güzel, Y.; Kaplan, I.; Kömek, H. 68Ga-FAPI Uptake of Thyroiditis in a Patient With Breast Cancer. Clin. Nucl. Med. 2021, 46, 683–685. [Google Scholar] [CrossRef]
- Deng, M.; Chen, Y.; Cai, L. Comparison of 68Ga-FAPI and 18F-FDG PET/CT in the Imaging of Pancreatic Cancer with Liver Metastases. Clin. Nucl. Med. 2021, 46, 589–591. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W.; Qin, C.; Liu, F.; Lan, X. Non-malignant findings of focal (68)Ga-FAPI-04 uptake in pancreas. Eur. J. Nucl. Med. Mol. Imaging. 2021, 48, 2635–2641. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.L.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of (68)Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2021, 62, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, Q.; Yang, H.; Peng, L.; Zhang, W.; Li, F. Fibroblast Activation Protein-Targeted PET/CT with (68)Ga-FAPI for Imaging IgG4-Related Disease: Comparison to (18)F-FDG PET/CT. J. Nucl. Med. 2021, 62, 266–271. [Google Scholar] [CrossRef]
- Roth, K.S.; Voltin, C.A.; van-Heek, L.; Wegen, S.; Schomaecker, K.; Fischer, T.; Marnitz, S.; Drzezga, A.E.; Kobe, C. Dual-tracer PET/CT protocol with [18F]-FDG and [68Ga]Ga-FAPI-46 for cancer imaging—A proof of concept. J. Nucl. Med. 2022. [Google Scholar] [CrossRef]
- Backhaus, P.; Burg, M.C.; Roll, W.; Büther, F.; Breyholz, H.-J.; Weigel, S.; Heindel, W.; Pixberg, M.; Barth, P.; Tio, J.; et al. Simultaneous FAPI PET/MRI Targeting the Fibroblast-Activation Protein for Breast Cancer. Radiology 2022, 302, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, M.; Leitz, D.; Glatting, F.M.; Wefers, A.K.; Weinheimer, O.; Flechsig, P.; Kahn, N.; Mall, M.A.; Giesel, F.L.; Kratochwil, C.; et al. Fibroblast Activation Protein-Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022, 63, 127–133. [Google Scholar] [CrossRef]
- Yang, T.; Ma, L.; Hou, H.; Gao, F.; Tao, W. FAPI PET/CT in the Diagnosis of Abdominal and Pelvic Tumors. Front. Oncol. 2021, 11, 797960. [Google Scholar] [CrossRef]
- Dendl, K.; Schlittenhardt, J.; Staudinger, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U.; Giesel, F.L. The Role of Fibroblast Activation Protein Ligands in Oncologic PET Imaging. PET Clin. 2021, 16, 341–351. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, S.; Zhang, J.; Yao, S.; Miao, W. Pleural Metastasis of Papillary Thyroid Cancer Depicted by 68Ga-FAPI PET/CT. Clin. Nucl. Med. 2022, 47, 467–468. [Google Scholar] [CrossRef]
