Fabrication, In Vitro and In Vivo Evaluation of Non-Ordered Mesoporous Silica-Based Ternary Solid Dispersions for Enhanced Solubility of Flurbiprofen
Abstract
1. Introduction
2. Results and Discussion
2.1. Pre-Formulation Studies
2.2. Physicochemical Properties
2.2.1. Percentage Yield
2.2.2. Flow Properties of Powders
2.2.3. Estimation of Drug Content
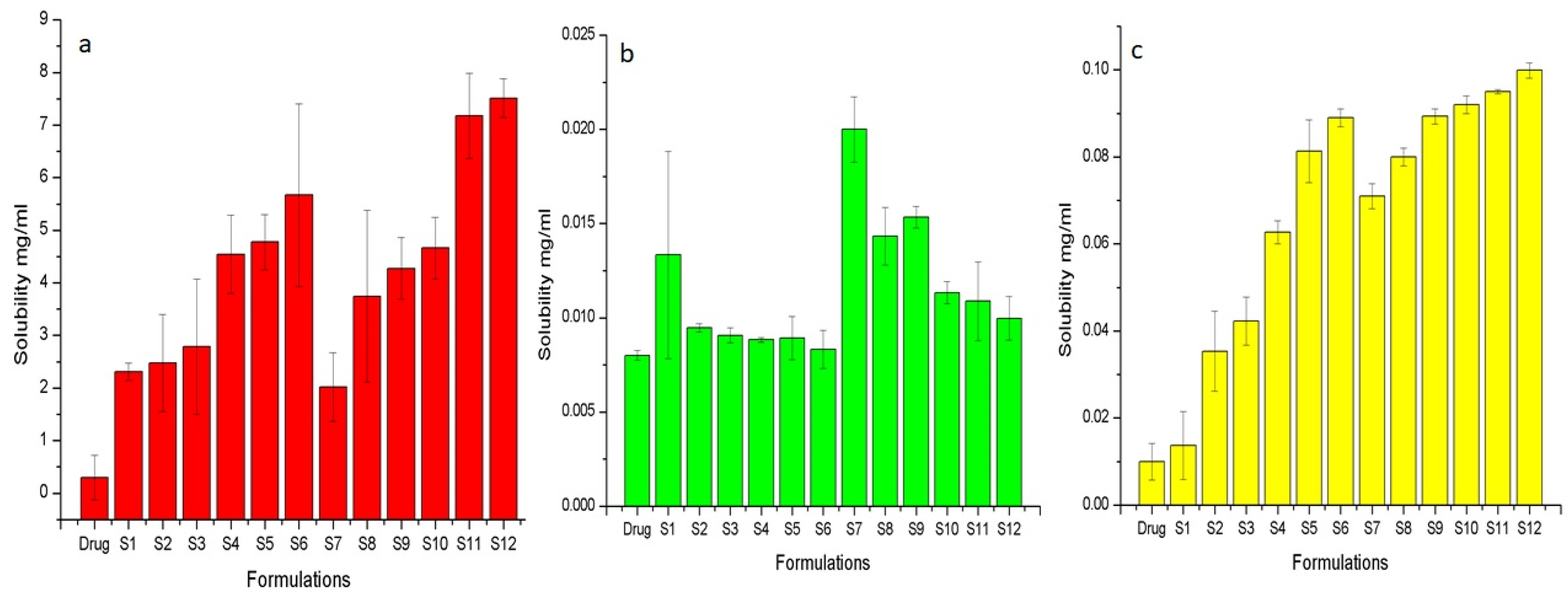
2.2.4. Solubility Studies
2.3. Solid State Characterisation
2.3.1. FTIR
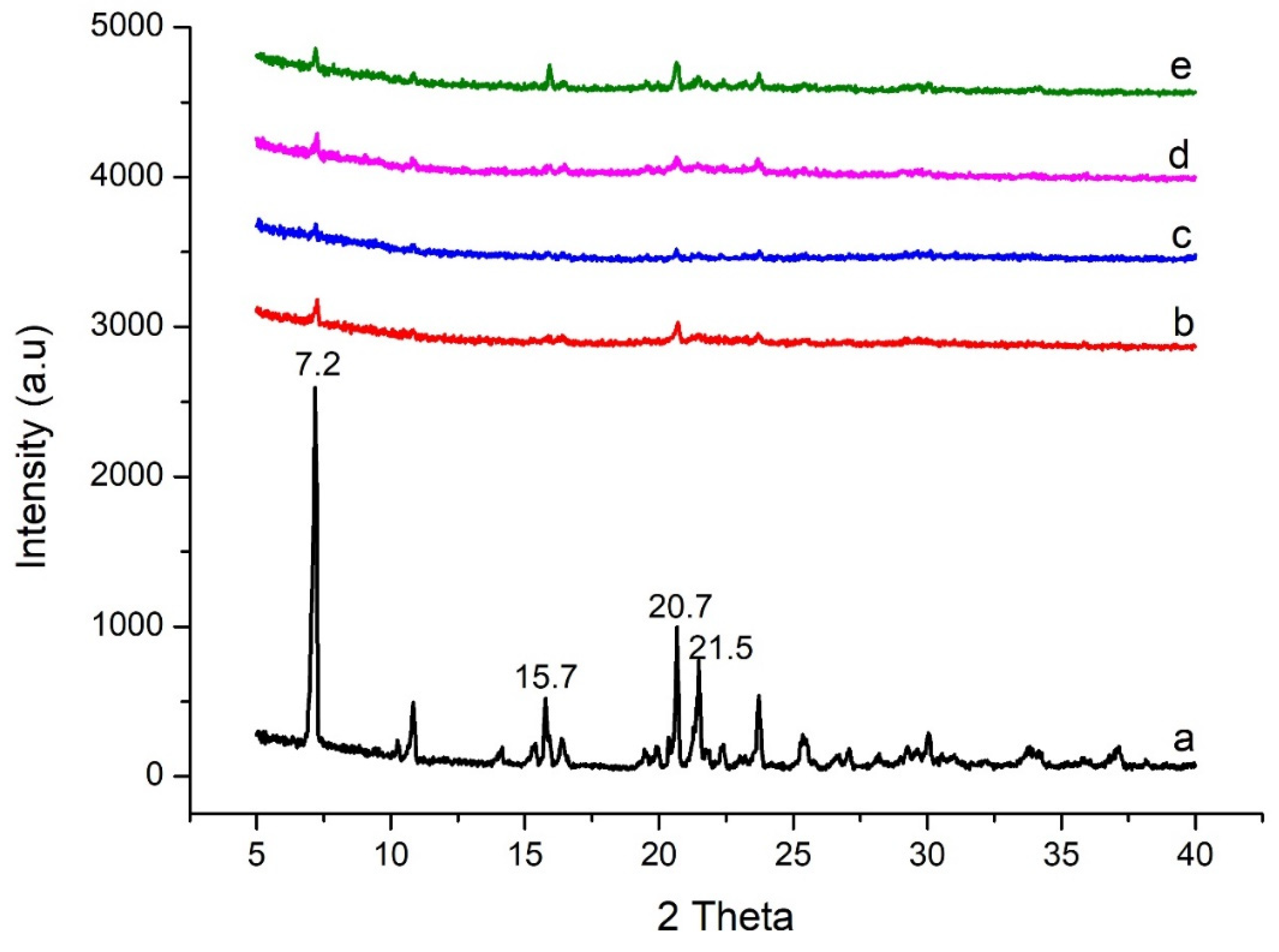
2.3.2. XRD
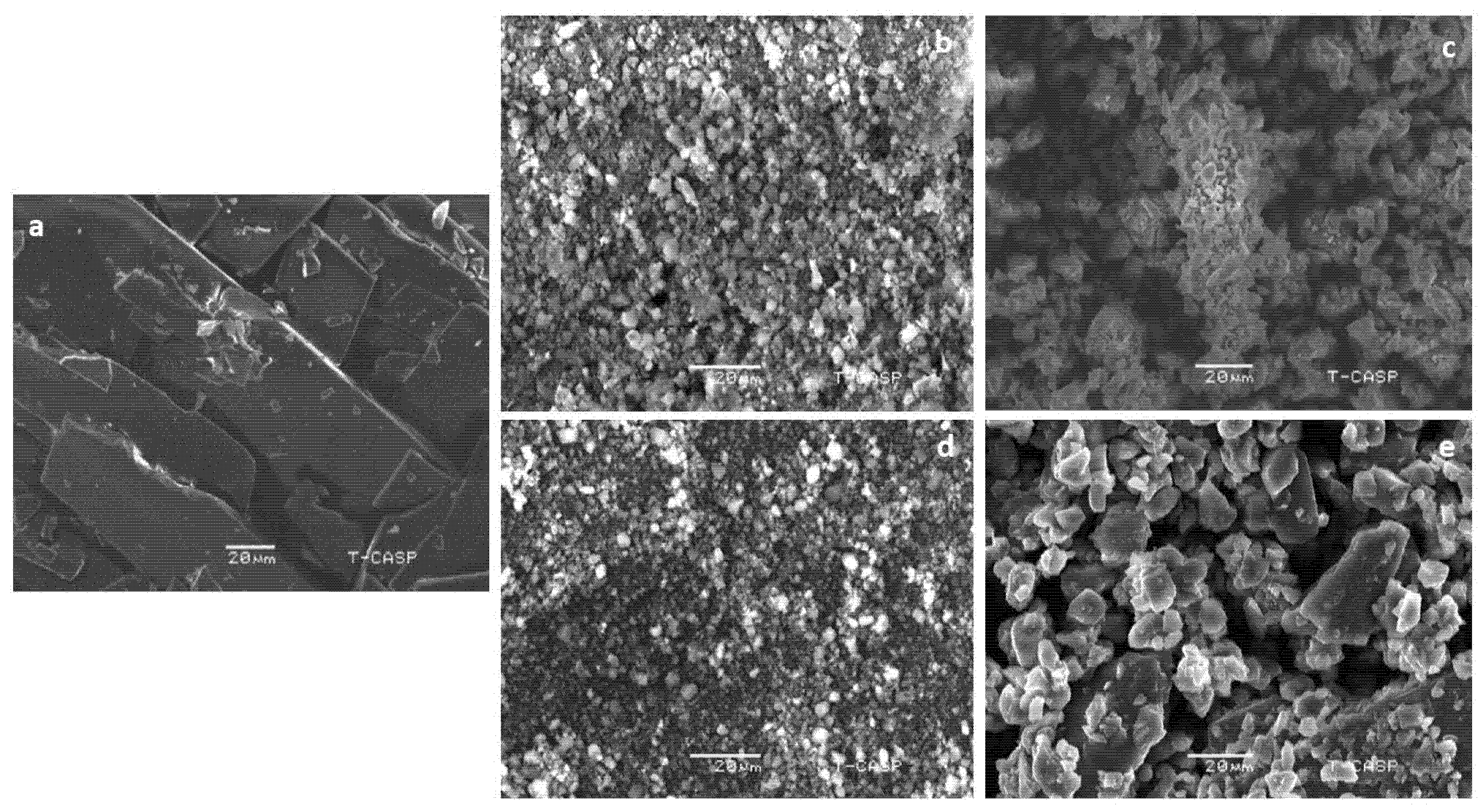
2.3.3. SEM
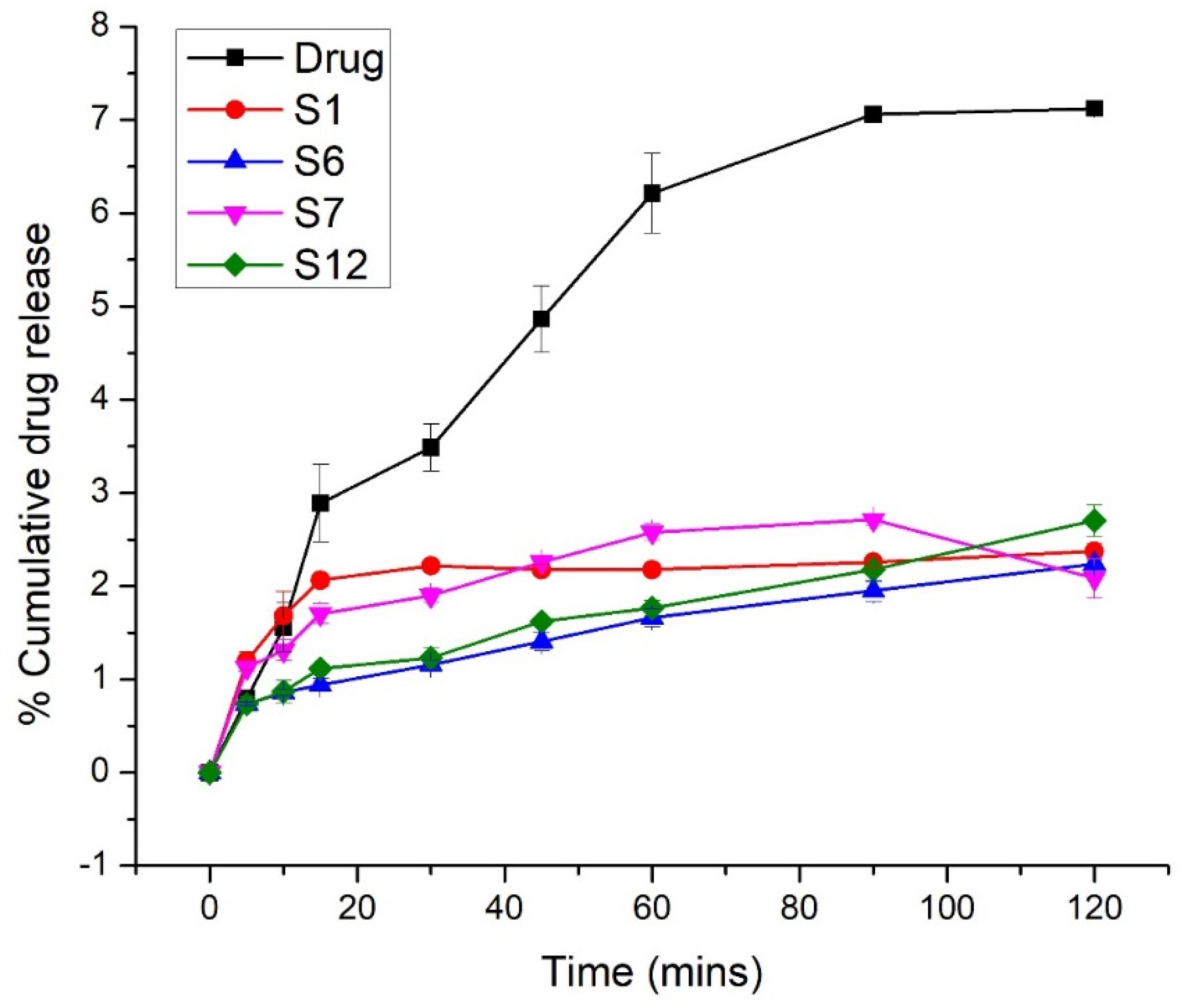
2.4. In Vitro Release
2.5. Estimation of Gastro-Protective Effect In Vivo
2.5.1. Macroscopic Scoring
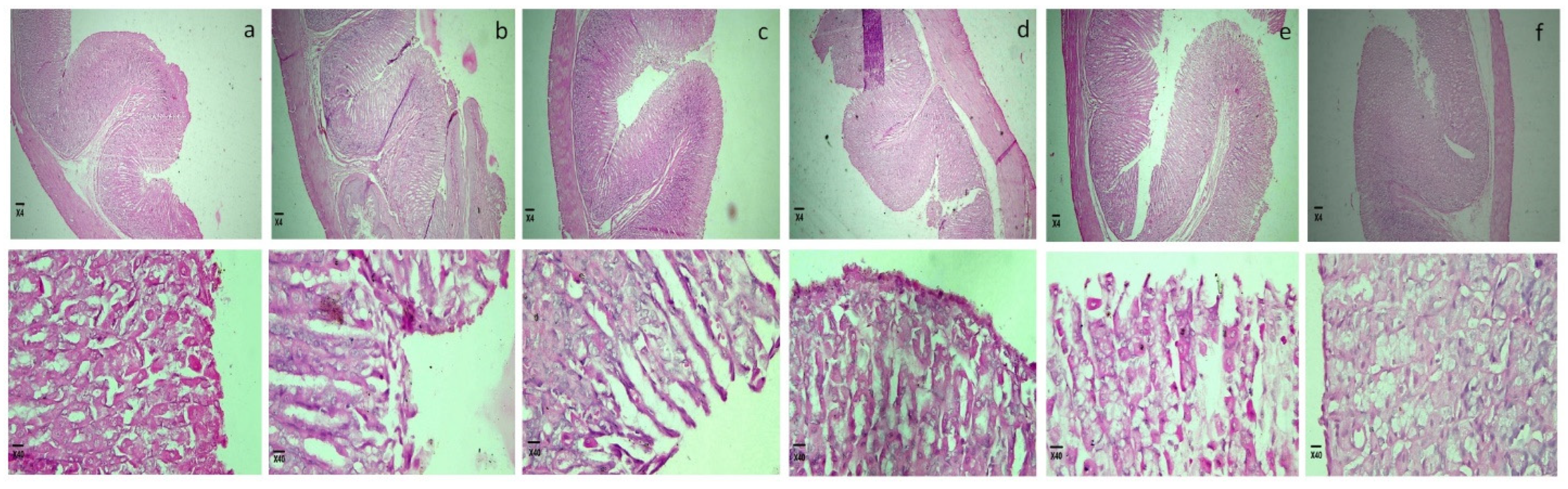
2.5.2. Microscopic Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Solid Dispersion
3.3. Pre-Formulation Studies
3.4. Physicochemical Properties
3.4.1. Practical Yield
3.4.2. Properties of the Powder
Density
Flowability
Angle of Repose
3.4.3. Estimation of Drug Content
3.4.4. Solubility Studies
3.5. Solid State Characterization
3.5.1. Fourier-Transform Infrared Spectrometry (FTIR)
3.5.2. X-ray Diffraction (XRD)
3.5.3. Shape and Surface Morphology
3.6. In Vitro Release
3.7. Estimation of Gastro-Protective Effect
3.7.1. Macroscopic Scoring
3.7.2. Microscopic Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery – a review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 195727. [Google Scholar] [CrossRef]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar] [CrossRef]
- Kim, K.-T.; Lee, J.-Y.; Lee, M.-Y.; Song, C.-K.; Choi, J.-H.; Kim, D.-D. Solid Dispersions as a Drug Delivery System. J. Pharm. Investig. 2011, 41, 125–142. [Google Scholar] [CrossRef]
