Suppressive Effects of Siegesbeckia orientalis Ethanolic Extract on Proliferation and Migration of Hepatocellular Carcinoma Cells through Promoting Oxidative Stress, Apoptosis and Inflammatory Responses
Abstract
:1. Introduction
2. Results
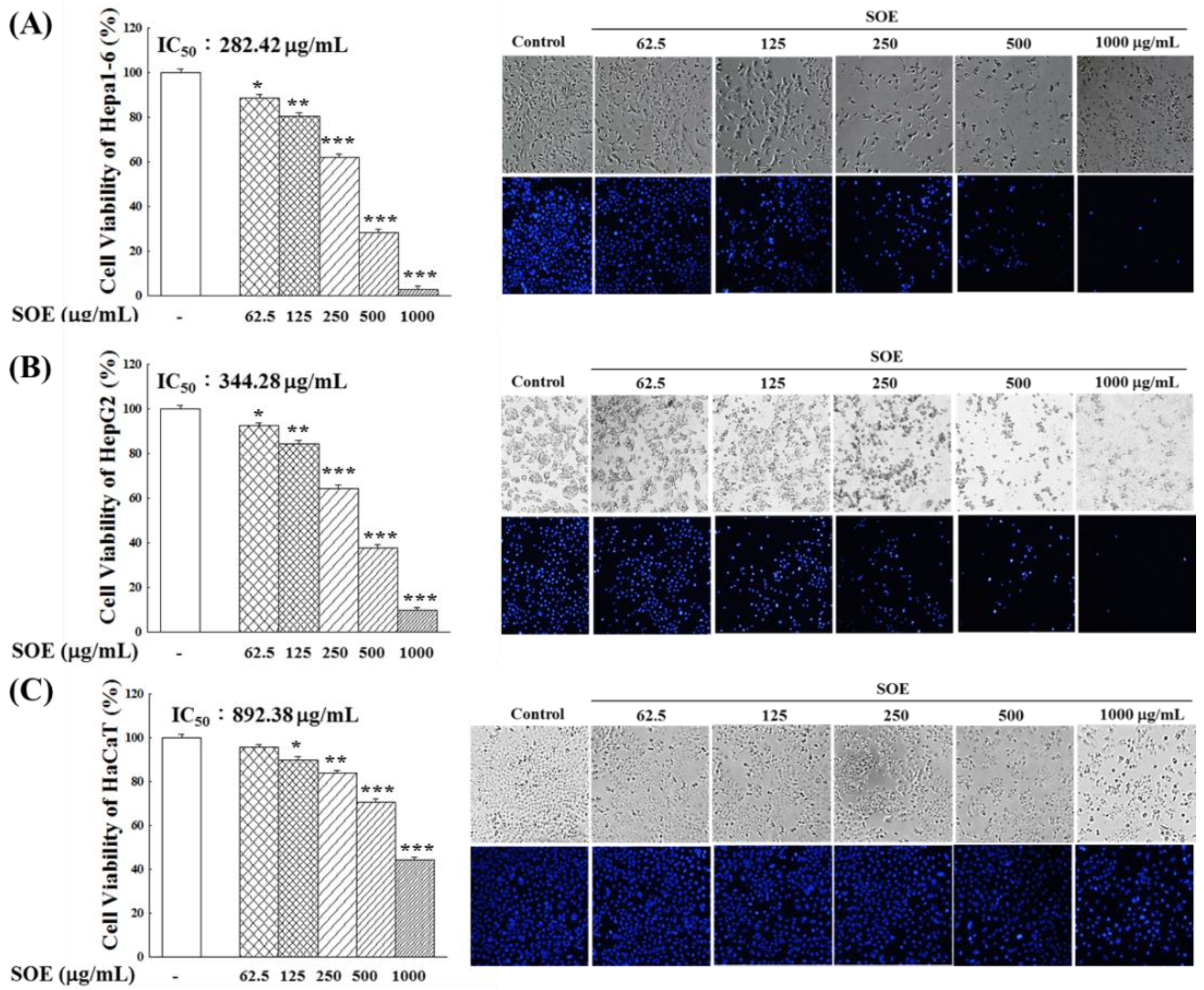
2.1. Effect of SOE Treatment on Proliferation of Hepatoma Cells and Keratinocyte Cells
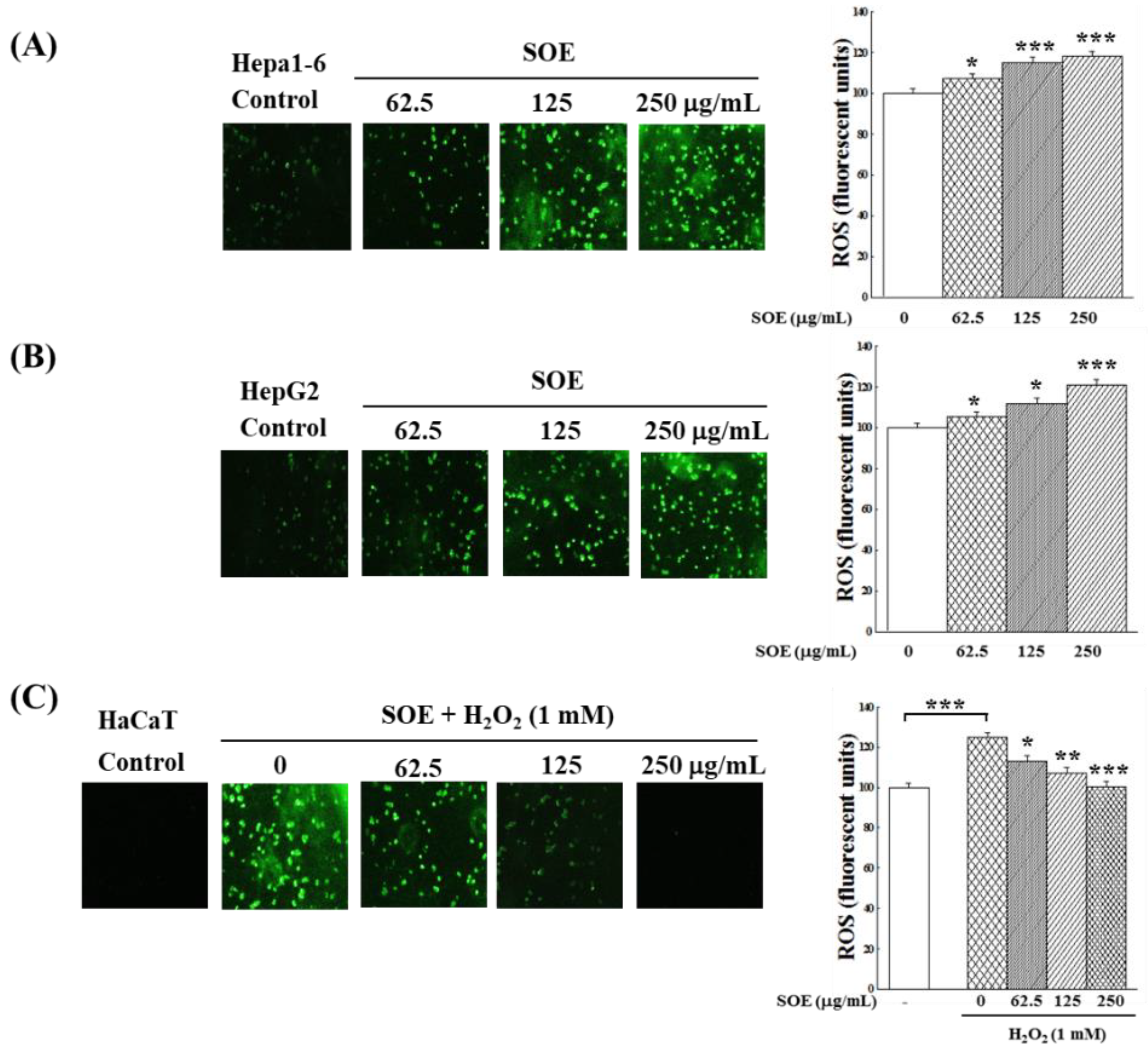
2.2. Effect of SOE Treatment on Oxidative System in Hepatoma and Keratinocyte Cells
2.2.1. Viability of Keratinocyte Cells under H2O2 Stimulation
2.2.2. Intracellular ROS Changes
2.2.3. Expression of Antioxidant Enzymes
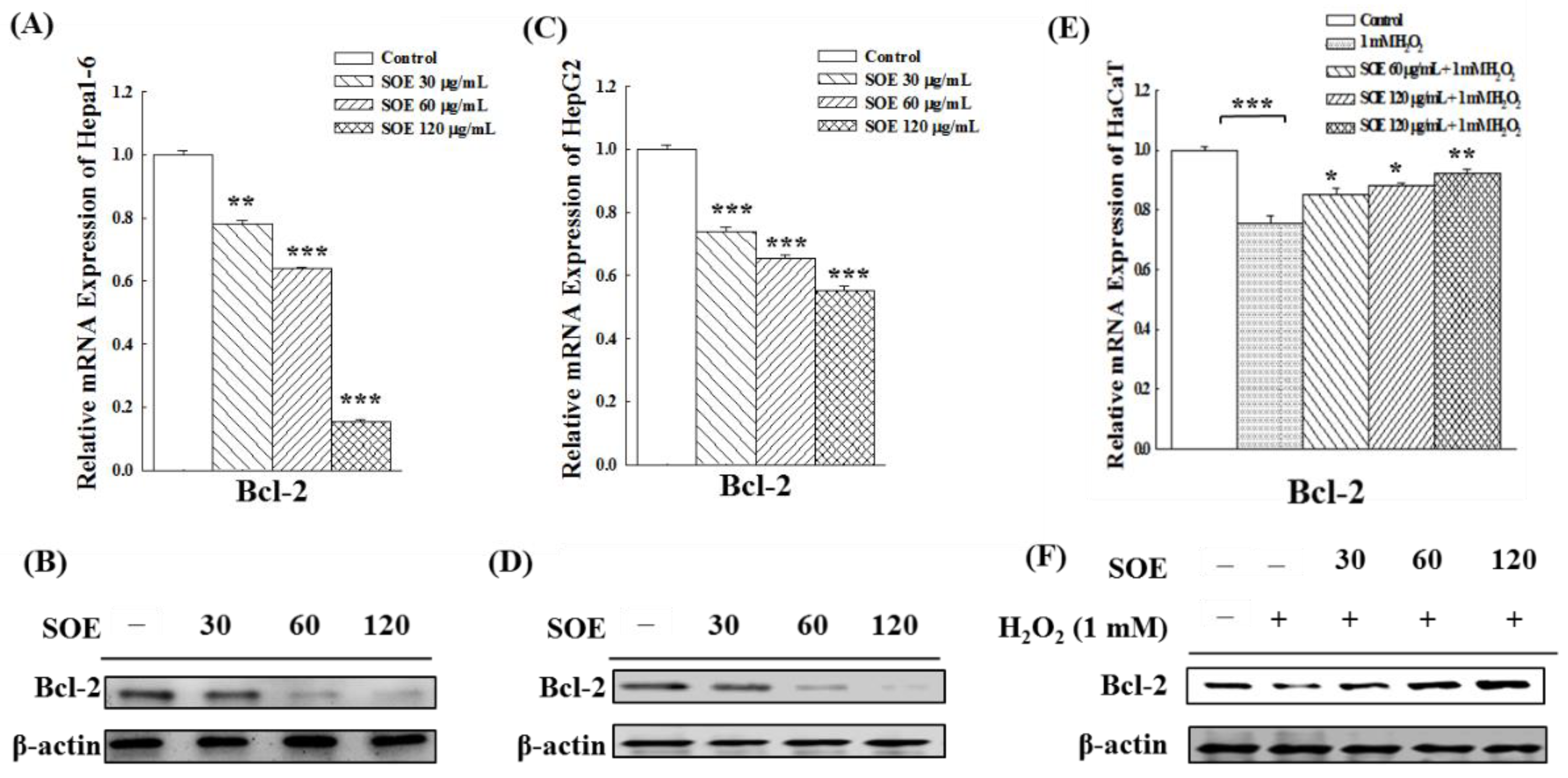
2.3. Effect of SOE on the Expression of Anti-Apoptotic Bcl-2 Gene and Protein
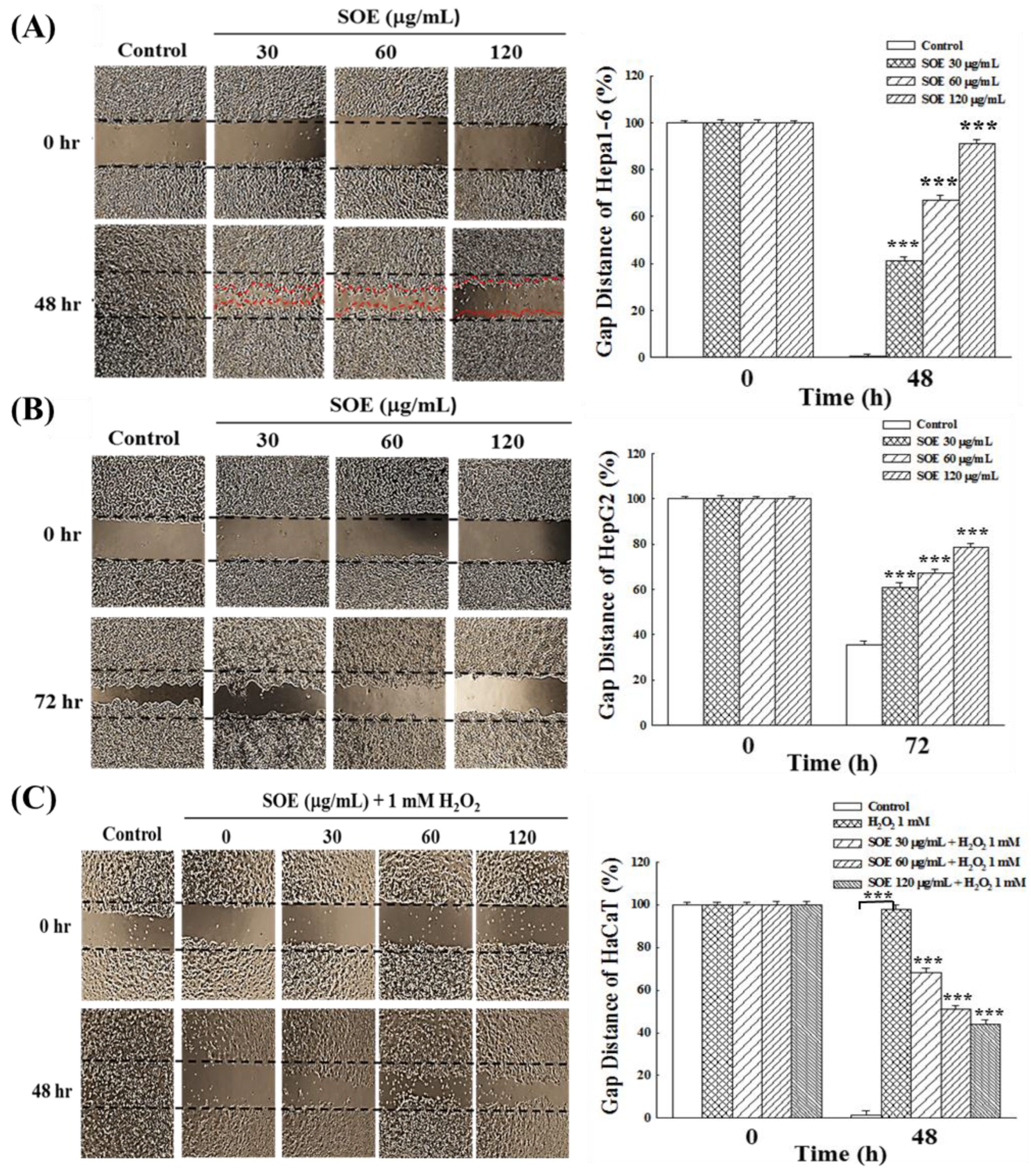
2.4. Effects of SOE on Migration of Hepatoma and Keratinocyte Cells
2.4.1. Wound Healing Assay
2.4.2. Expression of Migration-Related Proteins
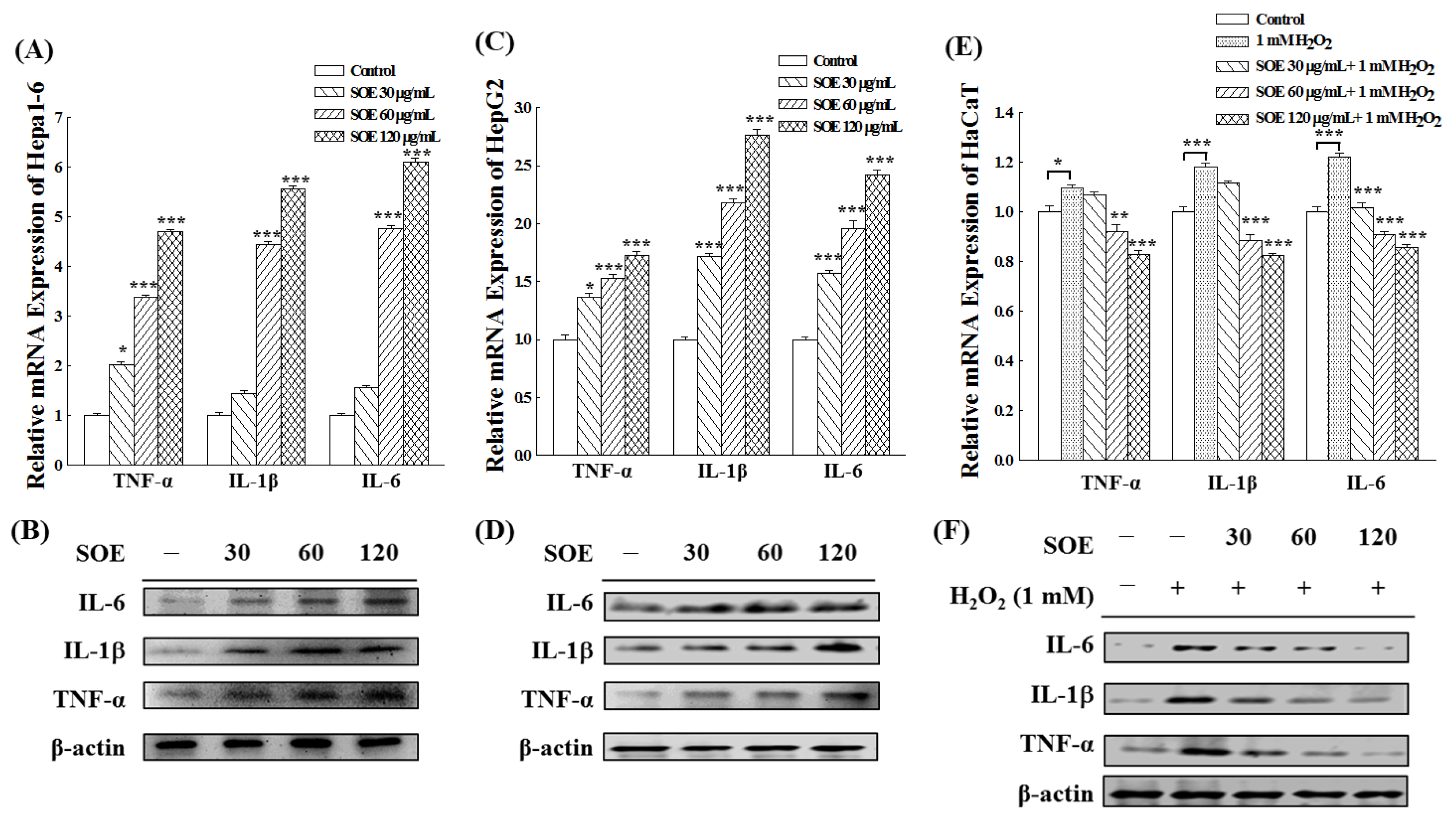
2.5. Effects of SOE on Inflammation in Hepatoma and Keratinocyte Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of SOE
4.2. Cell Culture and Viability Analysis
4.3. Assay of Intracellular ROS Level
4.4. Gene Expression Analysis with Quantitative RT-PCR
4.5. Western Blot Assay
4.6. Cell Migration by Wound Healing Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Severi, T.; van Malenstein, H.; Verslype, C.; van Pelt, J.F. Tumor initiation and progression in hepatocellular carcinoma: Risk factors, classification, and therapeutic targets. Acta. Pharmacol. Sin. 2010, 31, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 7. [Google Scholar] [CrossRef]
- Cordani, M.; Donadelli, M.; Strippoli, R.; Bazhin, A.V.; Sánchez-Álvarez, M. Interplay between ROS and autophagy in cancer and aging: From molecular mechanisms to novel therapeutic approaches. Oxid. Med. Cell. Longev. 2019, 2019, 8794612. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Ji, L.; Ruan, Y.; Wan, Z.; Lin, Z.; Xia, S.; Tao, L.; Zheng, J.; Cai, L.; Wang, Y.; et al. UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1β in hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 190. [Google Scholar] [CrossRef]
- Denisenko, T.V.; Gorbunova, A.S.; Zhivotovsky, B. Mitochondrial involvement in migration, invasion and metastasis. Front. Cell Dev. Biol. 2019, 7, 355. [Google Scholar] [CrossRef]
