Novel High-Throughput Fluorescence-Based Assay for the Identification of Nematocidal Compounds That Target the Blood-Feeding Pathway
Abstract
1. Introduction
2. Results
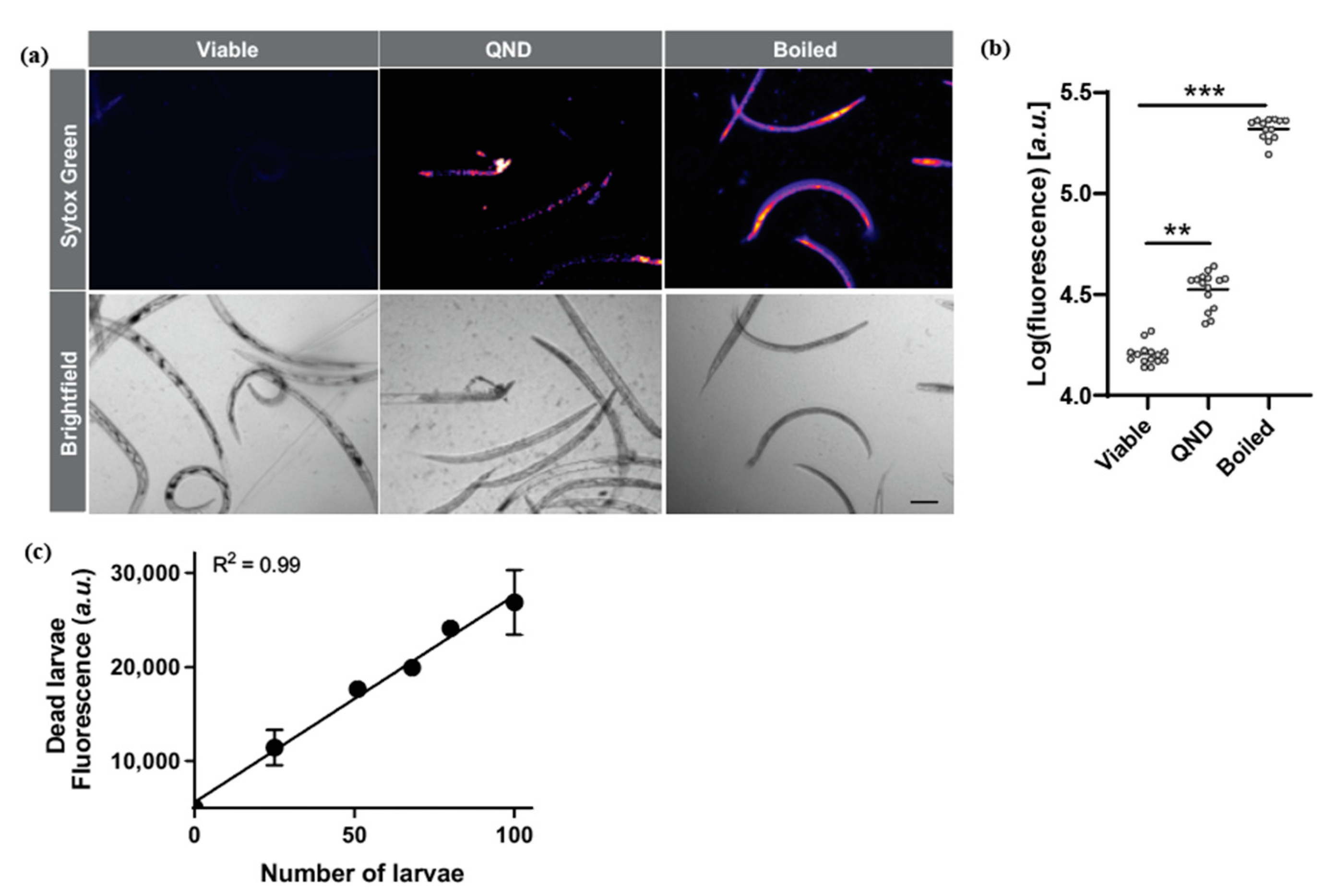
2.1. Sytox Green Is a Suitable Dye for the Screening of Drugs Targeting N. brasiliensis
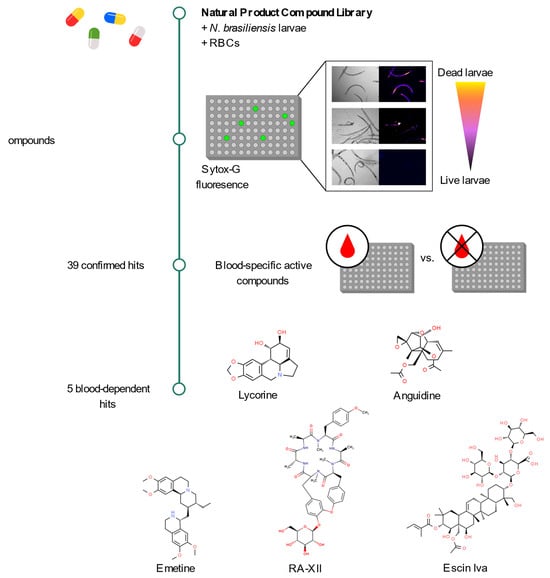
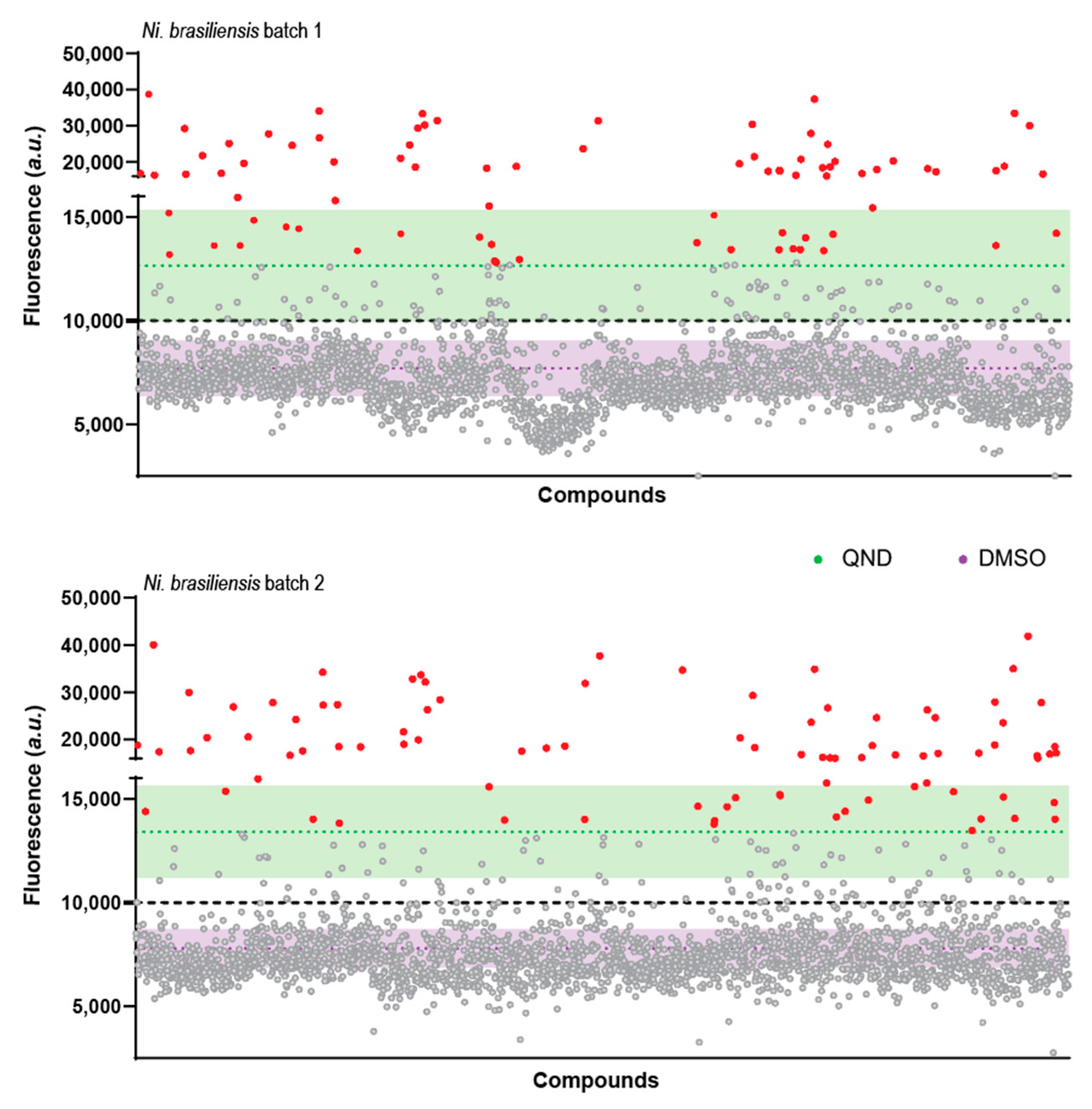
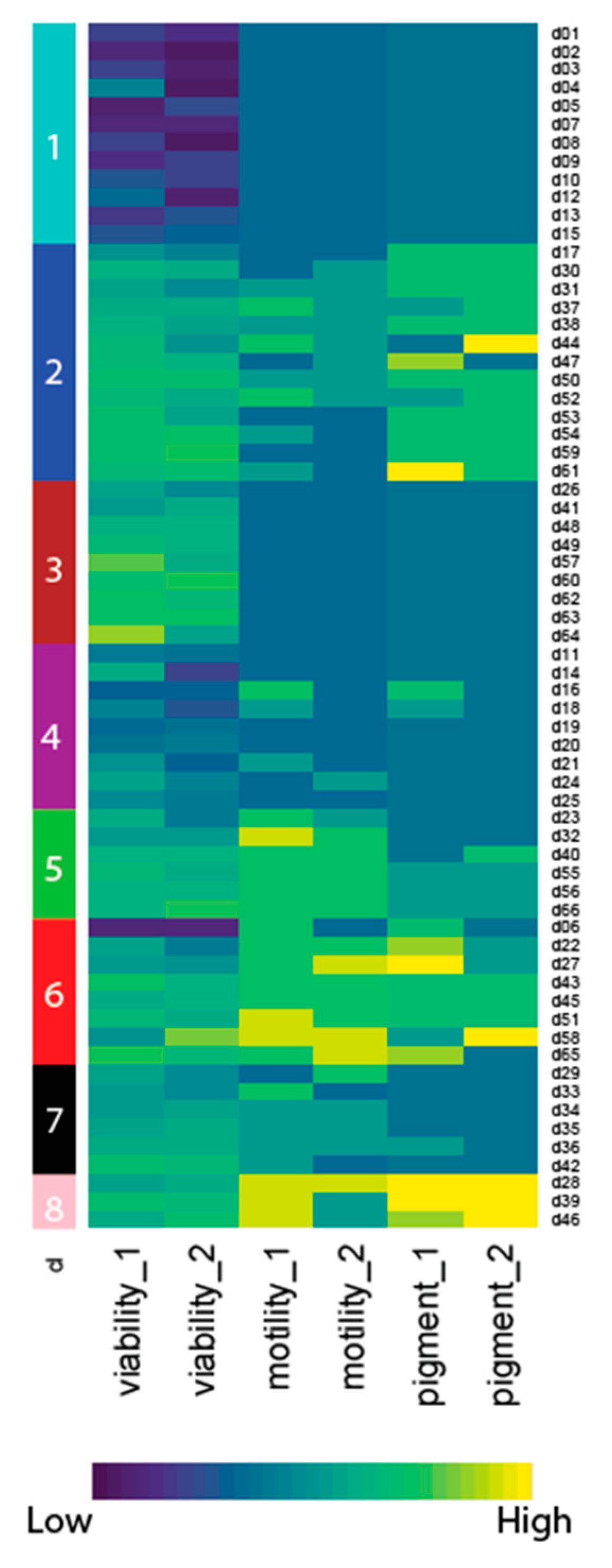
2.2. A Primary Screen Using Sytox Green Identifies 66 Compounds with Potential Anthelmintic Activity in the Presence of Blood
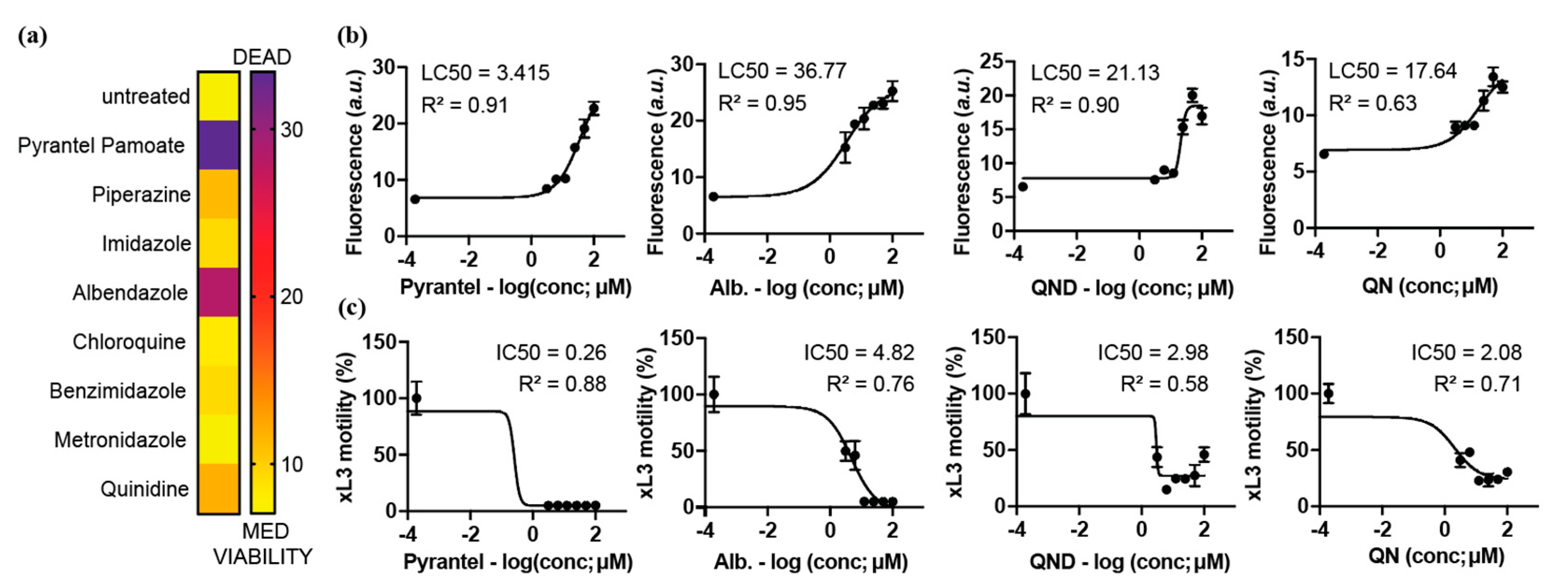
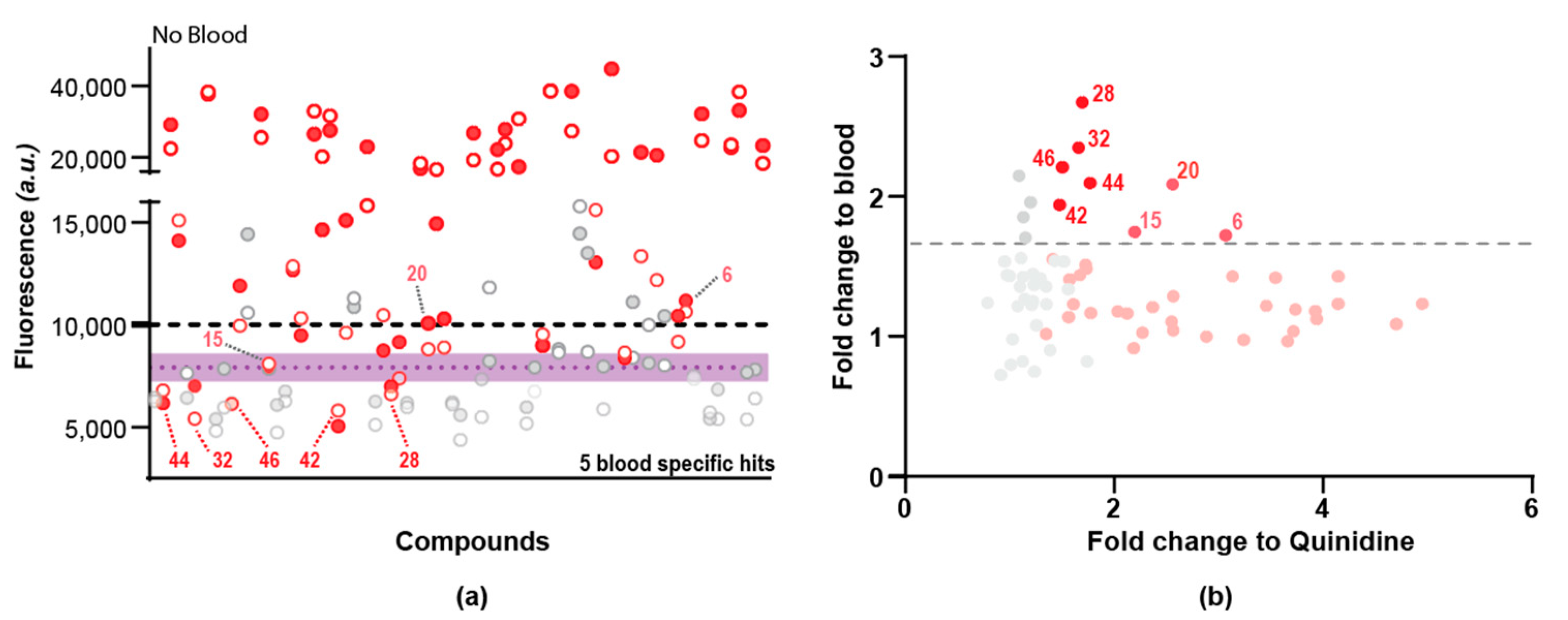
2.3. Secondary Screen Using Sytox Green Identify 5 Compounds with Specific Blood-Feeding Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of Compounds for Screening
4.2. Rats and Mice
4.3. Preparation and Isolation of N. brasiliensis
4.4. Conditions of Larval Culture with All Compounds
4.5. Sytox-Green Fluorescence Assessment
4.6. Pigmentation Assessment
4.7. Motility Assessment
4.8. Hit Identification
4.9. Statistical Assessment of the Assay Robustness
4.10. Phenotypic Clustering
4.11. Other Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Hotez, P.J.; Brooker, S.; Bethony, J.M.; Bottazzi, M.E.; Loukas, A.; Xiao, S. Hookworm infection. N. Engl. J. Med. 2004, 351, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Hotez, P.J.; Bundy, D.A. Hookworm-related anaemia among pregnant women: A systematic review. PLoS Negl. Trop. Dis. 2008, 2, e291. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Beaumier, C.M.; Gillespie, P.M.; Strych, U.; Hayward, T.; Bottazzi, M.E. Advancing a vaccine to prevent hookworm disease and anemia. Vaccine 2016, 34, 3001–3005. [Google Scholar] [CrossRef]
