Multifunctional Gel Films of Marine Polysaccharides Cross-Linked with Poly-Metal Ions for Wound Healing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation Mechanism of COS/SA/nM2+ PDF
2.2. Chemical Structure and Property Analysis of COS/SA/nM2+ PDF
2.2.1. Surface Appearance
2.2.2. FTIR Analysis
2.2.3. XRD Analysis
2.2.4. Contact Angle
2.2.5. Inflationary Behavior
2.2.6. Porosity
2.2.7. Thermal Stability
2.3. Mechanical Properties of COS/SA/nM2+ PDF
2.4. Antioxidant Activity of COS/SA/nM2+ PDF
2.5. Antibacterial Properties of COS/SA/nM2+ PDF
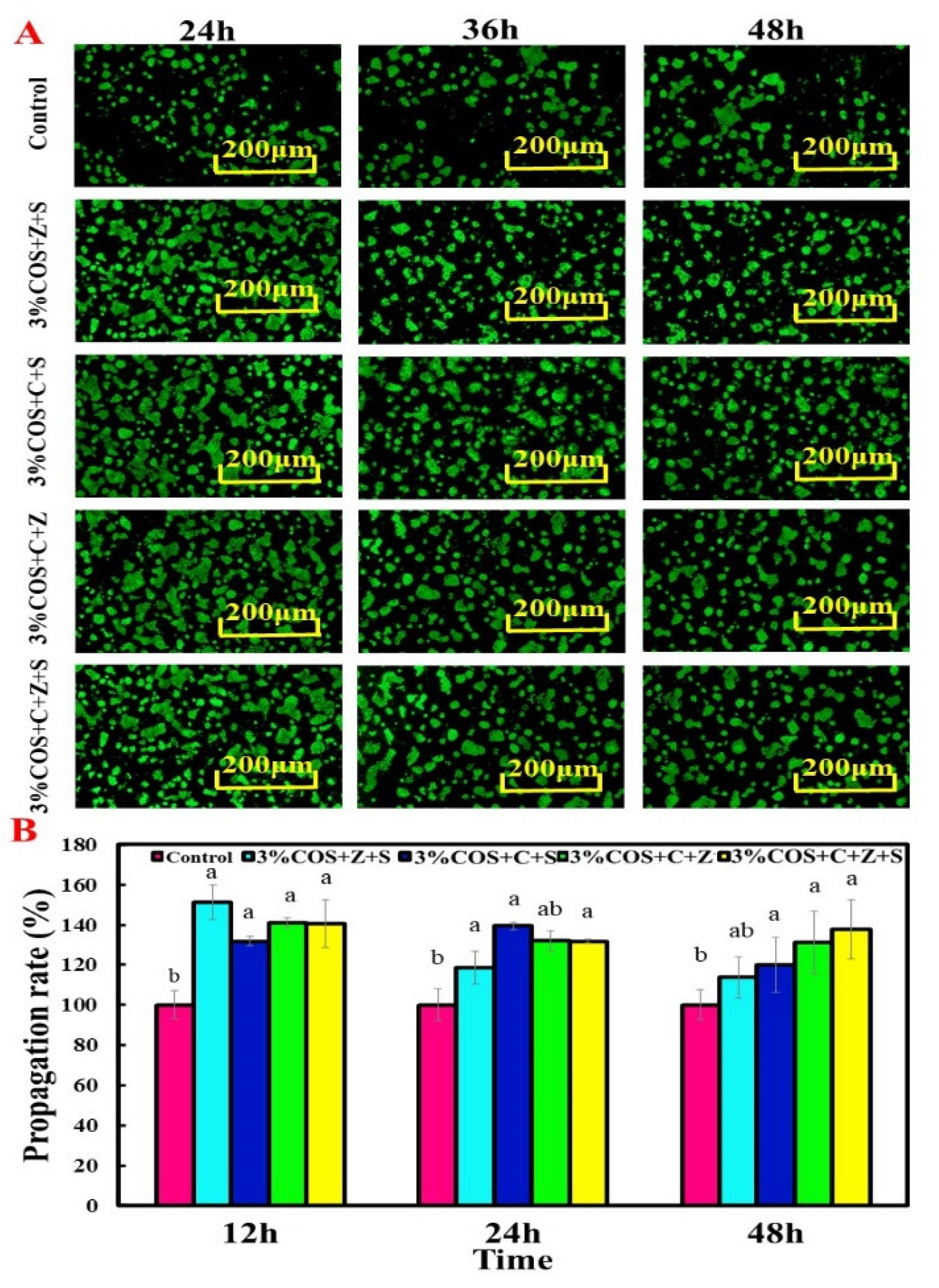
2.6. Cytocompatibility of COS/SA/nM2+ PDF
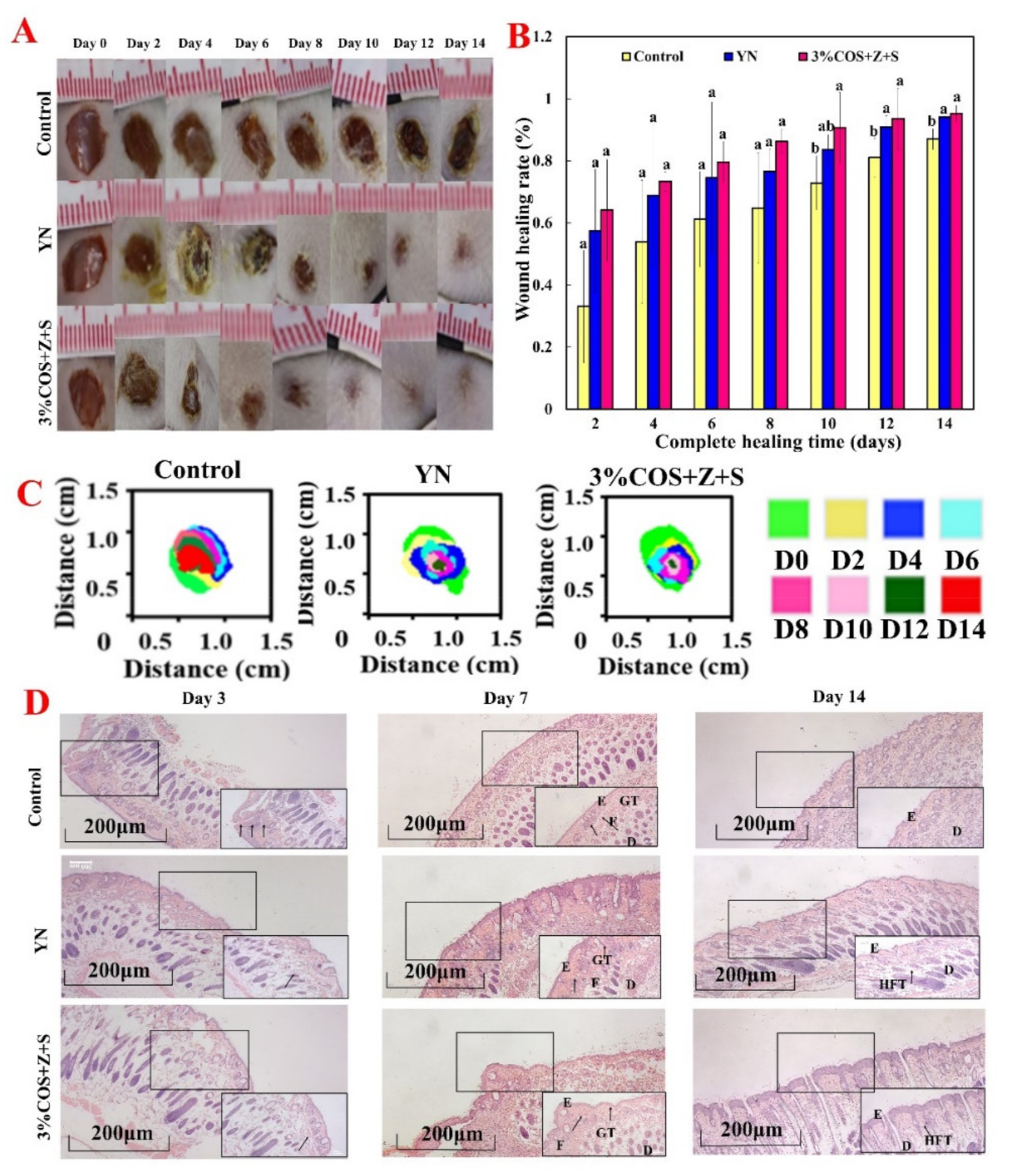
2.7. In Vivo Healing Promotion Experiment of COS/SA/nM2+ PDF
2.7.1. Wound Healing Assessment
2.7.2. Histological Evaluation of Regenerated Tissue
2.7.3. Expression of FGF and CD31 during Wound Healing
3. Materials and Methods
3.1. Materials
3.2. Preparation Process of nIon2+-COS/SA Gel Films
- (1)
- A certain amount of COS and SA was weighed and deionized water was added to prepare a mass fraction of 3% COS with a mass fraction of 2% SA.
