Perindopril/Ambrosin Combination Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice: Crosstalk between Toll-Like Receptor 4, the Pro-Inflammatory Pathways, and SIRT1/PPAR-γ Signaling
Abstract
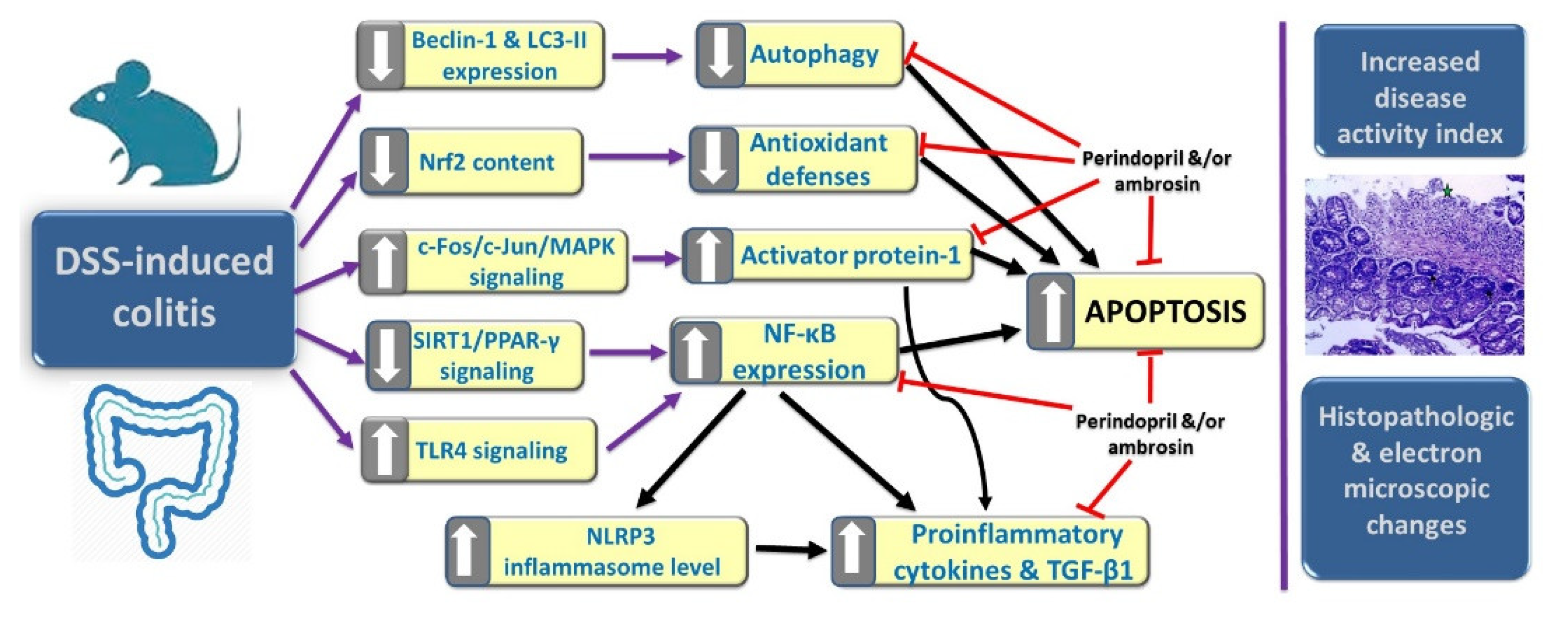
1. Introduction
2. Methods and Materials
2.1. Drugs and Chemicals
2.2. The Experimental Design
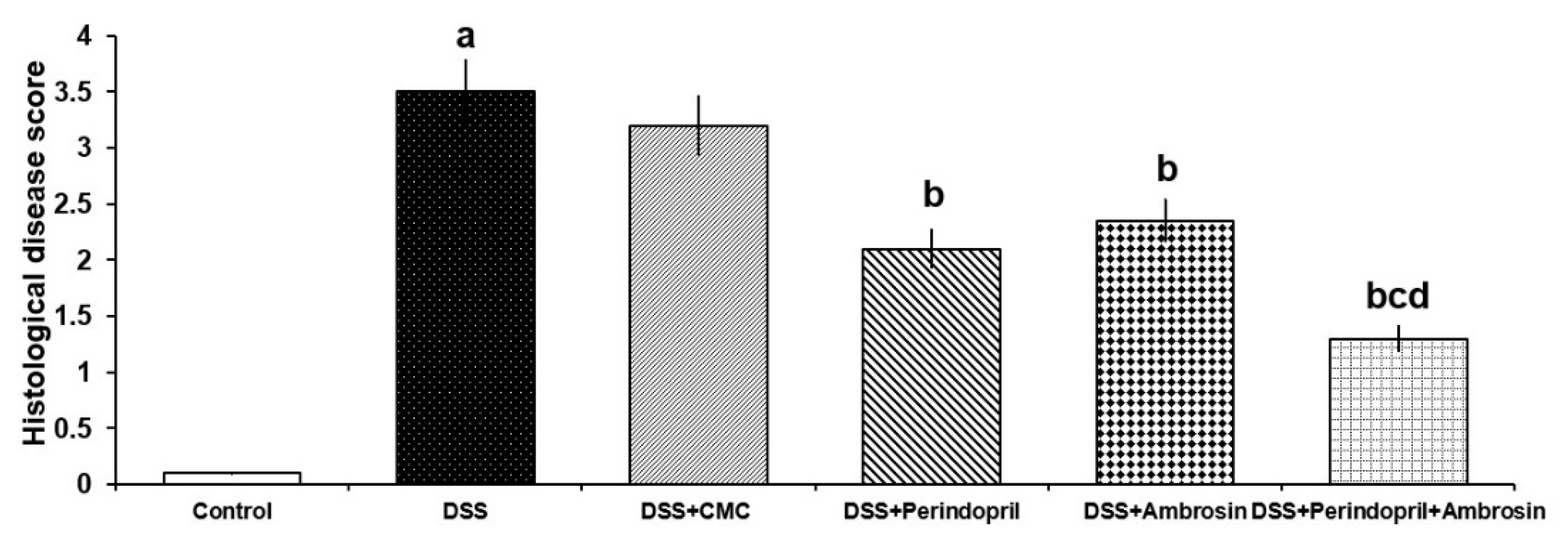
2.3. Evaluation of Colitis
2.4. Assessment of the Antioxidant Status of the Colonic Tissues
2.5. Determination of Nuclear Factor-Erythroid Factor 2-Related Factor 2 (Nrf2), Inducible Nitric Oxide Synthase (iNOS), Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ), and SIRT-1 in the Colonic Tissues
2.6. Assessment of the Inflammatory Microenvironment and NLR Family Pyrin Domain Containing 3 (NLRP3) Inflammasome in the Colonic Tissues
2.7. Determination of c-Fos, c-JUN, and the Phosphorylated Mitogen-Activated Protein Kinase (p38 MAPK) in the Colonic Tissues
2.8. Assessment of Colonic Tissue Beclin-1, LC3-II, Caspase-8, and Bcl-2
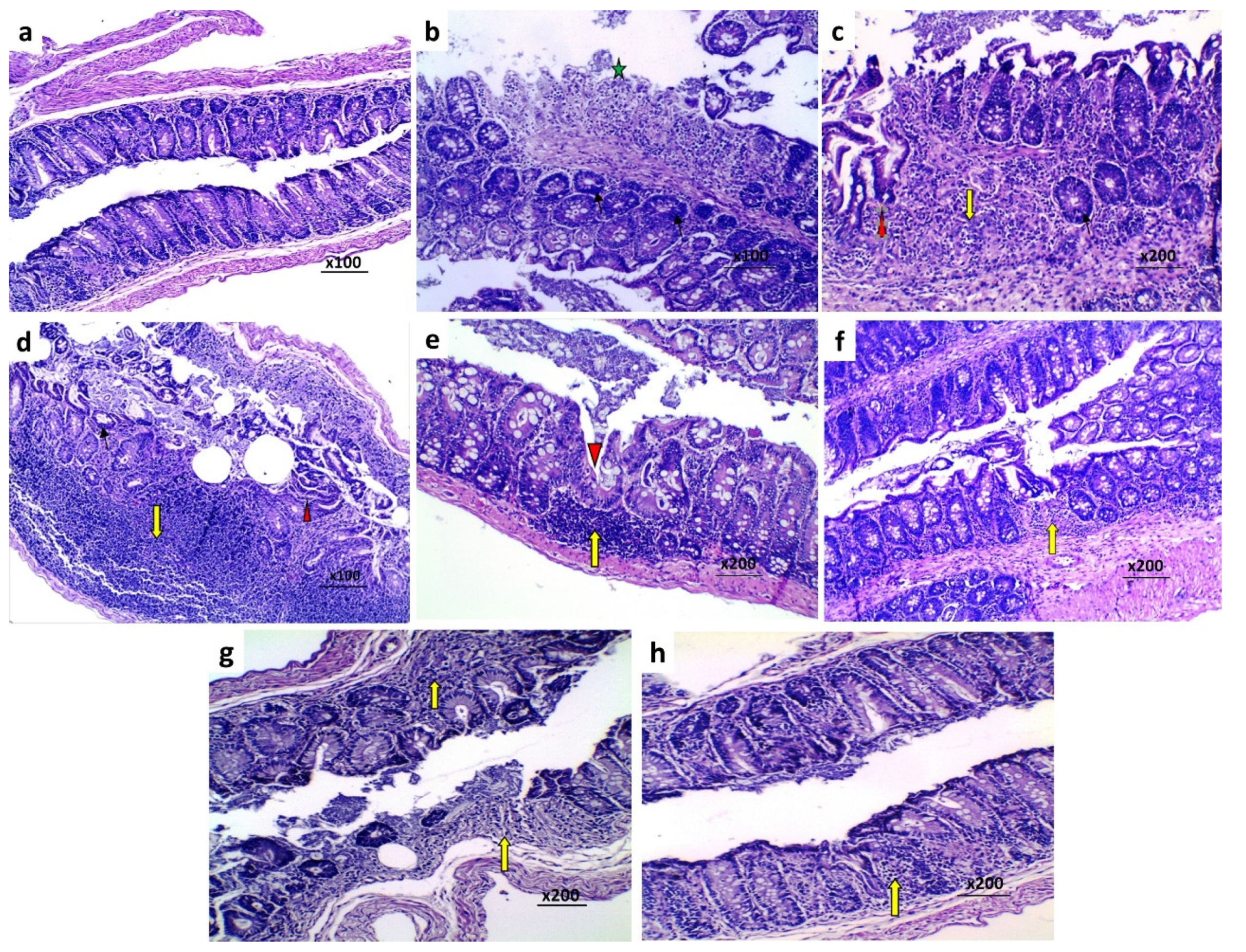
2.9. Evaluation of the Histopathologic Changes of the Colonic Tissues
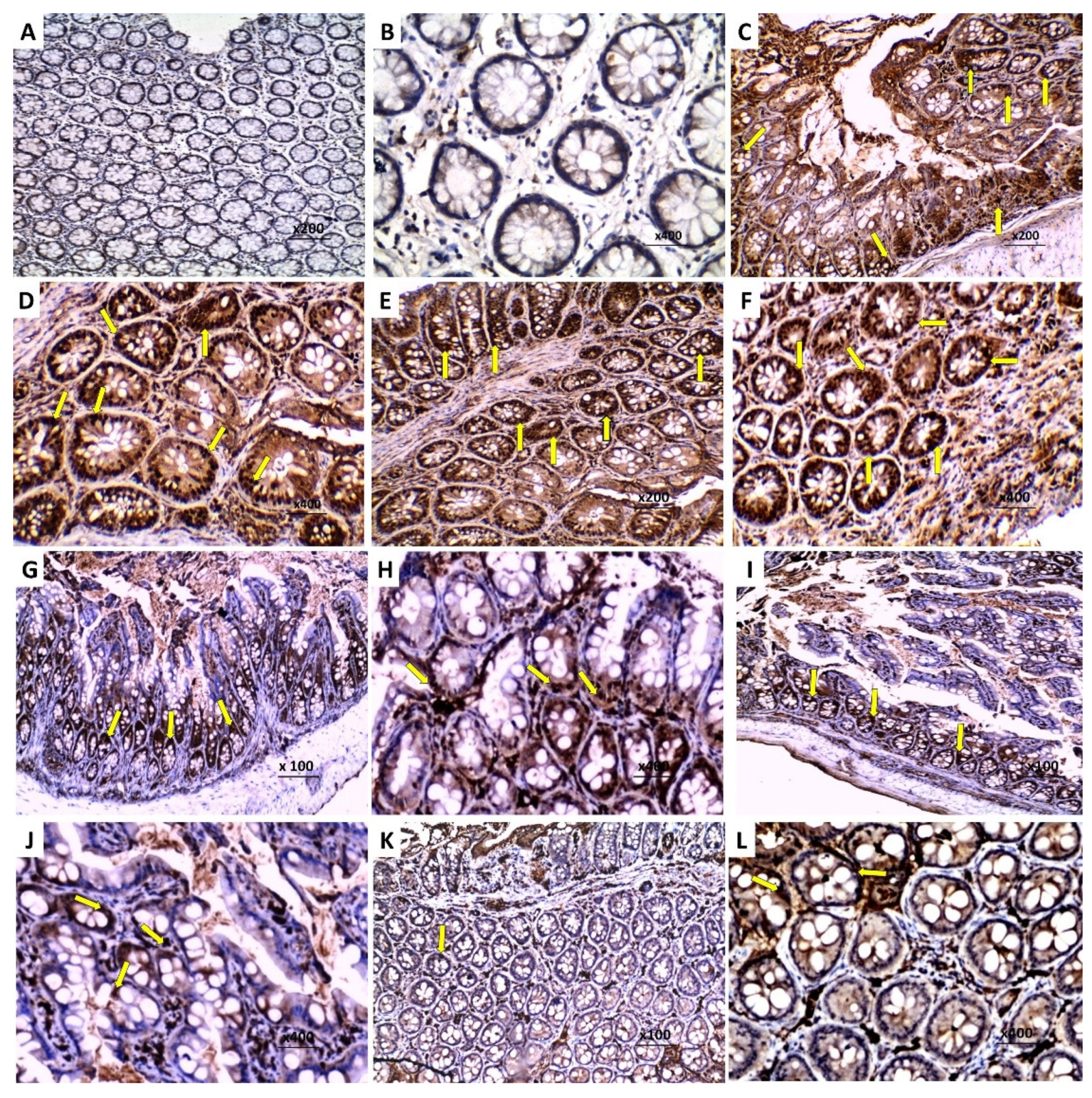
2.10. Assessment of NF-kB (p65) Immunostaining in the Colonic Tissues
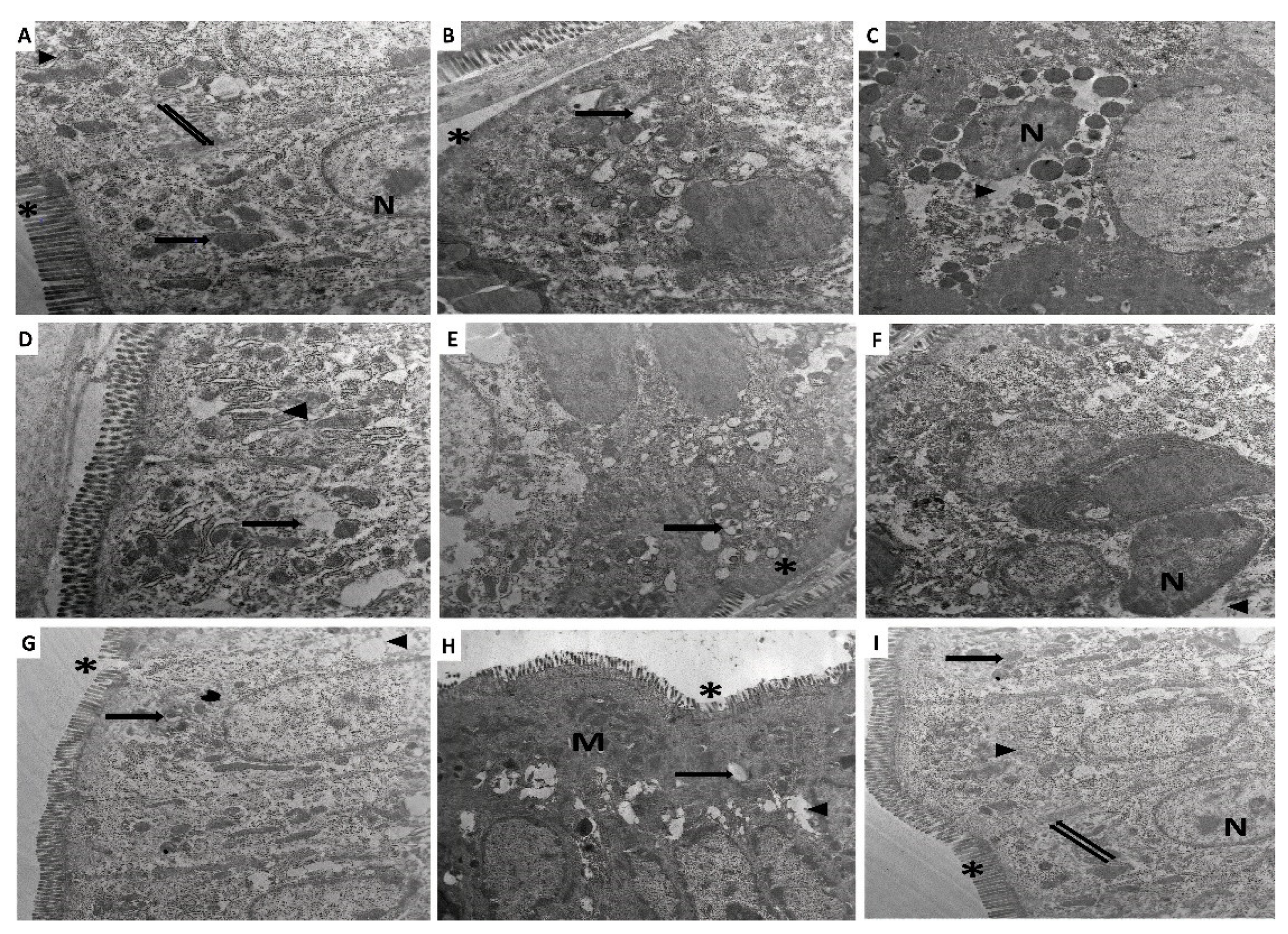
2.11. Evaluation of the Electron Microscopic Changes of the Colonic Tissues
2.12. Statistical Evaluation
3. Results
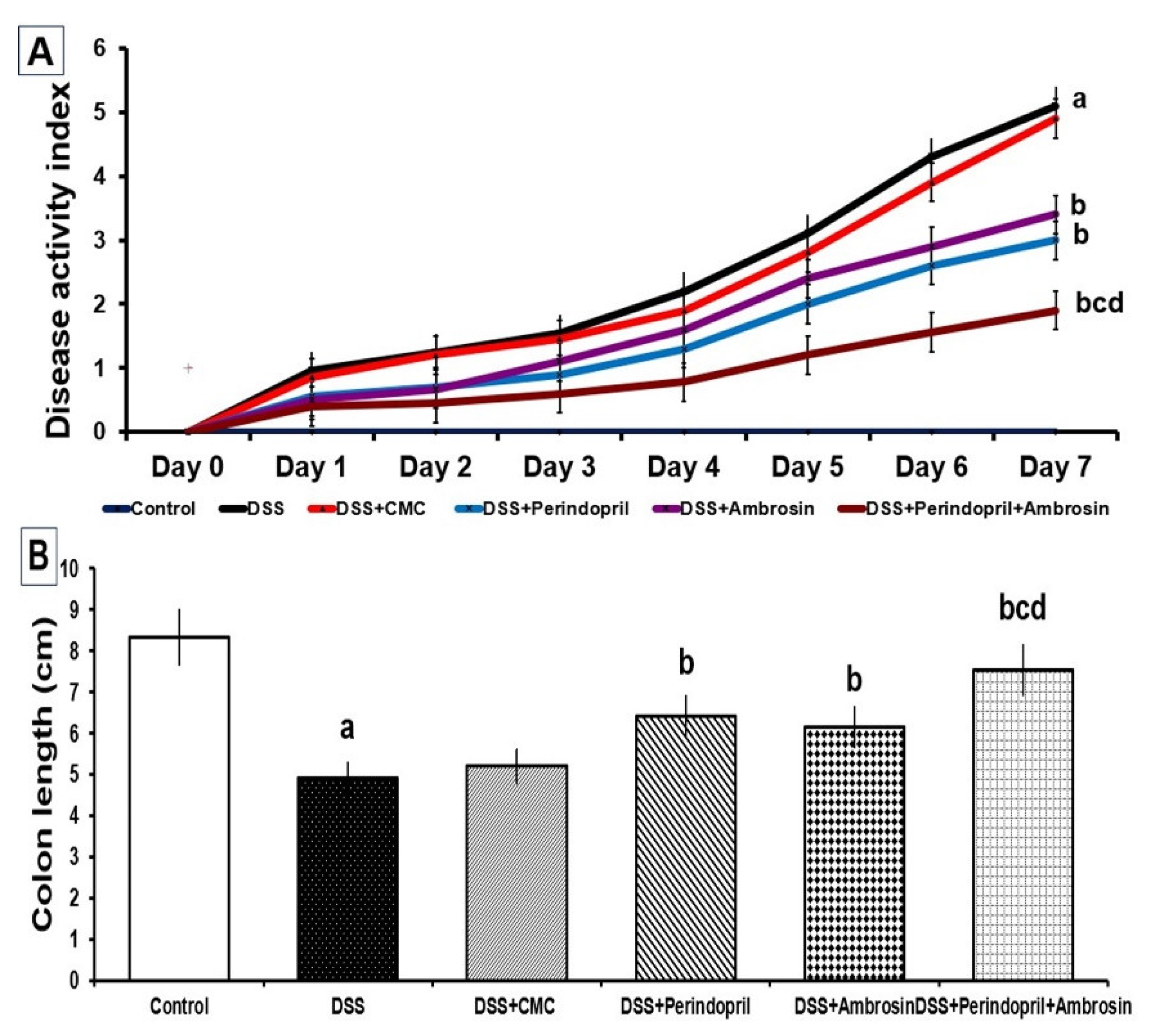
3.1. Perindopril and/or Ambrosin Significantly Mitigated the Changes in DAI and Colon Length in DSS-Treated Animals
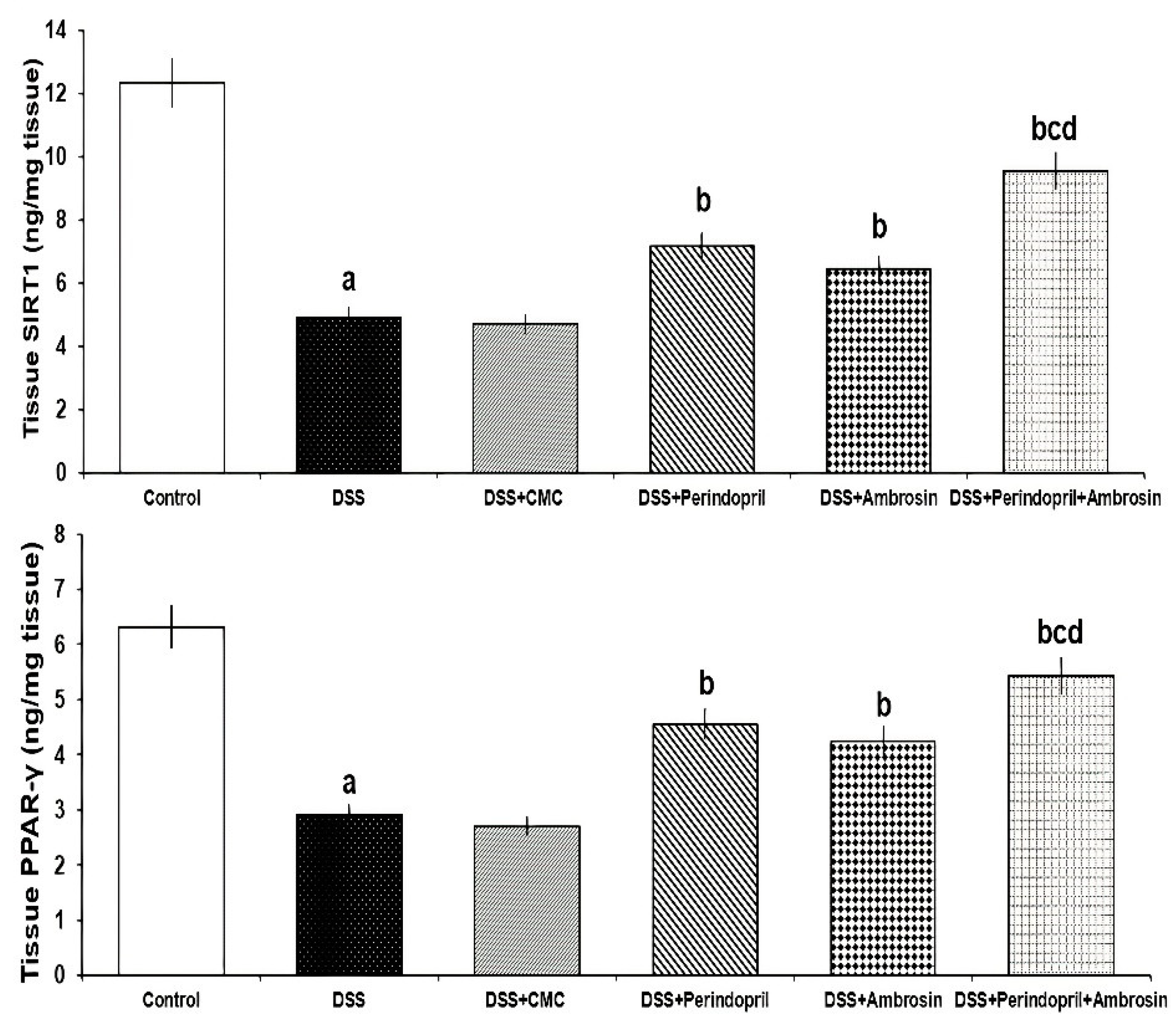
3.2. Perindopril and/or Ambrosin Significantly Activated SIRT1/PPAR-γ Signaling in the Colonic Tissues of DSS-Treated Animals
3.3. Perindopril and/or Ambrosin Restored the Pro-Oxidant/Antioxidant Balance and iNOS/Nrf2 Signaling in the Colonic Tissues of DSS-Treated Animals
3.4. Perindopril and/or Ambrosin Significantly Decreased the Levels of TLR4, IL-1β, IL-6, and TGF-β1 in the Colonic Tissues of DSS-Treated Animals
3.5. Perindopril and/or Ambrosin Mitigated p38 MAPK/c-Fos/c-Jun Pro-Inflammatory Pathways and Suppressed NLRP3 Inflammasome Levels in the Colonic Tissues of DSS-Treated Animals
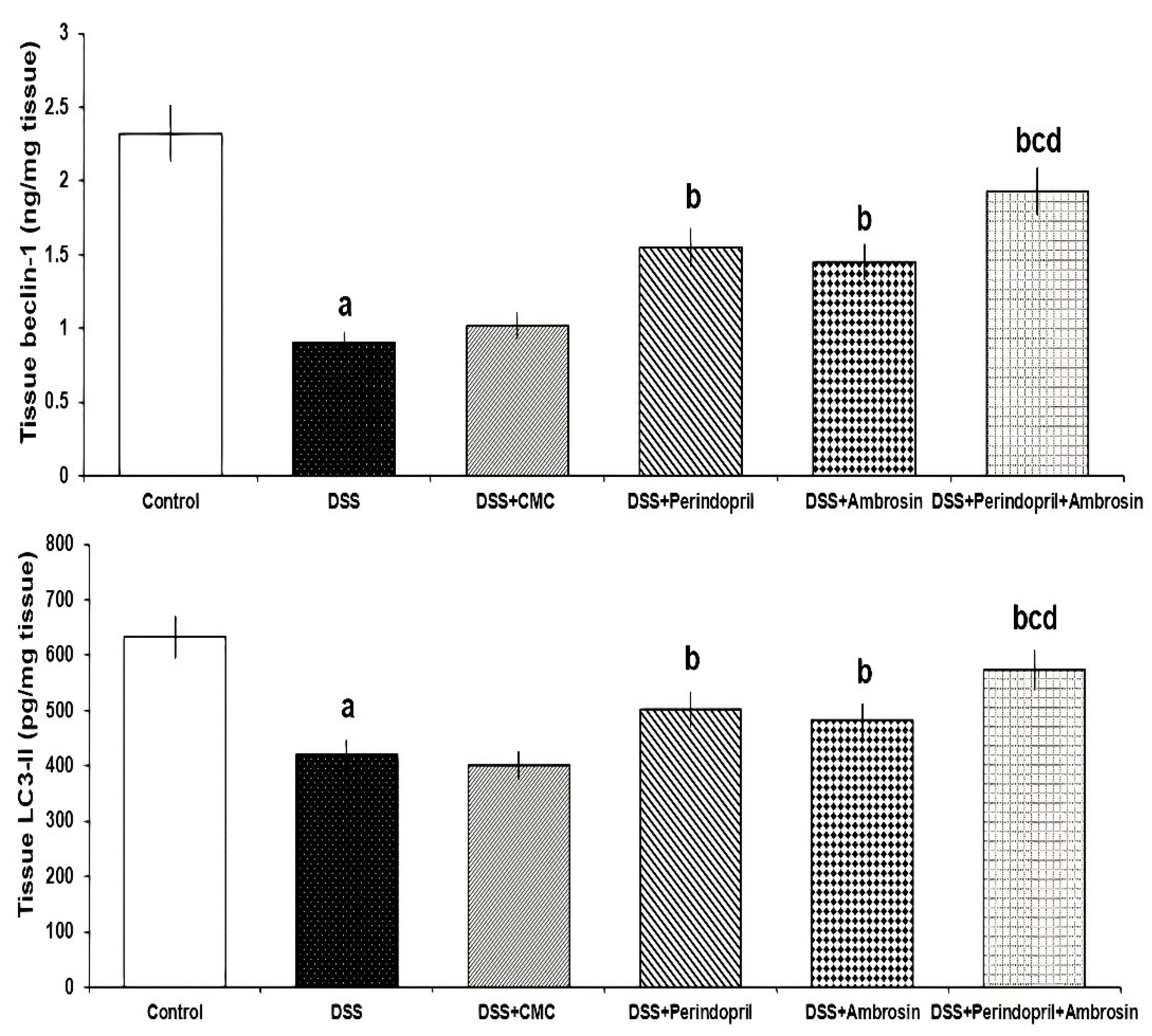
3.6. Effect of Perindopril and/or Ambrosin on Beclin-1 and LC3-II Levels in the Colonic Tissues of DSS-Treated Animals
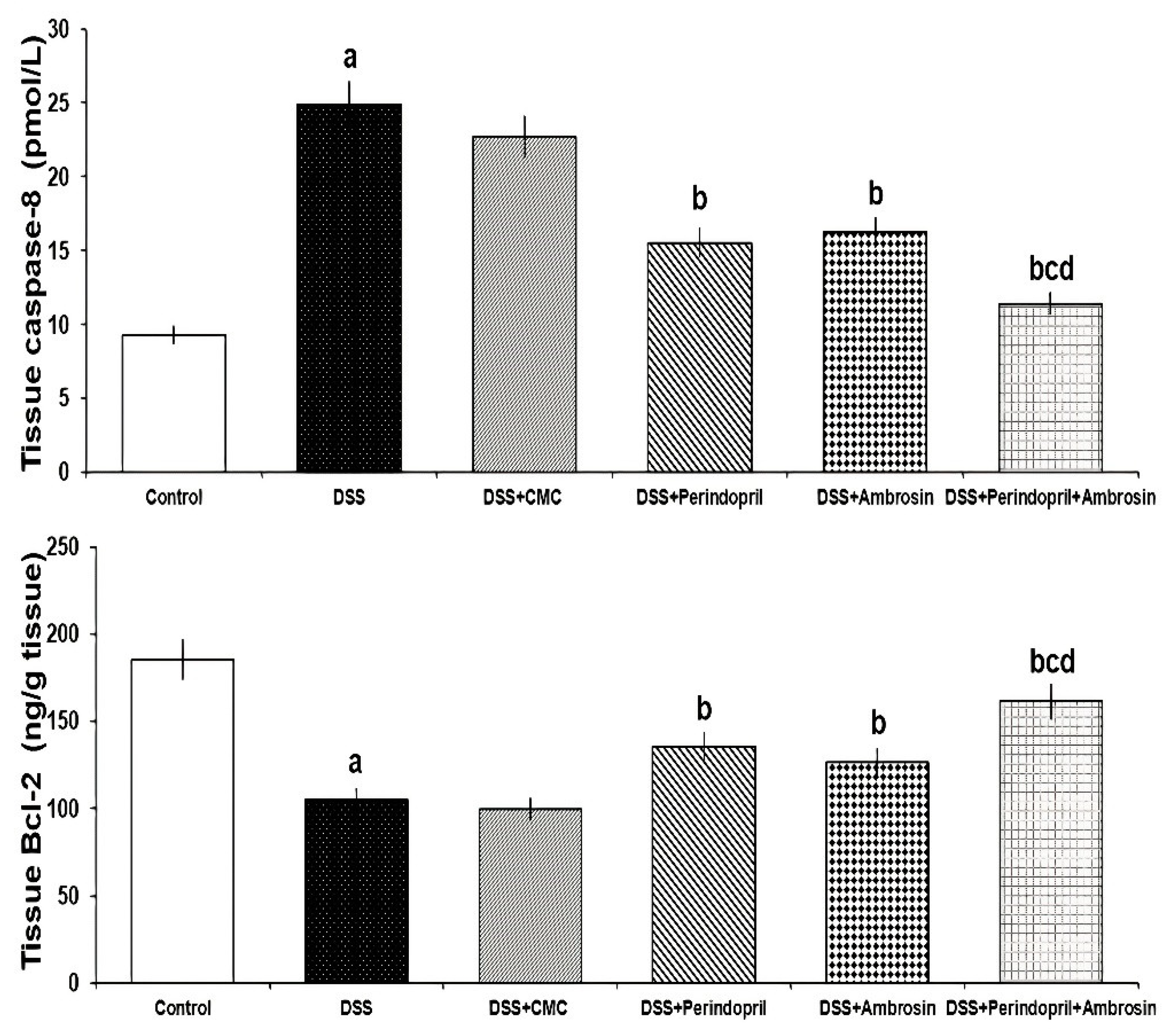
3.7. Effect of Perindopril and/or Ambrosin on Caspase-8 and Bcl-2 Levels in the Colonic Tissues of DSS-Treated Animals