- Loukas, A.; Bethony, J.; Brooker, S.; Hotez, P. Hookworm vaccines: Past, present, and future. Lancet Infect. Dis. 2006, 6, 733–741. [Google Scholar] [CrossRef]
- Lacey, E. Mode of action of benzimidazoles. Parasitol. Today 1990, 6, 112–115. [Google Scholar] [CrossRef]
- Lubega, G.W.; Prichard, R.K. Interaction of benzimidazole anthelmintics with Haemonchus contortus tubulin: Binding affinity and anthelmintic efficacy. Exp. Parasitol. 1991, 73, 203–213. [Google Scholar] [CrossRef]
- Pion, S.D.S.; Chesnais, C.B.; Weil, G.J.; Fischer, P.U.; Missamou, F.; Boussinesq, M. Effect of 3 years of biannual mass drug administration with albendazole on lymphatic filariasis and soil-transmitted helminth infections: A community-based study in Republic of the Congo. Lancet Infect. Dis. 2017, 17, 763–769. [Google Scholar] [CrossRef]
- Moser, W.; Schindler, C.; Keiser, J. Efficacy of recommended drugs against soil transmitted helminths: Systematic review and network meta-analysis. BMJ 2017, 358, j4307. [Google Scholar] [CrossRef]
- Humphries, D.; Nguyen, S.; Kumar, S.; Quagraine, J.E.; Otchere, J.; Harrison, L.M.; Wilson, M.; Cappello, M. Effectiveness of Albendazole for Hookworm Varies Widely by Community and Correlates with Nutritional Factors: A Cross-Sectional Study of School-Age Children in Ghana. Am. J. Trop. Med. Hyg. 2017, 96, 347–354. [Google Scholar] [CrossRef]
- Bungiro, R.; Cappello, M. Hookworm infection: New developments and prospects for control. Curr. Opin. Infect. Dis. 2004, 17, 421–426. [Google Scholar] [CrossRef]