- Tang, J.Y.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects—Involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef]
- Bai, X.; Ma, Y.; Zhang, G. Butein suppresses cervical cancer growth through the PI3K/AKT/mTOR pathway. Oncol. Rep. 2015, 33, 3085–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Kim, M.J.; Kim, E.J.; Yang, Y.; Lee, M.S.; Lim, J.S. Berberine-induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX-2 protein expression. Biochem. Pharmacol. 2012, 83, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.T.; Chen, X.H.; Gao, H.; Ye, J.L.; Wang, C.B. Physcion inhibits the metastatic potential of human colorectal cancer SW620 cells in vitro by suppressing the transcription factor SOX2. Acta. Pharmacol. Sin. 2016, 37, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Ciccarone, F.; Castelli, S.; Ciriolo, M.R. Oxidative stress-driven autophagy acROSs onset and therapeutic outcome in hepatocellular carcinoma. Oxid. Med. Cell Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Man, S.; Luo, C.; Yan, M.; Zhao, G.; Ma, L.; Gao, W. Treatment for liver cancer: From sorafenib to natural products. Eur. J. Med. Chem. 2021, 224, 113690. [Google Scholar] [CrossRef]
- Lu, M.; Fei, Z.; Zhang, G. Synergistic anticancer activity of 20(S)-ginsenoside Rg3 and sorafenib in hepatocellular carcinoma by modulating PTEN/Akt signaling pathway. Biomed. Pharmacother. 2018, 97, 1282–1288. [Google Scholar] [CrossRef]
- Catalogna, G.; Moraca, F.; D’Antona, L.; Dattilo, V.; Perrotti, G.; Lupia, A.; Costa, G.; Ortuso, F.; Iuliano, R.; Trapasso, F.; et al. Review about the multi-target profile of resveratrol and its implication in the SGK1 inhibition. Eur. J. Med. Chem. 2019, 183, 111675. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Barrera, J.; Queralt, B.; Menendez, J.A. Targeting STAT3 with silibinin to improve cancer therapeutics. Cancer Treat. Rev. 2017, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.Q.; Song, W.B.; Wang, W.Q.; Wei, J.J.; Li, J.; Liu, Y.; Xuan, L.J. New ent-kaurane and ent-pimarane diterpenoids from Siegesbeckia pubescens. Phytochem. Lett. 2017, 21, 273–277. [Google Scholar] [CrossRef]
- Su, T.; Yu, H.; Kwan, H.Y.; Ma, X.Q.; Cao, H.H.; Cheng, C.Y.; Leung, A.K.M.; Chan, C.L.; Li, W.D.; Cao, H.; et al. Comparisons of the chemical profiles, cytotoxicities and anti-inflammatory effects of raw and rice wine-processed herba Siegesbeckiae. J. Ethnopharmacol. 2014, 156, 365–369. [Google Scholar] [CrossRef]
