Future Prospects of Positron Emission Tomography–Magnetic Resonance Imaging Hybrid Systems and Applications in Psychiatric Disorders
Abstract
:1. Introduction
2. MRI-PET Fusion Imaging
2.1. MRI
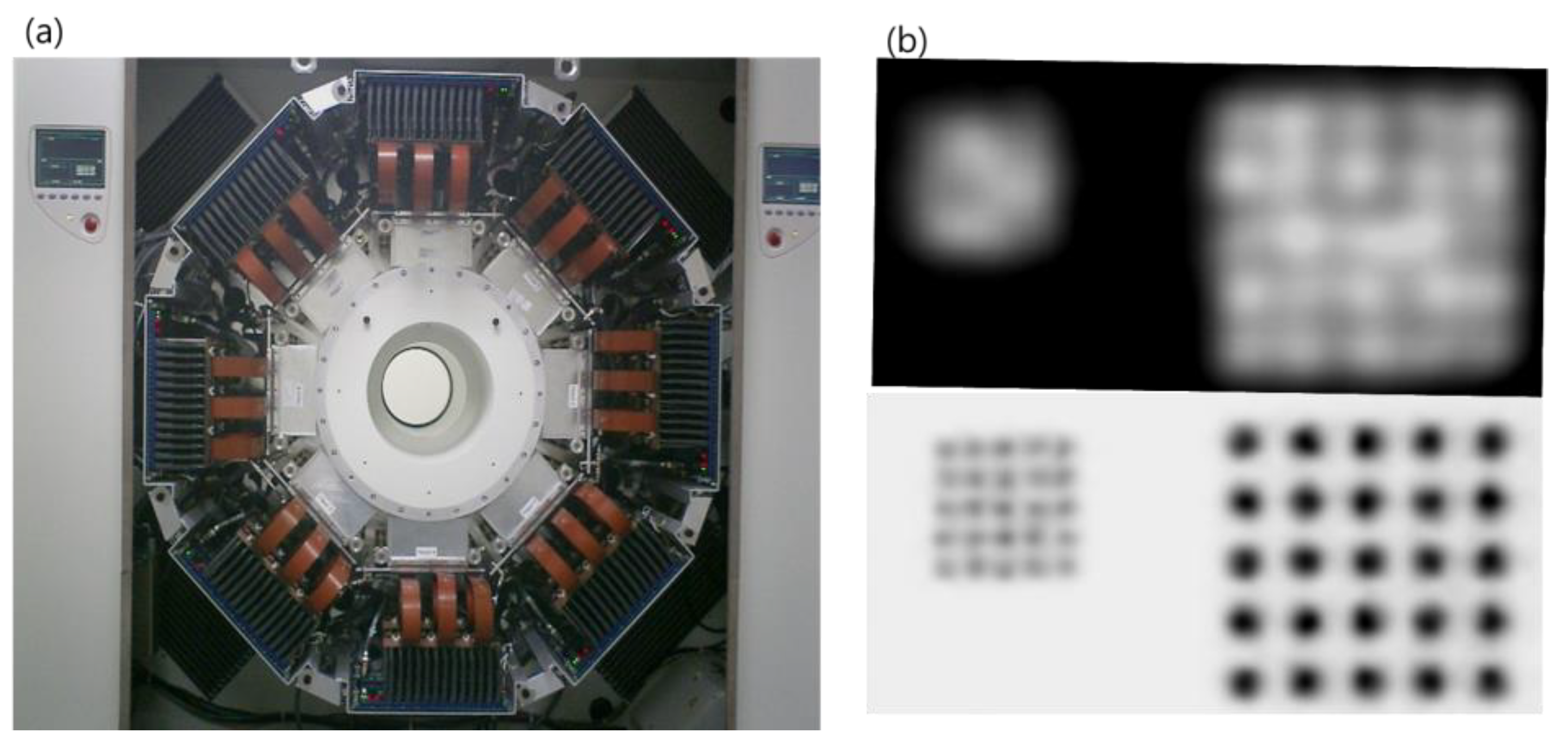
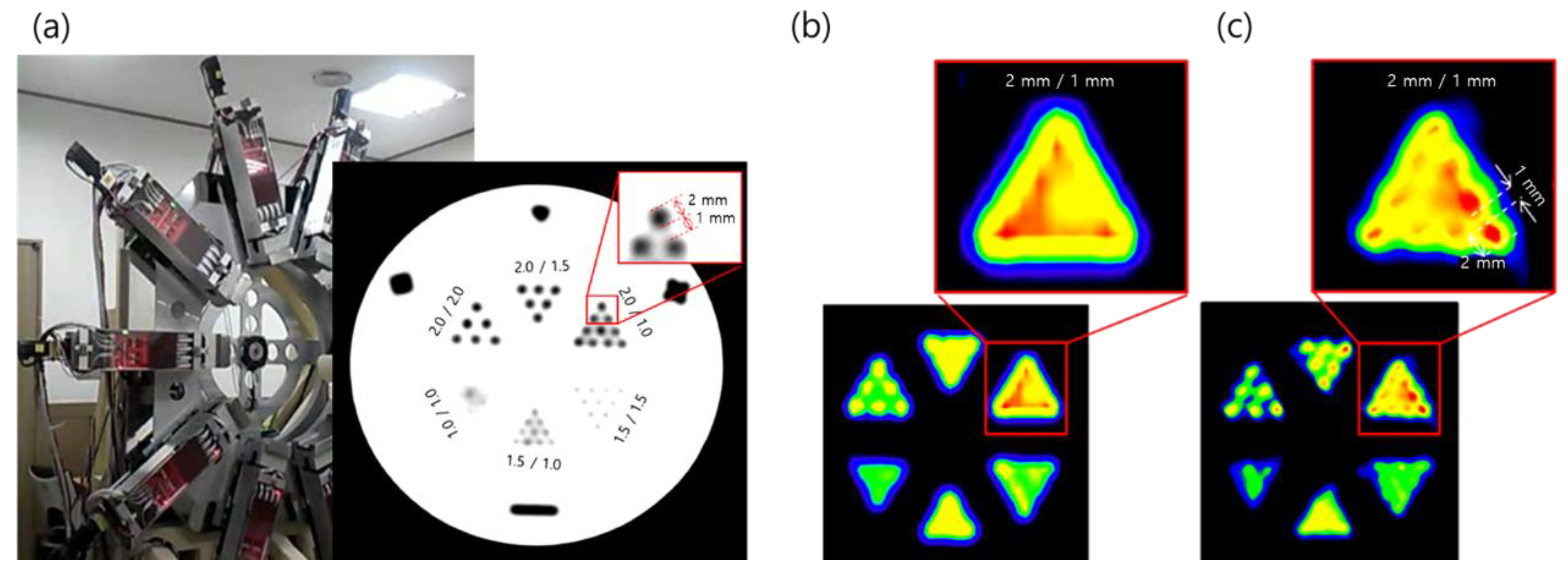
2.2. PET
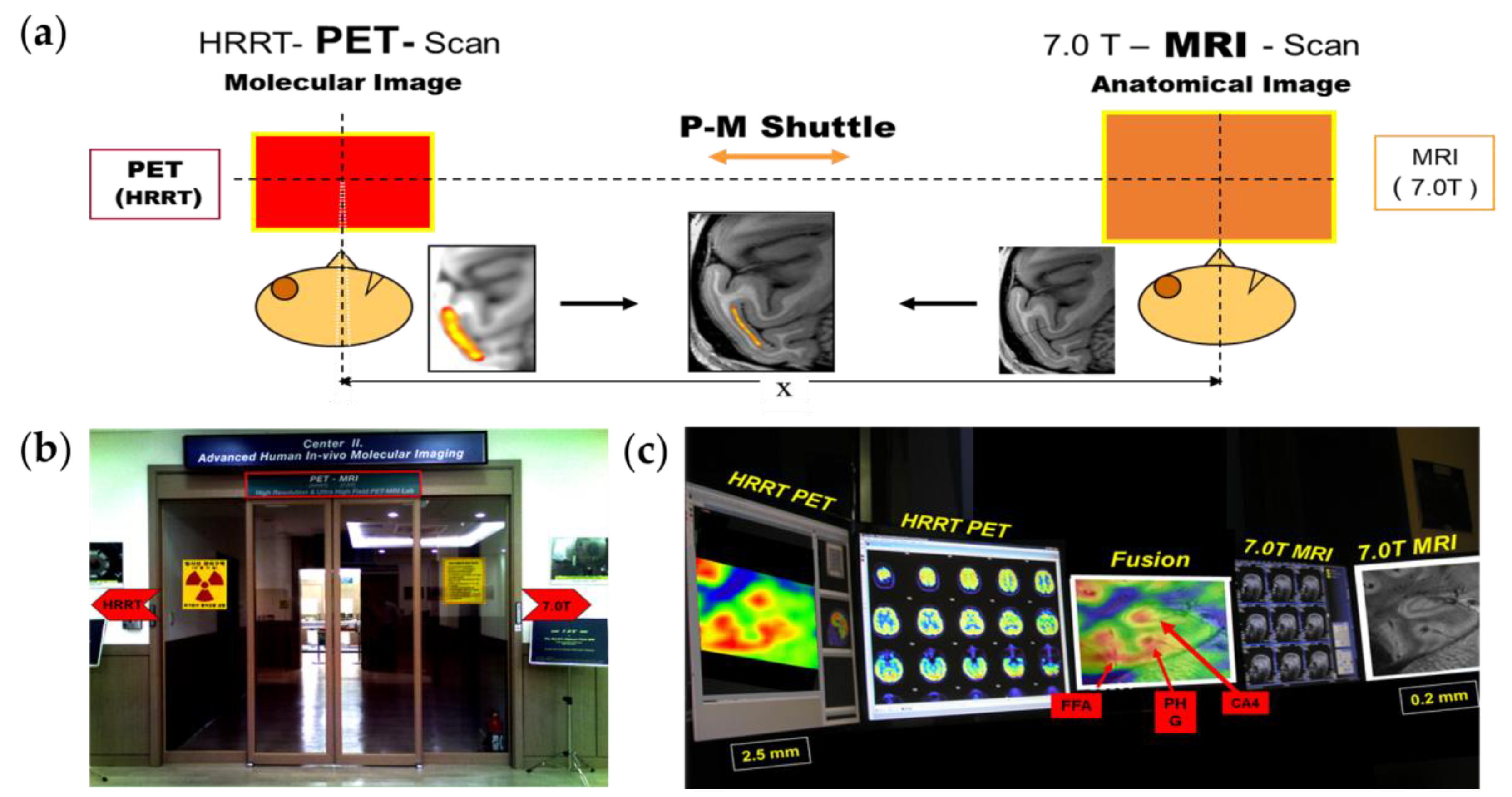
2.3. PET–MRI Fusion Imaging
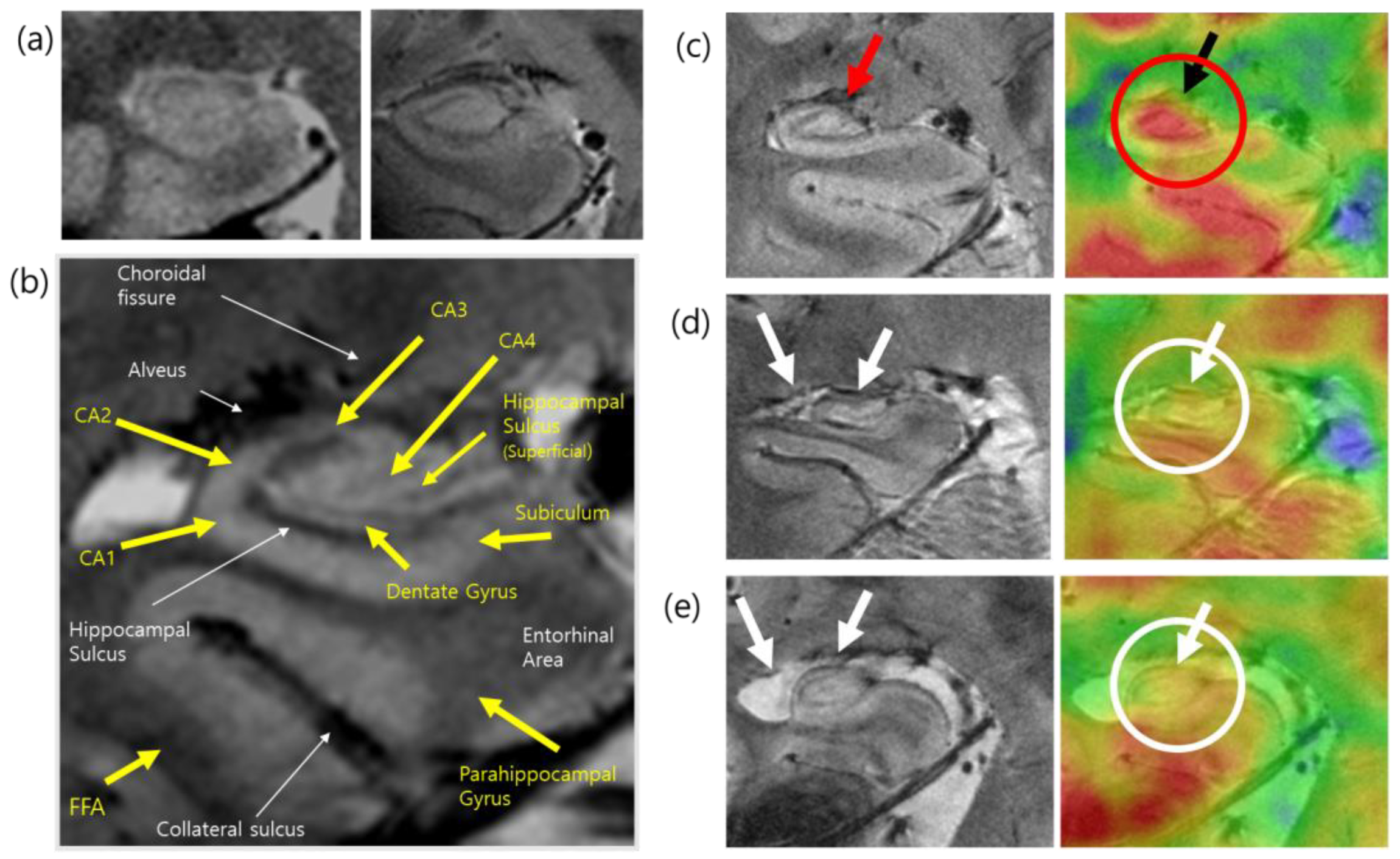
2.4. Applications of PET–MRI
3. Molecular Brain Imaging in Psychiatric Disorders
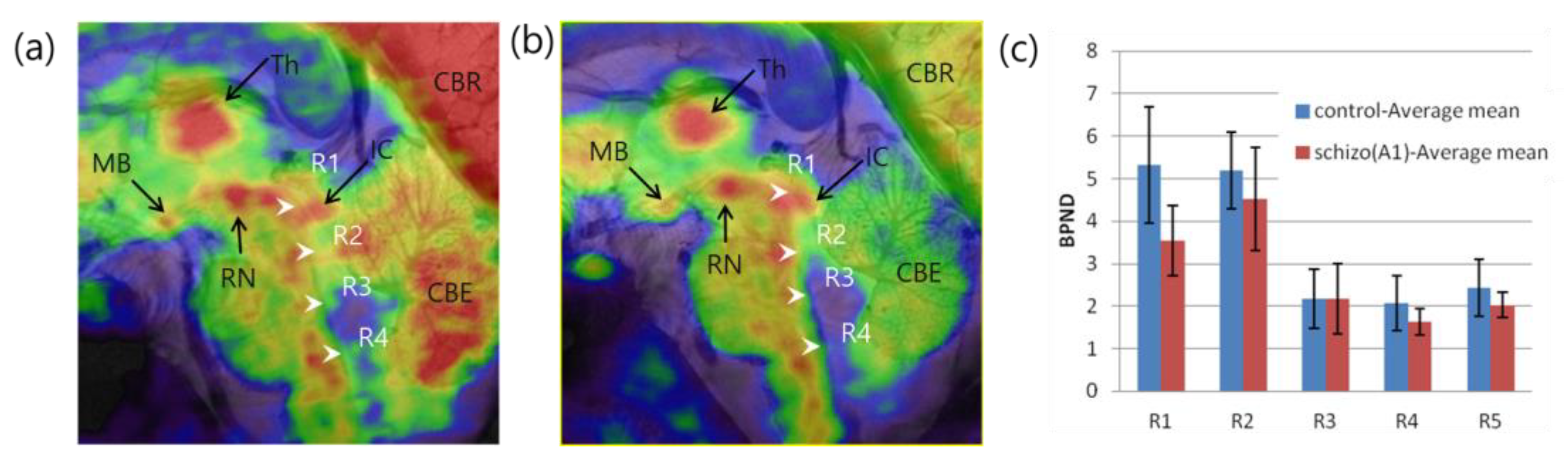
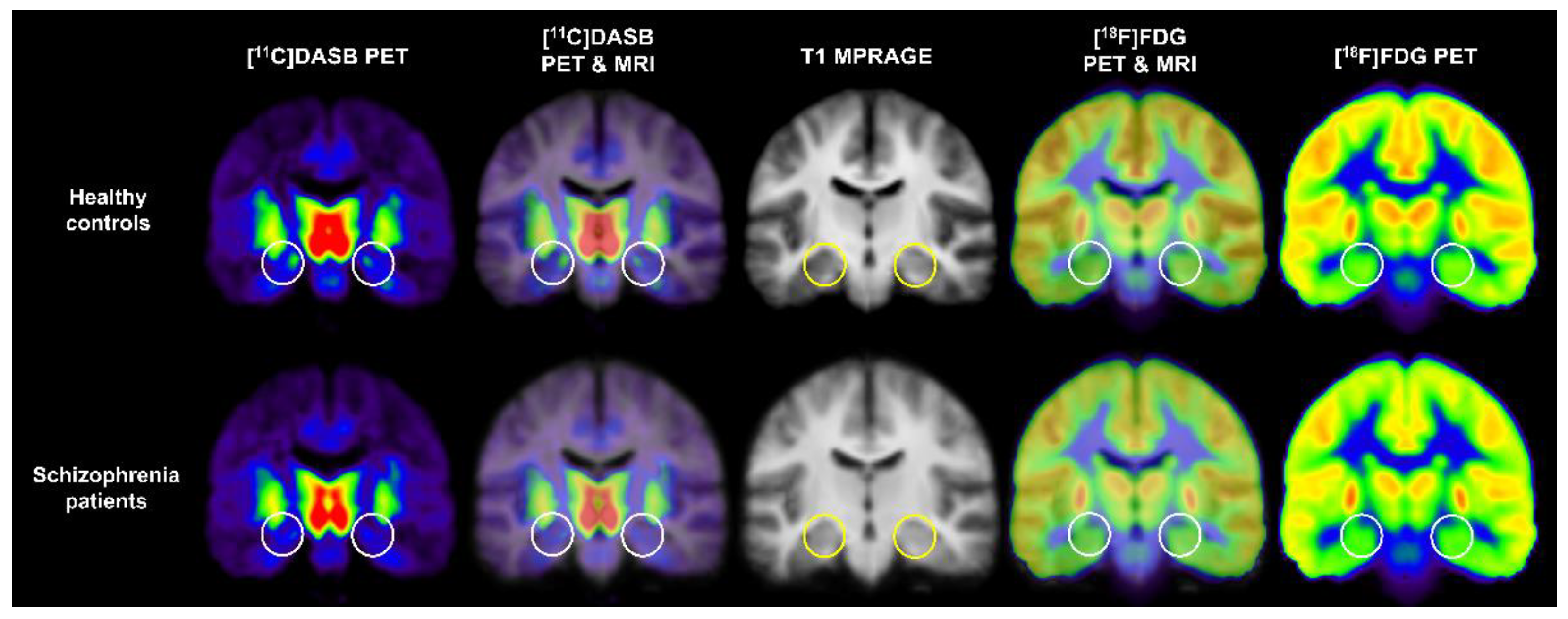
3.1. Schizophrenia
3.2. Major Depressive Disorder
4. PET–MRI Applications in Psychiatric Disorders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, Z.H. General Views on 3-D Image Reconstruction and Computerized Transverse Axial Tomography. IEEE Trans. Nucl. Sci. 1974, 21, 44–71. [Google Scholar] [CrossRef]
- Lauterbur, P.C. The Classic: Image Formation by Induced Local Interactions: Examples Employing Nuclear Magnetic Resonance. Clin. Orthop. Relat. Res. 1989, 244, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Phelps, M.E.; Hoffman, E.J.; Mullani, N.A.; Ter-Pogossian, M.M. Application of Annihilation Coincidence Detection to Transaxial Reconstruction Tomography. J. Nucl. Med. 1975, 16, 210–224. [Google Scholar] [PubMed]
- Cho, Z.H.; Chan, J.K.; Eriksson, L. Circular Ring Transverse Axial Positron Camera for 3-Dimensional Reconstruction of Radionuclides Distribution. IEEE Trans. Nucl. Sci. 1976, 23, 613–622. [Google Scholar] [CrossRef]
- Townsend, D.W.; Beyer, T.; Kinahan, P.E.; Brun, T.; Roddy, R.; Nutt, R.; Byars, L.G. The SMART Scanner: A Combined PET/CT Tomograph for Clinical Oncology. In Proceedings of the 1998 IEEE Nuclear Science Symposium Conference Record, 1998 IEEE Nuclear Science Symposium and Medical Imaging Conference (Cat. No.98CH36255), Toronto, ON, Canada, 8–14 November 1998; Volume 2, pp. 1170–1174. [Google Scholar]
- Musafargani, S.; Ghosh, K.K.; Mishra, S.; Mahalakshmi, P.; Padmanabhan, P.; Gulyas, B. PET/MRI: A frontier in era of complementary hybrid imaging. Eur. J. Hybrid Imag. 2018, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- van der Kolk, A.G.; Hendrikse, J.; Zwanenburg, J.J.M.; Visser, F.; Luijten, P.R. Clinical Applications of 7 T MRI in the Brain. Eur. J. Radiol. 2013, 82, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Cho, Z.-H.; Calamante, F.; Chi, J.-G. 7.0 Tesla MRI Brain White Matter Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-642-54392-0. [Google Scholar]