- Diawara, A.; Halpenny, C.M.; Churcher, T.S.; Mwandawiro, C.; Kihara, J.; Kaplan, R.M.; Streit, T.G.; Idaghdour, Y.; Scott, M.E.; Basanez, M.G.; et al. Association between response to albendazole treatment and beta-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl. Trop. Dis. 2013, 7, e2247. [Google Scholar] [CrossRef]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Shalaby, H.A. Anthelmintics Resistance; How to Overcome it? Iran. J. Parasitol. 2013, 8, 18–32. [Google Scholar] [PubMed]
- Waller, P.J. The development of anthelmintic resistance in ruminant livestock. Acta Trop. 1994, 56, 233–243. [Google Scholar] [CrossRef]
- Shepherd, C.; Wangchuk, P.; Loukas, A. Of dogs and hookworms: Man’s best friend and his parasites as a model for translational biomedical research. Parasit. Vectors 2018, 11, 59. [Google Scholar] [CrossRef]
- Bouchery, T.; Filbey, K.; Shepherd, A.; Chandler, J.; Patel, D.; Schmidt, A.; Camberis, M.; Peignier, A.; Smith, A.A.T.; Johnston, K.; et al. A novel blood-feeding detoxification pathway in Nippostrongylus brasiliensis L3 reveals a potential checkpoint for arresting hookworm development. PLoS Pathog. 2018, 14, e1006931. [Google Scholar] [CrossRef]
- Peak, E.; Hoffmann, K.F. Cross-disciplinary approaches for measuring parasitic helminth viability and phenotype. An. Acad. Bras. Cienc. 2011, 83, 649–662. [Google Scholar] [CrossRef]
- Bouchery, T.; Moyat, M.; Sotillo, J.; Silverstein, S.; Volpe, B.; Coakley, G.; Tsourouktsoglou, T.D.; Becker, L.; Shah, K.; Kulagin, M.; et al. Hookworms Evade Host Immunity by Secreting a Deoxyribonuclease to Degrade Neutrophil Extracellular Traps. Cell Host Microbe 2020, 27, 277–289.e276. [Google Scholar] [CrossRef]
