MgAl and ZnAl-Hydrotalcites as Materials for Cosmetic and Pharmaceutical Formulations: Study of Their Cytotoxicity on Different Cell Lines
Abstract
:1. Introduction
2. Results and Discussions
2.1. Cell Lines Choice
2.2. ICP-OES Analysis and pH Measurement
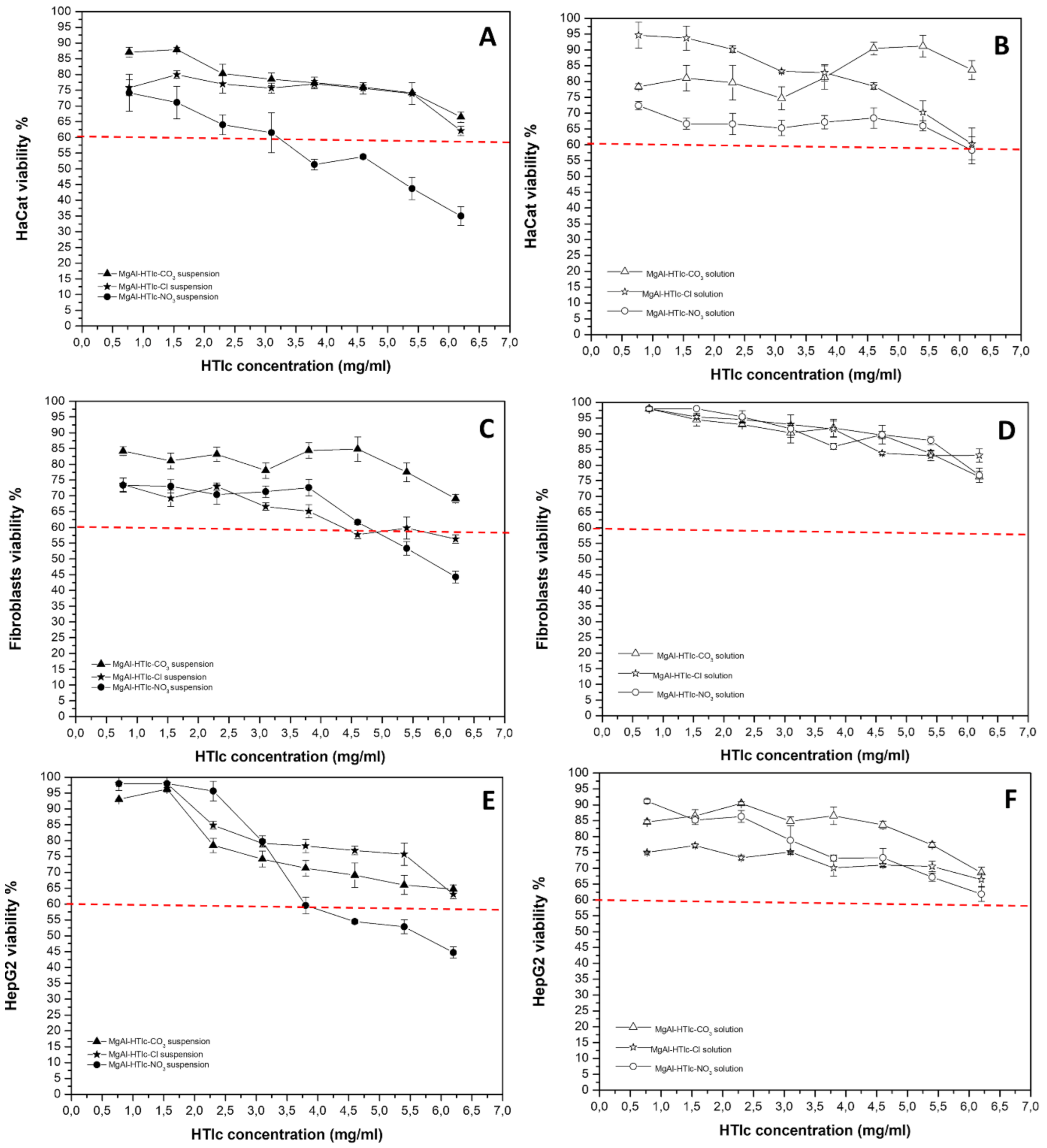
2.3. Cytotoxicity Studies on MgAl-HTlc Samples
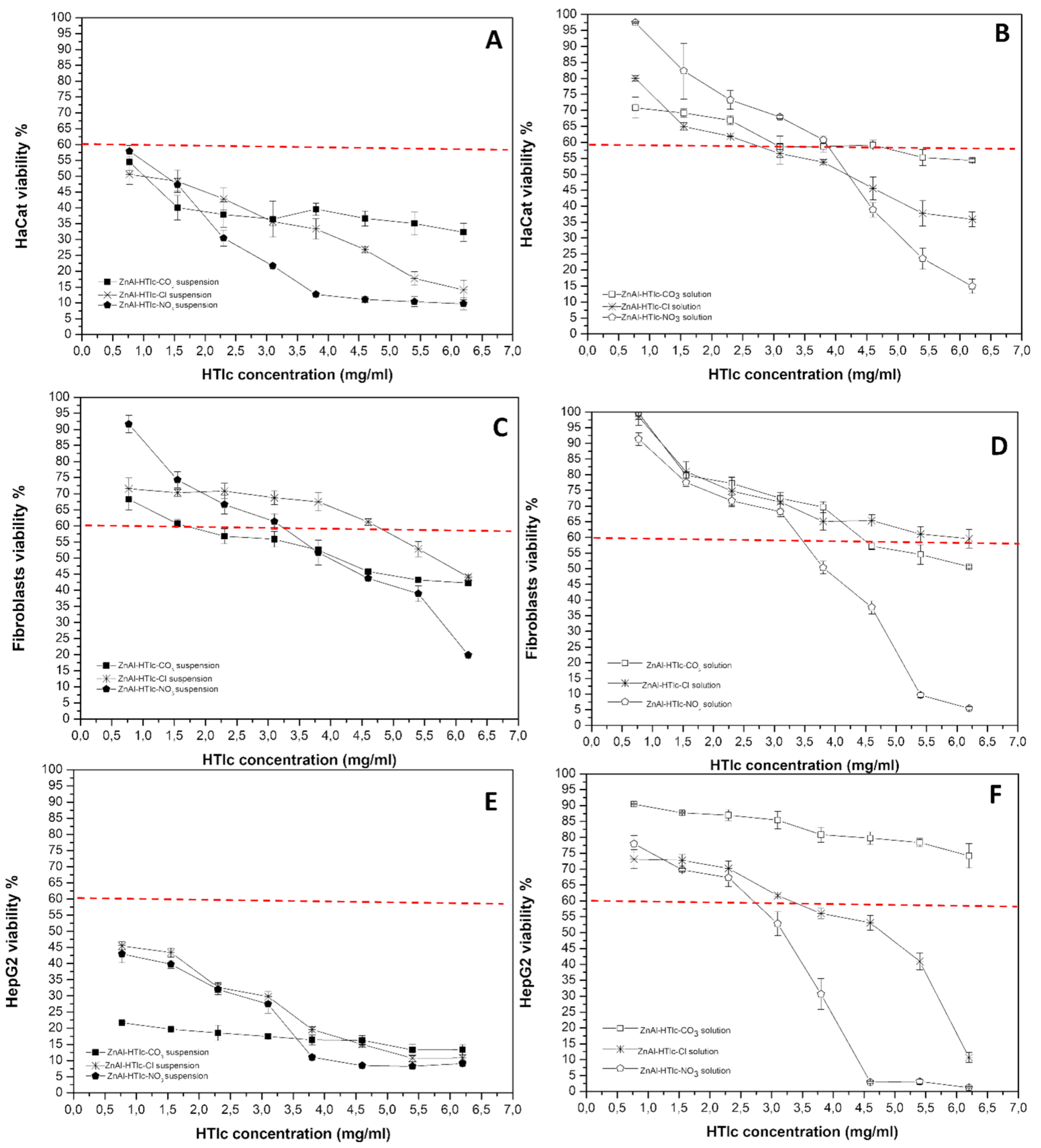
2.4. Cytotoxicity Studies on ZnAl-HTlc Samples
3. Materials and Methods
3.1. Materials
3.2. Hydrotalcites Syntheses
3.3. X-ray Analysis
3.4. Metal Composition and pH Measurement
3.5. Thermogravimetric Analysis (TGA)
3.6. Single Particle Optical Sensing Analysis
3.7. SEM and TEM Analyses
3.8. Samples Preparation
3.9. Cell Culture
3.10. Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Costantino, U.; Costantino, F.; Elisei, F.; Latterini, L.; Nocchetti, M. Coupling physical chemical techniques with hydrotalcite-like compounds to exploit their structural features and new multifunctional hybrids with luminescent properties. Phys. Chem. Chem. Phys. 2013, 15, 13254–13269. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Mosangi, D.; Pillai, S. Layered Double Hydroxide-Based Functional Nanohybrids as Controlled Release Carriers of Pharmaceutically Active Ingredients. Chem. Rec. 2018, 18, 913–927. [Google Scholar] [CrossRef]