- Cho, Z.-H.; Kang, C.-K.; Han, J.-Y.; Kim, S.-H.; Kim, K.-N.; Hong, S.-M.; Park, C.-W.; Kim, Y.-B. Observation of the Lenticulostriate Arteries in the Human Brain In Vivo Using 7.0T MR Angiography. Stroke 2008, 39, 1604–1606. [Google Scholar] [CrossRef] [Green Version]
- Cho, Z.-H.; Min, H.-K.; Oh, S.-H.; Han, J.-Y.; Park, C.-W.; Chi, J.-G.; Kim, Y.-B.; Paek, S.H.; Lozano, A.M.; Lee, K.H. Direct Visualization of Deep Brain Stimulation Targets in Parkinson Disease with the Use of 7-Tesla Magnetic Resonance Imaging: Clinical Article. J. Neurosurg. 2010, 113, 639–647. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Kim, J.-M.; Oh, S.-H.; Jeong, H.-J.; Park, S.-Y.; Oh, E.-S.; Chi, J.-G.; Kim, Y.-B.; Jeon, B.S.; Cho, Z.-H. Seven-Tesla Magnetic Resonance Images of the Substantia Nigra in Parkinson Disease. Ann. Neurol. 2012, 71, 267–277. [Google Scholar] [CrossRef]
- Jones, S.E.; Lee, J.; Law, M. Neuroimaging at 3T vs. 7T: Is It Really Worth It? Magn. Reason. Imaging Clin. N. Am. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Cho, Z.-H.; Kang, C.-K.; Son, Y.-D.; Choi, S.-H.; Lee, Y.-B.; Paek, S.H.; Park, C.-W.; Chi, J.-G.; Calamante, F.; Law, M.; et al. Pictorial Review of In Vivo Human Brain: From Anatomy to Molecular Imaging. World Neurosurg. 2014, 82, 72–95. [Google Scholar] [CrossRef] [PubMed]
- Cho, Z.-H.; Son, Y.-D.; Kim, H.-K.; Kim, N.-B.; Choi, E.-J.; Lee, S.-Y.; Chi, J.-G.; Park, C.-W.; Kim, Y.-B.; Ogawa, S. Observation of Glucose Metabolism in the Thalamic Nuclei by Fusion PET/MRI. J. Nucl. Med. 2011, 52, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourdias, T.; Saranathan, M.; Levesque, I.R.; Su, J.; Rutt, B.K. Visualization of Intra-Thalamic Nuclei with Optimized White-Matter-Nulled MPRAGE at 7T. Neuroimage 2014, 84, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanowski, M.; Voges, J.; Buentjen, L.; Stadler, J.; Heinze, H.-J.; Tempelmann, C. Direct Visualization of Anatomic Subfields within the Superior Aspect of the Human Lateral Thalamus by MRI at 7T. AJNR Am. J. Neuroradiol. 2014, 35, 1721–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, G.; Testud, B.; Girard, O.M.; Lehmann, P.; de Rochefort, L.; Besson, P.; Massire, A.; Ridley, B.; Girard, N.; Guye, M.; et al. Automatic Segmentation of Deep Grey Nuclei Using a High-Resolution 7T Magnetic Resonance Imaging Atlas-Quantification of T1 Values in Healthy Volunteers. Eur. J. Neurosci. 2022, 55, 438–460. [Google Scholar] [CrossRef] [PubMed]
