Abstract
The study of cytokine storm in COVID-19 has been having different edges in accordance with the knowledge of the disease. Various cytokines have been the focus, especially to define specific treatments; however, there are no conclusive results that fully support any of the options proposed for emergency treatment. One of the cytokines that requires a more exhaustive review is the tumor necrosis factor (TNF) and its receptors (TNFRs) as increased values of soluble formats for both TNFR1 and TNFR2 have been identified. TNF is a versatile cytokine with different impacts at the cellular level depending on the action form (transmembrane or soluble) and the receptor to which it is associated. In that sense, the triggered mechanisms can be diversified. Furthermore, there is the possibility of the joint action provided by synergism between one or more cytokines with TNF, where the detonation of combined cellular processes has been suggested. This review aims to discuss some roles of TNF and its receptors in the pro-inflammatory stage of COVID-19, understand its ways of action, and let to reposition this cytokine or some of its receptors as therapeutic targets.
1. Introduction
COVID-19 is an infectious disease caused by the Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2); it was declared a pandemic by the World Health Organization (WHO) in March 2020 [1,2,3]. On 18 March 2022, the WHO reported 462,58,117 confirmed cases worldwide and 6,056,725 deaths attributed to COVID-19 [4].
Epidemiological evolution shows that of those SARS-CoV-2 infected, about 80% present mild or moderate disease, 15% of patients developed a severe disease requiring oxygen, and near to 5% evolve to critical disease where complications such as respiratory failure, acute respiratory distress syndrome, sepsis, and septic shock, thromboembolism, and even multi-organ failure can manifest [5]. To date, five VOC (variants of concern) to SARS-CoV-2 have been described: Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529), reports indicate that the Omicron infection induces the lower risk of severe disease, but it has been considered highly transmissible [6,7]. Currently, millions of people receive vaccine doses worldwide (around 10.869.655.945 doses have been administered on 18 March 2022) [4], but unfortunately, we do not have an optimal therapy for COVID-19, mainly to use in moderate to critically ill patients. Therefore, scientists worldwide join forces to understand the clinical significance of molecular events that may lead some patients to display mild symptoms or even asymptomatic while others develop a severe clinical course that may culminate in death. Although diverse therapeutic proposals have been considered, the reports of adverse events attributable to the therapy are increasing [8]. Thus, the current emergency to control the evolution from moderate-severe COVID-19 to death should propose the use of therapeutic schemes beyond what concerns antivirals only.
Since the beginning of the pandemic, cytokine levels emerged as indicative of disease progression, suggesting that the pro-inflammatory cytokines play a determining role in the pathogenesis of COVID-19. The pro-inflammatory cytokines modify the immune system and, if it is out of control, in some cases, lead to fatal consequences [9]. The COVID-19 severity is associated mainly with the development of a phenomenon called "cytokine storm," characterized by an intense inflammatory process that fails to be regulated; this phenomenon could happen both under infectious or non-infectious contexts [9,10,11].
The absence of regulatory mechanisms to modulate the inflammatory process is responsible, at least partially, for favoring a cytokine storm that progresses to organ dysfunction and even death [9,12,13]. The multicomponent profile called "storm," typical of the innate viral immunity, includes cytokines, chemokines, and reactive oxygen species [14]. Tumor necrosis factor (TNF) is one of the most critical pro-inflammatory cytokines produced by several cells such as monocytes/macrophages, natural killer (NK), neutrophils, and T-cells, among others; it is expressed mainly in the acute phase of infections and is considered a key regulator of the immune response [15]. TNF triggers complex signaling where a low level benefits the health, but high levels can promote organ damage [15,16,17]. As in all cases of ligand-receptor interactions, the function of this cytokine is closely linked to its receptors. Thus, although the effect of one cytokine does not trigger COVID-19 complications, the TNF axis demands significant attention [18,19].
2. More than One Rout Favor the Cytokine Storm in COVID-19
The concept “cytokine storm” was published for the first time in 1993 in graft-versus-host processes; later, it was appropriated in viral and some bacterial infections [11]. It has been described in diverse infectious and non-infectious diseases, but in 2020 it gained attention in the context of the COVID-19 pandemic, alluding to an exaggerated release of pro-inflammatory cytokines that leads to tissue damage [14].
Under the context of non-infectious diseases, such as those with an autoimmune origin that display a robust inflammatory process such as systemic lupus erythematosus and systemic juvenile idiopathic arthritis, the cytokine storm is associated with macrophage activation syndrome (MAS) [20]. In this context, some of the cytokines that play an essential role as a member of the cytokine storm are interleukin (IL)-6, interferon-gamma (IFN-γ), TNF, IL-1β, and IL-18, as well as some chemokines [21].
In infectious diseases such as COVID-19, the cell entry used by the virus impacts the activation of the immune response; this is related in particular to the receptors used [13]. The angiotensin-converting enzyme 2 (ACE2) is the dominant SARS-CoV-2 receptor; although coronavirus infection influences the ACE2 expression, other host-dependent conditions may impact downregulation and malfunction, such as single nucleotide polymorphisms (SNPs) and a phenomenon of compartmentalization has also been suggested [22,23,24,25]. Once the SARS-CoV-2 arrives at the pulmonary alveoli, the head of the S protein is targeted by host proteases as the type II transmembrane serine protease (TMPRSS2), which generates S1 and S2 subunits; this activation leads the S1 subunit to interact with ACE2 [26]. Then, a conformational change in the S2 subunit leads to viral/host membranes fusion to enter the cell. Other proteases such as Neuropilin1 (NRP1), cathepsin L, cathepsin B, trypsin, factor X, elastase, and furin have been described as co-factors that facilitate this process. Here is important to mention that the alveolar epithelial type 2 cells express a higher level of ACE2 in the lung; consequently, they are predominantly infected [14,27,28,29,30].
ACE2 is shedding on the membrane cell surface by the effect of TMPRSS2 and the disintegrin and metalloprotease 17 (ADAM17), and this last also is responsible for TNF, TNF receptor 1 (TNFR1), and TNF receptor 2 (TNFR2) shedding [31,32]. Reports suggest that ADAM17 and TMPRSS2 compete for ACE2, although they are cleaved at different sites, and apparently, when ACE2 is cleaved by TMPRSS2, it favors the SARS-CoV-2 cell entry, while ADAM17-cleaved ACE2 has the opposite effect because seemingly ACE2 produces soluble ACE2 that may neutralize SARS-CoV-2 infection. However, ACE2-shedding by TMPRSS2 may block the ADAM17-cleavage activity [32,33,34,35]. It is well documented that for both SARS-CoV and SARS-CoV-2 infection, TMPRSS2 efficiently promotes virus-plasma membrane fusion [34], a mechanism more efficient for viral replication than endocytosis mediated by ADAM17-cleaved ACE2 [32].
Once the virus is inside the cell, the activation of cell death mechanisms such as apoptosis, necroptosis, pyroptosis, and PANoptosis induce the release of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), which are recognized by pattern recognition receptors (PRRs), mainly Toll-like receptors (TLR); molecules expressed on natural killer cells, dendritic cells, macrophages, and neutrophils [12,14,36,37] (Figure 1A).
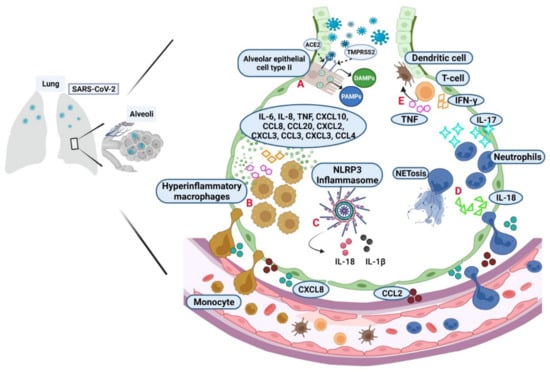
Figure 1.
Activation of the immune response during the SARS-CoV-2 infection establishment. (A) Alveolar epithelial cell type II express high levels of ACE2 used as the primary cell receptor for SARS-CoV-2 through the S protein activation by the protease TMPRSS2. PAMPs and DAMPs are the first signals to promote innate immune activation. The chemokines CXCL8 and CCL2 promote cell mobilization (CXCL refers to the C-X-C motif chemokine ligand number, where “C” is a cysteine, and “X” represents any amino acid; CCL refers to the C-C motif chemokine ligand number, where “C” is a cysteine). (B) In severe COVID-19, there are hyperinflammatory macrophages that promote the release of cytokines and chemokines like IL-6, IL-8, TNF, IFN-γ, IL-1β, CXCL10, CCL8, CCL20, CXCL2, CXCL3, CCL3, CXCL3, and CCL4. (C) PAMPs and DAMPs also favor inflammasome activation. The NLRP3 formation is the central platform for caspase-1 activation and the proteolytic maturation of IL-1β and IL-18. (D) IL-17 and IL-18 activate neutrophils in the alveolar space evolving into NETosis, which promotes more inflammation. (E) Together, all pro-inflammatory signals induce the T cell recruitment, which produces TNF and IFN-γ, and it impacts endothelial and dendritic cells. (Figure created with BioRender.com, adapted from “Cytokine storm template” by BioRender.com. Obtained with https://app.biorender.com/biorender-templates, accessed on 21 April 2022).