- Rives, V.; del Arco, M.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88, 239–269. [Google Scholar] [CrossRef]
- Miyata, S. Gastric ulcer therapeutic agent. US Patent US20110206779A1, 25 August 2011. [Google Scholar]
- Ballarin, B.; Mignani, A.; Mogavero, F.; Gabbanini, S.; Morigi, M. Hybrid material based on ZnAl hydrotalcite and silver nanoparticles for deodorant formulation. Appl. Clay Sci. 2015, 114, 303–308. [Google Scholar] [CrossRef]
- Khurana, I.S.; Kaur, S.; Kaur, H.; Khurana, R.K. Multifaceted role of clay minerals in pharmaceuticals. Futur. Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Li, Y.; Bi, H.Y.; Zhou, C.Y. Effects of Mg: Al molar ratio on the rheology of aqueous suspensions containing cationic starch and Mg/Al-hydrotalcite-like compounds. Russ. Chem. Bull. 2015, 64, 2422–2426. [Google Scholar] [CrossRef]
- Perioli, L.; Pagano, C.; Nocchetti, M.; Latterini, L. Development of Smart Semisolid Formulations to Enhance Retinoic Acid Topical Application. J. Pharm. Sci. 2015, 104, 3904–3912. [Google Scholar] [CrossRef]
- Baek, M.; Kim, I.-S.; Yu, J.; Chung, H.E.; Choy, J.-H.; Choi, S.-J. Effect of Different Forms of Anionic Nanoclays on Cytotoxicity. J. Nanosci. Nanotechnol. 2011, 11, 1803–1806. [Google Scholar] [CrossRef]