3.8. Effect of Perindopril and/or Ambrosin on the Histopathological Changes and NF-κB (p65) Immunostaining in the Colonic Tissues of Mice Treated with DSS
3.9. Perindopril and/or Ambrosin Combatted the Electron Microscopic Changes of the Colonic Tissues Induced by DSS Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, R.J.; Kalla, R.; Ho, G.T. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Research 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.A.T.; Chaves, F.C.; Chaves-Junior, N. The Importance of Colonoscopy in Inflammatory Bowel Diseases. Arq. Bras. Cir. Dig. 2018, 3, e1374. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Goggolidou, P. Ulcerative colitis: Understanding its cellular pathology could provide insights into novel therapies. J. Inflamm. 2020, 17, 15. [Google Scholar] [CrossRef]
- Gatti, S.; Gelzoni, G.; Catassi, G.N.; Catassi, C. The Clinical Spectrum of Inflammatory Bowel Disease Associated with Specific Genetic Syndromes: Two Novel Pediatric Cases and a Systematic Review. Front. Pediatr. 2021, 9, 742830. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Stringer, E.; Warner, N.; van Limbergen, J.; Vandersteen, A.; Muise, A.; Derfalvi, B. Multisystem Autoimmune Inflammatory Disease, Including Colitis, Due to Inborn Error of Immunity. Pediatrics 2021, 148, e2021050614. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Negreanu, L.; Voiosu, T.; State, M.; Voiosu, A.; Bengus, A.; Mateescu, B.R. Endoscopy in inflammatory bowel disease: From guidelines to real life. Therap. Adv. Gastroenterol. 2019, 12, 1756284819865153. [Google Scholar] [CrossRef]
- Ghosh, S.; Sanchez Gonzalez, Y.; Zhou, W.; Clark, R.; Xie, W.; Louis, E.; Loftus, E.V.; Panes, J.; Danese, S. Upadacitinib Treatment Improves Symptoms of Bowel Urgency and Abdominal Pain and Correlates with Quality of Life Improvements in Patients with Moderate to Severe Ulcerative Colitis. J. Crohns Colitis 2021, 15, 2022–2030. [Google Scholar] [CrossRef]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Mohammad Jafari, R.; Shayesteh, S.; Ala, M.; Yousefi-Manesh, H.; Rashidian, A.; Hashemian, S.M.; Sorouri, M.; Dehpour, A.R. Dapsone Ameliorates Colitis through TLR4/NF-kB Pathway in TNBS Induced Colitis Model in Rat. Arch. Med. Res. 2021, 52, 595–602. [Google Scholar] [CrossRef]