In the infection site, chemoattractants, such as CCL2 and CXCL8, are produced to help in cell mobilization as monocytes and neutrophils, which can also induce inflammatory mediators; epithelial and endothelial cells also contribute to both inflammation and induction of cell death mechanisms [37,38]. Single-cell immune analysis in bronchoalveolar lavage (BAL) suggested that mild COVID-19 has mainly anti-inflammatory macrophages; in contrast, in critical illness, hyperinflammatory macrophages promote the release of cytokines and chemokines like IL-6, IL-8, IL-1β, TNF, CXCL10, CCL8, CCL20, CXCL2, CXCL3, CCL3, CXCL3, and CCL4 [38,39] (Figure 1B).
Another consequence that maintains a constant inflammation status in the infection site is the inflammasome activation by the generated PAMPs and DAMPs. NLRP3 is the central protein platform for caspase-1 activation, leading to the proteolytic maturation of IL-1β and IL-18. The inflammasome induces an inflammatory cell death termed pyroptosis [12,36,40,41] (Figure 1C). IL-18 also acts as neutrophil-attracting to favor its continuous infiltration in infected lungs; thus, neutrophils are activated by IL-17, and they can form neutrophil extracellular trap (NET), a cell death type precisely in neutrophils, and it is called NETosis, where several intracellular components are released as neutrophil elastase, cathepsins, lactoferrin, myeloperoxidase, and granule proteins. Moreover, mitochondrial DNA, proteins derived from the cytoplasm and cytoskeletal, and DNA with histones may be present. NETosis has been reported as a relevant event to the immunopathology of thrombosis, apoptosis, and organ damage [42,43] (Figure 1D). The pro-inflammatory behavior produced by the events described helps T-cell recruitment, and these cells produce TNF and IFN-γ, impacting endothelial and dendritic cells [43] (Figure 1E).
Although the initial reports indicated a relationship between the severity of the disease and high inflammatory cytokine levels, recent evidence questions whether COVID-19 activates a cytokine storm. Some authors described that pro-inflammatory cytokines levels are not higher than in other pathologies with acute respiratory distress syndrome (ARDS), and others suggest that there are lower levels in COVID-19 than those observed in non-COVID-19 pathologies [10,44,45,46]. Mainly, TNF, IL-6, and IL-8 were lower in COVID-19 compared to patients with septic shock/ARDS, and due to IL-6 and IL-8 was near eightfold lower, it was suggested that cytokine storm does not characterize COVID-19, and it could be one of the reasons for the immunosuppressive therapies fail [45]. Even when there is a discrepancy in deciding whether patients with COVID-19 activate a true cytokine storm, the severity of the disease is characterized by an inflammatory process that needs to be modulated or stopped to have a better prognosis in patients.
3. Relevant Targets of the Inflammatory Response for Immunotherapy in COVID-19
Several efforts have focused on identifying cytokine profiles that predict a poor outcome to avoid mortality. In this regard, at the beginning of the pandemic, several reports converged in identifying increased levels of individual cytokines like IL-6 as potential biomarkers or targets for therapy. However, it later was proposed that the impact of the combined effect of two or more cytokines such as IL-6, IL-1β, TNF, IFN-γ, and CXCL10, among others, must be considered [9,12]. IL-6 has been one of the first references and therapeutic candidates [47]. Furthermore, various clinical protocols are carried out to evaluate the efficacy of anti-cytokine inhibitors as potential therapeutic options [1,48,49,50,51,52]. Nevertheless, the mechanism of action is not fully understood, in addition to the contradictory or adverse events observed [8].
The conventional approved immunotherapy to mediate neutralizing the inflammatory function through monoclonal antibodies (mAbs) has been used in different pathologies [53]. mAbs are produced by one B-cell clone and represent an efficient therapeutic intervention against several diseases because they offer strict specificity and a strong affinity to the target. As a kind of passive immunotherapy, mAbs act through different mechanisms, being the principal blockers of the target and neutralizing its function [54]. In the COVID-19 context, one of the first targets was IL-6 because reports showed that high serum levels of this cytokine correlated with severity [55]. IL-6 interacts with the IL-6 receptor (IL-6R) in the transmembrane or soluble form; after the ligand-receptor interaction, IL-6R complexes with gp130 membrane-bound to facilitate the signaling [55,56,57]. In addition, the IL-6/IL-6R interaction activates the Janus kinase (JAK) signaling downstream to mediate diverse functions in the immune cells. Thus, IL-6R represents an excellent therapeutic target; IL-6R-blocking mAbs such as tocilizumab and sarilumab have been included in the NIH COVID-19 Treatment Guidelines indicated in hospitalized adults that require oxygen delivery through a high-flow device or noninvasive ventilation [58]. IL-6R blockade may represent an essential strategy for interrupting the inflammatory process in COVID-19; however, the success has been limited [56]. In this regard, probably some of the reasons why this has been limited are because it has been reported that COVID-19 patients display variable levels of IL-6, and the association of secondary infections with immunomodulators such as tocilizumab also is a common risk that has been identified [47,56,57,59]. In summary, at present, we do not know exactly how is the mechanism of the inflammatory response against the SARS-CoV-2, and this is essential knowledge to develop therapies to suppress the uncontrolled inflammatory process without side effects.
Worldwide, there are around eleven antibodies targeting SARS-CoV-2 or modulators of the deregulated immune response that have received approvals both as emergency use or authorized [60]. The combined administration of anti-SARS-CoV-2 spike protein mAbs, bamlanivimab, and etesevimab, was indicated in mild-COVID-19 patients with a high risk of evolving into severe disease [60,61]. Sotrovimab is a human neutralizing anti-SARS-CoV-2 antibody approved in Australia for hospitalized patients with an increased risk of death [62,63]. Another antibody is regdanvimab (regkirona), which targets the SARS-CoV-2 viral spike protein and was approved in South Korea to treat mild-to-moderate COVID-19 in patients older than 50 with chronically diseases or immunosuppressed [60,64]. Two more combined mAbs with emergency use authorization in USA and Australia for high-risk outpatients non-hospitalized are casirivimab plus imdevimab for treatment and COVID-19 prevention [60,65].
Anakinra is another mAb used to modulate the pro-inflammatory response; it is an interleukin receptor antagonist that binds to the IL-1 type I receptor; it has been considered in combination with tocilizumab for COVID-19 clinical trials [66,67]. In the same way, canakinumab, a neutralizing mAb of IL-1β through competition for IL-1RI binding, is used to treat auto-inflammatory severe diseases and has been evaluated in COVID-19 patients [68,69,70,71]. Focusing on the signaling of cytokines implicated in COVID-19, the Janus-associated kinase (JAK)-inhibitor, baricitinib, has been suggested in combination with remdesivir in hospitalized COVID-19 patients [72,73]. Baricitinib is a synthetic inhibitor of the isoforms JAK1/JAK2; this drug shows few drug–drug interactions and is currently used in rheumatoid arthritis when the anti-TNF therapy fails [74]. Baricitinib affects the production of cytokines like IL-2, IL-6, IL-10, IFN-γ, and granulocyte-macrophage colony-stimulating factors during the inflammatory process and has been suggested that it may exert anti-viral effects inhibiting the endocytosis of SARS-CoV-2 [72,74]. The use of remdesivir in combination with baricitinib, on one side, prevents the hyperinflammatory response, and on the other, there is an advantage of the broad-spectrum antiviral that remdesivir presents. A double-blind study reported that COVID-19 hospitalized adults had higher safety than those who used remdesivir alone [73]. Interestingly, the incidence of adverse events such as thromboembolic was lower, and patients receiving high-flow oxygen or noninvasive mechanical ventilation improved faster with the combined therapy [72,73].
Thus far, therapeutic antibodies targeting the virus, some pro-inflammatory cytokines, or one of their receptors are highlighted as a focus of attention in COVID-19.
4. What Is TNF and Its Inhibitors?
TNF is one of the most critical pro-inflammatory cytokines of the innate immune response and mediates pleiotropic effects, which implies action on diverse cells subpopulations to mediate a wide range of activities such as the production of inflammatory mediators, cell proliferation, and cell death. TNF is produced by macrophages, T-, B- NK-, dendritic cells, and fibroblasts [75]. TNF is a versatile cytokine that acts as an alarm system in host defense, appearing in the first few minutes of damage [76]. TNF is a trimeric molecule that can be found as a transmembrane (tmTNF) or soluble form (sTNF) by the action of ADAM17 [77,78]. Both forms are bioactive molecules after interacting with one of its receptors, TNFR1 or TNFR2 [77,79]. Importantly, the soluble formats of both receptors can also be generated by the sheddase activity of ADAM17. Transmembrane TNFR1 and TNFR2 signaling to active nuclear factor-kappa B (NF-κB) or MAP kinase family inducing cell survival or cell death [80]. When tmTNF interacts with soluble TNFRs may trigger an activation called reverse signaling, which is activated in the tmTNF expressing subpopulation; in particular, sTNFR1 induces apoptosis through tmTNF by reverse signaling [78].