- Rapin, A.; Chuat, A.; Lebon, L.; Zaiss, M.M.; Marsland, B.J.; Harris, N.L. Infection with a small intestinal helminth, Heligmosomoides polygyrus bakeri, consistently alters microbial communities throughout the murine small and large intestine. Int. J. Parasitol. 2020, 50, 35–46. [Google Scholar] [CrossRef]
- Smyth, J.D. In Vitro Cultivation of Parasitic Helminths (1990); CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Cervoni, W.A.; Oliver-Gonzalez, J. Clinical evaluation of pyrantel pamoate in helminthiasis. Am. J. Trop. Med. Hyg. 1971, 20, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Breckenridge, A.M. Clinical pharmacokinetics of anthelmintic drugs. Clin. Pharmacokinet. 1988, 15, 67–93. [Google Scholar] [CrossRef]
- Frayha, G.J. The mode of action of certain drugs against helminth parasites of man. J. Med. Liban. 1972, 25, 507–520. [Google Scholar] [PubMed]
- Mann, P.H.; Harfenist, M.; De Beer, E.J. The effectiveness of piperazine citrate against intestinal helminths of the cat and dog. J. Parasitol. 1955, 41, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Cline, B.L.; Little, M.D.; Bartholomew, R.K.; Halsey, N.A. Larvicidal activity of albendazole against Necator americanus in human volunteers. Am. J. Trop. Med. Hyg. 1984, 33, 387–394. [Google Scholar] [CrossRef]
- Dominguez, L.; Saldana, J.; Chernin, J. Use of L4 larvae of Nippostrongylus brasiliensis for the in vivo screening of anthelmintic drugs. Can. J. Vet. Res. 2000, 64, 160–163. [Google Scholar]