- He, X.; Wei, Z.; Wang, J.; Kou, J.; Liu, W.; Fu, Y.; Yang, Z. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis. Sci. Rep. 2016, 20, 28370. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, S.; Luo, W.; Shen, Z.; Meng, X.; Xiao, M.; Tan, B.; Nie, K.; Tong, T.; Wang, X. Roseburia intestinalis-derived flagellin ameliorates colitis by targeting miR-223-3p-mediated activation of NLRP3 inflammasome and pyroptosis. Mol. Med. Rep. 2020, 22, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.A.; Alkahtani, S.A.; Alqahtani, A.A.; Hassanein, E.H.M. Umbelliferone ameliorates ulcerative colitis induced by acetic acid via modulation of TLR4/NF-κB-p65/iNOS and SIRT1/PPARγ signaling pathways in rats. Environ. Sci. Pollut. Res. Int. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Duarte, M.; Rodrigues-Pinto, T.; Sousa, T.; Faria, M.A.; Rocha, M.S.; Menezes-Pinto, D.; Esteves-Monteiro, M.; Magro, F.; Dias-Pereira, P.; Duarte-Araújo, M.; et al. Interaction between the Renin-Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis. Int. J. Mol. Sci. 2021, 22, 4836. [Google Scholar] [CrossRef]
- Jacobs, J.D.; Wagner, T.; Gulotta, G.; Liao, C.; Li, Y.C.; Bissonnette, M.; Pekow, J. Impact of Angiotensin II Signaling Blockade on Clinical Outcomes in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2019, 64, 1938–1944. [Google Scholar] [CrossRef]
- Santiago, O.I.; Rivera, E.; Ferder, L.; Appleyard, C.B. An angiotensin II receptor antagonist reduces inflammatory parameters in two models of colitis. Regul. Pept. 2008, 146, 250–259. [Google Scholar] [CrossRef]
- Svensson, D.; Lozano, M.; Almanza, G.R.; Nilsson, B.O.; Sterner, O.; Villagomez, R. Sesquiterpene lactones from Ambrosia arborescens Mill. inhibit pro-inflammatory cytokine expression and modulate NF-κB signaling in human skin cells. Phytomedicine 2018, 15, 118–126. [Google Scholar] [CrossRef]
- Khalil, M.N.A.; Choucry, M.A.; El Senousy, A.S.; Hassan, A.; El-Marasy, S.A.; El Awdan, S.A.; Omar, F.A. Ambrosin, a potent NF-κβ inhibitor, ameliorates lipopolysaccharide induced memory impairment, comparison to curcumin. PLoS ONE 2019, 14, e0219378. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer′s Disease Therapy. Biomolecules 2021, 25, 350. [Google Scholar] [CrossRef]
- Abouzid, S.; Elshahaat, A.; Ali, S.; Choudhary, M.I. Antioxidant activity of wild plants collected in Beni-Sueif governorate, Upper Egypt. Drug Discov. Ther. 2008, 2, 286–288. [Google Scholar]