In addition to the ADAM17 activity, the soluble formats of TNFR1 and TNFR2 may result from alternative splicing [81]. The soluble receptor variants have been associated with different biological effects. For example, in viral and bacterial infections, some pathogens can encode soluble homologs of receptors as a mechanism of immune evasion throughout the sequestration of the corresponding cytokine [82,83,84].
Anti-TNF therapy was approved by the U. S. Food & Drug Administration (FDA) in 1998, and currently, it is efficiently administered as a treatment for diverse inflammatory processes like rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, Crohn´s disease, plaque psoriasis, among others [15,76,85,86].
There are five types of biological TNF inhibitors approved for use; the difference between them is the nature of their origin (Figure 2). The first of these TNF inhibitors is infliximab, approved in August 1998, which is an anti-human mAb chimeric mouse-human IgG that inhibits soluble TNF in both the monomeric and trimeric form [77,87]. The second TNF inhibitor, etanercept, approved in November 1998, is a fully human recombinant molecule consisting of two subunits of the TNFR2 linked to a human IgG1 Fc and can block the trimeric form of TNF [77,88]. Third, Adalimumab and Golimumab are fully humanized IgG1, the first was FDA-approved in 2002, the second in 2009, and both neutralize tmTNF and sTNF [77,89]. Finally, Certolizumab-pegol is a PEGylated Fab fragment, which means an IgG1 mAb with a hinges region linked to two cross-linked chains of a 20-kDa of polyethylene glycol (PEG) but has lacked the Fc region. It was approved in 2008 and also can neutralize both tmTNF and sTNF [85,89,90].
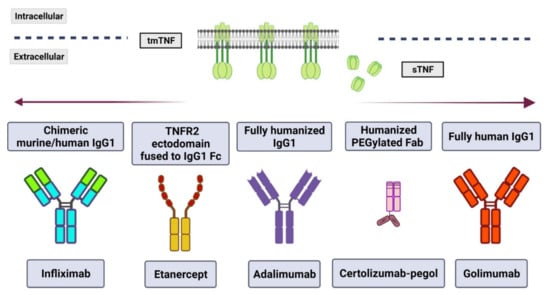
Figure 2.
TNF inhibitors approved for therapeutical use. Currently, there are five monoclonal antibodies used in anti-TNF therapy; each one has a specific structure and origin. However, all of them neutralize both transmembrane (tm) and soluble (s) TNF. (Figure created with BioRender.com, accessed on 21 April 2022).
5. Could TNF Be Considered a Target in COVID-19?
The anti-TNF biological agents target both tmTNF and sTNF, and all of them trigger diverse effects such as neutralization, apoptosis, and modulation of the immune system; several facts may affect the efficacy of every anti-TNF inhibitor as pharmacokinetic, tissue penetration, affinity, and avidity, among others [91], reasons by which more systematic reviews are required to understand the global impact of anti-TNF therapy in COVID-19. Despite the benefit of anti-TNF therapy in a broad panel of inflammatory diseases, it has been well documented that the TNF inhibitors may present controversial effects in non-responder patients; this means patients who previously responded to the treatment but posteriorly, they are treatment-refractory and susceptible to infection from some bacteria such as Legionella pneumonia and Listeria monocytogenes [15,85]. Even more, the side effects in some cases may also include Mycobacterium tuberculosis and hepatitis B reactivation, anti-TNF inhibitor antibodies, and neurological impact [15,92,93]. In some cases, the side effects may be overcome by stopping the administration or changing the inhibitor agent.
Various pronouncements have been made to support the administration of anti-TNF agents in COVID-19 patients; they are based on the principle that the increase of this cytokine has severe effects on diverse cell subpopulations and its function blocked with the anti-TNF therapy is efficient in diverse autoimmune diseases [18,19,48,94]. However, the first problem found is the discrepancy in the TNF levels identified in COVID-19 by several groups; some authors showed that COVID-19 patients display increased levels of this cytokine, but others cannot find it [44,95,96,97].
At present, there are ongoing clinical protocols to identify the efficiency of the anti-TNF therapy in COVID-19, some of them suggesting that COVID-19 patients treated with anti-TNF agents have a better prognosis [98,99]. In this regard, recently, it was suggested that patients with any inflammatory disease using TNF inhibitors had a lower probability of hospitalization or the development of severe COVID-19 compared to patients diagnosed with an inflammatory disease but another treatment [98,100]. However, despite the evidence, the use of these drugs is debatable in some cases, and probably it is favored because the knowledge about levels and regulation mechanisms of TNF, TNFR1, and TNFR2 in COVID-19 is limited, and other important factors call into question what side effects can leave the use of TNF immune modulators in viral infections [99].
In 2020, the Crohn´s & Colitis Foundation of America recommended that patients with Inflammatory Bowel Disease (IBD) that develop COVID-19 stop the anti-TNF administration (biologics or biosimilars) until they recover. It was supported by the National Scientific Advisory Committee of the USA, the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD), and the American Gastroenterological Association [101,102,103,104]. These recommendations were made because patients with chronic inflammation receive immunosuppressive or immune-modulator therapies that may represent a risk of viral infections. Additional to the lack of knowledge about what side effects can leave the use of TNF immune modulators in viral infections [103,104].
Recently, Keewan et al. suggested that PEG-, non-PEG-Certolizumab, and Adalimumab favor the infection of SARS-CoV-2 because the mode of action of these agents induces the Notch-1 signaling pathway promoting a pro-inflammatory environment [105]. In addition, previous reports documented that ADAM17 participates in the Notch signaling pathway, which also may lead to an inflammatory response [106,107]. In an in-vitro model of Mycobacterium avium subspecies paratuberculosis (MAP) infection has suggested that the Notch-1 signaling pathway induced by the anti-TNF therapy impacts IL-6 and MCL-1 expression in macrophages. Additionally, the authors showed that, in particular, adalimumab increases the TMPRSS2/ADAM17 ratio, suggesting the infection facilitated by TMPRSS2 [105].
Although successful cases of immunotherapy with TNF inhibitors have been well documented for years, indeed, some adverse events are also known. A meta-analysis documented that the use of adalimumab, golimumab, infliximab, certolizumab, and etanercept, significantly increases fungal, viral, and bacterial (including mycobacterial) infections [108]. Demyelinating disorders such as type I diabetes and psoriasis also have been reported as a side effect associated with the use of TNF inhibitors; it is the primary failure that occurs in one-third of patients [109].
Epigenetic control of the TNF gene might direct or predict the patient’s responsiveness to anti-TNF therapies, and TNF signaling pathways may be restricted to epigenetic regulation [110]. In particular, the presence of increased levels of H3K4me2 (histone H3 lysine 4 dimethylation) is a modification identified in promoters of expressed genes of TNF-producing cells [111]. The TNF-308G>A polymorphism in the promoter region could be associated with a poor response to TNF inhibitors [112]. At the level of the TNF locus, loss of CpG methylation has been reported as an age-associated factor in healthy donors [113]. In this region, a −238 position polymorphism disturbs a CpG motif, a fact that is also related to the severity in cases of arthritis rheumatoid [114].
In the case of COVID-19, there are two main unknowns related to TNF inhibitors: the risk of immunosuppression in the SARS-CoV-2 infection and the possibility that these agents could facilitate virus entry into target cells. Therefore, further molecular and clinical studies are necessary to understand the real potential of anti-TNF inhibitors in COVID-19. Meanwhile, novel treatment targets must be considered for evaluation, alone or combined with antiretrovirals [115]. In this regard, TNFRs deserve particular attention in COVID-19.
6. Could Be sTNFR1 a Treatment Target for COVID-19?
Scientific evidence shows that the TNF is not the alone-worker in COVID-19; data indicate that the couple TNF/IFN-γ synergizes to induce crosstalk of several types of cell death such as pyroptosis, necroptosis, and apoptosis (collectively named PANoptosis). It is regulated by signal transducer and activator of transcription 1 (STAT1), interferon regulatory factor 1 (IRF1), inducible nitric oxide synthase (iNOS), and nitric oxide (NO); and Caspase-8/RIPK3, all together induce an inflammatory cell death that is in detriment to the host. Under the COVID-19 context, it has also been identified that this TNF/IFN-γ synergism could be blocked with the mAbs co-administering [12].
At present, it is well known that TNFR1 signaling is related to apoptotic cell death, but additionally, this receptor also promotes the release of pro-inflammatory molecules, including cytokines, chemokines, adhesion molecules, and matrix metalloproteinases [116,117]. Thus, TNFR1 signaling is highly inflammatory, a high level of sTNFR1 in fluids has been previously proposed as a biomarker in several diseases with a pro-inflammatory profile as diabetic nephropathy, hyper-inflammation on the ocular surface, sepsis, ventricular dysfunction, and myocardial infarction, acute lung injury, smokers with microvascular complications, and acute graft-versus-host disease [116,118,119,120,121,122].