- Fu, L.; Huang, S.; Wu, H.; Dong, Y.; Xie, F.; Wu, R.; Zhou, K.; Tang, G.; Zhou, W. Superiority of [68Ga]Ga-FAPI-04/[18F]FAPI-42 PET/CT to [18F]FDG PET/CT in delineating the primary tumor and peritoneal metastasis in initial gastric cancer. Eur. Radiol. 2022, 1–10. [Google Scholar]
- Imlimthan, S.; Moon, E.S.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, S.E.; Lai, J.H.; Li, Y.; Jin, Z.; Liu, Y.; Wu, W.; Wu, Y.; Zhou, Y.; Sudmeier, J.L.; Sanford, D.G.; et al. Identification of selective and potent inhibitors of fibroblast activation protein and prolyl oligopeptidase. J. Med. Chem. 2013, 56, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.S.; Weerapana, E.; Cravatt, B.F. Strategies for discovering and derisking covalent, irreversible enzyme inhibitors. Future Med. Chem. 2010, 2, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Fabre, M.; Ferrer, C.; Domínguez-Hormaetxe, S.; Bockorny, B.; Murias, L.; Seifert, O.; Eisler, S.A.; Kontermann, R.E.; Pfizenmaier, K.; Lee, S.Y.; et al. OMTX705, a Novel FAP-Targeting ADC Demonstrates Activity in Chemotherapy and Pembrolizumab-Resistant Solid Tumor Models. Clin. Cancer Res. 2020, 26, 3420–3430. [Google Scholar] [CrossRef]
- Van Rymenant, Y.; Tanc, M.; Elzen, R.; Bracke, A.; de wever, O.; Augustyns, K.; Lambeir, A.M.; Kockx, M.; De Meester, I.; Van Der Veken, P. In Vitro and In Situ Activity-Based Labeling of Fibroblast Activation Protein with UAMC1110-Derived Probes. Front. Chem. 2021, 9, 640566. [Google Scholar] [CrossRef]
- De Decker, A.; Vliegen, G.; Van Rompaey, D.; Peeraer, A.; Bracke, A.; Verckist, L.; Jansen, K.; Geiss-Friedlander, R.; Augustyns, K.; De Winter, H.; et al. Novel Small Molecule-Derived, Highly Selective Substrates for Fibroblast Activation Protein (FAP). ACS Med. Chem. Lett. 2019, 10, 1173–1179. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Yamazaki, T.; Mccarty, K.; Phillips, M.; Alice, A.; Bambina, S.; Zebertavage, L.; Friedman, D.; Cottam, B.; Newell, P.; et al. Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma. PLoS ONE. 2019, 14, e0211117. [Google Scholar] [CrossRef]
- Ryabtsova, O.; Jansen, K.; Van Goethem, S.; Joossens, J.; Cheng, J.D.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; Van der Veken, P. Acylated Gly-(2-cyano)pyrrolidines as inhibitors of fibroblast activation protein (FAP) and the issue of FAP/prolyl oligopeptidase (PREP)-selectivity. Bioorganic Med. Chem. Lett. 2012, 22, 3412–3417. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Jansen, K.; De Winter, H.