- Dong, J.; Chen, Y.; Yang, F.; Zhang, W.; Wei, K.; Xiong, Y.; Wang, L.; Zhou, Z.; Li, C.; Wang, J.; et al. Naringin Exerts Therapeutic Effects on Mice Colitis: A Study Based on Transcriptomics Combined with Functional Experiments. Front. Pharmacol. 2021, 12, 729414. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Uchida, S.; Takahashi, S.; Takayama, M.; Nagata, Y.; Suzuki, N.; Shirakura, S.; Kanda, T. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer′s disease. Brain Res. 2010, 1352, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Künzli, B.M.; A-Rahim, Y.I.; Sevigny, J.; Berberat, P.O.; Enjyoji, K.; Csizmadia, E.; Friess, H.; Robson, S.C. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16788–16793. [Google Scholar] [CrossRef]
- Hirata, I.; Yasumoto, S.; Toshina, K.; Inoue, T.; Nishikawa, T.; Murano, N.; Murano, M.; Wang, F.Y.; Katsu, K. Evaluation of the effect of pyrrolidine dithiocarbamate in suppressing inflammation in mice with dextran sodium sulfate-induced colitis. World J. Gastroenterol. 2007, 13, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Andresen, L.; Jørgensen, V.L.; Perner, A.; Hansen, A.; Eugen-Olsen, J.; Rask-Madsen, J. Activation of nuclear factor KappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 2005, 54, 503–509. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers. 2020, 6, 74. [Google Scholar] [CrossRef]
- Samoilă, I.; Dinescu, S.; Costache, M. Interplay between Cellular and Molecular Mechanisms Underlying Inflammatory Bowel Diseases Development-A Focus on Ulcerative Colitis. Cells 2020, 9, 1647. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Wang, H.; Wang, T.; Weng, Y.; Zhang, Y.; Luo, Y.; Lu, Y.; Wang, Y. Therapeutic Targeting of Nrf2 Signaling by Maggot Extracts Ameliorates Inflammation-Associated Intestinal Fibrosis in Chronic DSS-Induced Colitis. Front. Immunol. 2021, 12, 670159. [Google Scholar] [CrossRef]
- Pompili, S.; Sferra, R.; Gaudio, E.; Viscido, A.; Frieri, G.; Vetuschi, A.; Latella, G. Can Nrf2 Modulate the Development of Intestinal Fibrosis and Cancer in Inflammatory Bowel Disease? Int. J. Mol. Sci. 2019, 20, 4061. [Google Scholar] [CrossRef]
- Liu, H.; Johnston, L.J.; Wang, F.; Ma, X. Triggers for the Nrf2/ARE Signaling Pathway and Its Nutritional Regulation: Potential Therapeutic Applications of Ulcerative Colitis. Int. J. Mol. Sci. 2021, 22, 11411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, H.; Chen, W.; Cui, Y.; Huang, A.; Qi, X. Perindopril Improves Cardiac Function by Enhancing the Expression of SIRT3 and PGC-1α in a Rat Model of Isoproterenol-Induced Cardiomyopathy. Front. Pharmacol. 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Ancion, A.; Tridetti, J.; Nguyen Trung, M.L.; Oury, C.; Lancellotti, P. A Review of the Role of Bradykinin and Nitric Oxide in the Cardioprotective Action of Angiotensin-Converting Enzyme Inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Ling, T.; Zhao, C.; Li, Y.; Geng, M.; Gai, S.; Qi, W.; Luo, X.; Chen, L.; et al. Chitosan Oligosaccharides Alleviate Colitis by Regulating Intestinal Microbiota and PPARγ/SIRT1-Mediated NF-κB Pathway. Mar. Drugs 2022, 20, 96. [Google Scholar] [CrossRef]
