Abstract
The present article reviewed the pharmacologic therapies of traumatic brain injury (TBI), including current and potential treatments. Pharmacologic therapies are an essential part of TBI care, and several agents have well-established effects in TBI care. In the acute phase, tranexamic acid, antiepileptics, hyperosmolar agents, and anesthetics are the mainstay of pharmacotherapy, which have proven efficacies. In the post-acute phase, SSRIs, SNRIs, antipsychotics, zolpidem and amantadine, as well as other drugs, have been used to manage neuropsychological problems, while muscle relaxants and botulinum toxin have been used to manage spasticity. In addition, increasing numbers of pre-clinical and clinical studies of pharmaceutical agents, including potential neuroprotective nutrients and natural therapies, are being carried out. In the present article, we classify the treatments into established and potential agents based on the level of clinical evidence and standard of practice. It is expected that many of the potential medicines under investigation will eventually be accepted as standard practice in the care of TBI patients.
1. Introduction
Traumatic brain injury (TBI) is a sudden injury that causes damage to the brain. Sixty-nine million individuals worldwide are estimated to sustain a TBI each year [1]. Pharmacologic therapies play important roles in mild to severe TBI. There are several pharmacologic therapies recommended by guidelines, which have proven efficacies and well-documented safety profiles for use in acute and post-acute TBI patients [2]. In addition, several new preclinical and clinical studies of pharmacologic therapies for TBI have been published recently, which could contribute to the addition of new agents into standard TBI management in the future. This review discusses current and potential pharmacologic therapies for TBI. We also summarize the pharmacologic therapies for TBI in Figure 1.
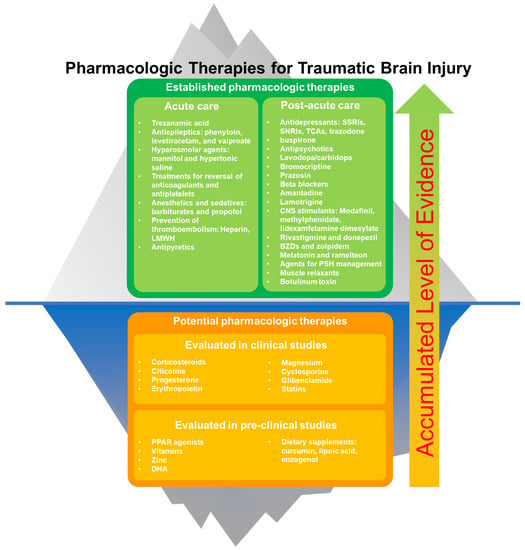
Figure 1.
Established and potential pharmacologic therapies for traumatic brain injury. Potential pharmaceutical agents may be accepted into standard practice (“go above the surface”) with the accumulation of clinical evidence.
2. Established Pharmacologic Therapies for TBI
Following traumatic brain injury, primary damage results from mechanical damage affecting cells and tissue. Hemorrhage and breakdown of the blood–brain barrier (BBB) also happen within seconds to minutes. Secondary damage develops within minutes, with the development of inflammation, ischemia and edema [3].
Subsequent processes, including delayed inflammation, vasospasm, cell death and genomic responses, develop within days. Cellular degeneration, neuropsychiatric comorbidities and muscle spasticity are noted over the next few weeks to months. Recent advances in biomarkers, including microRNA, have enhanced our understanding of the pathophysiologic process and could even help researchers to determine the time elapsed since an injury [3,4,5]. In current practice, pharmacological therapies are provided to treat both the acute and chronic effects of these pathological processes.
Published literature studying the effects of biological sex and gender shows mixed outcomes on TBI. A recent review of the literature found that women, after puberty but before menopause, were at higher risk of poor outcome, while postmenopausal women fared better than men of similar age [6]. Care pathways and treatment probably did not differ significantly between women and men [7]. More study is needed to support treatment strategies for different sexes.
2.1. Acute Treatments for TBI
2.1.1. Tranexamic Acid
Tranexamic acid reduces the risk of death in mild to moderate TBI patients when treatment is given within 3 h, in a loading dose of 1 g, followed by infusion of 1 g for 8 h, according to a recent CRASH-3 trial [8]. However, tranexamic acid does not reduce death in severe TBI patients who have extensive intracranial hemorrhage.
2.1.2. Treatments for Coagulopathies
Around one-third of severe TBI patients demonstrate coagulopathy, which may lead to hemorrhage enlargement and poor neurologic outcomes. Coagulopathy mostly results from existing medications, such as aspirin, clopidogrel, direct oral anticoagulants or warfarin. It has been demonstrated that direct oral anticoagulants do not increase the incidence of intracranial hemorrhage [9], and there are better outcomes for direct oral anticoagulant use compared to warfarin use, even with low use of the reversal strategy [10,11].
Patients taking warfarin could be managed with vitamin K and fresh frozen plasma (FFP) infusion, monitoring prothrombin time/international normalized ratio (INR) 30 min after transfusion or every 4 to 6 h to ensure INR < 1.4 [12].
In patients using anti-platelet agents or thrombocytopenia, a platelet count > 95,000/μL directly with platelet transfusion should be maintained. In one cohort study, a platelet count < 135,000/μL was associated with a 12.4 times higher risk of hemorrhage enlargement; patients with a platelet count < 95,000/μL were 31.5 times more likely to require neurosurgical intervention [13].
2.1.3. Hyperosmolar Agents
Mannitol and hypertonic saline are commonly used in the management of intracranial hypertension and cerebral edema. Mannitol at bolus doses of 0.25–1 g/kg every 4 to 6 h is effective in reducing brain volume, and thus lowering intracranial pressure (ICP) [2]. However, its diuretic effect should be monitored cautiously in hypotensive patients. Mannitol is not recommended in patients with systolic blood pressure < 90 mm Hg.
Hypertonic saline is also an effective hyperosmolar agent for lowering increased ICP [14,15]. Infusion of 3% hypertonic saline is administrated to achieve a sodium level goal of 145–155 mEq/L. There is less volume depletion and hypovolemia, which makes hypertonic saline safer in major trauma patients with ongoing volume loss and hypotension. When comparing these two hyperosmolar agents, there was no strong evidence to suggest the superiority of either in improving mortality or functional recovery [16,17].
2.1.4. Anesthetics and Sedatives
Anesthetics and sedatives are commonly used in acute stage TBI management in the intensive care unit (ICU) setting. Barbiturates and propofol have both been shown to depress cerebral metabolism, decrease oxygen consumption, lower ICP and prevent seizures. They are recommended as adjuvant therapy to control elevated ICP when refractory to maximum hyperosmolar therapy and surgical decompression. However, hemodynamic stability should be monitored during barbiturate or propofol therapy. Barbiturates result in a decrease in blood pressure in 25% of patients [18]. Body temperature is also significantly lower. Therefore, the duration and dose of barbiturate administration need to be carefully observed. It could be used under continuous monitoring of EEG to achieve optimal doses.
Within the ICU, propofol is even more widely used in acute TBI management. It is easier to control the treatment effects because of its rapid onset and short duration characteristics. However, caution is required, as high-dose propofol can result in morbidity. Propofol infusion syndrome could lead to hyperkalemia, metabolic acidosis, hyperlipidemia, myocardial failure and renal failure, which may result in death. Therefore, extreme caution must be taken when using propofol doses > 4 mg/kg/h, or when use exceeds 48 h. For refractory ICP elevation, pentobarbital and thiopental infusions may be used [18]. Nevertheless, the therapy may delay timely neurologic examination. It may also result in hypotension, ileus, ventilator-associated pneumonia and metabolic acidosis.