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; Van der Veken, P. Selective inhibitors of fibroblast activation protein (FAP) with a xanthine scaffold. MedChemComm 2014, 5, 1700–1707. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Da Pieve, C.; Costa Braga, M.; Turton, D.R.; Valla, F.A.; Cakmak, P.; Plate, K.H.; Kramer-Marek, G. New Fully Automated Preparation of High Apparent Molar Activity (68)Ga-FAPI-46 on a Trasis AiO Platform. Molecules 2022, 27, 675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, B.; Fang, J.; Pang, Y.; Li, S.; Xie, C.; Sun, L.; Zhang, X.; Guo, Z.; Lin, Q.; et al. Synthesis, preclinical evaluation, and a pilot clinical PET imaging study of (68)Ga-labeled FAPI dimer. J. Nucl. Med. 2021, 63, 862–868. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, K.; Fu, Z.; Zheng, J.; Mu, Z.; Zhao, C.; Liu, X.; Wang, S.; Yu, J.; Yuan, S. [18F]AlF-NOTA-FAPI-04 PET/CT uptake in metastatic lesions on PET/CT imaging might distinguish different pathological types of lung cancer. Eur. J. Nucl. Med. Mol. Imaging. 2022, 49, 1671–1681. [Google Scholar] [CrossRef]
- Hu, K.; Li, L.; Huang, Y.; Ye, S.; Zhong, J.; Yan, Q.; Zhong, Y.; Fu, L.; Feng, P.; Li, H. Radiosynthesis and Preclinical Evaluation of Bispecific PSMA/FAP Heterodimers for Tumor Imaging. Pharmaceuticals 2022, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, S.; Li, L.; Zhong, J.; Yan, Q.; Zhong, Y.; Feng, P.; Hu, K. 18F- or 177Lu-labeled bivalent ligand of fibroblast activation protein with high tumor uptake and retention. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2705–2715. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, J.; Ma, L.; Liu, X.; Xu, S.; Wang, S.; Jinli Pei Kai Cheng Shuanghu, Y.u.a.n.; Jinming, Y.u. [18F]AlF-NOTA-FAPI-04: FAP-targeting specificity, biodistribution, and PET/CT imaging of various cancers. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2761–2773. [Google Scholar] [CrossRef]
- Naka, S.; Watabe, T.; Lindner, T.; Cardinale, J.; Kurimoto, K.; Moore, M.; Tatsumi, M.; Mori, Y.; Shimosegawa, E.; Valla, F., Jr.; et al. One-pot and one-step automated radio-synthesis of [18F]AlF-FAPI-74 using a multi purpose synthesizer: A proof-of-concept experiment. EJNMMI Radiopharm. Chem. 2021, 6, 28. [Google Scholar] [CrossRef]
- Lindner, T.; Altmann, A.; Giesel, F.; Kratochwil, C.; Kleist, C.; Krämer, S.; Mier, W.; Cardinale, J.; Kauczor, H.-U.; Jäger, D.; et al. (18)F-labeled tracers targeting fibroblast activation protein. EJNMMI Radiopharm. Chem. 2021, 6, 26. [Google Scholar] [CrossRef]