- Decara, J.; Rivera, P.; López-Gambero, A.J.; Serrano, A.; Pavón, F.J.; Baixeras, E.; Rodríguez de Fonseca, F.; Suárez, J. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front. Pharmacol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Dou, X.; Xiao, J.; Jin, Z.; Zheng, P. Peroxisome proliferator-activated receptor-γ is downregulated in ulcerative colitis and is involved in experimental colitis-associated neoplasia. Oncol. Lett. 2015, 10, 1259–1266. [Google Scholar] [CrossRef]
- Sayed, A.M.; Abdel-Fattah, M.M.; Arab, H.H.; Mohamed, W.R.; Hassanein, E.H.M. Targeting inflammation and redox aberrations by perindopril attenuates methotrexate-induced intestinal injury in rats: Role of TLR4/NF-κB and c-Fos/c-Jun pro-inflammatory pathways and PPAR-γ/SIRT1 cytoprotective signals. Chem. Biol. Interact. 2022, 351, 109732. [Google Scholar] [CrossRef]
- Curciarello, R.; Docena, G.H.; MacDonald, T.T. The Role of Cytokines in the Fibrotic Responses in Crohn’s Disease. Front. Med. 2017, 4, 126. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Pei, C.; Liang, J.; Ding, P.; Chen, S.; Hou, S.Z. Monotropein alleviates secondary liver injury in chronic colitis by regulating TLR4/NF-κB signaling and NLRP3 inflammasome. Eur. J. Pharmacol. 2020, 883, 173358. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, K.; Nakase, H. Contradictory Effects of NLRP3 Inflammasome Regulatory Mechanisms in Colitis. Int. J. Mol. Sci. 2020, 21, 8145. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Langfermann, D.S.; Rössler, O.G.; Thiel, G. Stimulation of B-Raf increases c-Jun and c-Fos expression and upregulates AP-1-regulated gene transcription in insulinoma cells. Mol. Cell Endocrinol. 2018, 472, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Lertnimitphun, P.; Jiang, Y.; Kim, N.; Fu, W.; Zheng, C.; Tan, H.; Zhou, H.; Zhang, X.; Pei, W.; Lu, Y.; et al. Safranal Alleviates Dextran Sulfate Sodium-Induced Colitis and Suppresses Macrophage-Mediated Inflammation. Front. Pharmacol. 2019, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Koper, M.; Ufnal, M. Gut microbiota and renin-angiotensin system: A complex interplay at local and systemic levels. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G355–G366. [Google Scholar] [CrossRef] [PubMed]