- Hong, Y.H.; Weng, L.W.; Chang, C.C.; Hsu, H.F.; Wang, C.P.; Wang, S.W.; Houng, J.Y. Anti-inflammatory effects of Siegesbeckia orientalis ethanol extract in vitro and in vivo models. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar]
- Guo, H.; Zhang, Y.; Cheng, B.C.; Fu, X.; Zhu, P.; Chen, J.; Chan, Y.; Yin, C.; Wang, Y.; Hossen, M.; et al. An ethanolic extract of the aerial part of Siegesbeckia orientalis L. inhibits the production of inflammatory mediators regulated by AP-1, NF-κB and IRF3 in LPS-stimulated RAW 264.7 cells. Biosci. Trends 2018, 12, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wei, J.; Hong, L.; Fan, S.; Hu, G.; Jia, J. Comparative analysis of chemical composition, anti-inflammatory activity and antitumor activity in essential oils from Siegesbeckia orientalis, S. glabrescens and S. pubescens with an ITS sequence analysis. Molecules 2018, 23, 2185. [Google Scholar] [CrossRef] [Green Version]
- Engels, N.S.; Gierlikowska, B.; Waltenberger, B.; Chang, F.R.; Kiss, A.K.; Stuppner, H. A new diterpene and anti-inflammatory sesquiterpene lactones from Sigesbeckia orientalis. Planta Med. 2020, 86, 1108–1117. [Google Scholar] [CrossRef]
- Hwang, W.J.; Park, E.J.; Jang, C.H.; Han, S.W.; Oh, G.J.; Kim, N.S.; Kim, H.M. Inhibitory effect of immunoglobulin E production by jin-deuk-chal (Siegesbeckia orientalis). Immunopharmacol. Immunotoxicol. 2001, 23, 555–563. [Google Scholar] [CrossRef]
- Sun, H.X.; Wang, H. Immunosuppressive activity of the ethanol extract of Siegesbeckia orientalis on the immune responses to ovalbumin in mice. Chem. Biodivers. 2006, 3, 754–761. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Thuong, P.T.; Hwang, I.H.; Hoang, T.K.H.; Nguyen, M.K.; Nguyen, H.A.; Na, M. Anti-hyperuricemic, anti-inflammatory and analgesic effects of Siegesbeckia orientalis L. resulting from the fraction with high phenolic content. BMC Complement. Altern. Med. 2017, 17, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, W.C.; Ling, X.H.; Chang, C.C.; Hsu, H.F.; Wang, S.W.; Lee, Y.C.; Luo, C.; Lee, Y.T.; Houng, J.Y. Inhibitory effects of Siegesbeckia orientalis extracts on sdvanced glycation end product formation and key enzymes related to metabolic syndrome. Molecules 2017, 22, 1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Houng, J.Y.; Wang, S.W.; Hsuan, C.F.; Lu, Y.C.; Chang, T.H.; Chen, Y.L. Protective effect of Siegesbeckia orientalis on pancreatic β-cells under high glucose-induced glucotoxicity. Appl. Sci. 2021, 11, 10963. [Google Scholar] [CrossRef]