- Calamante, F.; Oh, S.-H.; Tournier, J.-D.; Park, S.-Y.; Son, Y.-D.; Chung, J.-Y.; Chi, J.-G.; Jackson, G.D.; Park, C.-W.; Kim, Y.-B.; et al. Super-Resolution Track-Density Imaging of Thalamic Substructures: Comparison with High-Resolution Anatomical Magnetic Resonance Imaging at 7.0T. Hum. Brain Mapp. 2013, 34, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.A.; Bertino, S.; Bramanti, A.; Ciurleo, R.; Anastasi, G.P.; Milardi, D.; Cacciola, A. In Vivo Super-Resolution Track-Density Imaging for Thalamic Nuclei Identification. Cereb. Cortex 2021, 31, 5613–5636. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Paek, S.H.; Kim, Y.-B.; Lee, H.; Cho, Z.-H. In Vivo 3D Reconstruction of the Human Pallidothalamic and Nigrothalamic Pathways With Super-Resolution 7T MR Track Density Imaging and Fiber Tractography. Front. Neuroanat. 2021, 15, 86. [Google Scholar] [CrossRef]
- Vandenberghe, S.; Marsden, P.K. PET–MRI: A Review of Challenges and Solutions in the Development of Integrated Multimodality Imaging. Phys. Med. Biol. 2015, 60, R115–R154. [Google Scholar] [CrossRef]
- Hernandez, D.; Kim, K.-N. A Review on the RF Coil Designs and Trends for Ultra High Field Magnetic Resonance Imaging. Investig. Magn. Reson. Imaging 2020, 24, 95–122. [Google Scholar] [CrossRef]
- Khalil, M. PET/MR: Basics and New Developments. In Basic Science of PET Imaging; Springer: Cham, Switzerland, 2017; pp. 199–228. ISBN 978-3-319-40068-6. [Google Scholar]
- Schug, D.; Lerche, C.; Weissler, B.; Gebhardt, P.; Goldschmidt, B.; Wehner, J.; Dueppenbecker, P.M.; Salomon, A.; Hallen, P.; Kiessling, F.; et al. Initial PET Performance Evaluation of a Preclinical Insert for PET/MRI with Digital SiPM Technology. Phys. Med. Biol. 2016, 61, 2851–2878. [Google Scholar] [CrossRef] [PubMed]
- Pratte, J.-F.; Nolet, F.; Parent, S.; Vachon, F.; Roy, N.; Rossignol, T.; Deslandes, K.; Dautet, H.; Fontaine, R.; Charlebois, S.A. 3D Photon-to-Digital Converter for Radiation Instrumentation: Motivation and Future Works. Sensors 2021, 21, 598. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, P.; Gundacker, S. SiPM Applications in Positron Emission Tomography: Toward Ultimate PET Time-of-Flight Resolution. Eur. Phys. J. Plus 2021, 136, 292. [Google Scholar] [CrossRef]