- Hu, K.; Li, J.; Wang, L.; Huang, Y.; Li, L.; Ye, S.; Han, Y.; Huang, S.; Wu, H.; Su, J.; et al. Preclinical evaluation and pilot clinical study of [18F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta Pharm. Sin. B. 2022, 12, 867–875. [Google Scholar] [CrossRef]
- Kou, Y.; Yao, Z.; Cheng, Z. Hepatic Lesion of Mucosa-Associated Lymphoid Tissue Lymphoma Revealed by Al18F-NOTA-FAPI-04 PET/CT. Clin. Nucl. Med. 2022, 47, e49–e51. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Yao, Z.; Cheng, Z. Al18F-NOTA-FAPI-04 Outperforms 18F-FDG PET/CT in Identifying the Primary Lesion and Rare Metastases From Gastric Cancer. Clin. Nucl. Med. 2021, 46, e570–e571. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Xu, X.; Ding, J.; Liu, S.; Hou, X.; Li, N.; Zhu, H.; Yang, Z. Clinical translational evaluation of Al(18)F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4259–4271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X.; Shen, T.; Yao, Y.; Chen, M.; Li, Z.; Li, X.; Shen, J.; Kou, Y.; Chen, S.; et al. FAPI-04 PET/CT Using [18F]AlF Labeling Strategy: Automatic Synthesis, Quality Control, and In Vivo Assessment in Patient. Front. Oncol. 2021, 11, 649148. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marme, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Wang, L.; Wu, H.; Huang, S.; Tian, Y.; Wang, Q.; Xiao, C.; Han, Y.; Tang, G. [18F]FAPI-42 PET imaging in cancer patients: Optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur. J Nucl. Med. Mol. Imaging 2022, 49, 2833–2843. [Google Scholar] [CrossRef]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: (64)Cu- and (225)Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Rathke, H.; Fink, R.; Dendl, K.; Debus, J.; Mier, W.; Jäger, D.; Lindner, T.; Haberkorn, U. [153Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur. J. Nucl. Med. Mol. Imaging. 2021, 48, 3011–3013. [Google Scholar] [CrossRef] [PubMed]
- Rathke, H.; Fuxius, S.; Giesel, F.L.; Lindner, T.; Debus, J.; Haberkorn, U.; Kratochwil, C. Two Tumors, One Target: Preliminary Experience With 90Y-FAPI Therapy in a Patient With Metastasized Breast and Colorectal Cancer. Clin. Nucl. Med. 2021, 46, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Kaghazchi, F.; Aghdam, R.A.; Haghighi, S.; Vali, R.; Adinehpour, Z. 177Lu-FAPI Therapy in a Patient With End-Stage Metastatic Pancreatic Adenocarcinoma. Clin. Nucl. Med. 2022, 47, e243–e245. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, P.; Ding, J.; Chen, J.; Huo, L.; Liu, Z. Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J. Nucl. Med. 2021, 63, 952–958. [Google Scholar] [CrossRef]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 871–880. [Google Scholar] [CrossRef]