- (2)
- The SA solution with a mass fraction of 2% was heated at 90°C for 3 h. The COS solution was adjusted to pH 5.6 with GDL and then stirred at room temperature for 1 h.
- (3)
- CaCl2-ZnCl2, CaCl2-SrCl2, SrCl2-ZnCl2, and CaCl2-ZnCl2-SrCl2 powders with each metal ion concentration of 1% were added to the 3% COS solution and stirred at room temperature for 5 min.
- (4)
- After that, 12 mL of SA solution with 2% mass fraction was poured into the prefabricated mold (The length, width and height were 10 cm, 5 cm and 1 cm respectively, for the Teflon plane) by the extended flow method.
- (5)
- The solution in step (4) was loaded into a medical spray bottle, left to remove air bubbles, and then sprayed evenly on the surface of the SA solution at a distance of 15–20 cm from the mold. The thickness of the composite hydrogel can be controlled by the sprayed solution due to the height limitation of the mold with the adjustment of the number of sprays. After extensive testing, it was determined that the number of coats required to fully cross-link the hydrogel and to form a more uniform film was 15.
- (6)
- After spraying, the molds were left to stand for 10 min, and the COS/SA/nM2+ PDFs were gradually formed. These gel films were named as 3%COS+C+Z, 3%COS+C+S, 3%COS+Z+S and 3%COS+C+Z+S, respectively.
3.3. SEM Morphological Analysis
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. XRD
3.6. Contact Angle
3.7. Inflationary Behavior
3.8. Porosity Measurements
3.9. Thermal Stability
3.10. Mechanical Properties
3.11. Antioxidant Activity Analysis
3.11.1. FRAP Measurement
3.11.2. DPPH Free Radical Scavenging Activity
3.11.3. ABTS Free Radical Scavenging Ability
3.12. Antibacterial Activity Analysis
3.13. Cellular Experiments
3.13.1. Cell Viability Assay
3.13.2. Cell Proliferation Assay
3.14. In Vivo Healing Promotion Experiment
3.14.1. Laboratory Animals
3.14.2. Trauma Modeling
3.14.3. Animal observation
3.14.4. Histological Assessment
3.14.5. Immunohistochemical Analysis
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coles, M.C.; Buckley, C.D. Ready-made cellular plugs heal skin wounds. Nature 2019, 576, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Parfejevs, V.; Debbache, J.; Shakhova, O.; Schaefer, S.M.; Glausch, M.; Wegner, M.; Suter, U.; Riekstina, U.; Werner, S.; Sommer, L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 2018, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lin, S.Y.; Tan, X.J.; Zhu, S.O.; Nie, F.F.; Zhen, Y.H.; Gu, L.S.; Zhang, C.L.; Wang, B.C.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Proliferat 2021, 54, e12993. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Rosca, I.; Morariu, S.; Mititelu-Tartau, L.; Marin, L. Iminoboronate-chitooligosaccharides hydrogels with strong antimicrobial activity for biomedical applications. Carbohydr. Polym. 2022, 276, 118727. [Google Scholar] [CrossRef]