Efforts have been made to identify if sTNFR1 could represent a potential biomarker in COVID-19. A first report indicated that COVID-19 patients in an intensive care unit (ICU) have a higher level of a pro-inflammatory profile (IL-1β, IL-6, IL-8), including high sTNFR1 levels [123]. Posteriorly, Mortaz et al. [124] identified high levels of sTNFR1 in patients with severe COVID-19, suggesting for the first time that this molecule could represent a biomarker of severity and mortality. In accordance, other authors identified that sTNFR1 is increased in severe COVID-19 compared with mild and moderate illness, and also it was associated with mortality [97]. However, Bowman et al. [125] reported that both TNFR1 and TNFR2 increased independently of severity.
The use of anti-TNF therapy requires an integrative comprehension considering the roles of TNFR1 and TNFR2, as well as the soluble or transmembrane formats of the proteins [79]. Based on the discussed evidence, we suggest that the clinical trials to evaluate the efficiency of the anti-TNF therapy should do an integral association between levels of TNF and its receptors. Moreover, a relevant question that has not been clarified is whether COVID-19 TNF is found mainly in transmembrane or soluble forms. In other pathologies, it has been deeply described that the TNF form is fundamental to determining its function as an activator or regulator of the inflammatory response [79,126].
Although the current TNF inhibitors are highly efficient, it has been well documented that total TNF inhibition may lead to severe side effects such as tuberculosis reactivation [127,128]. An advantage is that TNFR1 and TNFR2 mediate different cellular events, the reason why the option of selectively blocking one of them may result in fewer adverse effects [129,130]. As TNFR1 promotes inflammation and cell death, the specific inhibition can avoid side effects associated with TNF inhibitors and therefore constitute the next generation of immunomodulators even in viral infection as COVID-19 [131]. Various efforts are committed to the selective modulation of TNF receptors, with TNFR1 receiving greater attention due to the roles in which it has been identified [132,133]. This positions TNFR1 as a potential therapeutic target during SARS-CoV-2 infection. In Table 1, we summarized the human TNFR1 inhibitors that are currently under evaluation; this information is fundamental for developing effective therapeutic strategies for COVID-19.

Table 1.
Human TNFR1 inhibitors under progress.
7. What We Know from Preliminary Results about TNFR1-Selective Inhibitors under Progress?
TNFR1 inhibitors include a wide range of molecules, including antibodies, small molecules, and aptamers, that selectively inhibit TNFR1 but without affecting TNFR2 function. The first group of inhibitors includes derivatives of antagonist mAb that interact with the cysteine-rich domain 1 of TNFR1, competing with high affinity with the natural ligand [135,136]. To improve the ability of the Atrosab to binding to TNFR1, the mAb’ humanized variable domains were re-engineering, and the Fv13.7-Fc molecule displayed improved pharmacokinetic properties and antagonistic activity, but at present, this molecule has been not clinically evaluated [136]. Moreover, using inflammatory murine models, atrosimab was highly effective at suppressing the development of chronic diseases like arthritis, non-alcoholic steatohepatitis, and experimental autoimmune encephalomyelitis [151].
Muteins are mutant proteins of TNFR1-selective antagonists; structurally, they have peptide-linkers to enhance their stability and bioactivity and polyethylene glycol (PEG) modification to limit the reactive sites. These mutants showed good avidity and thermal stability for selective binding to TNFR1; using the arthritis murine model, muteins in plasma have an extended half-life, and regulatory T cells are increased compared to etanercept [138,139,140,141,142,152].
Though High-Throughput Screening (HTS) has been developed to identify selective small molecules TNFR1 [145,146]. Lo Ch et al. (2017) found non-competitive inhibitors, such as zafirlukast and triclabendazole, these molecules stabilize the nonfunctional conformational state of TNFR1, impacting IκBα degradation and NF-κB activation [145].
Another example of TNFR1 inhibitors is GSK1995057; it is a domain antibody (dAb) representing the minimal fraction of antigen-binding units, although it has a low molecular weight (10–13 kDa) is highly stable. One study reported the safety of the administration of GSK1995057 by nebulization; it was evaluated in both non-human primate models of acute lung injury and healthy subjects, suggesting that it could be a therapy for the prevention of acute respiratory distress syndrome [148].
Thus, the previous evidence supports the hypothesis that the TNFR1-selective inhibitors, which do not affect the immunomodulatory functions of TNFR2, have the potential to be used as therapy for diverse inflammatory diseases, including lung affections as COVID-19. Despite the clinical successes of the anti-TNF therapy, reports also indicate that it can induce side effects because TNFR1 mediates cell death; the inflammation process could be a novel drug candidate, although it is necessary to provide pre-clinical data to support further its clinical use.
8. Conclusions
Reports have shown diverse effects of anti-TNF agents in the context of COVID-19, some of which are beneficial, whereas others have suggested conflicting results based on the complexity of the nature of TNF and TNFRs pathways. More extensive research may be developed to understand the role of this pivotal axis in the innate immune response against SARS-CoV-2 infection. To support the use of the TNF axis in COVID-19 is imperative to include the dynamics and functions of the TNFRs in the development of selective and efficient therapies. Results suggest that sTNFR1 has an essential role in COVID-19 severity and mortality reason by which must represent a focus of attention in this pathology and a potential therapeutic target in future clinical studies.
Author Contributions
Conceptualization Y.P. and L.C.-G., writing—original draft preparation Y.P., Writing—review and editing Y.P. and L.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Y.P. was supported by Estancias Posdoctorales por México, CONACYT, México.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADAM17 | A Disintegrin and Metalloprotease 17 |
| ARDS | Acute Respiratory Distress Syndrome |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| BAL | Bronchoalveolar lavage |
| DAMPs | Danger-Associated Molecular Patterns |
| IL-6R | Interleukin 6 Receptor |
| IBD | Inflammatory Bowel Disease |
| iNOS | Inducible Nitric Oxide Synthase |
| IRF1 | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| mAbs | Monoclonal antibodies |
| MAP | Mycobacterium avium subspecies paratuberculosis |
| MAS | Macrophage Activation Syndrome |
| NET | Neutrophil Extracellular Trap |
| NF-κB | Nuclear Factor-kappa B |
| NK | Natural killer |
| NO | Nitric Oxide |
| NRP1 | Neuropilin 1 |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PANoptosis | Pyroptosis, Necroptosis, and Apoptosis |
| PLAD | Pre-ligand-binding assembly domain |
| PRRs | Pattern Recognition Receptors |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome coronavirus-2 |
| sTNFR1 | soluble TNFR1 |
| sTNF | soluble TNF |
| SNPs | Single nucleotide polymorphisms |
| TLR | Toll-like receptors |
| TNF | Tumor Necrosis Factor |
| tmTNF | Transmembrane-TNF |
| TMPRSS2 | Type II Transmembrane Serine Protease |
| TNFR1 | TNF receptor 1 |
| TNFR2 | TNF receptor 2 |
| TNFRs | TNF receptors |
| TNFRSF | TNF receptor superfamily |
| VOC | Variants of Concern |
References
- Yokota, S.; Miyamae, T.; Kuroiwa, Y.; Nishioka, K. Novel Coronavirus Disease 2019 (COVID-19) and Cytokine Storms for More Effective Treatments from an Inflammatory Pathophysiology. J. Clin. Med. 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 Pandemic: An Overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio Med. Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 18 March 2022).
- World Health Organization. COVID-19 Strategic Preparedness and Response Plan: Monitoring and Evaluation Framework. 2021. Available online: https://www.who.int/publications/i/item/WHO-WHE-2021.07-eng (accessed on 18 March 2022).