- Alwossabi, A.M.; Elamin, E.S.; Ahmed, E.M.; Abdelrahman, M. Solubility enhancement of some poorly soluble drugs by solid dispersion using Ziziphus spina-christi gum polymer. Saudi Pharm. J. 2022, 30, 711–725. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Ouyang, D. Investigating the molecular dissolution process of binary solid dispersions by molecular dynamics simulations. Asian J. Pharm. Sci. 2018, 13, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Chambin, O.; Jannin, V. Interest of multifunctional lipid excipients: Case of Gelucire® 44/14. Drug Dev. Ind. Pharm. 2005, 31, 527–534. [Google Scholar] [CrossRef]
- Limnell, T.; Santos, H.A.; Mäkilä, E.; Heikkilä, T.; Salonen, J.; Murzin, D.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J.T. Drug Delivery Formulations of Ordered and Nonordered Mesoporous Silica: Comparison of Three Drug Loading Methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Gupte, A. Mesoporous Silica as a Carrier for Amorphous Solid Dispersion. Br. J. Pharm. Res. 2017, 16, BJPR33553. [Google Scholar] [CrossRef]
- Davies, N.M. Clinical Pharmacokinetics of Flurbiprofen and its Enantiomers. Clin. Pharmacokinet. 1995, 28, 100–114. [Google Scholar] [CrossRef]
- Oh, D.H.; Park, Y.-J.; Kang, J.H.; Yong, C.S.; Choi, H.-G. Physicochemical characterization and in vivo evaluation of flurbiprofen-loaded solid dispersion without crystalline change. Drug Deliv. 2010, 18, 46–53. [Google Scholar] [CrossRef]
- Pradhan, R.; Tran, T.H.; Kim, S.Y.; Woo, K.B.; Choi, Y.J.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Preparation and characterization of fast dissolving flurbiprofen and esomeprazole solid dispersion using spray drying technique. Int. J. Pharm. 2016, 502, 38–46. [Google Scholar] [CrossRef]
- Soliman, M.S.; Khan, M.A. Preparation and in vitro characterization of a semi-solid dispersion of flurbiprofen with Gelucire 44/14 and Labrasol. Pharmazie 2005, 60, 288–293. [Google Scholar]