- Moses, W.W. Fundamental Limits of Spatial Resolution in PET. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. Nucl. Instrum. Methods Phys. Res. 2011, 648, S236–S240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K. Feasibility of High Spatial Resolution Working Modes for Clinical PET Scanner. Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2018, 7, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Cho, Z.H.; Chan, J.K.; Ericksson, L.; Singh, M.; Graham, S.; MacDonald, N.S.; Yano, Y. Positron Ranges Obtained from Biomedically Important Positron-Emitting Radionuclides. J. Nucl. Med. 1975, 16, 1174–1176. [Google Scholar]
- Levin, C.S.; Hoffman, E.J. Calculation of Positron Range and Its Effect on the Fundamental Limit of Positron Emission Tomography System Spatial Resolution. Phys. Med. Biol. 1999, 44, 781–799. [Google Scholar] [CrossRef]
- Cho, Z.H.; Juh, S.C.; Friedenberg, R.M.; Bunney, W.; Buchsbaum, M.; Wong, E. A New Approach to Very High Resolution Mini-Brain PET Using a Small Number of Large Detectors. IEEE Trans. Nucl. Sci. 1990, 37, 842–851. [Google Scholar] [CrossRef]
- Lowdon, M.; Martin, P.G.; Hubbard, M.W.J.; Taggart, M.P.; Connor, D.T.; Verbelen, Y.; Sellin, P.J.; Scott, T.B. Evaluation of Scintillator Detection Materials for Application within Airborne Environmental Radiation Monitoring. Sensors 2019, 19, 3828. [Google Scholar] [CrossRef] [Green Version]
- Wienhard, K.; Schmand, M.; Casey, M.E.; Baker, K.; Bao, J.; Eriksson, L.; Jones, W.F.; Knoess, C.; Lenox, M.; Lercher, M.; et al. The ECAT HRRT: Performance and First Clinical Application of the New High Resolution Research Tomograph. IEEE Trans. Nucl. Sci. 2002, 49, 104–110. [Google Scholar] [CrossRef]
- Cho, Z.-H.; Son, Y.-D.; Kim, H.-K.; Kwon, D.-H.; Joo, Y.-H.; Ra, J.B.; Choi, Y.; Kim, Y.-B. Development of Positron Emission Tomography With Wobbling and Zooming for High Sensitivity and High-Resolution Molecular Imaging. IEEE Trans. Med. Imaging 2019, 38, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Son, Y.-D.; Kwon, D.-H.; Joo, Y.; Cho, Z.-H. Wobbling and LSF-Based Maximum Likelihood Expectation Maximization Reconstruction for Wobbling PET. Radiat. Phys. Chem. 2016, 121, 1–9. [Google Scholar] [CrossRef]