- Broom, O.J.; Widjaya, B.; Troelsen, J.; Olsen, J.; Nielsen, O.H. Mitogen activated protein kinases: A role in inflammatory bowel disease? Clin. Exp. Immunol. 2009, 158, 272–280. [Google Scholar] [CrossRef]
- Shao, B.Z.; Yao, Y.; Zhai, J.S.; Zhu, J.H.; Li, J.P.; Wu, K. The Role of Autophagy in Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 621132. [Google Scholar] [CrossRef]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W.I. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar] [CrossRef]
- Kubota, M.; Kakimoto, K.; Nakagawa, T.; Koubayashi, E.; Nakazawa, K.; Tawa, H.; Hirata, Y.; Okada, T.; Kawakami, K.; Asai, A.; et al. Autophagy deficiency exacerbates colitis through excessive oxidative stress and MAPK signaling pathway activation. PLoS ONE 2019, 14, e0225066. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.; Deng, S.; Chen, J.; Gu, Q.; Su, H.; Wen, L.; Wang, S.; Lin, C.; Qi, C.; et al. Slit2/Robo1 Mitigates DSS-induced Ulcerative Colitis by Activating Autophagy in Intestinal Stem Cell. Int. J. Biol. Sci. 2020, 16, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, J.; Yan, Y.; Ruan, Z.; Su, Y.; Wang, J.; Huang, H.; Zhang, Y.; Wang, W.; Gao, J.; et al. Angiotensin-converting enzyme 2 regulates autophagy in acute lung injury through AMPK/mTOR signaling. Arch. Biochem. Biophys. 2019, 672, 108061. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.B.; Fu, P.Y.; Ky, N.; Zhu, H.S.; Feng, X.; Li, J.; Srinivasan, K.G.; Hamza, M.S.; Zhao, Y. NF-κB p65 repression by the sesquiterpene lactone, Helenalin, contributes to the induction of autophagy cell death. BMC Complement. Altern. Med. 2012, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, Z.; Wang, C.; Zhang, J.; Liu, C. Methane Alleviates Inflammation and Apoptosis of Dextran Sulfate Sodium-Induced Inflammatory Bowel Diseases by Inhibiting Toll-Like Receptor 4 (TLR4)/Myeloid Differentiation Factor 88 (MyD88)/Nuclear Translocation of Nuclear Factor-κB (NF-κB) and Endoplasmic Reticulum Stress Pathways in Mice. Med. Sci. Monit. 2020, 26, e922248. [Google Scholar] [CrossRef]
- Günther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef]