2.1.5. Drugs for Prevention of Thromboembolism
Heparin or low-molecular-weight heparin (LMWH) for the prevention of venous thromboembolism in TBI patients is generally safe if initiated within 24–48 h of injury [19].
2.1.6. Antiepileptics
The general incidence of post-traumatic seizure in hospitalized populations of TBI is about 3–5% [20,21]. In a study enrolling 5984 TBI patients in Minnesota from 1935 to 1984, the incidence of seizures ranged between 0.7 and 10% in five years of follow-up, correlating with the severity of TBI [22]. The use of antiepileptic drugs in the acute management of TBI has been proven to reduce the incidence of early seizures, but does not prevent the later development of epilepsy.
Furthermore, subclinical seizures detected from an electroencephalogram may be as high as 20–25% [23]. Thus, it is recommended to use prophylactic antiepileptic drugs to avoid early seizures after TBI (within 7 days of injury) [24]. Antiepileptics are recommended in the first seven days following injury in guidelines. Continued use of antiepileptics is recommended if there are electroencephalogram (EEG) discharges. Use of antiepileptics prevents post-traumatic seizures, but does not prevent later development of epilepsy [25,26].
Carbamazepine and valproate are also used as mood stabilizers for psychomotor aggregation after TBI, but the effects are controversial [27]. A newer antiepileptic drug, levetiracetam, is commonly used as there is less drug interaction and it has an equal effect as phenytoin in preventing early seizures [28]. The optimal duration of prophylactic antiepileptic drugs is uncertain and depends on the severity of brain injury. In the absence of early seizures, antiepileptic drugs are usually continued throughout the hospital stay and are discontinued within the first few weeks of discharge [29,30].
A recent meta-analysis study at World Neurosurgery, comparing the efficacy of phenytoin, levetiracetam and valproate in preventing early seizures in TBI patients, showed that phenytoin was the most studied drug. Phenytoin has level 2a evidence to decrease the incidence of early post-traumatic seizures [31]. However, more studies are needed to assess the efficacy of other antiepileptic drugs, such as levetiracetam and valproate. Currently, there is insufficient evidence to recommend levetiracetam or valproate over phenytoin.
2.1.7. Antipyretics
Fever could lead to worse outcomes after TBI, and antipyretics could be used to control fever in acute TBI. Maintenance of normothermia also improves ICP control [32] and brain tissue oxygenation [33].
2.2. Treatments for Post-TBI Neuropsychiatric Changes
Neuropsychiatric changes following TBI could cause significant distress in patients and long-term disability [34]. The choice of pharmacologic treatments could have a significant impact on post-acute TBI care, as well as the patient’s neurological recovery [35]. The present review further discusses pharmaceutical agents that have been studied for their use in post-acute TBI.
2.2.1. SSRIs and SNRIs
Several studies have been conducted on the efficacies of various selective serotonin reuptake inhibitors (SSRIs) in the treatment of depression. Sertraline [36], citalopram [36,37], and fluoxetine [36] have been shown to be beneficial in the treatment of post-TBI depression. Sertraline could even potentially prevent the later onset of depression [38,39]. Sertraline probably does not improve arousal in TBI patients [40].
SSRIs [41], including citalopram [42], sertraline [43] and paroxetine [44], could improve post-TBI pathological laughing and crying. Fluvoxamine and fluoxetine could probably improve apathy [45].
Serotonin and norepinephrine re-uptake inhibitors (SNRIs), such as milnacipran, have also been shown to be efficacious in the treatment of depression [46]. Another SNRI, atomoxetine, has failed to improve attention, speed of memory or working memory, compared to a placebo [47].
2.2.2. Trazodone
Trazodone may cause impaired attention and errors with memory tests [48], but has mixed results on sleep [49].
2.2.3. TCAs
Desipramine has been shown to improve depression in severe TBI [50]. However, tricyclic antidepressants (TCAs) are probably less effective than SSRIs in the treatment of post-TBI depression [51,52], and are associated with more complications [53].
2.2.4. Buspirone
Buspirone is a serotonin 1A receptor partial agonist that has been shown to reduce anxiety in patients with TBI [54].
2.2.5. Antipsychotics
Typical antipsychotics, including methotrimeprazine [55], droperidol, haloperidol [56] and loxapine [57], could improve agitation. Atypical antipsychotics, including quetiapine [58], clozapine [59], ziprasidone [60] and aripiprazole [61], have also been shown to improve agitation. Olanzapine has been shown to improve post-TBI psychosis [62,63]. Atypical antipsychotics are generally preferred over typical antipsychotics in post-TBI patients, due to their more favorable profile in safety and neurorecovery [64].
2.2.6. Levodopa/Carbidopa
Levodopa/carbidopa has been shown to improve consciousness [65].
2.2.7. Bromocriptine
Bromocriptine is a direct dopamine agonist at the D2 receptor. It could improve arousal [66], but probably could not improve attention [67].
2.2.8. Prazosin
Prazosin has been shown to reduce daytime sleepiness, improve headaches and improve cognition [68].
2.2.9. Beta Blockers
Beta blockers, such as propranolol and pindolol, have been shown to reduce post-TBI agitation in some studies [69]. Nevertheless, their hypotensive effect may limit the dose that could be applied.
2.2.10. Amantadine
Amantadine has been shown to improve the pace of functional recovery, as measured by the Disability Rating Scale (DRS) [70]. It has also been shown to improve early arousal in the acute phase of TBI [71,72,73].
2.2.11. Lamotrigine
Lamotrigine has been shown to reduce aggressive behavior in TBI patients [74].
2.2.12. Modafinil and Methylphenidate
Modafinil could probably improve excessive daytime sleepiness [75], but probably does not improve fatigue [76]. It might be able to improve sleep latency in patients with mild or moderate TBI [77]. Methylphenidate has been shown to improve post-TBI attention and processing speed [78,79,80,81].
2.2.13. Lisdexamfetamine Dimesylate
Lisdexamfetamine dimesylate has been shown to improve attention and working memory in a small-scale study [82].
2.2.14. Rivastigmine and Donepezil
Rivastigmine and donepezil are well known for their use in the treatment of Alzheimer’s disease. Donepezil is currently undergoing clinical studies to confirm its effect on memory, attention and processing speed [83]. Rivastigmine did not appear to improve cognition significantly [84], but it showed some improvement in memory in some subgroups of patients in a post hoc analysis of one study [85].
2.2.15. Benzodiazepines and Zolpidem
Benzodiazepines are associated with attentional and memory impairments in TBI, and are generally to be avoided [86,87]. They may impair coordination, leading to falls, increase sedation, negatively affect memory [49], and they may also lead to sleep–wake disturbances [88].
Interestingly, zolpidem has been shown to cause a temporary response in a fraction of patients with severe TBI [89]. It could probably cause attenuation of inter-hemispheric coherences on electroencephalograms [90], and improved cerebral perfusion was observed on single-photon emission computed tomography (SPECT) [91].
2.2.16. Melatonin and Ramelteon
Melatonin might be able to improve daytime sleepiness in TBI patients [92]. Ramelteon has been shown to improve total sleep time and could potentially improve cognition [93].
2.3. Other Pharmaceutical Agents for Post-Acute TBI Care
2.3.1. Muscle Relaxants
Spasticity is an important problem, particularly in moderate and severe TBI. Oral baclofen could improve the lower extremity Modified Ashworth Score [94]. Intrathecal baclofen might be able to improve muscle spasms even more than oral baclofen [95]. Oral tizanidine has been shown to reduce the Ashworth score, enhance motor strength and reduce muscle tone [96].