- Kuyumcu, S.; Kovan, B.; Sanli, Y.; Buyukkaya, F.; Simsek, D.H.; Özkan, Z.G.; Isik, E.G.; Ekenel, M.; Turkmen, C. Safety of Fibroblast Activation Protein-Targeted Radionuclide Therapy by a Low-Dose Dosimetric Approach Using 177Lu-FAPI04. Clin. Nucl. Med. 2021, 46, 641–646. [Google Scholar] [CrossRef]
- Assadi, M.; Rekabpour, S.J.; Jafari, E.; Divband, G.; Nikkholgh, B.; Amini, H.; Kamali, H.; Ebrahimi, S.; Shakibazad, N.; Jokar, N.; et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Almaguel, F.; Zboralski, D.; et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using (177)Lu-FAP-2286: First-in-Humans Results. J. Nucl. Med. 2022, 63, 415–423. [Google Scholar] [CrossRef]
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A.; Munteanu, C.R.; Cakebread, A.; Imberti, C.; Eykyn, T.R.; Young, J.D.; Paterson, B.M.; Blower, P.J.; et al. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599. [Google Scholar] [CrossRef]
- Fersing, C.; Masurier, N.; Rubira, L.; Deshayes, E.; Lisowski, V. AAZTA-Derived Chelators for the Design of Innovative Radiopharmaceuticals with Theranostic Applications. Pharmaceuticals 2022, 15, 234. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. New Insights in the Design of Bioactive Peptides and Chelating Agents for Imaging and Therapy in Oncology. Molecules 2017, 22, 1282. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Calabi, L.; Cavallotti, C.; Gianolio, E.; Giovenzana, G.B.; Losi, P.; Maiocchi, A.; Palmisano, G.; Sisti, M. [Gd-AAZTA]-: A new structural entry for an improved generation of MRI contrast agents. Inorg. Chem. 2004, 43, 7588–7590. [Google Scholar] [CrossRef] [PubMed]
- Waldron, B.P.; Parker, D.; Burchardt, C.; Yufit, D.S.; Zimny, M.; Roesch, F. Structure and stability of hexadentate complexes of ligands based on AAZTA for efficient PET labelling with gallium-68. Chem. Commun. 2013, 49, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA(5m) chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Chasák, J.; Šlachtová, V.; Urban, M.; Brulíková, L. Squaric acid analogues in medicinal chemistry. Eur. J. Med. Chem. 2021, 209, 112872. [Google Scholar] [CrossRef]
- Wurm, F.R.; Klok, H.A. Be squared: Expanding the horizon of squaric acid-mediated conjugations. Chem. Soc. Rev. 2013, 42, 8220–8236. [Google Scholar] [CrossRef]