- Kannan, S.; Shaik Syed Ali, P.; Sheeza, A. Omicron (B.1.1.529)-Variant of Concern-Molecular Profile and Epidemiology: A Mini Review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 8019–8022. [Google Scholar] [CrossRef]
- Nealon, J.; Cowling, B.J. Omicron Severity: Milder but Not Mild. Lancet Lond. Engl. 2022, 399, 412–413. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Mao, Y.C.; Liu, P.Y.; Lai, K.L. Pharmacology and Adverse Events of Emergency-Use Authorized Medication in Moderate to Severe COVID-19. Pharm. Basel Switz. 2021, 14, 955. [Google Scholar] [CrossRef]
- Buszko, M.; Nita-Lazar, A.; Park, J.-H.; Schwartzberg, P.L.; Verthelyi, D.; Young, H.A.; Rosenberg, A.S. Lessons Learned: New Insights on the Role of Cytokines in COVID-19. Nat. Immunol. 2021, 22, 404–411. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. MMBR 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2020, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine Storm and COVID-19: A Chronicle of pro-Inflammatory Cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef]
- Júnior, M.L.; de Souza, L.M.; Dutra, R.E.; de Melo Valente, R.G.; Melo, T.S. Review on Therapeutic Targets for COVID-19: Insights from Cytokine Storm. Postgrad. Med. J. 2021, 97, 391–398. [Google Scholar] [CrossRef]
- Steeland, S.; Libert, C.; Vandenbroucke, R.E. A New Venue of TNF Targeting. Int. J. Mol. Sci. 2018, 19, 1442. [Google Scholar] [CrossRef]
- Mootoo, A.; Stylianou, E.; Arias, M.A.; Reljic, R. TNF-Alpha in Tuberculosis: A Cytokine with a Split Personality. Inflamm. Allergy Drug Targets 2009, 8, 53–62. [Google Scholar] [CrossRef]
- Kollias, G.; Douni, E.; Kassiotis, G.; Kontoyiannis, D. The Function of Tumour Necrosis Factor and Receptors in Models of Multi-Organ Inflammation, Rheumatoid Arthritis, Multiple Sclerosis and Inflammatory Bowel Disease. Ann. Rheum. Dis. 1999, 58 (Suppl. S1), I32–I39. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of Anti-Tumour Necrosis Factor Therapy for COVID-19 Are Urgently Needed. Lancet 2020, 395, 1407–1409. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.L.; Liew, J.W.; Monaco, C.; Richards, D.; Shivakumar, S.; Tanner, H.L.; Feldmann, M. The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19. Med 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Ravelli, A.; Minoia, F.; Davì, S.; Horne, A.; Bovis, F.; Pistorio, A.; Aricò, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016, 68, 566–576. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.-D. The “Cytokine Storm”: Molecular Mechanisms and Therapeutic Prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Potdar, A.A.; Dube, S.; Naito, T.; Li, K.; Botwin, G.; Haritunians, T.; Li, D.; Casero, D.; Yang, S.; Bilsborough, J.; et al. Altered Intestinal ACE2 Levels Are Associated With Inflammation, Severe Disease, and Response to Anti-Cytokine Therapy in Inflammatory Bowel Disease. Gastroenterology 2021, 160, 809–822.e7. [Google Scholar] [CrossRef]
- Angeli, F.; Zappa, M.; Reboldi, G.; Trapasso, M.; Cavallini, C.; Spanevello, A.; Verdecchia, P. The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection: One Year Later. Eur. J. Intern. Med. 2021, 93, 28–34. [Google Scholar] [CrossRef]
- Li, X.Z.; Qiu, Y.; Jeffery, L.; Liu, F.; Feng, R.; He, J.S.; Tan, J.Y.; Ye, Z.Y.; Lin, S.N.; Ghosh, S.; et al. Down-Regulation of Colonic ACE2 Expression in Patients With Inflammatory Bowel Disease Responding to Anti-TNF Therapy: Implications for COVID-19. Front. Med. 2021, 7, 613475. [Google Scholar] [CrossRef]
- Aleksova, A.; Gagno, G.; Sinagra, G.; Beltrami, A.P.; Janjusevic, M.; Ippolito, G.; Zumla, A.; Fluca, A.L.; Ferro, F. Effects of SARS-CoV-2 on Cardiovascular System: The Dual Role of Angiotensin-Converting Enzyme 2 (ACE2) as the Virus Receptor and Homeostasis Regulator-Review. Int. J. Mol. Sci. 2021, 22, 4526. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the Receptor-Binding Domain (RBD) of 2019 Novel Coronavirus: Implication for Development of RBD Protein as a Viral Attachment Inhibitor and Vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the Human Innate Immune System. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Ambrocio-Ortiz, E.; Pérez-Rubio, G.; Del Ángel-Pablo, A.D.; Buendía-Roldán, I.; Chávez-Galán, L.; Hernández-Zenteno, R.D.; Ramírez-Venegas, A.; Rojas-Serrano, J.; Mejía, M.; Pérez-Padilla, R.; et al. Angiotensin-Converting Enzyme 2 (ACE2) in the Context of Respiratory Diseases and Its Importance in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Pharm. Basel Switz. 2021, 14, 805. [Google Scholar] [CrossRef]
- Calligaris, M.; Cuffaro, D.; Bonelli, S.; Spanò, D.P.; Rossello, A.; Nuti, E.; Scilabra, S.D. Strategies to Target ADAM17 in Disease: From Its Discovery to the IRhom Revolution. Mol. Basel Switz. 2021, 26, 944. [Google Scholar] [CrossRef]
- Xiao, L.; Sakagami, H.; Miwa, N. ACE2: The Key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses 2020, 12, 491. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by the Transmembrane Protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A Transmembrane Serine Protease Is Linked to the Severe Acute Respiratory Syndrome Coronavirus Receptor and Activates Virus Entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Channappanavar, R.; Kanneganti, T.-D. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol. 2020, 41, 1083–1099. [Google Scholar] [CrossRef]
- Medina-Quero, K.; Barreto-Rodriguez, O.; Mendez-Rodriguez, V.; Sanchez-Moncivais, A.; Buendia-Roldan, I.; Chavez-Galan, L. SARS-CoV-2 Infection: Understanding the Immune System Abnormalities to Get an Adequate Diagnosis. Bosn. J. Basic Med. Sci. 2021, 21, 503–514. [Google Scholar] [CrossRef]
- Paludan, S.R.; Mogensen, T.H. Innate Immunological Pathways in COVID-19 Pathogenesis. Sci. Immunol. 2022, 7, eabm5505. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wen, W.; Fan, X.; Hou, W.; Su, B.; Cai, P.; Li, J.; Liu, Y.; Tang, F.; Zhang, F.; et al. COVID-19 Immune Features Revealed by a Large-Scale Single-Cell Transcriptome Atlas. Cell 2021, 184, 1895–1913.e19. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Aktar, S.; Rahman, M.M.; Chowdhury, M.M.H. NLRP3 Inflammasome Activation in COVID-19: An Interlink between Risk Factors and Disease Severity. Microbes Infect. 2022, 24, 104913. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Ackermann, M.; Anders, H.-J.; Bilyy, R.; Bowlin, G.L.; Daniel, C.; De Lorenzo, R.; Egeblad, M.; Henneck, T.; Hidalgo, A.; Hoffmann, M.; et al. Patients with COVID-19: In the Dark-NETs of Neutrophils. Cell Death Differ. 2021, 28, 3125–3139. [Google Scholar] [CrossRef]
- Tomar, B.; Anders, H.-J.; Desai, J.; Mulay, S.R. Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19. Cells 2020, 9, 1383. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef]
- Wilson, J.G.; Simpson, L.J.; Ferreira, A.-M.; Rustagi, A.; Roque, J.; Asuni, A.; Ranganath, T.; Grant, P.M.; Subramanian, A.; Rosenberg-Hasson, Y.; et al. Cytokine Profile in Plasma of Severe COVID-19 Does Not Differ from ARDS and Sepsis. JCI Insight 2020, 5, e140289. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ippolito, M.; Greco, M.; Granone, V.; Protti, A.; Gregoretti, C.; Giarratano, A.; Einav, S.; Cecconi, M. Rationale and Evidence on the Use of Tocilizumab in COVID-19: A Systematic Review. Pulmonology 2021, 27, 52–66. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Anti-TNF Drug Adalimumab to Be Trialled for Patients in the Community. BMJ 2020, 371, m3847. [Google Scholar] [CrossRef]
- Aouba, A.; Baldolli, A.; Geffray, L.; Verdon, R.; Bergot, E.; Martin-Silva, N.; Justet, A. Targeting the Inflammatory Cascade with Anakinra in Moderate to Severe COVID-19 Pneumonia: Case Series. Ann. Rheum. Dis. 2020, 79, 1381–1382. [Google Scholar] [CrossRef]
- Atal, S.; Fatima, Z. IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy? Pharm. Med. 2020, 34, 223–231. [Google Scholar] [CrossRef]
- Huet, T.; Beaussier, H.; Voisin, O.; Jouveshomme, S.; Dauriat, G.; Lazareth, I.; Sacco, E.; Naccache, J.M.; Bézie, Y.; Laplanche, S.; et al. Anakinra for Severe Forms of COVID-19: A Cohort Study. Lancet Rheumatol. 2020, 2, e393–e400. [Google Scholar] [CrossRef]
- Lythgoe, M.P.; Middleton, P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol. Sci. 2020, 41, 363–382. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Rodrugues, E.B.; Farah, M.E.; Maia, M.; Penha, F.M.; Regatieri, C.; Melo, G.B.; Pinheiro, M.M.; Zanetti, C.r. Therapeutic monoclonal antibodies in ophthalmology. Prog. Retin. Eye Res. 2009, 28, 117–144. [Google Scholar] [CrossRef]
- Gautret, P.; Million, M.; Jarrot, P.-A.; Camoin-Jau, L.; Colson, P.; Fenollar, F.; Leone, M.; La Scola, B.; Devaux, C.; Gaubert, J.Y.; et al. Natural History of COVID-19 and Therapeutic Options. Expert Rev. Clin. Immunol. 2020, 16, 1159–1184. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Zhou, Z.; Guan, X.; Xiang, Y. Can We Use Interleukin-6 (IL-6) Blockade for Coronavirus Disease 2019 (COVID-19)-Induced Cytokine Release Syndrome (CRS)? J. Autoimmun. 2020, 111, 102452. [Google Scholar] [CrossRef]
- Le, T.T.; Karmouty-Quintana, H.; Melicoff, E.; Le, T.T.; Weng, T.; Chen, N.-Y.; Pedroza, M.; Zhou, Y.; Davies, J.; Philip, K.; et al. Blockade of IL-6 Trans Signaling Attenuates Pulmonary Fibrosis. J. Immunol. Baltim. Md. 1950 2014, 193, 3755–3768. [Google Scholar] [CrossRef]
- NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/ (accessed on 1 February 2022).