- Jin, W.; Lee, D.; Jeon, Y.; Park, D.H. Biocompatible hydrotalcite nanohybrids for medical functions. Minerals 2020, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Baek, M.; Chung, H.-E.; Choi, S.-J. Physicochemical properties affecting the potentialin vitro cytotoxicity of inorganic layered nanoparticles. Toxicol. Environ. Health Sci. 2010, 2, 149–152. [Google Scholar] [CrossRef]
- Choi, G.; Eom, S.; Vinu, A.; Choy, J.H. 2D Nanostructured Metal Hydroxides with Gene Delivery and Theranostic Functions; A Comprehensive Review. Chem. Rec. 2018, 18, 1033–1053. [Google Scholar] [CrossRef] [PubMed]
- Forano, C.; Bruna, F.; Mousty, C.; Prevot, V. Interactions between Biological Cells and Layered Double Hydroxides: Towards Functional Materials. Chem. Rec. 2018, 18, 1150–1166. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, K.; Zhi, P.X.; Gao, Q.L. Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin. Drug Deliv. 2009, 6, 907–922. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Structure-performance correlations of Mg-Al hydrotalcite catalysts for the isomerization of glucose into fructose. J. Catal. 2015, 327, 1–9. [Google Scholar] [CrossRef]
- Omonmhenle, S.I.; Shannon, I.J. Effect of Interlamelar Composition on ZnAl Hydrotalcites: Synthesis and Characterization. Eur. Sci. J. ESJ 2019, 15, 307–317. [Google Scholar] [CrossRef]
- Pagano, C.; Latterini, L.; Di Michele, A.; Luzi, F.; Puglia, D.; Ricci, M.; Iborra, C.A.V.; Perioli, L. Polymeric bioadhesive patch based on ketoprofen-hydrotalcite hybrid for local treatments. Pharmaceutics 2020, 12, 733. [Google Scholar] [CrossRef]