- Greifenstein, L.; Engelbogen, N.; Lahnif, H.; Sinnes, J.P.; Bergmann, R.; Bachmann, M.; Rösch, F. Synthesis, Labeling and Preclinical Evaluation of a Squaric Acid Containing PSMA Inhibitor Labeled with (68) Ga: A Comparison with PSMA-11 and PSMA-617. ChemMedChem 2020, 15, 695–704. [Google Scholar] [CrossRef]
- Greifenstein, L.; Grus, T.; Nagel, J.; Sinnes, J.P.; Rösch, F. Synthesis and labeling of a squaric acid containing PSMA-inhibitor coupled to AAZTA5 for versatile labeling with 44Sc, 64Cu, 68Ga and 177Lu. Appl. Radiat. Isot. 2020, 156, 108867. [Google Scholar] [CrossRef]
- Gauger, J.; Manecke, G. Kondensationsprodukte der Quadratsäure mit primären und sekundären Aminen, II. Chem. Berichte 1970, 103, 3553–3562. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging. 2021, 48, 942–944. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Bal, C. First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)(2). Pharmaceuticals 2021, 14, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ballal, M.S.; Yadav, M.P.; Moon, E.S.; Roesch, F.; Kumari, M.S.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Mangu, B.S.; Tupalli, A.; et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid 2022, 32, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; Stephan, S.; Bracke, A.; Van der Veken, P.; De Meester, I.; Roesch, F. Fibroblast Activation Protein (FAP) targeting homodimeric FAP inhibitor radiotheranostics: A step to improve tumor uptake and retention time. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 476–491. [Google Scholar] [PubMed]
- Kreppel, B.; Gonzalez-Carmona, M.A.; Feldmann, G.; Küppers, J.; Moon, E.S.; Marinova, M.; Bundschuh, R.A.; Kristiansen, G.; Essler, M.; Roesch, F.; et al. Fibroblast activation protein inhibitor (FAPi) positive tumour fraction on PET/CT correlates with Ki-67 in liver metastases of neuroendocrine tumours. Nuklearmedizin 2021, 60, 344–354. [Google Scholar] [CrossRef]
- Kreppel, B.; Gärtner, F.C.; Marinova, M.; Attenberger, U.; Meisenheimer, M.; Toma, M.; Kristiansen, G.; Feldmann, G.; Moon, E.S.; Roesch, F.; et al. [68Ga]Ga-DATA5m.SA.FAPi PET/CT: Specific Tracer-uptake in Focal Nodular Hyperplasia and potential Role in Liver Tumor Imaging. Nuklearmedizin 2020, 59, 387–389. [Google Scholar] [CrossRef]
- Glatting, F.M.; Hoppner, J.; Liew, D.P.; van Genabith, A.; Spektor, A.M.; Steinbach, L.; Hubert, A.; Kratochwil, C.; Giesel, F.L.; Dendl, K.; et al. Repetitive early FAPI-PET acquisition comparing FAPI-02, FAPI-46 and FAPI-74: Methodological and diagnostic implications for malignant, inflammatory and degenerative lesions. J. Nucl. Med. 2022; online ahead of print. [Google Scholar]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]