- Varma, M.M.; Pandi, J.K. Dissolution, Solubility, XRD, and DSC Studies on Flurbiprofen-Nicotinamide Solid Dispersions. Drug Dev. Ind. Pharm. 2005, 31, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Poovi, G.; Umamaheswari, M.; Sharmila, S.; Kumar, S.; Rajalakshmi, A. Development of domperidone solid dispersion powders using sodium alginate as carrier. Eur. J. Appl. Sci. 2013, 5, 36–42. [Google Scholar]
- Liu, L.; Li, J.; Zhao, M.-H.; Xu, H.; Li, L.-S.; Wang, S.-N. Loading of tacrolimus containing lipid based drug delivery systems into mesoporous silica for extended release. Asian J. Pharm. Sci. 2016, 11, 751–759. [Google Scholar] [CrossRef][Green Version]
- Daravath, B.; Tadikonda, R.R.; Vemula, S.K. Formulation and pharmacokinetics of gelucire solid dispersions of flurbiprofen. Drug Dev. Ind. Pharm. 2015, 41, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.B.; Tawakkul, M.A.; Khan, M. Comparative Evaluation of Flow for Pharmaceutical Powders and Granules. AAPS PharmSciTech 2008, 9, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; An, J.; Park, C.; Kim, D.; Lee, J. Design and characterization of phosphatidylcholine-based solid dispersions of aprepitant for enhanced solubility and dissolution. Pharmaceutics 2020, 12, 407. [Google Scholar] [CrossRef]
- LaBauve, A.E.; Rinker, T.E.; Noureddine, A.; Serda, R.E.; Howe, J.Y.; Sherman, M.B.; Rasley, A.; Brinker, C.J.; Sasaki, D.Y.; Negrete, O.A. Lipid-Coated Mesoporous Silica Nanoparticles for the Delivery of the ML336 Antiviral to Inhibit Encephalitic Alphavirus Infection. Sci. Rep. 2018, 8, 13990. [Google Scholar] [CrossRef]
- Hussain, T.; Waters, L.; Parkes, G.M.; Shahzad, Y. Microwave processed solid dispersions for enhanced dissolution of gemfibrozil using non-ordered mesoporous silica. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 428–435. [Google Scholar] [CrossRef]
- Bavnhøj, C.G.; Knopp, M.M.; Madsen, C.M.; Löbmann, K. The role interplay between mesoporous silica pore volume and surface area and their effect on drug loading capacity. Int. J. Pharm. X 2019, 1, 100008. [Google Scholar] [CrossRef]