- Ter-Pogossian, M.M.; Mullani, N.A.; Hood, J.T.; Higgins, C.S.; Ficke, D.C. Design Considerations for a Positron Emission Transverse Tomograph (PETT V) for Imaging of the Brain. J. Comput. Assist. Tomogr. 1978, 2, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Sank, V.J.; Talbert, A.J.; Di Chiro, G. Sampling Requirements and Detector Motion for Positron Emission Tomography. IEEE Trans. Nucl. Sci. 1979, 26, 2760–2763. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, X.; Wang, J.; Zhang, L.; Di, X.; Xu, Y. FDG-PET/CT Imaging for Tumor Staging and Definition of Tumor Volumes in Radiation Treatment Planning in Non-Small Cell Lung Cancer. Oncol. Lett. 2014, 7, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- Ming, Y.; Wu, N.; Qian, T.Y.; Li, X.; Wan, D.Q.; Li, C.Y.; Li, Y.L.; Wu, Z.H.; Wang, X.; Liu, J.Q.; et al. Progress and Future Trends in PET/CT and PET/MRI Molecular Imaging Approaches for Breast Cancer. Front. Oncol. 2020, 10, 1301. [Google Scholar] [CrossRef]
- Ehman, E.C.; Johnson, G.B.; Villanueva-Meyer, J.E.; Cha, S.; Leynes, A.P.; Larson, P.E.Z.; Hope, T.A. PET/MRI: Where Might It Replace PET/CT? J. Magn. Reson. Imaging 2017, 46, 1247–1262. [Google Scholar] [CrossRef] [Green Version]
- Grant, A.M.; Lee, B.J.; Chang, C.M.; Levin, C.S. Simultaneous PET/MR imaging with a radio frequency- penetrable PET insert. Med. Phys. 2017, 44, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.J.; Gonzalez-Montoro, A.; Vidal, L.F.; Barbera, J.; Aussenhofer, S.; Hernandez, L.; Moliner, L.; Sanchez, F.; Correcher, C.; Pincay, E.J.; et al. Initial Results of the MINDView PET Insert Inside the 3T mMR. IEEE Trans. Radiat. Plasma 2019, 3, 343–351. [Google Scholar] [CrossRef]
- Delso, G.; Furst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance Measurements of the Siemens mMR Integrated Whole-Body PET/MR Scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.G.; Gu, Y.S.; Yu, H.J.; Chen, X.; Cao, T.Y.; Hu, L.Z.; Shi, H.C. NEMA NU2-2012 performance measurements of the United Imaging uPMR790: An integrated PET/MR system. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1726–1735. [Google Scholar] [CrossRef]
- Disselhorst, J.A.; Bezrukov, I.; Kolb, A.; Parl, C.; Pichler, B.J. Principles of PET/MR Imaging. J. Nucl. Med. 2014, 55, 2s–10s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzic, R.F., Jr.; DiFilippo, F.P. Positron emission tomography-magnetic resonance imaging: Technical review. Semin. Roentgenol. 