- Holzer, B.R.; Frey, F.J. Differential efficacy of mebendazole and albendazole against Necator americanus but not for Trichuris trichiura infestations. Eur. J. Clin. Pharmacol. 1987, 32, 635–637. [Google Scholar] [CrossRef]
- Ibarra, O.F.; Jenkins, D.C. The relevance of in vitro anthelmintic screening tests employing the free-living stages of trichostrongylid nematodes. J. Helminthol. 1984, 58, 107–112. [Google Scholar] [CrossRef]
- Preston, S.; Jabbar, A.; Nowell, C.; Joachim, A.; Ruttkowski, B.; Baell, J.; Cardno, T.; Korhonen, P.K.; Piedrafita, D.; Ansell, B.R.; et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015, 45, 333–343. [Google Scholar] [CrossRef]
- Preston, S.; Jabbar, A.; Nowell, C.; Joachim, A.; Ruttkowski, B.; Cardno, T.; Hofmann, A.; Gasser, R.B. Practical and low cost whole-organism motility assay: A step-by-step protocol. Mol. Cell Probes. 2016, 30, 13–17. [Google Scholar] [CrossRef]
- Burns, A.R.; Luciani, G.M.; Musso, G.; Bagg, R.; Yeo, M.; Zhang, Y.; Rajendran, L.; Glavin, J.; Hunter, R.; Redman, E.; et al. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015, 6, 7485. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.S.; Olsen, A.; Sampayo, J.N.; Lithgow, G.J. An automated high-throughput assay for survival of the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 2003, 35, 558–565. [Google Scholar] [CrossRef]
- Partridge, F.A.; Brown, A.E.; Buckingham, S.D.; Willis, N.J.; Wynne, G.M.; Forman, R.; Else, K.J.; Morrison, A.A.; Matthews, J.B.; Russell, A.J.; et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 8–21. [Google Scholar] [CrossRef]