- Choudhari, Y.; Hoefer, H.; Libanati, C.; Monsuur, F.; McCarthy, W. Mesoporous silica drug delivery systems. In Amorphous Solid Dispersions; Springer: New York, NY, USA, 2014; pp. 665–693. [Google Scholar]
- Fernandez, S.; Rodier, J.-D.; Ritter, N.; Mahler, B.; Demarne, F.; Carrière, F.; Jannin, V. Lipolysis of the semi-solid self-emulsifying excipient Gelucire® 44/14 by digestive lipases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 367–375. [Google Scholar] [CrossRef]
- Yadav, B.; Tanwar, Y. Development, characterization and in vitro evaluation of flurbiprofen solid dispersions using polyethylene glycols as carrier. J. App. Pharm. Sci. 2016, 6, 60–66. [Google Scholar] [CrossRef][Green Version]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Yousaf, A.M.; Irfan, M.; Khalid, S.H.; Asghar, S.; Rasul, A.; Khan, N.R. Mesoporous Silica Nanoparticles-Based Bilayer Tablets: A New Strategy for Co-delivery of Velpatasvir and Sofosbuvir. Lat. Am. J. Pharm. 2022, 41, 283–293. [Google Scholar]
- Tahir, H.; Shahzad, Y.; Waters, L.; Hussain, T.; Yousaf, A.M.; Mahmood, T.; Sheikh, R. Impact of processing methods on the dissolution of artemether from two non-ordered mesoporous silicas. Eur. J. Pharm. Sci. 2018, 112, 139–145. [Google Scholar] [CrossRef]
- Karatas, A.; Bekmezci, S. Evaluation and enhancement of physical stability of semi-solid dispersions containing piroxicam into hard gelatin capsules. Acta Pol. Pharm. 2013, 70, 883–897. [Google Scholar]
- Fule, R.; Amin, P. Development and evaluation of lafutidine solid dispersion via hot melt extrusion: Investigating drug-polymer miscibility with advanced characterisation. Asian J. Pharm. Sci. 2014, 9, 92–106. [Google Scholar] [CrossRef]