2014, 49, 242–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehranian, A.; Arabi, H.; Zaidi, H. Vision 20/20: Magnetic resonance imaging-guided attenuation correction in PET/MRI: Challenges, solutions, and opportunities. Med. Phys. 2016, 43, 1130–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catana, C. Principles of Simultaneous PET/MR Imaging. Magn. Reason. Imaging Clin. N. Am. 2017, 25, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Z.H.; Son, Y.D.; Kim, H.K.; Kim, K.N.; Oh, S.H.; Han, J.Y.; Hong, I.K.; Kim, Y.B. A fusion PET–MRI system with a high-resolution research tomograph-PET and ultra-high field 7.0 T-MRI for the molecular-genetic imaging of the brain. Proteomics 2008, 8, 1302–1323. [Google Scholar] [CrossRef]
- Zaidi, H.; Ojha, N.; Morich, M.; Griesmer, J.; Hu, Z.; Maniawski, P.; Ratib, O.; Izquierdo-Garcia, D.; Fayad, Z.A.; Shao, L. Design and performance evaluation of a whole-body Ingenuity TF PET–MRI system. Phys. Med. Biol. 2011, 56, 3091–3106. [Google Scholar] [CrossRef] [Green Version]
- Van Reeth, E.; Tham, I.W.K.; Tan, C.H.; Poh, C.L. Super-resolution in magnetic resonance imaging: A review. Concept. Magn. Reson. A 2012, 40A, 306–325. [Google Scholar] [CrossRef]
- Cho, Z.-H.; Son, Y.-D.; Kim, H.-K.; Kim, S.-T.; Lee, S.-Y.; Chi, J.-G.; Park, C.-W.; Kim, Y.-B. Substructural Hippocampal Glucose Metabolism Observed on PET/MRI. J. Nucl. Med. 2010, 51, 1545–1548. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.-J.; Son, Y.-D.; Noh, Y.; Lee, H.; Kim, Y.-B.; Park, K.H. Glucose Hypometabolism in Hippocampal Subdivisions in Alzheimer’s Disease: A Pilot Study Using High-Resolution 18F-FDG PET and 7.0-T MRI. J. Clin. Neurol 2018, 14, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.-D.; Cho, Z.-H.; Kim, H.-K.; Choi, E.-J.; Lee, S.-Y.; Chi, J.-G.; Park, C.-W.; Kim, Y.-B. Glucose Metabolism of the Midline Nuclei Raphe in the Brainstem Observed by PET–MRI Fusion Imaging. NeuroImage 2012, 59, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Son, Y.-D.; Kim, J.-H.; Choi, E.-J.; Lee, S.-Y.; Joo, Y.-H.; Kim, Y.-B.; Cho, Z.-H. Self-Transcendence Trait and Its Relationship with in Vivo Serotonin Transporter Availability in Brainstem Raphe Nuclei: An Ultra-High Resolution PET–MRI Study. Brain Res. 2015, 1629, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H.; Du, L.; Palkovits, M.; Faludi, G.; Kovacs, G.G.; Szontagh-Kishazi, P.; Merali, Z.; Poulter, M.O. Serotonin Receptor Subtype and P11 MRNA Expression in Stress-Relevant Brain Regions of Suicide and Control Subjects. J. Psychiatry Neurosci. 2008, 33, 131–141. [Google Scholar] [PubMed]