- Costantino, U.; Ambrogi, V.; Nocchetti, M.; Perioli, L. Hydrotalcite-like compounds: Versatile layered hosts of molecular anions with biological activity. Microporous Mesoporous Mater. 2008, 107, 149–160. [Google Scholar] [CrossRef]
- Xu, Z.P.; Lu, G.Q. Layered double hydroxide nanomaterials as potential cellular drug delivery agents. Pure Appl. Chem. 2006, 78, 1771–1779. [Google Scholar] [CrossRef]
- Fischer, J.; Pröfrock, D.; Hort, N.; Willumeit, R.; Feyerabend, F. Improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng. B 2011, 176, 830–834. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Z.; Liu, X.; Huang, T.; Xi, T.; Zheng, Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng. C 2015, 46, 202–206. [Google Scholar] [CrossRef]
- Mailloux, R.; Lemire, J.; Appanna, V. Aluminum-Induced Mitochondrial Dysfunction Leads to Lipid Accumulation in Human Hepatocytes: A Link to Obesity. Cell. Physiol. Biochem. 2007, 20, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Kopač, I.; Sterle, M.; Marion, L. Electron microscopic analysis of the effects of chemical retraction agents on cultured rat keratinocytes. J. Prosthet. Dent. 2002, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Abraham, A.N.; Shukla, R.; Drummond, C.J.; Greaves, T.L. Cytotoxicity of protic ionic liquids towards the HaCat cell line derived from human skin. J. Mol. Liq. 2020, 314, 113602. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Foxx, D.N.; Ishaque, A.B.; Shen, E. Lead-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG2) cells. Mol. Cell. Biochem. 2004, 255, 161–170. [Google Scholar] [CrossRef]
- Holmes, A.M.; Mackenzie, L.; Roberts, M.S. Disposition and measured toxicity of zinc oxide nanoparticles and zinc ions against keratinocytes in cell culture and viable human epidermis. Nanotoxicology 2020, 14, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; James, S.A.; De jonge, M.D.; Turney, T.W.; Wright, P.F.A.; Feltis, B.N. Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle-exposed human immune cells. Toxicol. Sci. 2013, 136, 120–130. [Google Scholar] [CrossRef]
- Agren, M.S.; Mirastschijski, U. The release of zinc ions from and cytocompatibility of two zinc oxide dressings. J. Wound Care 2004, 13, 367–369. [Google Scholar]
- Yan, D.; Long, J.; Liu, J.; Cao, Y. The toxicity of ZnO nanomaterials to HepG2 cells: The influence of size and shape of particles. J. Appl. Toxicol. 2019, 39, 231–240. [Google Scholar] [CrossRef]
- Posati, T.; Bellezza, F.; Tarpani, L.; Perni, S.; Latterini, L.; Marsili, V.; Cipiciani, A. Selective internalization of ZnAl-HTlc nanoparticles in normal and tumor cells. A study of their potential use in cellular delivery. Appl. Clay Sci. 2012, 55, 62–69. [Google Scholar] [CrossRef]