- Baek, H.H.; Kwon, S.Y.; Rho, S.-J.; Lee, W.S.; Yang, H.-J.; Hah, J.-M.; Choi, H.-G.; Kim, Y.-R.; Yong, C.S. Enhanced solubility and bioavailability of flurbiprofen by cycloamylose. Arch. Pharmacal Res. 2011, 34, 391–397. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Dalwadi, S.J.; Thakkar, V.T.; Soni, T.G.; Gohel, M.C.; Gandhi, T.R. Improvement of dissolution rate of aceclofenac by solid dispersion technique. Powder Technol. 2011, 207, 47–54. [Google Scholar] [CrossRef]
- Chauhan, B.; Shimpi, S.; Paradkar, A. Preparation and evaluation of glibenclamide-polyglycolized glycerides solid dispersions with silicon dioxide by spray drying technique. Eur. J. Pharm. Sci. 2005, 26, 219–230. [Google Scholar] [CrossRef]
- PPatel, V.I.; Dave, R.H. Evaluation of Colloidal Solid Dispersions: Physiochemical Considerations and In Vitro Release Profile. AAPS PharmSciTech 2013, 14, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, K.C.; Patra, C.N.; Jena, G.K.; Ghose, D.; Jena, J.; Panda, S.K.; Sahu, M. Gelucire: A versatile polymer for modified release drug delivery system. Futur. J. Pharm. Sci. 2018, 4, 102–108. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; D’Argenio, G.; Tuccillo, C.; Loguercio, C.; Ritieni, A.; Morisco, F.; Blanco, C.D.V.; Fogliano, V.; Romano, M. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut 2005, 54, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, S.; Magierowska, K.; Śliwowski, Z.; Wójcik, D.; Magierowski, M.; Brzozowski, T. New insight into the mechanisms of gastroduodenal injury induced by nonsteroidal anti-inflammatory drugs: Practical implications. Pol. Arch. Intern. Med. 2015, 125, 191–198. [Google Scholar] [CrossRef]
- Chakraborty, S.; Stalin, S.; Das, N.; Choudhury, S.T.; Ghosh, S.; Swarnakar, S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials 2012, 33, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.-S.A.; Maghrabi, I.A. Diosmin Protects against Ethanol-Induced Gastric Injury in Rats: Novel Anti-Ulcer Actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef]