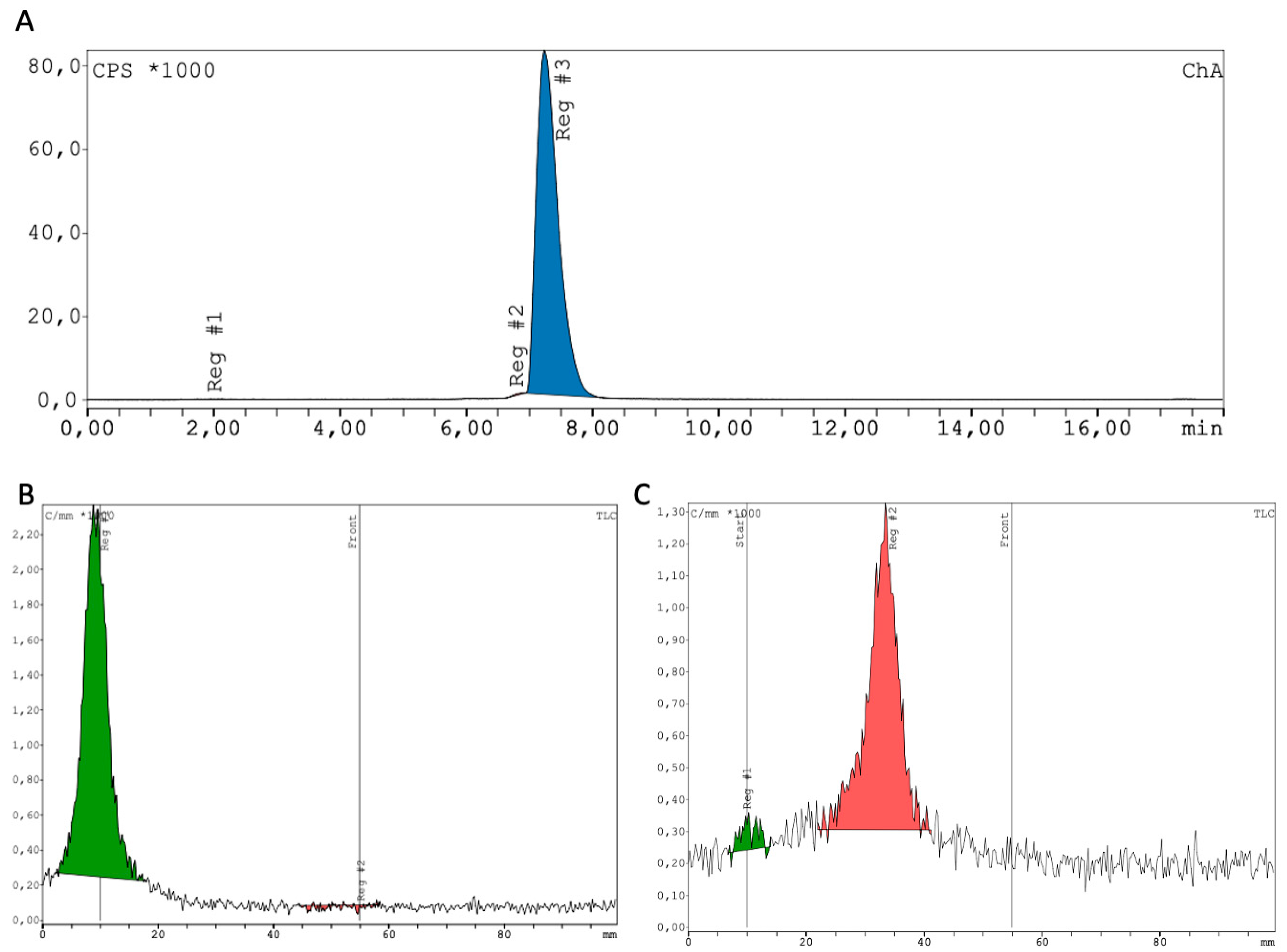
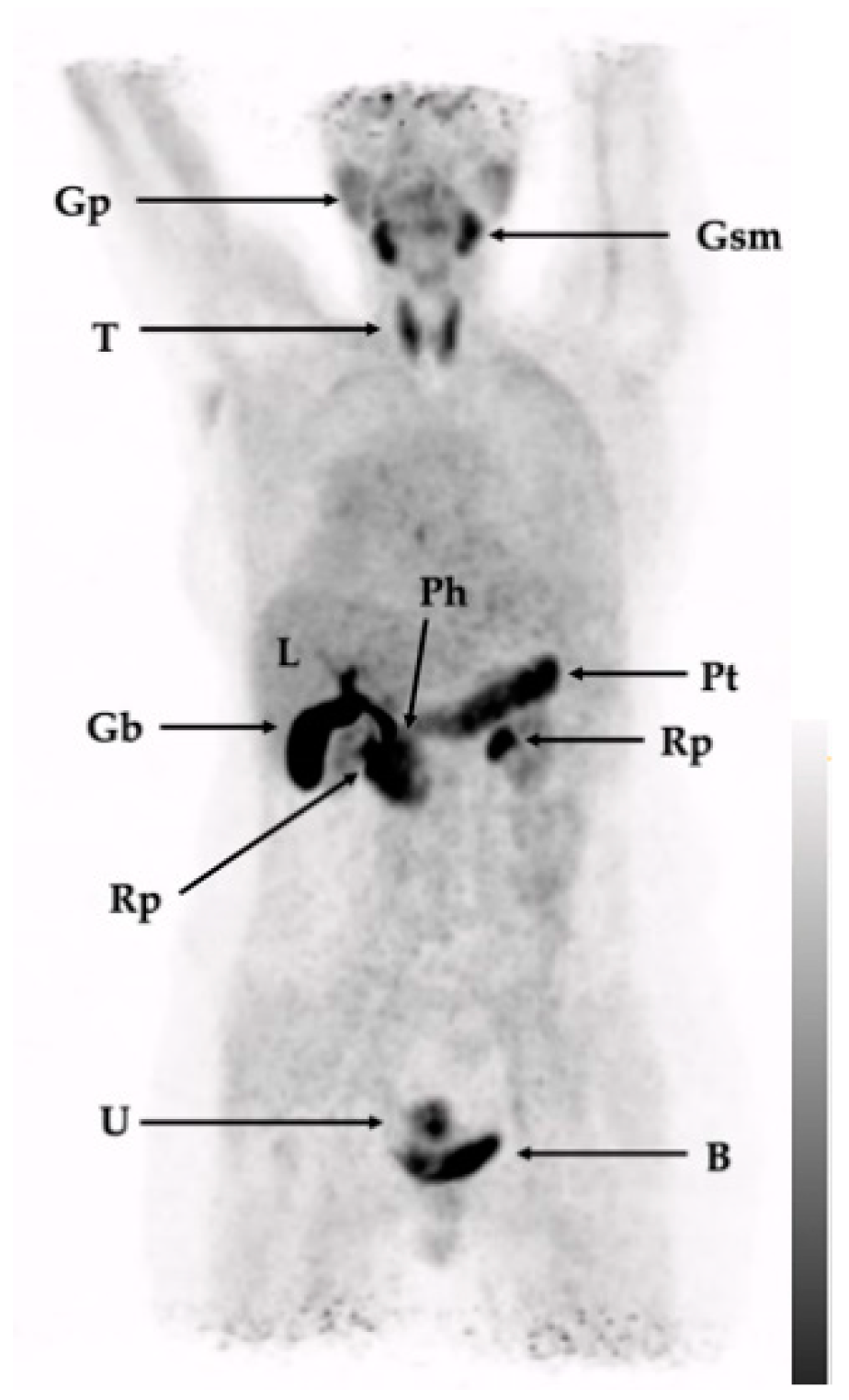
| Tissue/Organ | SUVmax [Average ± SD] | SUVmean [Average ± SD] |
|---|---|---|
| Spleen | 2.4 ± 0.5 | 1.6 ± 0.5 |
| Liver | 2.5 ± 0.4 | 1.9 ± 0.3 |
| Red marrow (vertebrae) | 2.3 ± 0.4 | 1.5 ± 0.5 |
| Kidney | 3.0 ± 1.1 | 2.4 ± 0.9 |
| Brain (frontal cortex) | 0.4 ± 0.2 | 0.1 ± 0.1 |
| Pituitary gland | 1.7 ± 0.6 | 1.0 ± 0.3 |
| Submandibular gland | 6.3 ± 1.2 | 4.3 ± 2.1 |
| Thyroid | 6.1 ± 1.8 | 3.9 ± 1.2 |
| Pancreas | 7.8 ± 2.5 | 4.5 ± 1.5 |
| Lung | 0.8 ± 0.3 | 0.6 ± 0.2 |
| Muscle (quadriceps) | 0.9 ± 0.2 | 0.6 ± 0.2 |
| Blood pool (aorta) | 1.8 ± 0.9 | 1.5 ± 0.7 |
| Patient | Disease | Pretreatments(Relevant) | Tracer Accumulation |
|---|---|---|---|
| 1 (Figure 3) | Sarcoidosis (left cervical lymph node manifestation), in the past inflammatory pulmonary activity, the activity of the sarcoidosis during image acquisition was not clear. History of DCIS in the left breast | Lymph node extirpation, modified radical mastectomy on left side, prophylactic radical modified mastectomy on right side | Only physiological tracer distribution with no further suspect tracer accumulations |
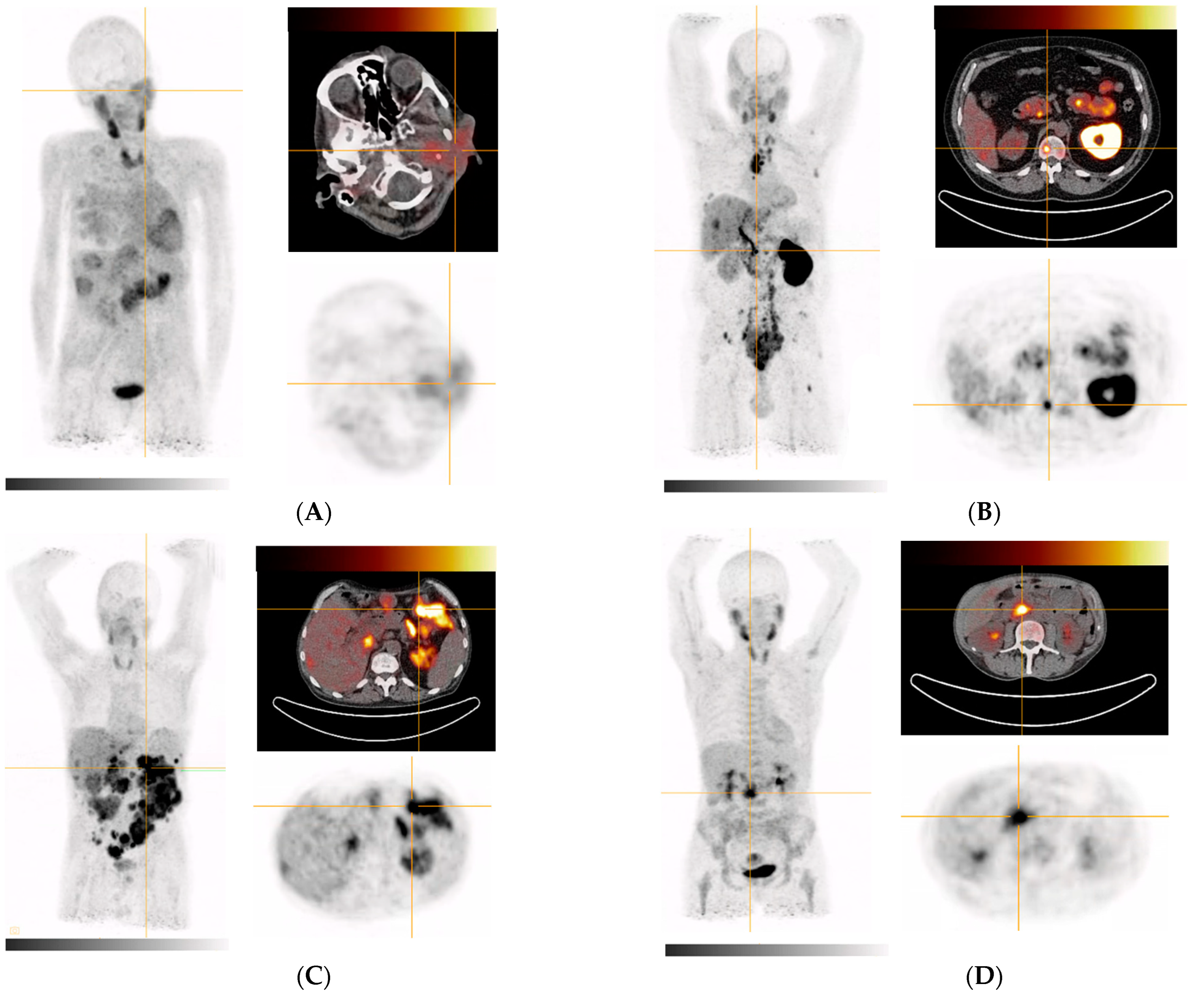
| 2 (Figure 4A) | Metastasized parotid gland tumor (adenoid-cystic subtype) | Subtotal parotidectomy (left), partial liver resection of segments II/III | Parotid gland, multiple masses in the right lung, accumulation in several liver segments |
| 3 (Figure 4B) | Metastasized prostate cancer (Gleason 4 + 3 = 7) | IMRT to prostate and seminal vesicles | Extensive bone and bone marrow involvement (including the extremities), large lymph node metastases in the retroperitoneum, mediastinum (bulky), and cervical region as well as in soft tissue. Additional findings: Severely impaired function of left kidney (slow wash out of activity with high parenchymal contrast and increased activity in the ureter and pelvis) |
| 4 (Figure 4C) | Metastasized liposarcoma | Tumor enucleation on the duodenum, pancreatic head resection, omentectomy, pancreaticogastrostomy | Extensive peritoneal tumor foci in all abdominal quadrants, caudal liver margin segment VI (or adjacent peritoneal foci) |