- Kaur, J.; Khalid, A.; Sartaj, A.; Prasad Panda, B.; Ali, A.; Zafar, A.; Kumar, V.; Jamal Gilani, S.; Kala, C.; Taleuzzaman, M. ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 1450. [Google Scholar] [CrossRef] [PubMed]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New Synthetic Routes to Hydrotalcite-Like Compounds − Characterisation and Properties of the Obtained Materials. Eur. J. Inorg. Chem. 1998, 1998, 1439–1446. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Vannini, S.; Cataldi, S.; Moretti, M.; Villarini, M.; Fioretti, B.; Albi, E.; Beccari, T.; Codini, M. In Vitro Protective Effects of Lycium barbarum Berries Cultivated in Umbria (Italy) on Human Hepatocellular Carcinoma Cells. Biomed Res. Int. 2016, 2016, 7529521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarini, M.R.; Codini, M.; Cataldi, S.; Vannini, S.; Lazzarini, A.; Floridi, A.; Moretti, M.; Villarini, M.; Fioretti, B.; Beccari, T.; et al. Acid sphingomyelinase as target of Lycium Chinense: Promising new action for cell health. Lipids Health Dis. 2016, 15, 183. [Google Scholar] [CrossRef] [Green Version]
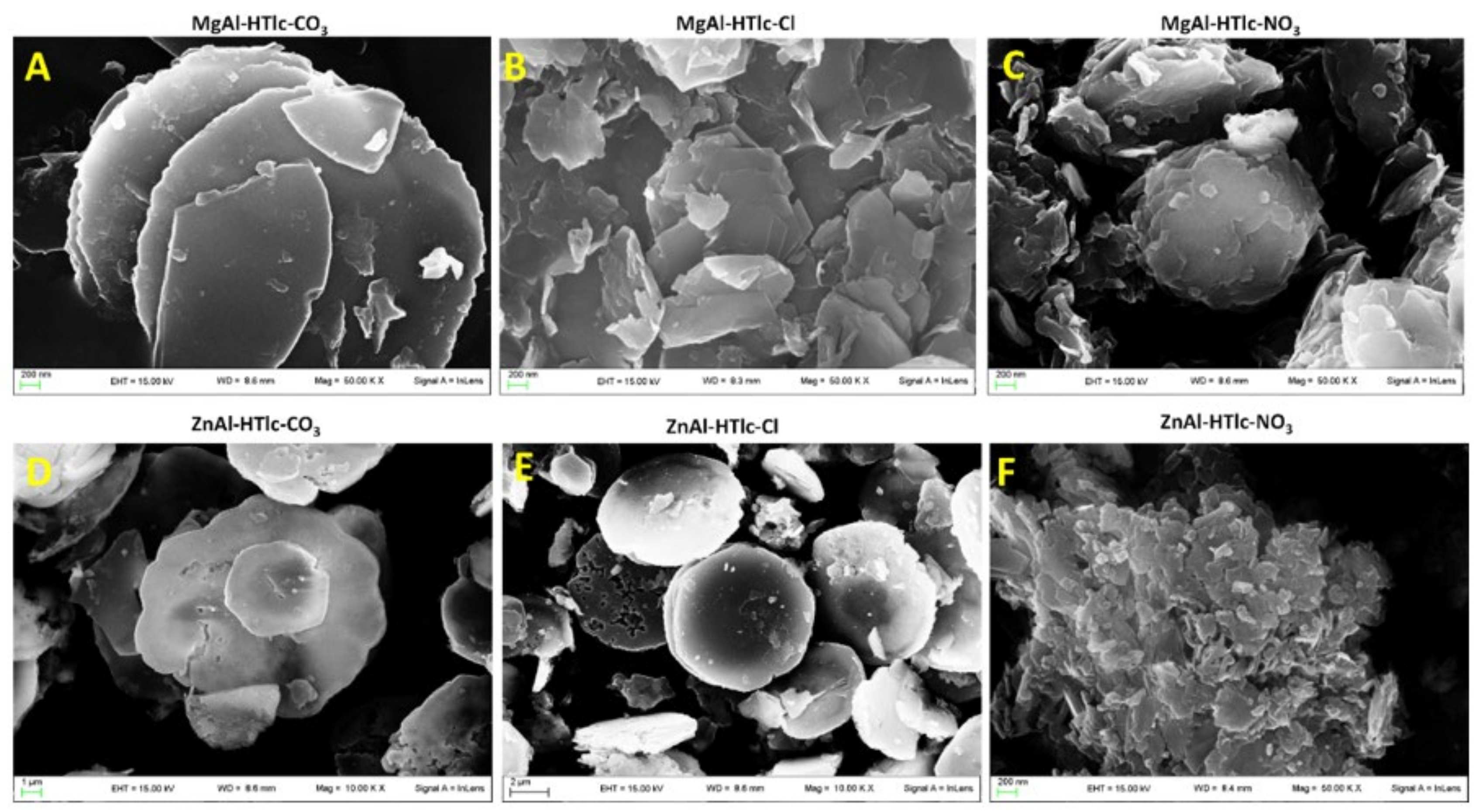
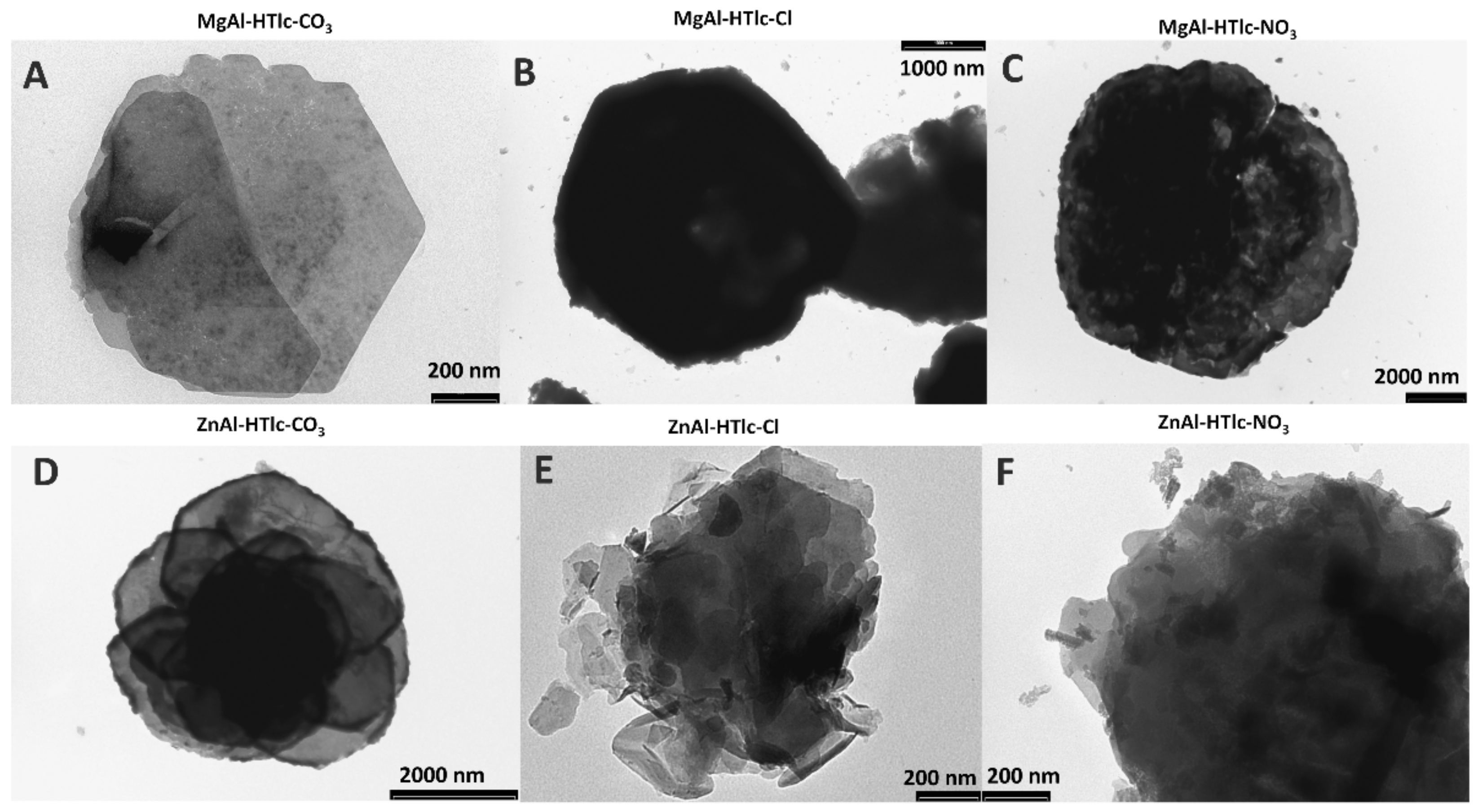
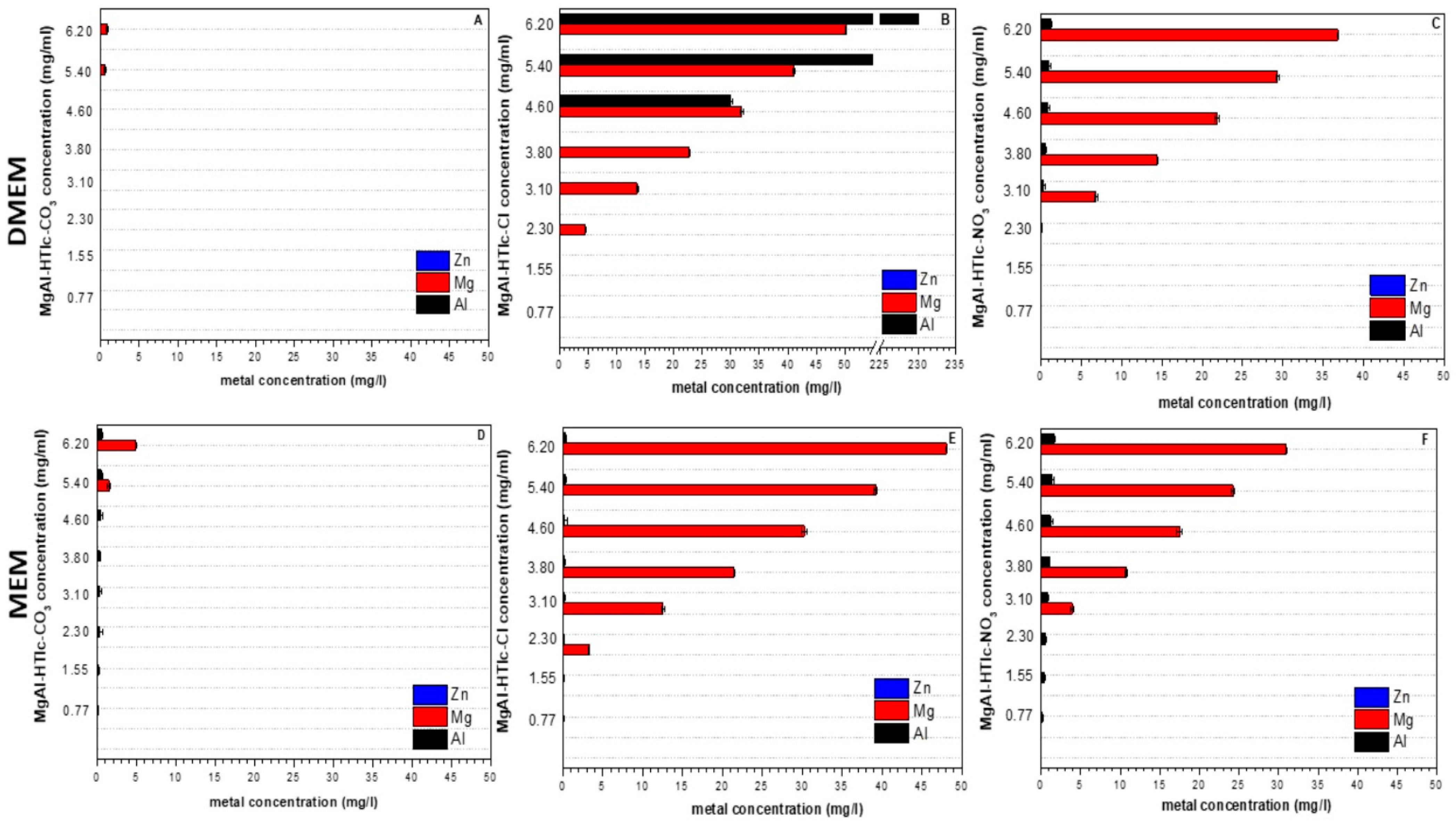