- Diarra, A.; Eissa, N.; Ghia, J. A212 Chromofungin Protects Against DSS-Induced Colitis by Regulating P-53 Apoptitic Pathway. J. Can. Assoc. Gastroenterol. 2020, 3, 84–85. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Shi, L.; Chen, L.; Zhang, B.; Ma, K.; Liu, Y.; Qian, Y. Protective effects of perindopril on d-galactose and aluminum trichloride induced neurotoxicity via the apoptosis of mitochondria-mediated intrinsic pathway in the hippocampus of mice. Brain Res. Bull. 2014, 109, 46–53. [Google Scholar] [CrossRef]
- Fan, S.; Cui, Y.; Hu, Z.; Wang, W.; Jiang, W.; Xu, H. Ambrosin sesquiterpene lactone exerts selective and potent anticancer effects in drug-resistant human breast cancer cells (MDA-MB-231) through mitochondrial mediated apoptosis, ROS generation and targeting Akt/β-Catenin signaling pathway. J. Buon. 2020, 25, 2221–2227. [Google Scholar]
- Wang, P.; Kong, C.H.; Zhang, C.X. Chemical composition and antimicrobial activity of the essential oil from Ambrosia trifida L. Molecules 2006, 11, 549–555. [Google Scholar] [CrossRef]
| Score | Weight Loss (%) | Stool Consistency | Hematochezia |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 0–10 | ------- | ------- |
| 2 | 11–15 | Loose | Hemoccult positive |
| 3 | 16–20 | ------- | ------- |
| 4 | >20 | Diarrhea | Gross bleeding |
| Parameters | Control (n = 8) | DSS (n = 8) | DSS + CMC (n = 8) | DSS + Perindopril (n = 8) | DSS + Ambrosin (n = 8) | DSS + Perindopril + Ambrosin (n = 8) |
|---|---|---|---|---|---|---|
| Tissue glutathione reductase (U/g tissue) | 152.25 ± 18.33 | 53.41 ± 7.89 a | 49.94 ± 7.19 | 93.41 ± 11.72 b | 86.27 ± 9.83 b | 126.84 ± 14.3 bcd |
| Tissue glutathione peroxidase (U/g tissue) | 35.31 ± 4.71 | 13.72 ± 1.85 a | 14.37 ± 1.89 | 24.13 ± 2.93 b | 21.81 ± 2.68 b | 30.39 ± 4.03 bcd |
| Tissue ROS (U/g tissue) | 56.47 ± 6.23 | 174.86 ± 19.27 a | 167.3 ± 18.51 | 98.46 ± 10.75 b | 112.81 ± 13.02 b | 74.37 ± 8.24 bcd |
| Tissue iNOS (ng/g tissue) | 21.46 ± 2.82 | 45.57 ± 5.61 a | 48.19 ± 5.92 | 33.67 ± 3.95 b | 38.56 ± 4.23 b | 26.76 ± 3.1 bcd |
| Tissue Nrf2 content (×10−1 ng/mg protein) | 0.47 ± 0.05 | 0.21 ± 0.03 a | 0.23 ± 0.03 | 0.34 ± 0.04 b | 0.31 ± 0.04 b | 0.41 ± 0.05 bcd |
| Parameters | Control (n = 8) | DSS (n = 8) | DSS + CMC (n = 8) | DSS + Perindopril (n = 8) | DSS + Ambrosin (n = 8) | DSS + Perindopril + Ambrosin (n = 8) |
|---|---|---|---|---|---|---|
| Tissue TLR-4 (ng/g tissue) | 321.47 ± 42.3 | 797.5 ± 87.13 a | 776.3 ± 82.17 | 547.7 ± 62.79 b | 595.3 ± 68.18 b | 427.8 ± 52.61 bcd |
| Tissue IL-1β (pg/mg protein) | 477.3 ± 54.5 | 1275.8 ± 146.7 a | 1298.3 ± 155.6 | 883.5 ± 97.4 b | 973.2 ± 112.36 b | 643.2 ± 75.23 bcd |
| Tissue IL-6 (pg/mg protein) | 231.64 ± 33.28 | 686.12 ± 75.76 a | 662.96 ± 72.57 | 432.49 ± 51.7 b | 474.38 ± 56.27 b | 331.67 ± 43.76 bcd |