- Helal, M.A.; Abdel-Gawad, A.M.; Kandil, O.M.; Khalifa, M.M.E.; Cave, G.W.V.; Morrison, A.A.; Bartley, D.J.; Elsheikha, H.M. Nematocidal Effects of a Coriander Essential Oil and Five Pure Principles on the Infective Larvae of Major Ovine Gastrointestinal Nematodes In Vitro. Pathogens 2020, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Petitte, J.M.; Lewis, M.H.; Witsil, T.K.; Huang, X.; Rice, J.W. High content analysis enables high-throughput nematicide discovery screening for measurement of viability and movement behavior in response to natural product samples. PLoS ONE 2019, 14, e0205619. [Google Scholar] [CrossRef] [PubMed]
- Tritten, L.; Silbereisen, A.; Keiser, J. In vitro and in vivo efficacy of Monepantel (AAD 1566) against laboratory models of human intestinal nematode infections. PLoS Negl. Trop. Dis. 2011, 5, e1457. [Google Scholar] [CrossRef]
- Sant’anna, V.; Vommaro, R.C.; de Souza, W. Caenorhabditis elegans as a model for the screening of anthelminthic compounds: Ultrastructural study of the effects of albendazole. Exp. Parasitol. 2013, 135, 1–8. [Google Scholar] [CrossRef]
- Loukas, A.; Gaze, S.; Mulvenna, J.P.; Gasser, R.B.; Brindley, P.J.; Doolan, D.L.; Bethony, J.M.; Jones, M.K.; Gobert, G.N.; Driguez, P.; et al. Vaccinomics for the major blood feeding helminths of humans. OMICS 2011, 15, 567–577. [Google Scholar] [CrossRef]
- Perner, J.; Gasser, R.B.; Oliveira, P.L.; Kopacek, P. Haem Biology in Metazoan Parasites—‘The Bright Side of Haem’. Trends Parasitol. 2019, 35, 213–225. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Mendes, T.A.; Bueno, L.L.; de Araujo, J.V.; Bartholomeu, D.C.; Fujiwara, R.T. A new methodology for evaluation of nematode viability. Biomed. Res. Int. 2015, 2015, 879263. [Google Scholar] [CrossRef]