- Chandika, P.; Kim, M.S.; Khan, F.; Kim, Y.M.; Heo, S.Y.; Oh, G.W.; Kim, N.G.; Jung, W.K. Wound healing properties of triple cross-linked poly (vinyl alcohol)/methacrylate kappa-carrageenan/chitooligosaccharide hydrogel. Carbohydr. Polym. 2021, 269, 118272. [Google Scholar] [CrossRef]
- Chen, Y.; Ling, Z.; Mamtimin, T.; Khan, A.; Peng, L.; Yang, J.; Ali, G.; Zhou, T.; Zhang, Q.; Zhang, J.; et al. Chitooligosaccharides production from shrimp chaff in chitosanase cell surface display system. Carbohydr. Polym. 2022, 277, 118894. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Y.; Huang, L.; Wang, S.; Hao, C.; Sun, L.; Zhang, Y.; Wang, W.; Li, C. The inhibition effects and mechanisms of sulfated chitooligosaccharides on influenza A virus in vitro and in vivo. Carbohydr. Polym. 2022, 286, 119316. [Google Scholar] [CrossRef]
- Ohm, Y.; Pan, C.F.; Ford, M.J.; Huang, X.N.; Liao, J.H.; Majidi, C. An electrically conductive silver-polyacrylamide-alginate hydrogel composite for soft electronics. Nat. Electron. 2021, 4, 185–192. [Google Scholar] [CrossRef]
- Suzuka, J.; Tsuda, M.; Wang, L.; Kohsaka, S.; Kishida, K.; Semba, S.; Sugino, H.; Aburatani, S.; Frauenlob, M.; Kurokawa, T.; et al. Rapid reprogramming of tumour cells into cancer stem cells on double-network hydrogels. Nat. Biomed. Eng. 2021, 5, 914–925. [Google Scholar] [CrossRef]
- Matsumae, G.; Terkawi, M.A.; Nonoyama, T.; Kurokawa, T.; Takahashi, D.; Shimizu, T.; Kadoya, K.; Gong, J.P.; Yasuda, K.; Iwasaki, N. Evaluation of biological responses to micro-particles derived from a double network hydrogel. Biomater. Sci. 2022, 10, 2182–2187. [Google Scholar] [CrossRef]
- Wu, S.; Lou, D.Y.; Wang, H.Y.; Jiang, D.Q.; Fang, X.; Meng, J.Q.; Sun, X.Y.; Li, J. One-pot synthesis of anti-freezing carrageenan/polyacrylamide double-network hydrogel electrolyte for low-temperature flexible supercapacitors (vol 435, 135057, 2022). Chem. Eng. J. 2022, 438, 135521. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Evaluation of alginate-biopolymers (protein, hydrocolloid, starch) composite microgels prepared by the spray aerosol technique as a carrier for green tea polyphenols. Food Chem. 2022, 371, 131382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.B.; Xiao, J.W.; Guan, S.W.; Geng, Z.J.; Zhao, R.F.; Gao, B.T. A hydrogen-bonded antibacterial curdlan-tannic acid hydrogel with an antioxidant and hemostatic function for wound healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Ma, J.; Liu, C.; Mao, X.; Li, J. The microbial stress responses of Escherichia coli and Staphylococcus aureus induced by chitooligosaccharide. Carbohydr. Polym. 2022, 287, 119325. [Google Scholar] [CrossRef]