- Wang, J.P.; Zhou, Y.M.; Zhang, Y.H. Kirenol production in hairy root culture of Siegesbeckea orientalis and its antimicrobial activity. Pharmacogn. Mag. 2012, 8, 149–155. [Google Scholar]
- Yang, Y.; Chen, H.; Lei, J.; Yu, J. Biological activity of extracts and active compounds isolated from Siegesbeckia orientalis L. Ind. Crops. Prod. 2016, 94, 288–293. [Google Scholar] [CrossRef]
- Chang, C.C.; Hsu, H.F.; Huang, K.H.; Wu, J.M.; Kuo, S.M.; Ling, X.H.; Houng, J.Y. Anti-proliferative effects of Siegesbeckia orientalis ethanol extract on human endometrial RL-95 cancer cells. Molecules 2014, 19, 19980–19994. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.P.; Luo, Q.; Ruan, J.L.; Chen, Y.A.; Chen, M.X. Effect of Siegesbeckia orientalis L. on cervical cancer HeLa cell in vitro. Yiyao. Daobao. 2009, 1, 45–46. [Google Scholar]
- Liu, N.; Wu, C.; Yu, J.H.; Zhu, K.K.; Song, M.N.; Yang, F.Y.; Feng, R.I.; Zhang, Y.Y.; Chang, W.Q.; Zhang, H. Germacrane-type sesquiterpenoids with cytotoxic activity from Sigesbeckia orientalis. Bioorg. Chem. 2019, 92, 103196. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Ling, X.H.; Hsu, H.F.; Wu, J.M.; Wang, C.P.; Yang, J.F.; Fang, L.W.; Houng, J.Y. Siegesbeckia orientalis extract inhibits TGFβ1-induced migration and invasion of endometrial cancer cells. Molecules 2016, 21, 1021. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.P.; Zhou, Y.M.; Ye, Y.J.; Shang, X.M.; Cai, Y.I.; Xiong, C.M.; Wu, Y.X.; Xu, H.X. Topical anti-inflammatory and analgesic activity of kirenol isolated from Siegesbeckia orientalis. J. Ethnopharmacol. 2011, 137, 1089–1094. [Google Scholar] [CrossRef]
- Tang, X.D.; Zhao, Q.; Lan, X.F.; Ge, N.N.; Tang, Z.H.; Fan, C.H. Effect of Siegesbeckia orientalis on cartilage damage in knee osteoarthritis rats by regulating sirt1/FOXO1 pathway. Chin. J. Immunol. 2020, 36, 439–444. [Google Scholar]