2.3.2. Botulinum Toxin
A botulinum toxin injection might also be beneficial in the treatment of spasticity in TBI patients [97]. Botulinum toxin might also improve chronic post-traumatic headache [98].
2.3.3. Agents for Paroxysmal Sympathetic Hyperactivity Management
The various drugs discussed above are used for the prevention and/or abortion of paroxysmal sympathetic hyperactivity (PSH), which occurs in up to 10% of patients with severe TBI. Drugs that have been studied for the treatment of PSH in TBI include beta blockers, benzodiazepines, bromocriptine [99], dantrolene [100] and gabapentin [101].
3. Potential Therapies for TBI
3.1. Neuroprotective Approaches Previously Evaluated in Clinical Studies
Several pharmaceutical agents have been evaluated in clinical studies for their potential efficacies in the treatment of TBI. So far, the routine use of most of these agents in the management of TBI has not been justified. Nevertheless, future evidence may arise to support their use in the care of TBI patients.
3.1.1. Corticosteroids
Corticosteroid was one of the first agents studied for its neuroprotective effect in TBI. The use of corticosteroids has been studied in the Medical Research Council’s Corticosteroid Randomization after Significant Head Injury study [102,103]. This large-scale study found that treatment with glucocorticoids increased mortality.
3.1.2. Citicoline
Citicoline is a cholinergic agent that increases the formation of ATP. It was evaluated in a multi-center, double-blind, randomized phase III controlled trial, The Citicoline Brain Injury Treatment Trial (COBRIT), but it did not improve outcomes [104].
3.1.3. Progesterone
Despite the potential benefits shown in two older, small-scale studies [105,106], progesterone has been evaluated by two large-scale clinical trials: SyNAPSe and ProTECT III [107,108], but did not demonstrate clinical benefit in patient mortality and functional outcomes. Some clinical studies suggested that progesterone might be neuroprotective [109,110].
3.1.4. Erythropoietin
One randomized controlled trial showed that erythropoietin treatment results in lower mortality, but that result is not statistically significant [111]. Two meta-analyses of trials also suggested that erythropoietin might lower mortality, but not reduce poor functional outcomes [112,113]. Other studies have not revealed evidence of improved outcomes from erythropoietin use [114].
3.1.5. Magnesium
The use of magnesium has been evaluated in a number of heterogeneous clinical studies [115,116]. A meta-analysis concluded that while all-cause mortality did not improve in the treatment group, the GCS might have improved [115].
3.1.6. Cyclosporine
Cyclosporine has been evaluated in a few small-scale clinical trials, and did not appear to contribute to a favorable outcome [117,118].
3.1.7. Glibenclamide
Glibenclamide is an antagonist of sulfonylurea receptor 1 (SUR1). It has been evaluated in several small-scale clinical studies and showed favorable outcomes, such as an improved Glasgow Coma Scale (GCS) score and improved Glasgow Outcome Scale (GOS) score [119,120,121,122].
3.1.8. Statins
Clinical studies of statins in TBI patients suggested that statin use might improve functional outcomes. It might also lead to a reduction in pro-inflammatory mediators [123,124,125].
3.2. Neuroprotective Approaches and Natural Therapies Previously Evaluated in Pre-Clinical Studies
3.2.1. PPAR Agonists
Peroxisome proliferator-activated receptor (PPAR) agonists, such as rosiglitazone pioglitazone, play a role in the regulation of gene transcriptions, which are essential in metabolic processes and cell differentiation. Its neuroprotective properties were suggested in several pre-clinical studies [126]. It might exert such an effect by decreasing axonal injury, decreasing apoptosis, decreasing autophagy and/or decreasing microglial activation [126,127,128].
3.2.2. Vitamins
Vitamin D could reduce inflammation biomarkers and prevent neuron death in animal models when it is used together with progesterone [129]. Vitamin E has been reported to enhance the neuroprotective effects of progesterone [130]. Water-soluble nicotinamides aggregate the functional recovery of TBI rodents [131,132]. An equivocal effect of folic acid has been documented [133,134]. However, there is an overall lack of clinical trials on vitamins for TBI patients.
3.2.3. Zinc
Zinc has been shown to have double effects on both anti-inflammation and anti-oxidative damage [135]. High zinc supplements might decrease neuropsychiatric symptoms in TBI patients, based on animal experiment results [136,137,138].
3.2.4. DHA
Docosahexaenoic (DHA) is a fatty acid that exists in phospholipids of the neuron membrane. It can be released to counteract glutamate overactivity after brain damage [139]. DHA can also reduce endoplasmic reticulum (ER) stress and prevent abnormal protein accumulation in the TBI model [140]. DHA is quite a safe and accessible food supplement and might be beneficial for neuroprotection in traumatic brain injury; however, human clinical studies are necessary.
3.2.5. Dietary Supplements
Curcumin has been reported to improve the motor and learning ability in TBI animal models [141]. Resveratrol has been shown to reduce reactive oxygen species (ROS), inhibit excitotoxicity and decrease inflammation in cortical injury models of TBI [142]. Lipoic acid could stabilize plasma membranes and prevent NADPH (nicotinamide adenine dinucleotide phosphate) oxidative stress in mild TBI rats. In a clinical trial [143], Enzogenol has been shown to take advantage of the cognitive function in TBI patients. Both nutrients and pharmacological treatment are important for the recovery of TBI. A low nutrient intake in TBI is correlated with poor outcomes [144].
4. Conclusions
Figure 2 shows the use of each type of pharmacologic agent, in various phases of alteration within central nervous system (CNS) physiology, following traumatic brain injury. Table 1 shows current pharmaceutical therapies for TBI, based on the timing of use, and main effects on the CNS.
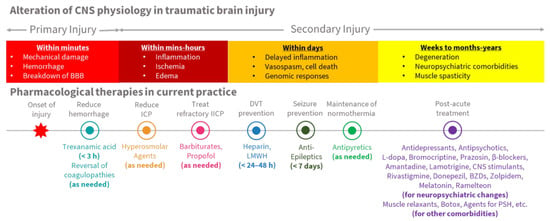
Figure 2.
Alteration of CNS physiology and pharmacological therapies in TBI.

Table 1.
Current pharmaceutical therapies for TBI.
Various pharmacological treatments could affect the pathophysiology of TBI; proper treatment can reduce the detrimental effect of brain trauma in the acute and post-acute phases, and improve the overall prognosis. In this review, we have summarized medications based on clinical evidence and usage, though more clinical studies should be carried out for potential pharmacologic therapies. We expect that the accumulation of clinical evidence on newer agents would eventually lead to new therapeutic strategies that eventually improve the quality of TBI care.