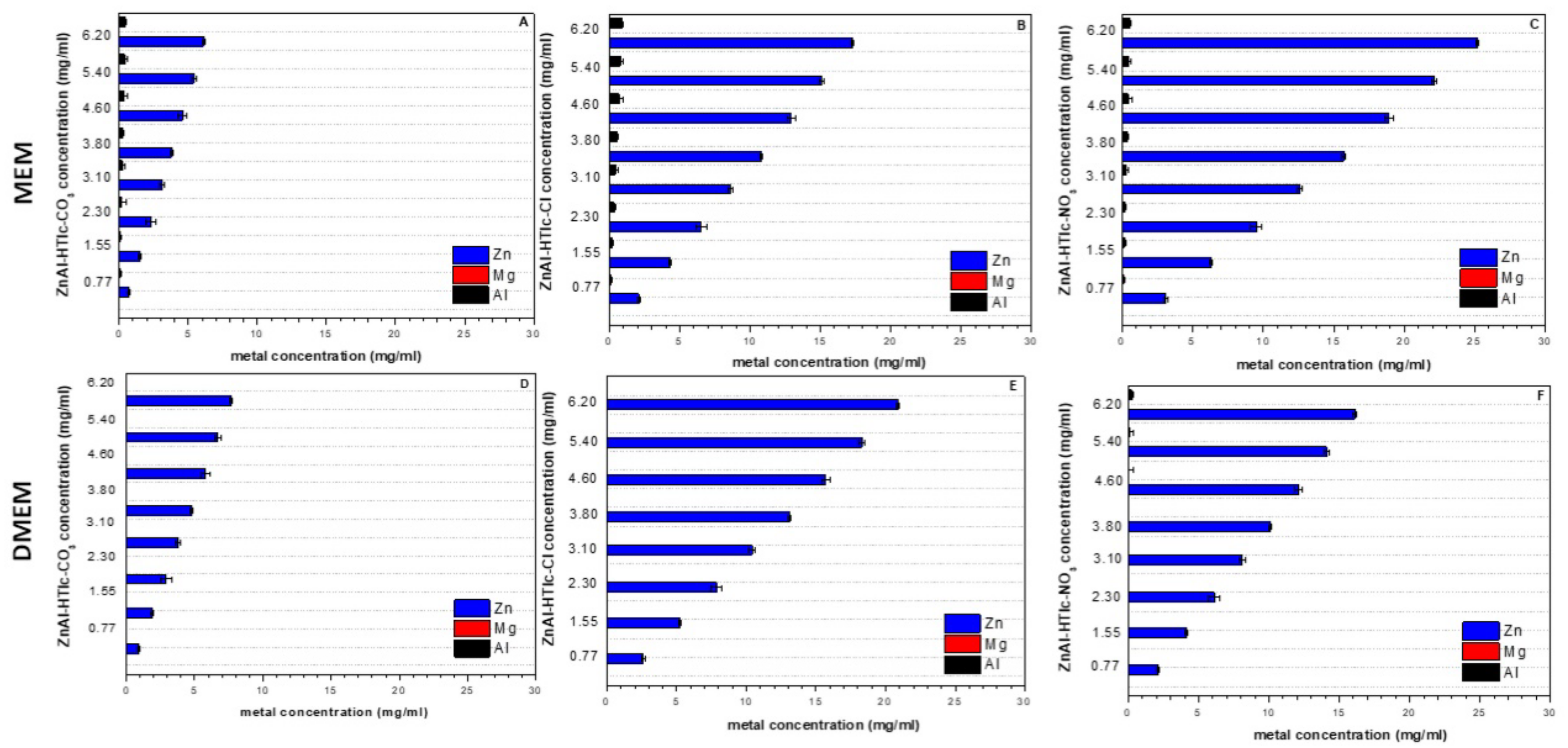
| Sample | Interlayer Distance (Å) | Formula |
|---|---|---|
| ZnAl-HTlc-CO3 | 7.5 | [Zn0.65Al0.35(OH)2](CO3)0.175•0.4 H2O |
| ZnAl-HTlc-Cl | 7.8 | [Zn0.62Al0.38(OH)2](Cl)0.38•0.6 H2O |
| ZnAl-HTlc-NO3 | 8.9 | [Zn0.69Al0.31(OH)2](NO3)0.31•0.5 H2O |
| MgAl-HTlc-CO3 | 7.5 | [Mg0.67Al0.33(OH)2](CO3)0.165•0.4 H2O |
| MgAl-HTlc-Cl | 7.8 | [Mg0.60Al0.40(OH)2](Cl)0.40•0.6 H2O |
| MgAl-HTlc-NO3 | 8.9 | [Mg0.63Al0.37(OH)2](NO3)0.37•0.5 H2O |
| Sample | SPOS | |||
|---|---|---|---|---|
| D10 (µm) ± SD | D50 (µm) ± SD | D90 (µm) ± SD | Mode (µm) ± SD | |
| MgAl-HTlc-CO3 | 3.99 ± 0.03 | 8.50 ± 0.11 | 13.93 ± 0.03 | 9.47 ± 0.01 |
| MgAl-HTlc-Cl | 2.33 ± 0.15 | 8.50 ± 0.13 | 16.24 ± 0.17 | 8.97 ± 0.11 |
| MgAl-HTlc-NO3 | 3.78 ± 0.23 | 9.47 ± 0.05 | 35.68 ± 0.19 | 8.50 ± 0.02 |
| ZnAl-HTlc-CO3 | 8.50 ± 0.13 | 14.42 ± 0.08 | 23.70 ± 0.08 | 13.82 ± 0.05 |
| ZnAl-HTlc-Cl | 4.45 ± 0.19 | 7.63 ± 0.12 | 13.09 ± 0.09 | 7.63 ± 0.03 |
| ZnAl-HTlc-NO3 | 3.80 ± 0.22 | 5.99 ± 0.02 | 9.01 ± 0.05 | 5.99 ± 0.06 |
| Sample | pH (MEM) | pH (DMEM) |
|---|---|---|
| MgAl-HTlc-CO3 | 7.50 ±0.01 | 8.05 ± 0.04 |
| MgAl-HTlc-Cl | 7.40 ± 0.02 | 7.40 ± 0.02 |
| MgAl-HTlc-NO3 | 7.27 ± 0.03 | 7.45 ± 0.01 |
| ZnAl-HTlc-CO3 | 7.51 ±0.01 | 8.16 ± 0.03 |
| ZnAl-HTlc-Cl | 7.40 ± 0.01 | 7.45 ± 0.02 |