- Vela, D.; Vela-Gaxha, Z.; Rexhepi, M.; Olloni, R.; Hyseni, V.; Nallbani, R. Efficacy and Safety of Tocilizumab versus Standard Care/Placebo in Patients with COVID-19; a Systematic Review and Meta-analysis of Randomized Clinical Trials. Br. J. Clin. Pharmacol. 2022, 88, 1955–1963. [Google Scholar] [CrossRef]
- Kaplon, H.; Chenoweth, A.; Crescioli, S.; Reichert, J.M. Antibodies to Watch in 2022. mAbs 2022, 14, 2014296. [Google Scholar] [CrossRef]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. COMET-ICE Investigators. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. Australian Public Assessment Report for Sotrovimab, Proprietary Product Name: Xevudy; Sponsor: GlaxoSmithKline Australia Pty Ltd. Available online: https://www.tga.gov.au/sites/default/files/auspar-sotrovimab-210820.pdf (accessed on 13 February 2022).
- Syed, Y.Y. Regdanvimab: First Approval. Drugs 2021, 81, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Casirivimab/Imdevimab: First Approval. Drugs 2021, 81, 2047–2055. [Google Scholar] [CrossRef]
- Bachove, I.; Chang, C. Anakinra and Related Drugs Targeting Interleukin-1 in the Treatment of Cryopyrin-Associated Periodic Syndromes. Open Access Rheumatol. Res. Rev. 2014, 6, 15–25. [Google Scholar] [CrossRef][Green Version]
- Maes, B.; Bosteels, C.; De Leeuw, E.; Declercq, J.; Van Damme, K.; Delporte, A.; Demeyere, B.; Vermeersch, S.; Vuylsteke, M.; Willaert, J.; et al. Treatment of Severely Ill COVID-19 Patients with Anti-Interleukin Drugs (COV-AID): A Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 2020, 21, 468. [Google Scholar] [CrossRef]
- Hwang, Y.-C.; Lu, R.-M.; Su, S.-C.; Chiang, P.-Y.; Ko, S.-H.; Ke, F.-Y.; Liang, K.-H.; Hsieh, T.-Y.; Wu, H.-C. Monoclonal Antibodies for COVID-19 Therapy and SARS-CoV-2 Detection. J. Biomed. Sci. 2022, 29, 1. [Google Scholar] [CrossRef]
- Kedor, C.; Listing, J.; Zernicke, J.; Weiß, A.; Behrens, F.; Blank, N.; Henes, J.C.; Kekow, J.; Rubbert-Roth, A.; Schulze-Koops, H.; et al. Canakinumab for Treatment of Adult-Onset Still’s Disease to Achieve Reduction of Arthritic Manifestation (CONSIDER): Phase II, Randomised, Double-Blind, Placebo-Controlled, Multicentre, Investigator-Initiated Trial. Ann. Rheum. Dis. 2020, 79, 1090–1097. [Google Scholar] [CrossRef]
- Sfriso, P.; Bindoli, S.; Doria, A.; Feist, E.; Galozzi, P. Canakinumab for the Treatment of Adult-Onset Still’s Disease. Expert Rev. Clin. Immunol. 2020, 16, 129–138. [Google Scholar] [CrossRef]
- Caricchio, R.; Abbate, A.; Gordeev, I.; Meng, J.; Hsue, P.Y.; Neogi, T.; Arduino, R.; Fomina, D.; Bogdanov, R.; Stepanenko, T.; et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized With Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 230–239. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Tse, C.L.Y.; Burry, L.; Dresser, L.D. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacotherapy 2020, 40, 843–856. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Biddle, K.; White, J.; Sofat, N. What Is the Full Potential of Baricitinib in Treating Patients with COVID-19? Expert Rev. Clin. Immunol. 2022, 1–5. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour Necrosis Factor Signalling in Health and Disease. F1000Research 2019, 8. F1000 Faculty Rev-111. [Google Scholar] [CrossRef]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF Therapy: Past, Present and Future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-Alpha: Structure, Function and Interaction with Anti-TNF Agents. Rheumatol. Oxf. Engl. 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Ruiz, A.; Palacios, Y.; Garcia, I.; Chavez-Galan, L. Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections. Int. J. Mol. Sci. 2021, 22, 5461. [Google Scholar] [CrossRef]
- Pimentel-Muiños, F.X.; Seed, B. Regulated Commitment of TNF Receptor Signaling: A Molecular Switch for Death or Activation. Immunity 1999, 11, 783–793. [Google Scholar] [CrossRef]
- Lainez, B.; Fernandez-Real, J.M.; Romero, X.; Esplugues, E.; Cañete, J.D.; Ricart, W.; Engel, P. Identification and Characterization of a Novel Spliced Variant That Encodes Human Soluble Tumor Necrosis Factor Receptor 2. Int. Immunol. 2004, 16, 169–177. [Google Scholar] [CrossRef]
- Hernaez, B.; Alcamí, A. Virus-Encoded Cytokine and Chemokine Decoy Receptors. Curr. Opin. Immunol. 2020, 66, 50–56. [Google Scholar] [CrossRef]
- Levine, S.J. Mechanisms of Soluble Cytokine Receptor Generation. J. Immunol. Baltim. Md 1950 2004, 173, 5343–5348. [Google Scholar] [CrossRef]
- Sultana, S.; Bishayi, B. Neutralization of TNFR-1 and TNFR-2 Modulates S. Aureus Induced Septic Arthritis by Regulating the Levels of pro Inflammatory and Anti Inflammatory Cytokines during the Progression of the Disease. Immunol. Lett. 2018, 196, 33–51. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Drug Safety Communication: Drug Labels for the Tumor Necrosis Factor-Alpha (TNFα) Blockers Now Include Warnings about Infection with Legionella and Listeria Bacteria. FDA 2011. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-drug-labels-tumor-necrosis-factor-alpha-tnfa-blockers-now-include (accessed on 12 March 2022).
- Lim, H.; Lee, S.H.; Lee, H.T.; Lee, J.U.; Son, J.Y.; Shin, W.; Heo, Y.-S. Structural Biology of the TNFα Antagonists Used in the Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 768. [Google Scholar] [CrossRef]
- Caporali, R.; Allanore, Y.; Alten, R.; Combe, B.; Durez, P.; Iannone, F.; Nurmohamed, M.T.; Lee, S.J.; Kwon, T.S.; Choi, J.S.; et al. Efficacy and Safety of Subcutaneous Infliximab versus Adalimumab, Etanercept and Intravenous Infliximab in Patients with Rheumatoid Arthritis: A Systematic Literature Review and Meta-Analysis. Expert Rev. Clin. Immunol. 2021, 17, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Wiens, A.; Correr, C.J.; Pontarolo, R.; Venson, R.; Quinalha, J.V.; Otuki, M.F. A Systematic Review and Meta-Analysis of the Efficacy and Safety of Etanercept for Treating Rheumatoid Arthritis. Scand. J. Immunol. 2009, 70, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular Mechanisms of Action of Anti-TNF-α Agents-Comparison among Therapeutic TNF-α Antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, Y.; Chen, X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Kaymakcalan, Z.; Sakorafas, P.; Bose, S.; Scesney, S.; Xiong, L.; Hanzatian, D.K.; Salfeld, J.; Sasso, E.H. Comparisons of Affinities, Avidities, and Complement Activation of Adalimumab, Infliximab, and Etanercept in Binding to Soluble and Membrane Tumor Necrosis Factor. Clin. Immunol. Orlando Fla 2009, 131, 308–316. [Google Scholar] [CrossRef]
- Sherman, M.; Tsynman, D.N.; Kim, A.; Arora, J.; Pietras, T.; Messing, S.; St Hilaire, L.; Yoon, S.; Decross, A.; Shah, A.; et al. Sustained Improvement in Health-Related Quality of Life Measures in Patients with Inflammatory Bowel Disease Receiving Prolonged Anti-Tumor Necrosis Factor Therapy. J. Dig. Dis. 2014, 15, 174–179. [Google Scholar] [CrossRef]
- van Schouwenburg, P.A.; Rispens, T.; Wolbink, G.J. Immunogenicity of Anti-TNF Biologic Therapies for Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2013, 9, 164–172. [Google Scholar] [CrossRef]
- Patel, S.; Wadhwa, M. Therapeutic Use of Specific Tumour Necrosis Factor Inhibitors in Inflammatory Diseases Including COVID-19. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 140, 111785. [Google Scholar] [CrossRef]
- Liuzzo, G.; Patrono, C. COVID 19: In the Eye of the Cytokine Storm. Eur. Heart J. 2021, 42, 150–151. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Palacios, Y.; Ruiz, A.; Ramón-Luing, L.A.; Ocaña-Guzman, R.; Barreto-Rodriguez, O.; Sánchez-Monciváis, A.; Tecuatzi-Cadena, B.; Regalado-García, A.G.; Pineda-Gudiño, R.D.; García-Martínez, A.; et al. Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality. Int. J. Mol. Sci. 2021, 22, 8423. [Google Scholar] [CrossRef]
- Baslılar, S.; Pehlivan, O. Evaluation of Factors Affecting the Frequency and Clinical Course of COVID-19 in Patients Using Anti-TNF-Alpha Agents. Rev. Assoc. Med. Bras. 1992 2021, 67, 1286–1292. [Google Scholar] [CrossRef]
- Izadi, Z.; Brenner, E.J.; Mahil, S.K.; Dand, N.; Yiu, Z.Z.N.; Yates, M.; Ungaro, R.C.; Zhang, X.; Agrawal, M.; Colombel, J.-F.; et al. Association Between Tumor Necrosis Factor Inhibitors and the Risk of Hospitalization or Death Among Patients With Immune-Mediated Inflammatory Disease and COVID-19. JAMA Netw. Open 2021, 4, e2129639. [Google Scholar] [CrossRef]
- Kokkotis, G.; Kitsou, K.; Xynogalas, I.; Spoulou, V.; Magiorkinis, G.; Trontzas, I.; Trontzas, P.; Poulakou, G.; Syrigos, K.; Bamias, G. Systematic Review with Meta-Analysis: COVID-19 Outcomes in Patients Receiving Anti-TNF Treatments. Aliment. Pharmacol. Ther. 2022, 55, 154–167. [Google Scholar] [CrossRef]
- Foundation CsaC. Resources for IBD Healthcare Professionals: 2019 Novel Coronavirus (COVID-19) (2020). Available online: https://www.crohnscolitisfoundation.org/coronavirus/ibd-medication (accessed on 18 March 2022).