Author Contributions
Conceptualization, J.T., C.-J.H., and J.-Y.S.; data review, J.T., Y.-T.W., C.-J.H., and J.-Y.S.; writing, J.T., Y.-T.W., C.-J.H., and J.-Y.S.; supervision, C.-J.H. and J.-Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology (MOST), Taiwan (Grant no: 107-2314-B-038-078-, 108-2314-B-038-049-, 110-2314-B-038-091-) and Taipei Medical University-Wan Fang Hospital, Taipei, Taiwan (Grant no: 105TMU-WFH-02, 110-phd-02. 110TMU-WFH-13).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- dell’Aquila, M.; Maiese, A.; de Matteis, A.; Viola, R.V.; Arcangeli, M.; la Russa, R.; Fineschi, V. Traumatic brain injury: Estimate of the age of the injury based on neuroinflammation, endothelial activation markers and adhesion molecules. Histol. Histopathol. 2021, 36, 795–806. [Google Scholar] [PubMed]
- Rocchi, A.; Chiti, E.; Maiese, A.; Turillazzi, E.; Spinetti, I. MicroRNAs: An Update of Applications in Forensic Science. Diagnostics 2020, 11, 32. [Google Scholar] [CrossRef]
- Pinchi, E.; Frati, A.; Cantatore, S.; D’Errico, S.; Russa, R.; Maiese, A.; Palmieri, M.; Pesce, A.; Viola, R.V.; Frati, P.; et al. Acute Spinal Cord Injury: A Systematic Review Investigating miRNA Families Involved. Int. J. Mol. Sci. 2019, 20, 1841. [Google Scholar] [CrossRef] [Green Version]
- Biegon, A. Considering Biological Sex in Traumatic Brain Injury. Front. Neurol. 2021, 12, 576366. [Google Scholar] [CrossRef] [PubMed]
- Mikolić, A.; van Klaveren, D.; Groeniger, J.O.; Wiegers, E.J.A.; Lingsma, H.F.; Zeldovich, M.; von Steinbüchel, N.; Maas, A.I.R.; van Lennep, J.E.R.; Polinder, S. Differences between Men and Women in Treatment and Outcome after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Crash, T. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet 2019, 394, 1713–1723. [Google Scholar]
- Santing, J.A.L.; van den Brand, C.L.; Jellema, K. Traumatic Brain Injury in Patients Receiving Direct Oral Anticoagulants. J. Emerg. Med. 2021, 60, 285–291. [Google Scholar] [CrossRef]
- Frontera, J.A.; Lewin, J.J., 3rd; Rabinstein, A.A.; Aisiku, I.P.; Alexandrov, A.W.; Cook, A.M.; del Zoppo, G.J.; Kumar, M.A.; Peerschke, E.I.; Stiefel, M.F.; et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocritical Care 2016, 24, 6–46. [Google Scholar] [CrossRef]
- Shin, S.S.; Marsh, E.B.; Ali, H.; Nyquist, P.A.; Hanley, D.F.; Ziai, W.C. Comparison of Traumatic Intracranial Hemorrhage Expansion and Outcomes Among Patients on Direct Oral Anticoagulants Versus Vitamin k Antagonists. Neurocritical Care 2020, 32, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Yasaka, M.; Sakata, T.; Minematsu, K.; Naritomi, H. Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complication. Thromb. Res. 2002, 108, 25–30. [Google Scholar] [CrossRef]
- Joseph, B.; Pandit, V.; Meyer, D.; Butvidas, L.; Kulvatunyou, N.; Khalil, M.; Tang, A.; Zangbar, B.; O’Keeffe, T.; Gries, L.; et al. The significance of platelet count in traumatic brain injury patients on antiplatelet therapy. J. Trauma Acute Care Surg. 2014, 77, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Alnemari, A.M.; Krafcik, B.M.; Mansour, T.R.; Gaudin, D. A Comparison of Pharmacologic Therapeutic Agents Used for the Reduction of Intracranial Pressure After Traumatic Brain Injury. World Neurosurg. 2017, 106, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Abu-Laban, R.B.; Slavik, R.S.; Vu, E.N.; Zed, P.J. A Systematic Review of Randomized Controlled Trials Comparing Hypertonic Sodium Solutions and Mannitol for Traumatic Brain Injury: Implications for Emergency Department Management. Ann. Pharm. 2016, 50, 291–300. [Google Scholar] [CrossRef]
- Sakellaridis, N.; Pavlou, E.; Karatzas, S.; Chroni, D.; Vlachos, K.; Chatzopoulos, K.; Dimopoulou, E.; Kelesis, C.; Karaouli, V. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J. Neurosurg. 2011, 114, 545–548. [Google Scholar] [CrossRef]
- Cottenceau, V.; Masson, F.; Mahamid, E.; Petit, L.; Shik, V.; Sztark, F.; Zaaroor, M.; Soustiel, J.F. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J. Neurotrauma 2011, 28, 2003–2012. [Google Scholar] [CrossRef]
- Roberts, I.; Sydenham, E. Barbiturates for acute traumatic brain injury. Cochrane Database Syst. Rev. 2012, 12, Cd000033. [Google Scholar] [CrossRef]
- Margolick, J.; Dandurand, C.; Duncan, K.; Chen, W.; Evans, D.C.; Sekhon, M.S.; Garraway, N.; Griesdale, D.E.G.; Gooderham, P.; Hameed, S.M. A Systematic Review of the Risks and Benefits of Venous Thromboembolism Prophylaxis in Traumatic Brain Injury. Can. J. Neurol. Sci. 2018, 45, 432–444. [Google Scholar] [CrossRef]
- Chen, J.W.; Ruff, R.L.; Eavey, R.; Wasterlain, C.G. Posttraumatic epilepsy and treatment. J. Rehabil. Res. Dev. 2009, 46, 685–696. [Google Scholar] [CrossRef]
- Vaaramo, K.; Puljula, J.; Tetri, S.; Juvela, S.; Hillbom, M. Predictors of new-onset seizures: A 10-year follow-up of head trauma subjects with and without traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2014, 85, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Annegers, J.F.; Hauser, W.A.; Coan, S.P.; Rocca, W.A. A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 1998, 338, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.H.; Lerner, J.T.; Matsumoto, J.H.; Madikians, A.; Yudovin, S.; Valino, H.; McArthur, D.L.; Wu, J.Y.; Leung, M.; Buxey, F.; et al. Subclinical early posttraumatic seizures detected by continuous EEG monitoring in a consecutive pediatric cohort. Epilepsia 2013, 54, 1780–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, K.; Pohlmann-Eden, B.; Campbell, L.A.; Abel, H. Pharmacological treatments for preventing epilepsy following traumatic head injury. Cochrane Database Syst. Rev. 2015, 8, Cd009900. [Google Scholar] [CrossRef]
- Temkin, N.R.; Dikmen, S.S.; Wilensky, A.J.; Keihm, J.; Chabal, S.; Winn, H.R. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N. Engl. J. Med. 1990, 323, 497–502. [Google Scholar] [CrossRef]
- Schierhout, G.; Roberts, I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst. Rev. 2001, 4, Cd000173. [Google Scholar]
- Hicks, A.J.; Clay, F.J.; Hopwood, M.; James, A.C.; Jayaram, M.; Perry, L.A.; Batty, R.; Ponsford, J.L. The Efficacy and Harms of Pharmacological Interventions for Aggression After Traumatic Brain Injury-Systematic Review. Front. Neurol. 2019, 10, 1169. [Google Scholar] [CrossRef]