| ZnAl-HTlc-NO3 | 7.40 ± 0.02 | 7.46 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarini, M.R.; Puccetti, M.; Pagano, C.; Nocchetti, M.; Beccari, T.; di Michele, A.; Ricci, M.; Perioli, L. MgAl and ZnAl-Hydrotalcites as Materials for Cosmetic and Pharmaceutical Formulations: Study of Their Cytotoxicity on Different Cell Lines. Pharmaceuticals 2022, 15, 784. https://doi.org/10.3390/ph15070784
Ceccarini MR, Puccetti M, Pagano C, Nocchetti M, Beccari T, di Michele A, Ricci M, Perioli L. MgAl and ZnAl-Hydrotalcites as Materials for Cosmetic and Pharmaceutical Formulations: Study of Their Cytotoxicity on Different Cell Lines. Pharmaceuticals. 2022; 15(7):784. https://doi.org/10.3390/ph15070784
Chicago/Turabian StyleCeccarini, Maria Rachele, Matteo Puccetti, Cinzia Pagano, Morena Nocchetti, Tommaso Beccari, Alessandro di Michele, Maurizio Ricci, and Luana Perioli. 2022. "MgAl and ZnAl-Hydrotalcites as Materials for Cosmetic and Pharmaceutical Formulations: Study of Their Cytotoxicity on Different Cell Lines" Pharmaceuticals 15, no. 7: 784. https://doi.org/10.3390/ph15070784
APA StyleCeccarini, M. R., Puccetti, M., Pagano, C., Nocchetti, M., Beccari, T., di Michele, A., Ricci, M., & Perioli, L. (2022). MgAl and ZnAl-Hydrotalcites as Materials for Cosmetic and Pharmaceutical Formulations: Study of Their Cytotoxicity on Different Cell Lines. Pharmaceuticals, 15(7), 784. https://doi.org/10.3390/ph15070784