| Tissue TGF-β1 (pg/mg protein) | 19.35 ± 2.93 | 78.38 ± 8.31 a | 83.1 ± 8.83 | 48.53 ± 5.64 b | 55.26 ± 6.23 b | 33.78 ± 4.52 bcd |
| Parameters | Control (n = 8) | DSS (n = 8) | DSS + CMC (n = 8) | DSS + Perindopril (n = 8) | DSS + Ambrosin (n = 8) | DSS + Perindopril + Ambrosin (n = 8) |
|---|---|---|---|---|---|---|
| Tissue phospho-p38 MAPK (% change from the control) | 100.00 ± 12.11 | 178.65 ± 19.34 a | 183.94 ± 19.88 | 141.75 ± 15.84 b | 149.45 ± 16.15 b | 123.17 ± 14.93 bcd |
| Tissue c-Fos (% change from the control) | 100.00 ± 9.23 | 229.75 ± 25.73 a | 234.24 ± 28.16 | 173.41 ± 19.8 b | 188.74 ± 21.03 b | 144.72 ± 17.36 bcd |
| Tissue c-Jun (% change from the control) | 100.00 ± 11.65 | 257.36 ± 29.45 a | 249.72 ± 28.15 | 181.43 ± 20.47 b | 196.74 ± 21.42 b | 153.78 ± 17.79 bcd |
| Tissue NLRP3 inflammasome (pg/mg protein) | 186.3 ± 20.45 | 572.5 ± 63.48 a | 589.7 ± 68.33 | 364.5 ± 41.76 b | 391.92 ± 45.8 b | 277.56 ± 34.8 bcd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabel, A.M.; Atef, A.; Borg, H.M.; El-Sheikh, A.A.K.; Al Khabbaz, H.J.; Arab, H.H.; Estfanous, R.S. Perindopril/Ambrosin Combination Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice: Crosstalk between Toll-Like Receptor 4, the Pro-Inflammatory Pathways, and SIRT1/PPAR-γ Signaling. Pharmaceuticals 2022, 15, 600. https://doi.org/10.3390/ph15050600
Kabel AM, Atef A, Borg HM, El-Sheikh AAK, Al Khabbaz HJ, Arab HH, Estfanous RS. Perindopril/Ambrosin Combination Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice: Crosstalk between Toll-Like Receptor 4, the Pro-Inflammatory Pathways, and SIRT1/PPAR-γ Signaling. Pharmaceuticals. 2022; 15(5):600. https://doi.org/10.3390/ph15050600
Chicago/Turabian StyleKabel, Ahmed M., Aliaa Atef, Hany M. Borg, Azza A. K. El-Sheikh, Hana J. Al Khabbaz, Hany H. Arab, and Remon S. Estfanous. 2022. "Perindopril/Ambrosin Combination Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice: Crosstalk between Toll-Like Receptor 4, the Pro-Inflammatory Pathways, and SIRT1/PPAR-γ Signaling" Pharmaceuticals 15, no. 5: 600. https://doi.org/10.3390/ph15050600
APA StyleKabel, A. M., Atef, A., Borg, H. M., El-Sheikh, A. A. K., Al Khabbaz, H. J., Arab, H. H., & Estfanous, R. S. (2022). Perindopril/Ambrosin Combination Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice: Crosstalk between Toll-Like Receptor 4, the Pro-Inflammatory Pathways, and SIRT1/PPAR-γ Signaling. Pharmaceuticals, 15(5), 600. https://doi.org/10.3390/ph15050600