| 5 (Figure 4D) | Primary pancreatic head adenocarcinoma, moderately differentiated, ductal | ERCP with stenting | Inhomogeneous in the pancreas with emphasis in pancreatic head, peritoneal (extensively in the left mid to lower abdomen), anterior margin of liver (peritoneal or lymph nodes), mammaria interna lymph node, segment VI of the liver (most likely biliary excreted tracer), uterus (nonspecific/physiological), muscle attachments at the hip joints (nonspecific/inflammatory/bursitis) |
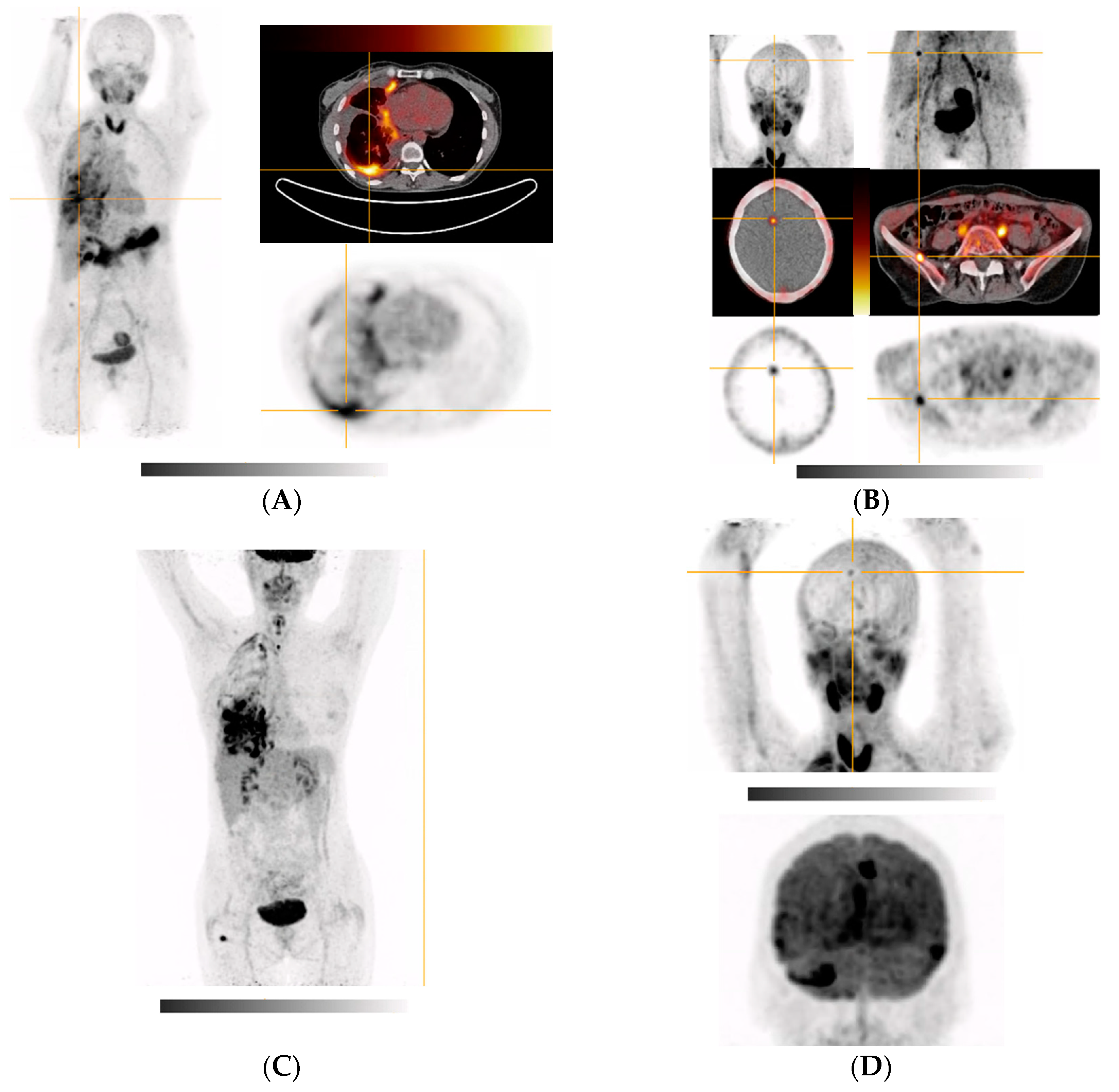
| 6 (Figure 5A,B) | Poorly differentiated NSCLC of right lower lobe (pleural, cerebral, hepatic, and osseous metastases) | VATS with partial pleurectomy and talc pleurodesis | Right hemithorax and right lung, diffuse cerebral (mainly focal in the right frontal cortex in the region of the great longitudinal fissure and periventricular), right and right ilium, left sacrum, right thigh (after surgical removal of a hibernoma), uterus (possible myoma) FDG-PET (Figure 5C,D): whole right hemithorax (especially basolateral, in the myelon, intracranially in the area of the meninges, (around the temporal poles and on the tentorium), in the right frontal cortex in the area of the great longitudinal fissure, right thigh (after surgical removement of a hibernoma) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greifenstein, L.; Kramer, C.S.; Moon, E.S.; Rösch, F.; Klega, A.; Landvogt, C.; Müller, C.; Baum, R.P. From Automated Synthesis to In Vivo Application in Multiple Types of Cancer—Clinical Results with [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals 2022, 15, 1000. https://doi.org/10.3390/ph15081000
Greifenstein L, Kramer CS, Moon ES, Rösch F, Klega A, Landvogt C, Müller C, Baum RP. From Automated Synthesis to In Vivo Application in Multiple Types of Cancer—Clinical Results with [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals. 2022; 15(8):1000. https://doi.org/10.3390/ph15081000
Chicago/Turabian StyleGreifenstein, Lukas, Carsten S. Kramer, Euy Sung Moon, Frank Rösch, Andre Klega, Christian Landvogt, Corinna Müller, and Richard P. Baum. 2022. "From Automated Synthesis to In Vivo Application in Multiple Types of Cancer—Clinical Results with [68Ga]Ga-DATA5m.SA.FAPi" Pharmaceuticals 15, no. 8: 1000. https://doi.org/10.3390/ph15081000
APA StyleGreifenstein, L., Kramer, C. S., Moon, E. S., Rösch, F., Klega, A., Landvogt, C., Müller, C., & Baum, R. P. (2022). From Automated Synthesis to In Vivo Application in Multiple Types of Cancer—Clinical Results with [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals, 15(8), 1000. https://doi.org/10.3390/ph15081000