- Saxena, A.; Mishra, A.K.; Verma, N.; Bhattacharya, S.S.; Ghosh, A.; Verma, A.; Pandit, J.K. Gelucire BasedIn SituGelling Emulsions: A Potential Carrier for Sustained Stomach Specific Delivery of Gastric Irritant Drugs. BioMed Res. Int. 2013, 436932. [Google Scholar] [CrossRef][Green Version]
- Gupta, M.K.; Goldman, D.; Bogner, R.H.; Tseng, Y.-C. Enhanced Drug Dissolution and Bulk Properties of Solid Dispersions Granulated with a Surface Adsorbent. Pharm. Dev. Technol. 2001, 6, 563–572. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G. Dissolution Modulating Mechanism of Flurbiprofen Solid Dispersions: Characterization, Physical Stability and in vivo Performance: Formulation Considerations and optimization study. Pharm. Methods 2017, 8, 127–135. [Google Scholar] [CrossRef]
- Damian, F.; Blaton, N.; Naesens, L.; Balzarini, J.; Kinget, R.; Augustijns, P.; Van den Mooter, G. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur. J. Pharm. Sci. 2000, 10, 311–322. [Google Scholar] [CrossRef]
- Teng, X.W.; Wang, S.W.; Davies, N.M. Stereospecific high-performance liquid chromatographic analysis of flurbiprofen: Application to pharmacokinetic studies. J. Pharm. Biomed. Anal. 2003, 33, 95–100. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Khan, H.; Naseem, S. Encapsulation and characterization of controlled release flurbiprofen loaded microspheres using beeswax as an encapsulating agent. J. Mater. Sci. Mater. Med. 2010, 21, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, Z.; Kanwal, M.; Hassan, M.; Abdullah, S.; Waheed, M.; Ahsan, H.; Kim, S.J. Flurbiprofen–antioxidant mutual prodrugs as safer nonsteroidal anti-inflammatory drugs: Synthesis, pharmacological investigation, and computational molecular modeling. Drug Des. Dev. Ther. 2016, 10, 2401. [Google Scholar]