- Crohn´s and Colitis Foundation. Crohn’s and Colitis Foundation. National Scientific Advisory Committee (NSAC). Available online: https://www.crohnscolitisfoundation.org/about/national-scientific-advisory-committee (accessed on 18 March 2022).
- Rubin, D.T.; Abreu, M.T.; Rai, V.; Siegel, C.A. Management of Patients With Crohn’s Disease and Ulcerative Colitis During the Coronavirus Disease-2019 Pandemic: Results of an International Meeting. Gastroenterology 2020, 159, 6–13.e6. [Google Scholar] [CrossRef]
- Rubin, D.T.; Feuerstein, J.D.; Wang, A.Y.; Cohen, R.D. AGA Clinical Practice Update on Management of Inflammatory Bowel Disease During the COVID-19 Pandemic: Expert Commentary. Gastroenterology 2020, 159, 350–357. [Google Scholar] [CrossRef]
- Keewan, E.; Beg, S.; Naser, S.A. Anti-TNF-α Agents Modulate SARS-CoV-2 Receptors and Increase the Risk of Infection Through Notch-1 Signaling. Front. Immunol. 2021, 12, 1662. [Google Scholar] [CrossRef]
- Kopan, R. Notch Signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011213. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Sun, X.; Zhang, Y.; Wang, L.; Suo, J. ADAM17 Promotes Lymph Node Metastasis in Gastric Cancer via Activation of the Notch and Wnt Signaling Pathways. Int. J. Mol. Med. 2019, 43, 914–926. [Google Scholar] [CrossRef]
- Minozzi, S.; Bonovas, S.; Lytras, T.; Pecoraro, V.; González-Lorenzo, M.; Bastiampillai, A.J.; Gabrielli, E.M.; Lonati, A.C.; Moja, L.; Cinquini, M.; et al. Risk of Infections Using Anti-TNF Agents in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Expert Opin. Drug Saf. 2016, 15 (Suppl. S1), 11–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ziring, D.; Desai, S.; Kim, S.; Wong, M.; Korin, Y.; Braun, J.; Reed, E.; Gjertson, D.; Singh, R.R. TNFalpha blockade in human diseases: An overview of efficacy and safety. Clin immunol. 2008, 126, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.; Wilson, A.G.; Barton, A. Genetic and Epigenetic Predictors of Responsiveness to Treatment in RA. Nat. Rev. Rheumatol. 2014, 10, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Kamal, M.; Lindblad-Toh, K.; Bekiranov, S.; Bailey, D.K.; Huebert, D.J.; McMahon, S.; Karlsson, E.K.; Kulbokas, E.J.; Gingeras, T.R.; et al. Genomic Maps and Comparative Analysis of Histone Modifications in Human and Mouse. Cell 2005, 120, 169–181. [Google Scholar] [CrossRef]
- O’Rielly, D.D.; Roslin, N.M.; Beyene, J.; Pope, A.; Rahman, P. TNF-Alpha-308 G/A Polymorphism and Responsiveness to TNF-Alpha Blockade Therapy in Moderate to Severe Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Pharm. J. 2009, 9, 161–167. [Google Scholar] [CrossRef]
- Gowers, I.R.; Walters, K.; Kiss-Toth, E.; Read, R.C.; Duff, G.W.; Wilson, A.G. Age-Related Loss of CpG Methylation in the Tumour Necrosis Factor Promoter. Cytokine 2011, 56, 792–797. [Google Scholar] [CrossRef]
- Brinkman, B.M.; Huizinga, T.W.; Kurban, S.S.; van der Velde, E.A.; Schreuder, G.M.; Hazes, J.M.; Breeddveld, F.C.; Verweij, C.L. Tumour necrosis factor alpha gene polymorphisms in rheumatoid arthritis: Association with susceptibility to, or severity of, disease? Br. J. Rheumatol. 1997, 36, 516–521. [Google Scholar] [CrossRef]
- Kawazoe, M.; Kihara, M.; Nanki, T. Antirheumatic Drugs against COVID-19 from the Perspective of Rheumatologists. Pharm. Basel Switz. 2021, 14, 1256. [Google Scholar] [CrossRef]
- Sakimoto, T.; Ohnishi, T.; Ishimori, A. Significance of Ectodomain Shedding of TNF Receptor 1 in Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2419–2423. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Gohda, T.; Tomino, Y. Novel Biomarkers for the Progression of Diabetic Nephropathy: Soluble TNF Receptors. Curr. Diab. Rep. 2013, 13, 560–566. [Google Scholar] [CrossRef]
- Nilsson, L.; Szymanowski, A.; Swahn, E.; Jonasson, L. Soluble TNF Receptors Are Associated with Infarct Size and Ventricular Dysfunction in ST-Elevation Myocardial Infarction. PLoS ONE 2013, 8, e55477. [Google Scholar] [CrossRef][Green Version]
- Parsons, P.E.; Matthay, M.A.; Ware, L.B.; Eisner, M.D.; National Heart, Lung, Blood Institute. Acute Respiratory Distress Syndrome Clinical Trials Network. Elevated Plasma Levels of Soluble TNF Receptors Are Associated with Morbidity and Mortality in Patients with Acute Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L426–L431. [Google Scholar] [CrossRef]
- Zoppini, G.; Faccini, G.; Muggeo, M.; Zenari, L.; Falezza, G.; Targher, G. Elevated Plasma Levels of Soluble Receptors of TNF-Alpha and Their Association with Smoking and Microvascular Complications in Young Adults with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 3805–3808. [Google Scholar] [CrossRef][Green Version]
- Paczesny, S.; Krijanovski, O.I.; Braun, T.M.; Choi, S.W.; Clouthier, S.G.; Kuick, R.; Misek, D.E.; Cooke, K.R.; Kitko, C.L.; Weyand, A.; et al. A Biomarker Panel for Acute Graft-versus-Host Disease. Blood 2009, 113, 273–278. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ní Choileáin, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Jamaati, H.; Dalil Roofchayee, N.; KakaDezfuli, N.; Hashemian, S.M.; Moniri, A.; Marjani, M.; Malekmohammd, M.; Manosuri, D.; et al. Increased Serum Levels of Soluble TNF-α Receptor Is Associated with ICU Mortality in COVID-19 Patients. Front Immunol. 2021, 12, 592727. [Google Scholar] [CrossRef]
- Bowman, E.R.; Cameron, C.M.A.; Avery, A.; Gabriel, J.; Kettelhut, A.; Hecker, M.; Ute Sontich, C.; Tamilselvan, B.; Nichols, C.N.; Richardson, B.; et al. Levels of Soluble CD14 and Tumor Necrosis Factor Receptors 1 and 2 May Be Predictive of Death in Severe Coronavirus Disease 2019 (COVID-19). J. Infect. Dis. 2021, 223, 805–810. [Google Scholar] [CrossRef]
- Aderka, D.; Engelmann, H.; Maor, Y.; Brakebusch, C.; Wallach, D. Stabilization of the Bioactivity of Tumor Necrosis Factor by Its Soluble Receptors. J. Exp. Med. 1992, 175, 323–329. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wu, X.; Zhou, J.; Meng, D.; Zhu, P. Risk of Adverse Events After Anti-TNF Treatment for Inflammatory Rheumatological Disease. A Meta-Analysis. Front. Pharmacol. 2021, 12, 3010. [Google Scholar] [CrossRef]
- Robert, M.; Miossec, P. Reactivation of Latent Tuberculosis with TNF Inhibitors: Critical Role of the Beta 2 Chain of the IL-12 Receptor. Cell. Mol. Immunol. 2021, 18, 1644–1651. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Davies, M.; Mistry, P.; Green, P.; Giddins, G.; Feldmann, M.; Stoop, A.A.; Brennan, F.M. Selective Blockade of Tumor Necrosis Factor Receptor I Inhibits Proinflammatory Cytokine and Chemokine Production in Human Rheumatoid Arthritis Synovial Membrane Cell Cultures. Arthritis Rheum. 2013, 65, 2262–2273. [Google Scholar] [CrossRef]