- Chu, J.M.T.; Xiong, W.; Linghu, K.G.; Liu, Y.; Zhang, Y.; Zhao, G.D.; Irwin, M.G.; Wong, G.T.C.; Yu, H. Siegesbeckia Orientalis L. extract attenuates postoperative cognitive dysfunction, systemic inflammation, and neuroinflammation. Exp. Neurobiol. 2018, 27, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Kim, C.; Hwang, J.K. High hydrostatic pressure extract of Siegesbeckia orientalis inhibits adipogenesis through the activation of the Wnt/β-catenin signaling pathway. Food Sci. Biotechnol. 2020, 29, 977–985. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Kamijo, T.; Koike, K.; Noda, T. ARF tumor suppressor induces mitochondria-dependent apoptosis by modulation of mitochondrial Bcl-2 family proteins. J. Biol. Chem. 2003, 278, 27888–27895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; He, Y.; Zhao, M.; Jiang, J. Collective cell migration: Implications for wound healing and cancer invasion. Burns Trauma 2013, 1, 21–26. [Google Scholar]
- Clevers, H. Wnt/β-Catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wang, J.; Zhuang, J.; Ma, X.; Zheng, N.; Song, Y.; Xia, W. P4HB modulates epithelial-mesenchymal transition and the β-catenin/Snail pathway influencing chemoresistance in liver cancer cells. Oncol. Lett. 2020, 20, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Chuang, G.C.; Xia, H.; Mahne, S.E.; Varner, K.J. Environmentally persistent free radicals cause apoptosis in HL-1 cardiomyocytes. Cardiovasc. Toxicol. 2017, 17, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhou, C.; Huang, S.; Jiang, C. Selenium Polysaccharide SPMP-2a from Pleurotus geesteranus alleviates H2O2-induced oxidative damage in HaCaT Cells. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar]
- Chen, Y.; Liu, J.M.; Xiong, X.X.; Qiu, X.Y.; Pan, F.; Liu, D.; Lan, S.J.; Jin, S.; Yu, S.B.; Chen, X.Q. Piperlongumine selectively kills hepatocellular carcinoma cells and preferentially inhibits their invasion via ROS-ER-MAPKs-CHOP. Oncotarget 2015, 6, 6406–6421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant activities and protective effects of dendropachol, a new bisbibenzyl compound from Dendrobium pachyglossum, on hydrogen peroxide-induced oxidative stress in HaCaT keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chippada-Venkata, U.; Oh, W. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers 2014, 6, 1298–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, C.; Yu, N.; Si, L.; Zhu, L.; Zeng, A.; Liu, Z.; Wang, X. INF2 regulates oxidative stress-induced apoptosis in epidermal HaCaT cells by modulating the HIF1 signaling pathway. Biomed. Pharmacother. 2019, 111, 151–161. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, Y.M.; Song, S.; Lee, Y.Y.; Yeum, K.J. Black soybeans protect human keratinocytes from oxidative stress-induced cell death. Food Sci. Nutr. 2018, 6, 2423–2430. [Google Scholar] [CrossRef]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Wang, W.; Qin, D.; Jia, C.; Yuan, M.; Wang, H.; Guo, Y.; Zhu, J.; Zhou, Y.; et al. Emodin suppresses the migration and invasion of melanoma cells. Biol. Pharm. Bull. 2021, 44, 771–779. [Google Scholar] [CrossRef]
- Ciborowski, P.; Finn, O.J. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: Potential role in metastasis. Clin. Exp. Metastasis 2002, 19, 339–345. [Google Scholar] [CrossRef]
- Huang, M.; Wu, S.; Hu, Q.; Wu, H.; Wei, S.; Xie, H.; Sun, K.; Li, X.; Fang, L. Agkihpin, a novel SVAE may inhibit the migration and invasion of liver cancer cells associated with the inversion of EMT induced by Wnt/β-catenin signaling inhibition. Biochem. Biophys. Res. Commun. 2016, 479, 283–289. [Google Scholar] [CrossRef]
- Gao, J.Z.; Li, J.; Du, J.L.; Li, X.L. Long non-coding RNA HOTAIR is a marker for hepatocellular carcinoma progression and tumor recurrence. Oncol. Lett. 2016, 11, 1791–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.F.; Shi, F.; Huang, T.; Huang, C.; Shen, L.; Wu, P.; Li, W. ASTN1 is associated with immune infiltrates in hepatocellular carcinoma, and inhibits the migratory and invasive capacity of liver cancer via the Wnt/β-catenin signaling pathway. Oncol. Rep. 2020, 44, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.L.; Pei, X.F.; Qiao, X.; Yu, J.; Ye, H.; Xi, C.L.; Wang, P.Y.; Gong, Z.L. SERPINA3 silencing inhibits the migration, invasion, and liver metastasis of colon cancer cells. Dig. Dis. Sci. 2018, 63, 2309–2319. [Google Scholar] [CrossRef]
- Lin, X.l.; Li, K.; Yang, Z.; Chen, B.; Zhang, T. Dulcitol suppresses proliferation and migration of hepatocellular carcinoma via regulating SIRT1/p53 pathway. Phytomedicine 2020, 66, 153112. [Google Scholar] [CrossRef]
- Liu, H.; Liao, W.; Fan, L.; Zheng, Z.; Liu, D.; Zhang, Q.W.; Yang, A.; Liu, F. Ethanol extract of Ophiorrhiza pumila suppresses liver cancer cell proliferation and migration. Chinese Med. 2020, 15, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.Y.; Da, C.M.; Liao, B.; Zhang, H.H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef]