- Zafar, S.N.; Khan, A.A.; Ghauri, A.A.; Shamim, M.S. Phenytoin versus Leviteracetam for seizure prophylaxis after brain injury—A meta analysis. BMC Neurol. 2012, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Chang, B.S.; Lowenstein, D.H. Practice parameter: Antiepileptic drug prophylaxis in severe traumatic brain injury: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2003, 60, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Perron, A.D.; Brady, W.J.; Huff, J.S. Concussive convulsions: Emergency department assessment and management of a frequently misunderstood entity. Acad. Emerg. Med. 2001, 8, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Wat, R.; Mammi, M.; Paredes, J.; Haines, J.; Alasmari, M.; Liew, A.; Lu, V.M.; Arnaout, O.; Smith, T.R.; Gormley, W.B.; et al. The Effectiveness of Antiepileptic Medications as Prophylaxis of Early Seizure in Patients with Traumatic Brain Injury Compared with Placebo or No Treatment: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 122, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Puccio, A.M.; Fischer, M.R.; Jankowitz, B.T.; Yonas, H.; Darby, J.M.; Okonkwo, D.O. Induced normothermia attenuates intracranial hypertension and reduces fever burden after severe traumatic brain injury. Neurocritical Care 2009, 11, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Nduom, E.; Frangos, S.; MacKenzie, L.; Chen, I.; Maloney-Wilensky, E.; Kofke, W.A.; Levine, J.M.; LeRoux, P.D. Acute lung injury is an independent risk factor for brain hypoxia after severe traumatic brain injury. Neurosurgery 2010, 67, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Selassie, A.W.; Zaloshnja, E.; Langlois, J.A.; Miller, T.; Jones, P.; Steiner, C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma. Rehabil. 2008, 23, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haarbauer-Krupa, J.; Pugh, M.J.; Prager, E.M.; Harmon, N.; Wolfe, J.; Yaffe, K. Epidemiology of Chronic Effects of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3235–3247. [Google Scholar] [CrossRef]
- Ashman, T.A.; Cantor, J.B.; Gordon, W.A.; Spielman, L.; Flanagan, S.; Ginsberg, A.; Engmann, C.; Egan, M.; Ambrose, F.; Greenwald, B. A Randomized Controlled Trial of Sertraline for the Treatment of Depression in Persons With Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2009, 90, 733–740. [Google Scholar] [CrossRef]
- Rapoport, M.J.; Chan, F.; Lanctot, K.; Herrmann, N.; McCullagh, S.; Feinstein, A. An open-label study of citalopram for major depression following traumatic brain injury. J. Psychopharmacol. 2008, 22, 860–864. [Google Scholar] [CrossRef]
- Novack, T.A.; Baños, J.H.; Brunner, R.; Renfroe, S.; Meythaler, J.M. Impact of early administration of sertraline on depressive symptoms in the first year after traumatic brain injury. J. Neurotrauma 2009, 26, 1921–1928. [Google Scholar] [CrossRef]
- Jorge, R.E.; Acion, L.; Burin, D.I.; Robinson, R.G. Sertraline for Preventing Mood Disorders Following Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 1041–1047. [Google Scholar] [CrossRef]
- Meythaler, J.M.; Depalma, L.; Devivo, M.J.; Guin-Renfroe, S.; Novack, T.A. Sertraline to improve arousal and alertness in severe traumatic brain injury secondary to motor vehicle crashes. Brain Inj. 2001, 15, 321–331. [Google Scholar] [CrossRef]
- Wortzel, H.S.; Oster, T.J.; Anderson, C.A.; Arciniegas, D.B. Pathological Laughing and Crying. CNS Drugs 2008, 22, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Kaschka, W.P.; Meyer, A.; Schier, K.R.; Fröscher, W. Treatment of Pathological Crying with Citalopram. Pharmacopsychiatry 2001, 34, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Nahas, Z.; Arlinghaus, K.A.; Kotrla, K.J.; Clearman, R.R.; George, M.S. Rapid response of emotional incontinence to selective serotonin reuptake inhibitors. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Murai, T.; Bauer-Wittmund, T.; von Cramon, D.Y. Paroxetine versus citalopram treatment of pathological crying after brain injury. Brain Inj. 1999, 13, 805–811. [Google Scholar] [CrossRef]
- Hoehn-Saric, R.; Lipsey, J.R.; McLeod, D.R. Apathy and indifference in patients on fluvoxamine and fluoxetine. J. Clin. Psychopharmacol. 1990, 10, 343–345. [Google Scholar] [CrossRef]
- Kanetani, K.; Kimura, M.; Endo, S. Therapeutic effects of milnacipran (serotonin noradrenalin reuptake inhibitor) on depression following mild and moderate traumatic brain injury. J. Nippon. Med. Sch. 2003, 70, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Ripley, D.L.; Morey, C.E.; Gerber, D.; Harrison-Felix, C.; Brenner, L.A.; Pretz, C.R.; Cusick, C.; Wesnes, K. Atomoxetine for attention deficits following traumatic brain injury: Results from a randomized controlled trial. Brain Inj. 2014, 28, 1514–1522. [Google Scholar] [CrossRef]
- Curran, H.V.; Sakulsriprong, M.; Lader, M. Antidepressants and human memory: An investigation of four drugs with different sedative and anticholinergic profiles. Psychopharmacology 1988, 95, 520–527. [Google Scholar] [CrossRef]
- Larson, E.B.; Zollman, F.S. The effect of sleep medications on cognitive recovery from traumatic brain injury. J. Head Trauma. Rehabil. 2010, 25, 61–67. [Google Scholar] [CrossRef]
- Wroblewski, B.A.; Joseph, A.B.; Cornblatt, R.R. Antidepressant pharmacotherapy and the treatment of depression in patients with severe traumatic brain injury: A controlled, prospective study. J. Clin. Psychiatry 1996, 57, 582–587. [Google Scholar] [CrossRef]
- Dinan, T.G.; Mobayed, M. Treatment resistance of depression after head injury: A preliminary study of amitriptyline response. Acta Psychiatr. Scand. 1992, 85, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.R.; Hart, T.; Schomer, K.G. Treatment for depression after traumatic brain injury: A systematic review. J. Neurotrauma 2009, 26, 2383–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wroblewski, B.A.; McColgan, K.; Smith, K.; Whyte, J.; Singer, W.D. The incidence of seizures during tricyclic antidepressant drug treatment in a brain-injured population. J. Clin. Psychopharmacol. 1990, 10, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Berson, A.; Cook, T.; Bollegala, N.; Seto, E.; Tursanski, S.; Kim, J.; Sockalingam, S.; Rajput, A.; Krishnadev, N.; et al. Treatment of agitation following traumatic brain injury: A review of the literature. NeuroRehabilitation 2005, 20, 279–306. [Google Scholar] [CrossRef]
- Maryniak, O.; Manchanda, R.; Velani, A. Methotrimeprazine in the treatment of agitation in acquired brain injury patients. Brain Inj. 2001, 15, 167–174. [Google Scholar] [CrossRef]
- Stanislav, S.W.; Childs, A. Evaluating the usage of droperidol in acutely agitated persons with brain injury. Brain Inj. 2000, 14, 261–265. [Google Scholar]
- Krieger, D.; Hansen, K.; McDermott, C.; Matthews, R.; Mitchell, R.; Bollegala, N.; Bhalerao, S. Loxapine versus olanzapine in the treatment of delirium following traumatic brain injury. NeuroRehabilitation 2003, 18, 205–208. [Google Scholar] [CrossRef]
- Kim, E.; Bijlani, M. A pilot study of quetiapine treatment of aggression due to traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2006, 18, 547–549. [Google Scholar] [CrossRef]
- Michals, M.L.; Crismon, M.L.; Roberts, S.; Childs, A. Clozapine response and adverse effects in nine brain-injured patients. J. Clin. Psychopharmacol. 1993, 13, 198–203. [Google Scholar] [CrossRef]