- Yue, H.Y.; Shang, Z.J.; Xu, P.; Feng, D.Y.; Li, X.X. Preparation of EDTA modified chitooligosaccharide/sodium alginate/Ca2+ physical double network hydrogel by using of high-salinity oilfield produced water for adsorption of Zn2+, Ni2+ and Mn2+. Sep. Purif. Technol. 2022, 280, 119767. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.N.; Han, W.W.; Jiang, T.Z.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Lee, H.; Seo, Y.J.; Kim, J.; Bae, M.J.; Hwang, S.; Bae, J.G.; Lee, W.B.; Yoon, H. Function transformation of polymeric films through morphing of surface shapes. Chem. Eng. J. 2022, 434, 134665. [Google Scholar] [CrossRef]
- Griveau, L.; Lafont, M.; le Goff, H.; Drouglazet, C.; Robbiani, B.; Berthier, A.; Sigaudo-Roussel, D.; Latif, N.; Visage, C.L.; Gache, V.; et al. Design and characterization of an in vivo injectable hydrogel with effervescently generated porosity for regenerative medicine applications. Acta Biomater. 2022, 140, 324–337. [Google Scholar] [CrossRef]
- Wang, X.Y.; Kim, H.J. Ultra-stretchable dual-network ionic hydrogel strain sensor with moistening and anti-freezing ability. Prog. Org. Coat. 2022, 166, 106784. [Google Scholar] [CrossRef]
- Rettke, D.; Danneberg, C.; Neuendorf, T.A.; Kuhn, S.; Friedrichs, J.; Hauck, N.; Werner, C.; Thiele, J.; Pompe, T. Microfluidics-assisted synthesis and functionalization of monodisperse colloidal hydrogel particles for optomechanical biosensors. J. Mater. Chem. B 2022, 10, 1663–1674. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Bakhsh, E.M.; Chani, M.T.S. Preparation, Characterization, and Biological Features of Cactus Coated Bacterial Cellulose Hydrogels. Gels 2022, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Lakouraj, M.M.; Mojerlou, F.; Zare, E.N. Nanogel and superparamagnetic nanocomposite based on sodium alginate for sorption of heavy metal ions. Carbohydr. Polym. 2014, 106, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Vu, T.T.; Karthika, V.; Jo, S.H.; Jo, Y.J.; Seo, J.W.; Oh, C.W.; Park, S.H.; Lim, K.T. Dual cross-linked chitosan/alginate hydrogels prepared by Nb-Tz ‘click’ reaction for pH responsive drug delivery. Carbohydr. Polym. 2022, 288, 119389. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Haque, M.O.; Ahmed, F.; Pervez, M.N.; Naddeo, V.; Ahmed, M.B. Super-Adsorptive Biodegradable Hydrogel from Simply Treated Sugarcane Bagasse. Gels 2022, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.C.; Gao, K.P.; Zhao, N.; Yi, Z.R.; Jiang, C.P.; Yang, B.; Liu, J.Q. A novel flexible hydrogel electrode with a strong moisturizing ability for long-term EEG recording. J. Neural Eng. 2021, 18, 066047. [Google Scholar] [CrossRef] [PubMed]
- Kapanya, A.; Somsunan, R.; Phasayavan, W.; Molloy, R.; Jiranusornkul, S. Effect of molecular weight of poly(ethylene glycol) as humectant in interpenetrating polymer network hydrogels based on poly(sodium AMPS) and gelatin for wound dressing applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 496–506. [Google Scholar] [CrossRef]