- Charvat, S.; Chignol, M.C.; Souchier, C.; Le Griel, C.; Schmitt, D.; Serres, M. Cell migration and MMP-9 secretion are increased by epidermal growth factor in HaCaT-ras transfected cells. Exp. Dermatol. 1998, 7, 184–190. [Google Scholar] [CrossRef]
- Manosalva, C.; Alarcón, P.; González, K.; Soto, J.; Igor, K.; Peña, F.; Medina, G.; Burgos, R.A.; Hidalgo, M.A. Free fatty acid receptor 1 signaling contributes to migration, MMP-9 activity, and expression of IL-8 induced by linoleic acid in HaCaT cells. Front. Pharmacol. 2020, 11, 595. [Google Scholar] [CrossRef]
- Yang, C.; Luo, L.; Bai, X.; Shen, K.; Liu, K.; Wang, J.; Hu, D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020, 681, 108259. [Google Scholar] [CrossRef] [PubMed]
- Lachenmayer, A.; Alsinet, C.; Savic, R.; Cabellos, L.; Toffanin, S.; Hoshida, Y.; Villanueva, A.; Minguez, B.; Newell, P.; Tsai, H.W.; et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin. Cancer Res. 2012, 18, 4997–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Cheon, S.S.; Wei, Q.; Gurung, A.; Youn, A.; Bright, T.; Poon, R.; Whetstone, H.; Guha, A.; Alman, B.A. β-Catenin regulates wound size and mediates the effect of TGF-β in cutaneous healing. FASEB J. 2006, 20, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Tsai, Y.C.; Korivi, M.; Chang, C.T.; Hseu, Y.C. Lucidone promotes the cutaneous wound healing process via activation of the PI(3)K/AKT, Wnt/β-catenin and NF-κB signaling pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 151–168. [Google Scholar] [CrossRef]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef]
- Kim, D.; Ku, B.; Choi, E.M. Se-methylselenocysteine stimulates migration and antioxidant response in HaCaT keratinocytes: Implications for wound healing. J. Trace Elem. Med. Biol. 2020, 58, 126426. [Google Scholar] [CrossRef]
- Ritter, B.; Greten, F.R. Modulating inflammation for cancer therapy. J. Exp. Med. 2019, 216, 1234–1243. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226. [Google Scholar] [PubMed]
- Hsu, H.F.; Chen, Z.H.; Chang, S.F.; Wang, C.P.; Chiou, S.J.; Yen, J.H.; Chang, C.C.; Tsai, Y.D.; Fang, L.W.; Houng, J.Y. Evaluating the anti-metastatic potential of Anisomeles indica extract by using human oral squamous carcinoma FaDu cells. Afr. J. Pharm. Pharmacol. 2012, 6, 1782–1791. [Google Scholar]



| Primer | Sequence |
|---|---|
| Catalase | 5′-GCCATTGCCACAGGAAAGTA-3′ |
| 5′-CCTTGGTGAGATCGAATGGA-3′ | |