- Noé, E.; Ferri, J.; Trénor, C.; Chirivella, J. Efficacy of ziprasidone in controlling agitation during post-traumatic amnesia. Behav. Neurol. 2007, 18, 7–11. [Google Scholar] [CrossRef]
- Umene-Nakano, W.; Yoshimura, R.; Okamoto, T.; Hori, H.; Nakamura, J. Aripiprazole improves various cognitive and behavioral impairments after traumatic brain injury: A case report. Gen. Hosp. Psychiatry 2013, 35, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.V. Diurnal variation in Cotard’s syndrome (copresent with Capgras delusion) following traumatic brain injury. Aust. N. Z. J. Psychiatry 2000, 34, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Bde, M.V.; Prais, H.A.; Nicolato, R.; Caramelli, P. Posttraumatic brain injury psychosis successfully treated with olanzapine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 233–235. [Google Scholar]
- Arciniegas, D.B.; Harris, S.N.; Brousseau, K.M. Psychosis following traumatic brain injury. Int. Rev. Psychiatry 2003, 15, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Krimchansky, B.Z.; Keren, O.; Sazbon, L.; Groswasser, Z. Differential time and related appearance of signs, indicating improvement in the state of consciousness in vegetative state traumatic brain injury (VS-TBI) patients after initiation of dopamine treatment. Brain Inj. 2004, 18, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Passler, M.A.; Riggs, R.V. Positive outcomes in traumatic brain injury-vegetative state: Patients treated with bromocriptine. Arch. Phys. Med. Rehabil. 2001, 82, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Vaccaro, M.; Grieb-Neff, P.; Hart, T.; Polansky, M.; Coslett, H.B. The effects of bromocriptine on attention deficits after traumatic brain injury: A placebo-controlled pilot study. Am. J. Phys. Med. Rehabil. 2008, 87, 85–99. [Google Scholar] [CrossRef]
- Ruff, R.L.; Riechers, R.G., II.; Wang, X.F.; Piero, T.; Ruff, S.S. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J. Rehabil. Res. Dev. 2012, 49, 1305–1320. [Google Scholar] [CrossRef]
- Fleminger, S.; Greenwood, R.J.; Oliver, D.L. Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database Syst. Rev. 2006, Cd003299. [Google Scholar] [CrossRef]
- Rappaport, M.; Hall, K.M.; Hopkins, K.; Belleza, T.; Cope, D.N. Disability rating scale for severe head trauma: Coma to community. Arch. Phys. Med. Rehabil. 1982, 63, 118–123. [Google Scholar]
- Zafonte, R.D.; Watanabe, T.; Mann, N.R. Amantadine: A potential treatment for the minimally conscious state. Brain Inj. 1998, 12, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Brunner, R.C.; Johnson, A.; Novack, T.A. Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: A pilot double-blind randomized trial. J. Head Trauma Rehabil. 2002, 17, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, E.; Mauro, L.S.; Ohlinger, M.J. Amantadine enhancement of arousal and cognition after traumatic brain injury. Ann. Pharm. 2008, 42, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Pachet, A.; Friesen, S.; Winkelaar, D.; Gray, S. Beneficial behavioural effects of lamotrigine in traumatic brain injury. Brain Inj. 2003, 17, 715–722. [Google Scholar] [CrossRef]
- Kaiser, P.R.; Valko, P.O.; Werth, E.; Thomann, J.; Meier, J.; Stocker, R.; Bassetti, C.L.; Baumann, C.R. Modafinil ameliorates excessive daytime sleepiness after traumatic brain injury. Neurology 2010, 75, 1780–1785. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.; Weintraub, A.; Allshouse, A.; Morey, C.; Cusick, C.; Kittelson, J.; Harrison-Felix, C.; Whiteneck, G.; Gerber, D. A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J. Head Trauma Rehabil. 2008, 23, 52–63. [Google Scholar] [CrossRef]
- Menn, S.J.; Yang, R.; Lankford, A. Armodafinil for the treatment of excessive sleepiness associated with mild or moderate closed traumatic brain injury: A 12-week, randomized, double-blind study followed by a 12-month open-label extension. J. Clin. Sleep Med. 2014, 10, 1181–1191. [Google Scholar] [CrossRef] [Green Version]
- Whyte, J.; Hart, T.; Vaccaro, M.; Grieb-Neff, P.; Risser, A.; Polansky, M.; Coslett, H.B. Effects of methylphenidate on attention deficits after traumatic brain injury: A multidimensional, randomized, controlled trial. Am. J. Phys. Med. Rehabil. 2004, 83, 401–420. [Google Scholar] [CrossRef]
- Willmott, C.; Ponsford, J. Efficacy of methylphenidate in the rehabilitation of attention following traumatic brain injury: A randomised, crossover, double blind, placebo controlled inpatient trial. J. Neurol. Neurosurg. Psychiatry 2009, 80, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Sivan, M.; Neumann, V.; Kent, R.; Stroud, A.; Bhakta, B.B. Pharmacotherapy for treatment of attention deficits after non-progressive acquired brain injury. A systematic review. Clin. Rehabil. 2010, 24, 110–121. [Google Scholar] [CrossRef]
- McAllister, T.W.; Zafonte, R.; Jain, S.; Flashman, L.A.; George, M.S.; Grant, G.A.; He, F.; Lohr, J.B.; Andaluz, N.; Summerall, L.; et al. Stein, Randomized Placebo-Controlled Trial of Methylphenidate or Galantamine for Persistent Emotional and Cognitive Symptoms Associated with PTSD and/or Traumatic Brain Injury. Neuropsychopharmacology 2016, 41, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tramontana, M.G.; Cowan, R.L.; Zald, D.; Prokop, J.W.; Guillamondegui, O. Traumatic brain injury-related attention deficits: Treatment outcomes with lisdexamfetamine dimesylate (Vyvanse). Brain Inj. 2014, 28, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, J.; Spitz, G.; Hicks, A.J. Highlights in traumatic brain injury research in 2021. Lancet Neurol. 2022, 21, 5–6. [Google Scholar] [CrossRef]
- Tenovuo, O.; Alin, J.; Helenius, H. A randomized controlled trial of rivastigmine for chronic sequels of traumatic brain injury-what it showed and taught? Brain Inj. 2009, 23, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.M.; Koumaras, B.; Meng, X.; Potkin, S.G.; Reyes, P.F.; Harvey, P.D.; Katz, D.I.; Gunay, I.; Arciniegas, D.B. Long-term effects of rivastigmine capsules in patients with traumatic brain injury. Brain Inj. 2009, 23, 123–132. [Google Scholar] [CrossRef]
- Barker, M.J.; Greenwood, K.M.; Jackson, M.; Crowe, S.F. Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: A meta-analysis. Arch. Clin. Neuropsychol. 2004, 19, 437–454. [Google Scholar] [CrossRef] [Green Version]
- Barker, M.J.; Greenwood, K.M.; Jackson, M.; Crowe, S.F. Cognitive effects of long-term benzodiazepine use: A meta-analysis. CNS Drugs 2004, 18, 37–48. [Google Scholar] [CrossRef]
- Ouellet, M.C.; Beaulieu-Bonneau, S.; Morin, C.M. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015, 14, 746–757. [Google Scholar] [CrossRef]
- Whyte, J.; Rajan, R.; Rosenbaum, A.; Katz, D.; Kalmar, K.; Seel, R.; Greenwald, B.; Zafonte, R.; Demarest, D.; Brunner, R.; et al. Zolpidem and restoration of consciousness. Am. J. Phys. Med. Rehabil. 2014, 93, 101–113. [Google Scholar] [CrossRef]