- Tiger, M.; Farde, L.; Rück, C.; Varrone, A.; Forsberg, A.; Lindefors, N.; Halldin, C.; Lundberg, J. Low Serotonin1B Receptor Binding Potential in the Anterior Cingulate Cortex in Drug-Free Patients with Recurrent Major Depressive Disorder. Psychiatry Res. Neuroimaging 2016, 253, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Murrough, J.W.; Henry, S.; Hu, J.; Gallezot, J.-D.; Planeta-Wilson, B.; Neumaier, J.F.; Neumeister, A. Reduced Ventral Striatal/Ventral Pallidal Serotonin1B Receptor Binding Potential in Major Depressive Disorder. Psychopharmacology 2011, 213, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; Matuskey, D.; Nabulsi, N.; Gaiser, E.; Gallezot, J.-D.; Henry, S.; Planeta, B.; Lin, S.; Ropchan, J.; Huang, Y.; et al. In Vivo 5-HT6 and 5-HT2A Receptor Availability in Antipsychotic Treated Schizophrenia Patients vs. Unmedicated Healthy Humans Measured with [11C]GSK215083 PET. Psychiatry Res. Neuroimaging 2020, 295, 111007. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-H.; Son, Y.-D.; Joo, Y.-H.; Lee, S.-Y.; Kim, H.-K.; Woo, M.-K. Altered Interregional Correlations between Serotonin Transporter Availability and Cerebral Glucose Metabolism in Schizophrenia: A High-Resolution PET Study Using [11C]DASB and [18F]FDG. Schizophr. Res. 2017, 182, 55–65. [Google Scholar] [CrossRef]
- Yeh, Y.-W.; Ho, P.-S.; Chen, C.-Y.; Kuo, S.-C.; Liang, C.-S.; Ma, K.-H.; Shiue, C.-Y.; Huang, W.-S.; Cheng, C.-Y.; Wang, T.-Y.; et al. Incongruent Reduction of Serotonin Transporter Associated with Suicide Attempts in Patients with Major Depressive Disorder: A Positron Emission Tomography Study with 4-[18F]-ADAM. Int. J. Neuropsychopharmacol. 2015, 18, pyu065. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Sacchet, M.D.; Hjørnevik, T.; Chin, F.T.; Shen, B.; Kämpe, R.; Park, J.H.; Knutson, B.D.; Williams, L.M.; Borg, N.; et al. Striatal Dopamine Deficits Predict Reductions in Striatal Functional Connectivity in Major Depression: A Concurrent 11C-Raclopride Positron Emission Tomography and Functional Magnetic Resonance Imaging Investigation. Transl. Psychiatry 2018, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Caravaggio, F.; Borlido, C.; Wilson, A.; Graff-Guerrero, A. Examining Endogenous Dopamine in Treated Schizophrenia Using [11C]-(+)-PHNO Positron Emission Tomography: A Pilot Study. Clin. Chim. Acta 2015, 449, 60–62. [Google Scholar] [CrossRef] [Green Version]