- Blanchard, A.; Guegnard, F.; Charvet, C.L.; Crisford, A.; Courtot, E.; Sauve, C.; Harmache, A.; Duguet, T.; O’Connor, V.; Castagnone-Sereno, P.; et al. Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: When novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLoS Pathog. 2018, 14, e1006996. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Robertson, A.P. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology 2007, 134, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Robertson, A.P.; Buxton, S.K.; Beech, R.N.; Charvet, C.L.; Neveu, C. Levamisole receptors: A second awakening. Trends Parasitol. 2012, 28, 289–296. [Google Scholar] [CrossRef]
- Oelkers, H.A. Studies on anthelmintics. Arzneimittelforschung 1962, 12, 810–812. [Google Scholar]
- Panic, G.; Duthaler, U.; Speich, B.; Keiser, J. Repurposing drugs for the treatment and control of helminth infections. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Conner, M.W.; de Camargo, J.; Punyarit, P.; Riengropitak, S.; Rogers, A.E.; Newberne, P.M. Toxicity of anguidine in mice. Fundam. Appl. Toxicol. 1986, 7, 153–164. [Google Scholar] [CrossRef]
- Campbell, S.; Soman-Faulkner, K. Antiparasitic Drugs. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Camberis, M.; Le Gros, G.; Urban, J., Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. 2003, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Rajwa, B. Effect-Size Measures as Descriptors of Assay Quality in High-Content Screening: A Brief Review of Some Available Methodologies. Assay Drug Dev. Technol. 