- Pal, V.K.; Roy, S. Cooperative Metal Ion Coordination to the Short Self-Assembling Peptide Promotes Hydrogelation and Cellular Proliferation. Macromol. Biosci. 2022, 22, 2100462. [Google Scholar] [CrossRef]
- Cao, L.Q.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y.P. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Siboro, S.A.P.; Anugrah, D.S.B.; Ramesh, K.; Park, S.H.; Kim, H.R.; Lim, K.T. Tunable porosity of covalently crosslinked alginate-based hydrogels and its significance in drug release behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef]
- Lin, Z.K.; Yang, Y.R.; Liang, Z.Z.; Zeng, L.; Zhang, A.P. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef]
- Shaheen, A.; Maswal, M.; Dar, A.A. Synergistic effect of various metal ions on the mechanical, thixotropic, self-healing, swelling and water retention properties of bimetallic hydrogels of alginate. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 627, 127223. [Google Scholar] [CrossRef]
- Wang, C.C.; Yang, Y.L. Tunable volume memory poly(acrylic acid sodium) hydrogel by metal ionsd. Funct. Mater. Lett. 2022, 15, 2250010. [Google Scholar] [CrossRef]
- Shang, K.; Tao, L.X.; Jiang, S.Y.; Yan, J.H.; Hu, S.K.; Yang, G.W.; Ma, C.; Cheng, S.; Wang, X.F.; Yin, J. Highly flexible hydrogel dressing with efficient antibacterial, antioxidative, and wound healing performances. Biomater. Sci. 2022, 10, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, O.; Salinas-Moreno, Y.; Santillan-Fernandez, A.; Sumaya-Martinez, M.T. Screening antioxidant capacity of Mexican maize (Zea mays L.) landraces with colored grain using ABTS, DPPH and FRAP methods. Cereal Res. Commun. 2021, 1–9. [Google Scholar] [CrossRef]
- Jo, Y.J.; Cho, H.S.; Chun, J.Y. Antioxidant activity of beta-cyclodextrin inclusion complexes containing trans-cinnamaldehyde by DPPH, ABTS and FRAP. Food. Sci. Biotechnol. 2021, 30, 807–814. [Google Scholar] [CrossRef]
- Kim, S.; Ko, J.; Choi, J.H.; Kang, J.Y.; Lim, C.; Shin, M.; Lee, D.W.; Kim, J.W. Antigen-Antibody Interaction-Derived Bioadhesion of Bacterial Cellulose Nanofibers to Promote Topical Wound Healing. Adv. Funct. Mater. 2022, 32, 2110557. [Google Scholar] [CrossRef]
- Pei, M.J.; Peng, X.T.; Wan, T.T.; Fan, P.H.; Yang, H.J.; Liu, X.; Xu, W.L.; Zhou, Y.S.; Xiao, P. Double cross-linked poly(vinyl alcohol) microcomposite hydrogels with high strength and cell compatibility. Eur. Polym. J. 2021, 160, 110786. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.M.; Zhang, J.Q.; Qin, G.W.; Zhang, E.L. Construction of a TiO2/Cu2O multifunctional coating on Ti-Cu alloy and its influence on the cell compatibility and antibacterial properties. Surf. Coat. Technol. 2021, 421, 127438. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Cai, X.Q.; Xiang, Y.Y.; Zhao, Y.; Zhang, X.G.; Wu, Z.M. Cross-linked antifouling polysaccharide hydrogel coating as extracellular matrix mimics for wound healing. J. Mater. Chem. B 2017, 5, 2989–2999. [Google Scholar] [CrossRef]