- Williams, S.T.; Conte, M.M.; Goldfine, A.M.; Noirhomme, Q.; Gosseries, O.; Thonnard, M.; Beattie, B.; Hersh, J.; Katz, D.I.; Victor, J.D.; et al. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury. Elife 2013, 2, e01157. [Google Scholar] [CrossRef]
- Du, B.; Shan, A.; Zhang, Y.; Zhong, X.; Chen, D.; Cai, K. Zolpidem arouses patients in vegetative state after brain injury: Quantitative evaluation and indications. Am. J. Med. Sci. 2014, 347, 178–182. [Google Scholar] [CrossRef]
- Kemp, S.; Biswas, R.; Neumann, V.; Coughlan, A. The value of melatonin for sleep disorders occurring post-head injury: A pilot RCT. Brain Inj. 2004, 18, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Lequerica, A.; Jasey, N.; Tremont, J.N.P.; Chiaravalloti, N.D. Pilot Study on the Effect of Ramelteon on Sleep Disturbance After Traumatic Brain Injury: Preliminary Evidence From a Clinical Trial. Arch. Phys. Med. Rehabil. 2015, 96, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Clayton, W.; Davis, L.K.; Guin-Renfroe, S.; Brunner, R.C. Orally delivered baclofen to control spastic hypertonia in acquired brain injury. J. Head Trauma Rehabil. 2004, 19, 101–108. [Google Scholar] [CrossRef]
- Posteraro, F.; Calandriello, B.; Galli, R.; Logi, F.; Iardella, L.; Bordi, L. Timing of intrathecal baclofen therapy in persons with acquired brain injury: Influence on outcome. Brain Inj. 2013, 27, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Guin-Renfroe, S.; Johnson, A.; Brunner, R.M. Prospective assessment of tizanidine for spasticity due to acquired brain injury. Arch. Phys Med. Rehabil. 2001, 82, 1155–1163. [Google Scholar] [CrossRef]
- Yablon, S.A.; Agana, B.T.; Ivanhoe, C.B.; Boake, C. Botulinum toxin in severe upper extremity spasticity among patients with traumatic brain injury: An open-labeled trial. Neurology 1996, 47, 939–944. [Google Scholar] [CrossRef]
- Yerry, J.A.; Kuehn, D.; Finkel, A.G. Onabotulinum toxin a for the treatment of headache in service members with a history of mild traumatic brain injury: A cohort study. Headache 2015, 55, 395–406. [Google Scholar] [CrossRef]
- Lump, D.; Moyer, M. Paroxysmal sympathetic hyperactivity after severe brain injury. Curr. Neurol. Neurosci. Rep. 2014, 14, 494. [Google Scholar] [CrossRef]
- Blackman, J.A.; Patrick, P.D.; Buck, M.L.; Rust, R.S., Jr. Paroxysmal autonomic instability with dystonia after brain injury. Arch. Neurol. 2004, 61, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Baguley, I.J.; Heriseanu, R.E.; Gurka, J.A.; Nordenbo, A.; Cameron, I.D. Gabapentin in the management of dysautonomia following severe traumatic brain injury: A case series. J. Neurol. Neurosurg. Psychiatry 2007, 78, 539–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, I.; Yates, D.; Sandercock, P.; Farrell, B.; Wasserberg, J.; Lomas, G.; Cottingham, R.; Svoboda, P.; Brayley, N.; Mazairac, G.; et al. Effect of intravenous corticosteroids on death within 14 days in 10,008 adults with clinically significant head injury (MRC CRASH trial): Randomised placebo-controlled trial. Lancet 2004, 364, 1321–1328. [Google Scholar] [PubMed]
- Edwards, P.; Arango, M.; Balica, L.; Cottingham, R.; El-Sayed, H.; Farrell, B.; Fernandes, J.; Gogichaisvili, T.; Golden, N.; Hartzenberg, B.; et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005, 365, 1957–1959. [Google Scholar] [PubMed]
- Zafonte, R.D.; Bagiella, E.; Ansel, B.M.; Novack, T.A.; Friedewald, W.T.; Hesdorffer, D.C.; Timmons, S.D.; Jallo, J.; Eisenberg, H.; Hart, T. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). Jama 2012, 308, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.W.; Kellermann, A.L.; Hertzberg, V.S.; Clark, P.L.; Frankel, M.; Goldstein, F.C.; Salomone, J.P.; Dent, L.L.; Harris, O.A.; Ander, D.S.; et al. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007, 49, 391–402. [Google Scholar] [CrossRef]
- Xiao, G.; Wei, J.; Yan, W.; Wang, W.; Lu, Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit. Care 2008, 12, R61. [Google Scholar] [CrossRef] [Green Version]
- Skolnick, B.E.; Maas, A.I.; Narayan, R.K.; van der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.W.; Yeatts, S.D.; Silbergleit, R.; Palesch, Y.Y.; Hertzberg, V.S.; Frankel, M.; Goldstein, F.C.; Caveney, A.F.; Howlett-Smith, H.; Bengelink, E.M.; et al. Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 2014, 371, 2457–2466. [Google Scholar] [CrossRef] [Green Version]
- Soltani, Z.; Shahrokhi, N.; Karamouzian, S.; Khaksari, M.; Mofid, B.; Nakhaee, N.; Reihani, H. Does progesterone improve outcome in diffuse axonal injury? Brain Inj. 2017, 31, 16–23. [Google Scholar] [CrossRef]
- Sinha, S.; Raheja, A.; Samson, N.; Goyal, K.; Bhoi, S.; Selvi, A.; Sharma, P.; Sharma, B.S. A randomized placebo-controlled trial of progesterone with or without hypothermia in patients with acute severe traumatic brain injury. Neurol. India 2017, 65, 1304–1311. [Google Scholar] [CrossRef]
- Nichol, A.; French, C.; Little, L.; Haddad, S.; Presneill, J.; Arabi, Y.; Bailey, M.; Cooper, D.J.; Duranteau, J.; Huet, O.; et al. Erythropoietin in traumatic brain injury (EPO-TBI): A double-blind randomised controlled trial. Lancet 2015, 386, 2499–2506. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.; Choi, K.S.; Kim, W.; Jang, B.H.; Shin, H.; Ahn, C.; Lim, T.H.; Yi, H.J. Efficacy and safety of erythropoietin in patients with traumatic brain injury: A systematic review and meta-analysis. Am. J. Emerg. Med. 2019, 37, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, A.J.; Chen, Y.; Zhao, G.; Jiang, Z.; Wang, X.; Shi, D.; Zhang, T.; Sun, B.; He, H.; et al. Efficacy and safety of erythropoietin for traumatic brain injury. BMC Neurol. 2020, 20, 399. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.F.; Gao, Y.K. Recombinant human erythropoietin for treating severe traumatic brain injury. Medicine 2018, 97, e9532. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.W.H.; Blackshaw, W.J. Does magnesium sulfate have a role in the management of severe traumatic brain injury in civilian and military populations? A systematic review and meta-analysis. J. R. Army Med. Corp. 2018, 164, 442–449. [Google Scholar] [CrossRef]
- Temkin, N.R.; Anderson, G.D.; Winn, H.R.; Ellenbogen, R.G.; Britz, G.W.; Schuster, J.; Lucas, T.; Newell, D.W.; Mansfield, P.N.; Machamer, J.E.; et al. Magnesium sulfate for neuroprotection after traumatic brain injury: A randomised controlled trial. Lancet Neurol. 2007, 6, 29–38. [Google Scholar] [CrossRef]
- Hatton, J.; Rosbolt, B.; Empey, P.; Kryscio, R.; Young, B. Dosing and safety of cyclosporine in patients with severe brain injury. J. Neurosurg. 2008, 109, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Aminmansour, B.; Fard, S.A.; Habibabadi, M.R.; Moein, P.; Norouzi, R.; Naderan, M. The efficacy of Cyclosporine-A on Diffuse Axonal Injury after Traumatic Brain Injury. Adv. Biomed. Res. 2014, 3, 35. [Google Scholar]