2017, 15, 15–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchand, A.; Van Bree, J.W.M.; Taki, A.C.; Moyat, M.; Turcatti, G.; Chambon, M.; Smith, A.A.T.; Doolan, R.; Gasser, R.B.; Harris, N.L.; et al. Novel High-Throughput Fluorescence-Based Assay for the Identification of Nematocidal Compounds That Target the Blood-Feeding Pathway. Pharmaceuticals 2022, 15, 669. https://doi.org/10.3390/ph15060669
Marchand A, Van Bree JWM, Taki AC, Moyat M, Turcatti G, Chambon M, Smith AAT, Doolan R, Gasser RB, Harris NL, et al. Novel High-Throughput Fluorescence-Based Assay for the Identification of Nematocidal Compounds That Target the Blood-Feeding Pathway. Pharmaceuticals. 2022; 15(6):669. https://doi.org/10.3390/ph15060669
Chicago/Turabian StyleMarchand, Anthony, Joyce W. M. Van Bree, Aya C. Taki, Mati Moyat, Gerardo Turcatti, Marc Chambon, Adam Alexander Thil Smith, Rory Doolan, Robin B. Gasser, Nicola Laraine Harris, and et al. 2022. "Novel High-Throughput Fluorescence-Based Assay for the Identification of Nematocidal Compounds That Target the Blood-Feeding Pathway" Pharmaceuticals 15, no. 6: 669. https://doi.org/10.3390/ph15060669
APA StyleMarchand, A., Van Bree, J. W. M., Taki, A. C., Moyat, M., Turcatti, G., Chambon, M., Smith, A. A. T., Doolan, R., Gasser, R. B., Harris, N. L., & Bouchery, T. (2022). Novel High-Throughput Fluorescence-Based Assay for the Identification of Nematocidal Compounds That Target the Blood-Feeding Pathway. Pharmaceuticals, 15(6), 669. https://doi.org/10.3390/ph15060669