- Zou, C.Y.; Lei, X.X.; Hu, J.J.; Jiang, Y.L.; Li, Q.J.; Song, Y.T.; Zhang, Q.Y.; Li-Ling, J.; Xie, H.Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef]
- Banza, M.; Rutto, H. Continuous fixed-bed column study and adsorption modeling removal of Ni2+, Cu2+, Zn2+ and Cd2+ ions from synthetic acid mine drainage by nanocomposite cellulose hydrogel. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2022, 57, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Rajalekshmy, G.P.; Rekha, M.R. Strontium ion cross-linked alginate-g-poly (PEGMA) xerogels for wound healing applications: In vitro studies. Carbohydr. Polym. 2021, 251, 117119. [Google Scholar] [CrossRef]
- Nardone, V.; Zonefrati, R.; Mavilia, C.; Romagnoli, C.; Ciuffi, S.; Fabbri, S.; Palmini, G.; Galli, G.; Tanini, A.; Brandi, M.L. In Vitro Effects of Strontium on Proliferation and Osteoinduction of Human Preadipocytes. Stem Cells Int. 2015, 2015, 871863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankari, L.; Fernandes, B.L.; Rebelatto, C.L.K.; Brofman, P.R.S. Evaluation of PVA hydrogel as an extracellular matrix for in vitro study of fibroblast proliferation. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 653–658. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, L.L.; Wang, T.; Wang, H.N. A pH-responsive hydrogel system based on cellulose and dopamine with controlled hydrophobic drug delivery ability and long-term bacteriostatic property. Colloid Polym. Sci. 2019, 297, 705–717. [Google Scholar] [CrossRef]
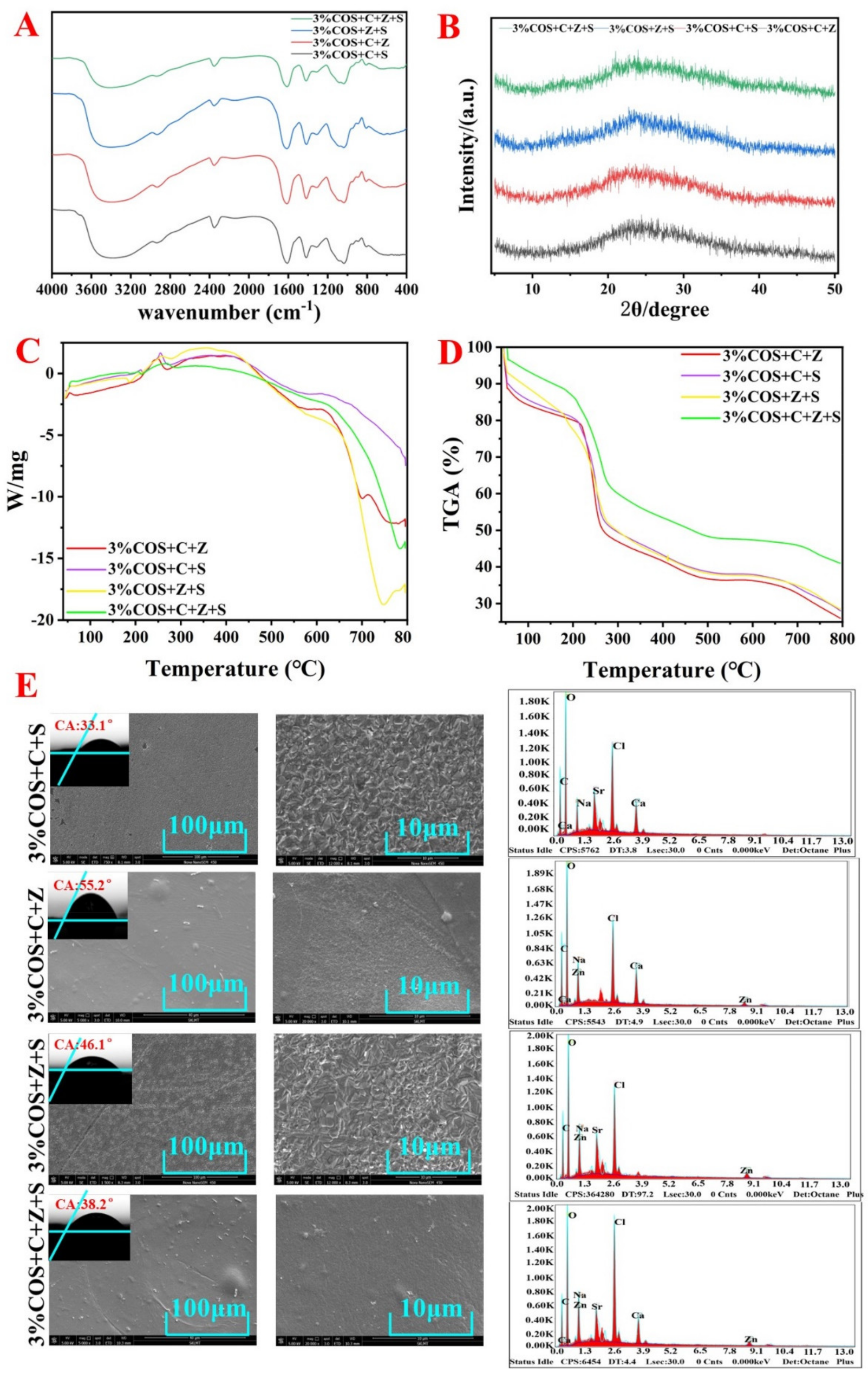



| Group | Contact Angle |
|---|---|
| 3%COS+C+S | 33.1° |
| 3%COS+C+Z | 55.2° |
| 3%COS+Z+S | 46.1° |
| 3%COS+C+Z+S | 38.2° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Shi, C.; Guo, T.; Zhang, K.; Cui, S.; Chen, L.; Yang, F.; Chen, J. Multifunctional Gel Films of Marine Polysaccharides Cross-Linked with Poly-Metal Ions for Wound Healing. Pharmaceuticals 2022, 15, 750. https://doi.org/10.3390/ph15060750
Zhao D, Shi C, Guo T, Zhang K, Cui S, Chen L, Yang F, Chen J. Multifunctional Gel Films of Marine Polysaccharides Cross-Linked with Poly-Metal Ions for Wound Healing. Pharmaceuticals. 2022; 15(6):750. https://doi.org/10.3390/ph15060750
Chicago/Turabian StyleZhao, Di, Chao Shi, Tingting Guo, Kun Zhang, Shenghao Cui, Liqi Chen, Faming Yang, and Jingdi Chen. 2022. "Multifunctional Gel Films of Marine Polysaccharides Cross-Linked with Poly-Metal Ions for Wound Healing" Pharmaceuticals 15, no. 6: 750. https://doi.org/10.3390/ph15060750
APA StyleZhao, D., Shi, C., Guo, T., Zhang, K., Cui, S., Chen, L., Yang, F., & Chen, J. (2022). Multifunctional Gel Films of Marine Polysaccharides Cross-Linked with Poly-Metal Ions for Wound Healing. Pharmaceuticals, 15(6), 750. https://doi.org/10.3390/ph15060750