- Jha, R.M.; Bell, J.; Citerio, G.; Hemphill, J.C.; Kimberly, W.T.; Narayan, R.K.; Sahuquillo, J.; Sheth, K.N.; Simard, J.M. Role of Sulfonylurea Receptor 1 and Glibenclamide in Traumatic Brain Injury: A Review of the Evidence. Int. J. Mol. Sci. 2020, 21, 409. [Google Scholar] [CrossRef] [Green Version]
- Zafardoost, P.; Ghasemi, A.A.; Salehpour, F.; Piroti, C.; Ziaeii, E. Evaluation of the Effect of Glibenclamide in Patients With Diffuse Axonal Injury Due to Moderate to Severe Head Trauma. Trauma Mon. 2016, 21, e25113. [Google Scholar] [CrossRef] [Green Version]
- Khalili, H.; Derakhshan, N.; Niakan, A.; Ghaffarpasand, F.; Salehi, M.; Eshraghian, H.; Shakibafard, A.; Zahabi, B. Effects of Oral Glibenclamide on Brain Contusion Volume and Functional Outcome of Patients with Moderate and Severe Traumatic Brain Injuries: A Randomized Double-Blind Placebo-Controlled Clinical Trial. World Neurosurg. 2017, 101, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, H.M.; Shenton, M.E.; Pasternak, O.; Simard, J.M.; Okonkwo, D.O.; Aldrich, C.; He, F.; Jain, S.; Hayman, E.G. Magnetic Resonance Imaging Pilot Study of Intravenous Glyburide in Traumatic Brain Injury. J. Neurotrauma 2020, 37, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, W.; Sapkota, A.; Khurshid, H.; Qureshi, I.A.; Jahan, N.; Went, T.R.; Dominic, J.L.; Win, M.; Kannan, A.; Tara, A.; et al. Statins’ Effect on Cognitive Outcome After Traumatic Brain Injury: A Systematic Review. Cureus 2021, 13, e16953. [Google Scholar] [CrossRef] [PubMed]
- Mansi, I.A.; English, J.L.; Alvarez, C.A.; Mortensen, E.M.; Pugh, M.J. Statins in survivors of traumatic brain injury: A propensity score-matched analysis. Brain Inj. 2020, 34, 1367–1374. [Google Scholar] [CrossRef]
- Gruenbaum, S.E.; Zlotnik, A.; Gruenbaum, B.F.; Hersey, D.; Bilotta, F. Pharmacologic Neuroprotection for Functional Outcomes After Traumatic Brain Injury: A Systematic Review of the Clinical Literature. CNS Drugs 2016, 30, 791–806. [Google Scholar] [CrossRef]
- Lerouet, D.; Marchand-Leroux, C.; Besson, V.C. Neuropharmacology in traumatic brain injury: From preclinical to clinical neuroprotection? Fundam. Clin. Pharmacol. 2021, 35, 524–538. [Google Scholar] [CrossRef]
- Wen, L.; You, W.; Wang, H.; Meng, Y.; Feng, J.; Yang, X. Polarization of Microglia to the M2 Phenotype in a Peroxisome Proliferator-Activated Receptor Gamma-Dependent Manner Attenuates Axonal Injury Induced by Traumatic Brain Injury in Mice. J. Neurotrauma 2018, 35, 2330–2340. [Google Scholar] [CrossRef]
- Yi, J.H.; Park, S.W.; Brooks, N.; Lang, B.T.; Vemuganti, R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008, 1244, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Cekic, M.; Cutler, S.M.; VanLandingham, J.W.; Stein, D.G. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol. Aging 2011, 32, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Conte, V.; Uryu, K.; Fujimoto, S.; Yao, Y.; Rokach, J.; Longhi, L.; Trojanowski, J.Q.; Lee, V.M.; McIntosh, T.K.; Praticò, D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J. Neurochem. 2004, 90, 758–764. [Google Scholar] [CrossRef]
- Peterson, T.C.; Hoane, M.R.; McConomy, K.S.; Farin, F.M.; Bammler, T.K.; MacDonald, J.W.; Kantor, E.D.; Anderson, G.D. A Combination Therapy of Nicotinamide and Progesterone Improves Functional Recovery following Traumatic Brain Injury. J. Neurotrauma 2015, 32, 765–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffus, A.M.; Anderson, G.D.; Hoane, M. Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxidative Med. Cell. Longev. 2010, 3, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Haar, C.V.; Emery, M.A.; Hoane, M.R. Chronic folic acid administration confers no treatment effects in either a high or low dose following unilateral controlled cortical impact injury in the rat. Restor. Neurol. Neurosci. 2012, 30, 291–302. [Google Scholar]
- Naim, M.Y.; Friess, S.; Smith, C.; Ralston, J.; Ryall, K.; Helfaer, M.A.; Margulies, S.S. Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev. Neurosci. 2010, 32, 466–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scrimgeour, A.G.; Condlin, M.L. Nutritional treatment for traumatic brain injury. J. Neurotrauma 2014, 31, 989–999. [Google Scholar] [CrossRef]
- McClain, C.J.; Twyman, D.L.; Ott, L.G.; Rapp, R.P.; Tibbs, P.A.; Norton, J.A.; Kasarskis, E.J.; Dempsey, R.J.; Young, B. Serum and urine zinc response in head-injured patients. J. Neurosurg. 1986, 64, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Cope, E.C.; Morris, D.R.; Scrimgeour, A.G.; Levenson, C.W. Use of zinc as a treatment for traumatic brain injury in the rat: Effects on cognitive and behavioral outcomes. Neurorehabilit. Neural Repair 2012, 26, 907–913. [Google Scholar] [CrossRef]
- Cope, E.C.; Morris, D.R.; Gower-Winter, S.D.; Brownstein, N.C.; Levenson, C.W. Effect of zinc supplementation on neuronal precursor proliferation in the rat hippocampus after traumatic brain injury. Exp. Neurol. 2016, 279, 96–103. [Google Scholar] [CrossRef]
- Ménard, C.; Patenaude, C.; Gagné, A.M.; Massicotte, G. AMPA receptor-mediated cell death is reduced by docosahexaenoic acid but not by eicosapentaenoic acid in area CA1 of hippocampal slice cultures. J. Neurosci. Res. 2009, 87, 876–886. [Google Scholar] [CrossRef]
- Begum, G.; Yan, H.Q.; Li, L.; Singh, A.; Dixon, C.E.; Sun, D. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J. Neurosci. 2014, 34, 3743–3755. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Ying, Z.; Schubert, D.; Gomez-Pinilla, F. Brain and spinal cord interaction: A dietary curcumin derivative counteracts locomotor and cognitive deficits after brain trauma. Neurorehabil. Neural. Repair 2011, 25, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.J.; Chen, T.H.; Yang, L.Y.; Shih, C.M. Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death Dis. 2014, 5, e1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theadom, A.; Mahon, S.; Barker-Collo, S.; McPherson, K.; Rush, E.; Vandal, A.C.; Feigin, V.L. Enzogenol for cognitive functioning in traumatic brain injury: A pilot placebo-controlled RCT. Eur. J. Neurol. 2013, 20, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, G.A.; Pate, A.J.; Panigrahi, B.; LaBoy, S.; Prosniak, R.; Mody, A.; Chendrasekhar, A. Malnutrition as measured by albumin and prealbumin on admission is associated with poor outcomes after severe traumatic brain injury. Am. Surg. 2015, 81, E61-3. [Google Scholar] [CrossRef] [PubMed]
- Horsfield, S.A.; Rosse, R.B.; Tomasino, V.; Schwartz, B.L.; Mastropaolo, J.; Deutsch, S.I. Fluoxetine’s effects on cognitive performance in patients with traumatic brain injury. Int. J. Psychiatry Med. 2002, 32, 337–344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).