- Fischer, R.; Kontermann, R.E.; Pfizenmaier, K. Selective Targeting of TNF Receptors as a Novel Therapeutic Approach. Front. Cell Dev. Biol. 2020, 8, 401. [Google Scholar] [CrossRef]
- Van Hauwermeiren, F.; Vandenbroucke, R.E.; Grine, L.; Lodens, S.; Van Wonterghem, E.; De Rycke, R.; De Geest, N.; Hassan, B.; Libert, C. TNFR1-Induced Lethal Inflammation Is Mediated by Goblet and Paneth Cell Dysfunction. Mucosal Immunol. 2015, 8, 828–840. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Zhao, Y. Selective Inhibition of Tumor Necrosis Factor Receptor-1 (TNFR1) for the Treatment of Autoimmune Diseases. Cytokine Growth Factor Rev. 2020, 55, 80–85. [Google Scholar] [CrossRef]
- Steeland, S.; Puimège, L.; Vandenbroucke, R.E.; Van Hauwermeiren, F.; Haustraete, J.; Devoogdt, N.; Hulpiau, P.; Leroux-Roels, G.; Laukens, D.; Meuleman, P.; et al. Generation and Characterization of Small Single Domain Antibodies Inhibiting Human Tumor Necrosis Factor Receptor 1. J. Biol. Chem. 2015, 290, 4022–4037. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Münkel, S.; Neumeyer, J.; Müller, D.; Branschädel, M.; Scheurich, P.; Pfizenmaier, K. A Humanized Tumor Necrosis Factor Receptor 1 (TNFR1)-Specific Antagonistic Antibody for Selective Inhibition of Tumor Necrosis Factor (TNF) Action. J. Immunother. Hagerstown Md. 1997 2008, 31, 225–234. [Google Scholar] [CrossRef]
- Williams, S.K.; Fairless, R.; Maier, O.; Liermann, P.C.; Pichi, K.; Fischer, R.; Eisel, U.L.M.; Kontermann, R.; Herrmann, A.; Weksler, B.; et al. Anti-TNFR1 Targeting in Humanized Mice Ameliorates Disease in a Model of Multiple Sclerosis. Sci. Rep. 2018, 8, 13628. [Google Scholar] [CrossRef]
- Richter, F.; Zettlitz, K.A.; Seifert, O.; Herrmann, A.; Scheurich, P.; Pfizenmaier, K.; Kontermann, R.E. Monovalent TNF Receptor 1-Selective Antibody with Improved Affinity and Neutralizing Activity. mAbs 2019, 11, 166–177. [Google Scholar] [CrossRef]
- Richter, F.; Seifert, O.; Herrmann, A.; Pfizenmaier, K.; Kontermann, R.E. Improved Monovalent TNF Receptor 1-Selective Inhibitor with Novel Heterodimerizing Fc. mAbs 2019, 11, 653–665. [Google Scholar] [CrossRef]
- Shibata, H.; Yoshioka, Y.; Ohkawa, A.; Minowa, K.; Mukai, Y.; Abe, Y.; Taniai, M.; Nomura, T.; Kayamuro, H.; Nabeshi, H.; et al. Creation and X-Ray Structure Analysis of the Tumor Necrosis Factor Receptor-1-Selective Mutant of a Tumor Necrosis Factor-Alpha Antagonist. J. Biol. Chem. 2008, 283, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Yoshioka, Y.; Ohkawa, A.; Abe, Y.; Nomura, T.; Mukai, Y.; Nakagawa, S.; Taniai, M.; Ohta, T.; Mayumi, T.; et al. The Therapeutic Effect of TNFR1-Selective Antagonistic Mutant TNF-Alpha in Murine Hepatitis Models. Cytokine 2008, 44, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Shibata, H.; Nakamura, T.; Yoshioka, Y.; Abe, Y.; Nomura, T.; Taniai, M.; Ohta, T.; Ikemizu, S.; Nakagawa, S.; et al. Structure-Function Relationship of Tumor Necrosis Factor (TNF) and Its Receptor Interaction Based on 3D Structural Analysis of a Fully Active TNFR1-Selective TNF Mutant. J. Mol. Biol. 2009, 385, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Nakamura, T.; Yoshioka, Y.; Shibata, H.; Abe, Y.; Nomura, T.; Taniai, M.; Ohta, T.; Nakagawa, S.; Tsunoda, S.; et al. Fast Binding Kinetics and Conserved 3D Structure Underlie the Antagonistic Activity of Mutant TNF: Useful Information for Designing Artificial Proteo-Antagonists. J. Biochem. 2009, 146, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Ando, D.; Kamada, H.; Taki, S.; Niiyama, M.; Mukai, Y.; Tadokoro, T.; Maenaka, K.; Nakayama, T.; Kado, Y.; et al. A Trimeric Structural Fusion of an Antagonistic Tumor Necrosis Factor-α Mutant Enhances Molecular Stability and Enables Facile Modification. J. Biol. Chem. 2017, 292, 6438–6451. [Google Scholar] [CrossRef]
- Steeland, S.; Van Ryckeghem, S.; Van Imschoot, G.; De Rycke, R.; Toussaint, W.; Vanhoutte, L.; Vanhove, C.; De Vos, F.; Vandenbroucke, R.E.; Libert, C. TNFR1 Inhibition with a Nanobody Protects against EAE Development in Mice. Sci. Rep. 2017, 7, 13646. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.H.; Lv, D.Y.; Chen, X.F.; Chen, L.D.; Zhu, Z.Y.; Chai, Y.F.; Zhang, J.P. Identification of a Ligand for Tumor Necrosis Factor Receptor from Chinese Herbs by Combination of Surface Plasmon Resonance Biosensor and UPLC-MS. Anal. Bioanal. Chem. 2016, 408, 5359–5367. [Google Scholar] [CrossRef]
- Lo, C.H.; Vunnam, N.; Lewis, A.K.; Chiu, T.-L.; Brummel, B.E.; Schaaf, T.M.; Grant, B.D.; Bawaskar, P.; Thomas, D.D.; Sachs, J.N. An Innovative High-Throughput Screening Approach for Discovery of Small Molecules That Inhibit TNF Receptors. SLAS Discov. Adv. Life Sci. R D 2017, 22, 950–961. [Google Scholar] [CrossRef]
- Lo, C.H.; Schaaf, T.M.; Grant, B.D.; Lim, C.K.-W.; Bawaskar, P.; Aldrich, C.C.; Thomas, D.D.; Sachs, J.N. Noncompetitive Inhibitors of TNFR1 Probe Conformational Activation States. Sci. Signal. 2019, 12, eaav5637. [Google Scholar] [CrossRef]
- Huang, X.W.; Yang, J.; Dragovic, A.F.; Zhang, H.; Lawrence, T.S.; Zhang, M. Antisense Oligonucleotide Inhibition of Tumor Necrosis Factor Receptor 1 Protects the Liver from Radiation-Induced Apoptosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 2849–2855. [Google Scholar] [CrossRef][Green Version]
- Proudfoot, A.; Bayliffe, A.; O’Kane, C.M.; Wright, T.; Serone, A.; Bareille, P.J.; Brown, V.; Hamid, U.I.; Chen, Y.; Wilson, R.; et al. Novel Anti-Tumour Necrosis Factor Receptor-1 (TNFR1) Domain Antibody Prevents Pulmonary Inflammation in Experimental Acute Lung Injury. Thorax 2018, 73, 723–730. [Google Scholar] [CrossRef]
- Tao, S.; Song, P.; Zhang, X.; Zhang, L.; Chu, C.-Q. Single-Stranded DNA Aptamers Against TNF and Their Potential Applications. Methods Mol. Biol. Clifton NJ 2020, 2108, 181–196. [Google Scholar] [CrossRef]
- Chan, F.K.; Chun, H.J.; Zheng, L.; Siegel, R.M.; Bui, K.L.; Lenardo, M.J. A Domain in TNF Receptors That Mediates Ligand-Independent Receptor Assembly and Signaling. Science 2000, 288, 2351–2354. [Google Scholar] [CrossRef]
- Richter, F.; Williams, S.K.; John, K.; Huber, C.; Vaslin, C.; Zanker, H.; Fairless, R.; Pichi, K.; Marhenke, S.; Vogel, A.; et al. The TNFR1 Antagonist Atrosimab Is Therapeutic in Mouse Models of Acute and Chronic Inflammation. Front. Immunol. 2021, 12, 705485. [Google Scholar] [CrossRef]
- Inoue, M.; Tsuji, Y.; Yoshimine, C.; Enomoto, S.; Morita, Y.; Osaki, N.; Kunishige, M.; Miki, M.; Amano, S.; Yamashita, K.; et al. Structural Optimization of a TNFR1-Selective Antagonistic TNFα Mutant to Create New-Modality TNF-Regulating Biologics. J. Biol. Chem. 2020, 295, 9379–9391. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).