| GPx | 5′-CCAAGCTCATCACCTGGTCT-3′ |
| 5′-TCGATGTCAATGGTCTGGAA-3′ | |
| SOD | 5′-TGGCCGATGTGTCTATTGAA-3′ |
| 5′-CACCTTTGCCCAAGTCATCT-3′ | |
| Bcl-2 | 5′-CTGAGTACCTGAACCGGCA-3′ |
| 5′-GAGAAATCAAACAGAGGCCG-3′ | |
| β-Catenin | 5′-ATTGATTCGAAACCTTGCCC-3′ |
| 5′-AGCTCCAGTACACCCTTCTA-3′ | |
| MMP-2 | 5′-AGAACTTCCGATTATCCCATGATGA-3′ |
| 5′-TGACAGGTCCCAGTGTTGGTG-3′ | |
| MMP-7 | 5′-GGCGGAGATGCTCACTTTGAC-3′ |
| 5′-AATTCATGGGTGGCAGCAAAC-3′ | |
| MMP-9 | 5′-GCCCTGGAACTCACACGACA-3′ |
| 5′-TTGGAAACTCACACGCCAGAAG-3′ | |
| IL-6 | 5′-TGGAGTACCATAGCTACCTGGAGT-3′ |
| 5′-TCCTTAGCCACTCCTTCTGTGACT-3′ | |
| IL-1β | 5′-GGTCAAAGGTTTGGAAGCAG-3′ |
| 5′-TGTGAAATGCCACCTTTTGA-3′ | |
| TNF-α | 5′-CAGGTTCTGTCCCTTTCACTCACT-3′ |
| 5′-GTTCAGTAGACAGAAGAGCGTGGT-3′ | |
| GAPDH | 5′-TGCACCACCAACTGCTTAGC-3′ |
| 5′-GGCATGGACTGTGGTCATGAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-H.; Chang, C.-C.; Houng, J.-Y.; Chang, T.-H.; Chen, Y.-L.; Hsu, C.-C.; Chang, L.-S. Suppressive Effects of Siegesbeckia orientalis Ethanolic Extract on Proliferation and Migration of Hepatocellular Carcinoma Cells through Promoting Oxidative Stress, Apoptosis and Inflammatory Responses. Pharmaceuticals 2022, 15, 826. https://doi.org/10.3390/ph15070826
Chen T-H, Chang C-C, Houng J-Y, Chang T-H, Chen Y-L, Hsu C-C, Chang L-S. Suppressive Effects of Siegesbeckia orientalis Ethanolic Extract on Proliferation and Migration of Hepatocellular Carcinoma Cells through Promoting Oxidative Stress, Apoptosis and Inflammatory Responses. Pharmaceuticals. 2022; 15(7):826. https://doi.org/10.3390/ph15070826
Chicago/Turabian StyleChen, Tzu-Hua, Chi-Chang Chang, Jer-Yiing Houng, Tzu-Hsien Chang, Ya-Ling Chen, Chia-Chang Hsu, and Long-Sen Chang. 2022. "Suppressive Effects of Siegesbeckia orientalis Ethanolic Extract on Proliferation and Migration of Hepatocellular Carcinoma Cells through Promoting Oxidative Stress, Apoptosis and Inflammatory Responses" Pharmaceuticals 15, no. 7: 826. https://doi.org/10.3390/ph15070826
APA StyleChen, T.-H., Chang, C.-C., Houng, J.-Y., Chang, T.-H., Chen, Y.-L., Hsu, C.-C., & Chang, L.-S. (2022). Suppressive Effects of Siegesbeckia orientalis Ethanolic Extract on Proliferation and Migration of Hepatocellular Carcinoma Cells through Promoting Oxidative Stress, Apoptosis and Inflammatory Responses. Pharmaceuticals, 15(7), 826. https://doi.org/10.3390/ph15070826