- Sanka, K.; Munjulury, V.S.; Mohd, A.B.; Diwan, P. Enhancement of solubility, dissolution release profile and reduction in ulcerogenicity of piroxicam by inclusion complex with skimmed milk. Drug Deliv. 2014, 21, 560–570. [Google Scholar] [CrossRef] [PubMed]




| Sr. No. | Code | Grade of Silica | Drug: Silica (w/w) | Gelucire 44/14 (w/w) |
|---|---|---|---|---|
| 1 | S1 | Syloid 244 FP | 1:1 | --- |
| 2 | S2 | Syloid 244 FP | 1:1 | 5% |
| 3 | S3 | Syloid 244 FP | 1:1 | 10% |
| 4 | S4 | Syloid 244 FP | 1:1 | 15% |
| 5 | S5 | Syloid 244 FP | 1:1 | 20% |
| 6 | S6 | Syloid 244 FP | 1:1 | 25% |
| 7 | S7 | Syloid AL1 FP | 1:1 | --- |
| 8 | S8 | Syloid AL1 FP | 1:1 | 5% |
| 9 | S9 | Syloid AL1 FP | 1:1 | 10% |
| 10 | S10 | Syloid AL1 FP | 1:1 | 15% |
| 11 | S11 | Syloid AL1 FP | 1:1 | 20% |
| 12 | S12 | Syloid AL1 FP | 1:1 | 25% |
| Sr. No. | Code | Composition | Solubility (mg/mL) in pH 6.8 (PBS) | Times Increase in Solubility |
|---|---|---|---|---|
| 1 | Flurbiprofen | ---- | 0.03 | ---- |
| 2 | SA | Flurbiprofen:Syloid 244FP (1:1) | 2.74 | 91 |
| 3 | SB | Flurbiprofen:Syloid 244FP (1:3) | 3.10 | 103 |
| 4 | SC | Flurbiprofen:Syloid 244FP (1:5) | 3.34 | 111 |
| 5 | SD | Flurbiprofen:Syloid 244FP (1:9) | 2.94 | 98 |
| 5 | SE | Flurbiprofen:Syloid Al1FP (1:1) | 2.75 | 92 |
| 6 | SF | Flurbiprofen:Syloid Al1FP (1:3) | 3.16 | 105 |
| 7 | SG | Flurbiprofen:Syloid Al1FP (1:5) | 3.23 | 108 |
| 8 | SH | Flurbiprofen:Syloid Al1FP (1:9) | 3.01 | 100 |
| Sr. No. | Code | % Yield | % Drug | Bulk Density gm/mL | Tapped Denstiy gm/mL | Carr’s Index | Hausner’s Ratio | Angle of Repose (°) | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | S1 | 99 | 80.72 | 0.178 | 0.278 | 35.71 | 1.56 | 61 | Very poor |
| 2 | S2 | 98.4 | 93.24 | 0.178 | 0.263 | 32.14 | 1.47 | 56 | Very poor |
| 3 | S3 | 98 | 97.10 | 0.192 | 0.263 | 26.92 | 1.36 | 52 | Poor |
| 4 | S4 | 98.8 | 98.74 | 0.208 | 0.263 | 20.84 | 1.26 | 45 | Passable |
| 5 | S5 | 98.2 | 99.30 | 0.208 | 0.25 | 16.67 | 1.2 | 39 | Fair |
| 6 | S6 | 97.6 | 99.79 | 0.217 | 0.25 | 13.04 | 1.15 | 35 | Good |
| 7 | S7 | 99.5 | 87.31 | 0.5 | 0.769 | 35 | 1.53 | 59 | Very poor |
| 8 | S8 | 98.8 | 89.74 | 0.454 | 0.714 | 36.36 | 1.57 | 57 | Very poor |
| 9 | S9 | 97.5 | 91.00 | 0.5 | 0.714 | 30 | 1.42 | 51 | Poor |
| 10 | S10 | 98 | 93.68 | 0.5 | 0.667 | 25 | 1.34 | 43 | Passable |
| 11 | S11 | 98.7 | 99.47 | 0.34 | 0.4167 | 20 | 1.25 | 37 | Fair |
| 12 | S12 | 99 | 80.72 | 0.38 | 0.4167 | 14.28 | 1.67 | 32 | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, M.U.; Ikraam, M.; Nadeem, M.; Khalid, S.H.; Asghar, S.; Khalid, I.; Irfan, M.; Islam, N.; Ajaz, N.; Khan, I.U. Fabrication, In Vitro and In Vivo Evaluation of Non-Ordered Mesoporous Silica-Based Ternary Solid Dispersions for Enhanced Solubility of Flurbiprofen. Pharmaceuticals 2022, 15, 856. https://doi.org/10.3390/ph15070856
Munir MU, Ikraam M, Nadeem M, Khalid SH, Asghar S, Khalid I, Irfan M, Islam N, Ajaz N, Khan IU. Fabrication, In Vitro and In Vivo Evaluation of Non-Ordered Mesoporous Silica-Based Ternary Solid Dispersions for Enhanced Solubility of Flurbiprofen. Pharmaceuticals. 2022; 15(7):856. https://doi.org/10.3390/ph15070856
Chicago/Turabian StyleMunir, Muhammad Usman, Mahnoor Ikraam, Muhammad Nadeem, Syed Haroon Khalid, Sajid Asghar, Ikrima Khalid, Muhammad Irfan, Nayyer Islam, Nyla Ajaz, and Ikram Ullah Khan. 2022. "Fabrication, In Vitro and In Vivo Evaluation of Non-Ordered Mesoporous Silica-Based Ternary Solid Dispersions for Enhanced Solubility of Flurbiprofen" Pharmaceuticals 15, no. 7: 856. https://doi.org/10.3390/ph15070856
APA StyleMunir, M. U., Ikraam, M., Nadeem, M., Khalid, S. H., Asghar, S., Khalid, I., Irfan, M., Islam, N., Ajaz, N., & Khan, I. U. (2022). Fabrication, In Vitro and In Vivo Evaluation of Non-Ordered Mesoporous Silica-Based Ternary Solid Dispersions for Enhanced Solubility of Flurbiprofen. Pharmaceuticals, 15(7), 856. https://doi.org/10.3390/ph15070856