- Veselinović, T.; Vernaleken, I.; Janouschek, H.; Cumming, P.; Paulzen, M.; Mottaghy, F.M.; Gründer, G. The Role of Striatal Dopamine D2/3 Receptors in Cognitive Performance in Drug-Free Patients with Schizophrenia. Psychopharmacology 2018, 235, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Berretta, S.; Wooten, D.; Goer, F.; Pilobello, K.T.; Kumar, P.; Murray, L.; Beltzer, M.; Boyer-Boiteau, A.; Alpert, N.; et al. Assessment of Striatal Dopamine Transporter Binding in Individuals With Major Depressive Disorder: In Vivo Positron Emission Tomography and Postmortem Evidence. JAMA Psychiatry 2019, 76, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Moriya, H.; Tiger, M.; Tateno, A.; Sakayori, T.; Masuoka, T.; Kim, W.; Arakawa, R.; Okubo, Y. Low Dopamine Transporter Binding in the Nucleus Accumbens in Geriatric Patients with Severe Depression. Psychiatry Clin. Neurosci. 2020, 74, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Artiges, E.; Leroy, C.; Dubol, M.; Prat, M.; Pepin, A.; Mabondo, A.; de Beaurepaire, R.; Beaufils, B.; Korwin, J.-P.; Galinowski, A.; et al. Striatal and Extrastriatal Dopamine Transporter Availability in Schizophrenia and Its Clinical Correlates: A Voxel-Based and High-Resolution PET Study. Schizophr. Bull. 2017, 43, 1134–1142. [Google Scholar] [CrossRef]
- Guerra, A.D.; Ahmad, S.; Avram, M.; Belcari, N.; Berneking, A.; Biagi, L.; Bisogni, M.G.; Brandl, F.; Cabello, J.; Camarlinghi, N.; et al. TRIMAGE: A Dedicated Trimodality (PET/MR/EEG) Imaging Tool for Schizophrenia. Eur. Psychiatry 2018, 50, 7–20. [Google Scholar] [CrossRef]
- Moriguchi, S.; Yamada, M.; Takano, H.; Nagashima, T.; Takahata, K.; Yokokawa, K.; Ito, T.; Ishii, T.; Kimura, Y.; Zhang, M.-R.; et al. Norepinephrine Transporter in Major Depressive Disorder: A PET Study. Am. J. Psychiatry 2017, 174, 36–41. [Google Scholar] [CrossRef]
- Arakawa, R.; Stenkrona, P.; Takano, A.; Svensson, J.; Andersson, M.; Nag, S.; Asami, Y.; Hirano, Y.; Halldin, C.; Lundberg, J. Venlafaxine ER Blocks the Norepinephrine Transporter in the Brain of Patients with Major Depressive Disorder: A PET Study Using [18F]FMeNER-D2. Int. J. Neuropsychopharmacol. 2019, 22, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Marques, T.R.; Natesan, S.; Niccolini, F.; Politis, M.; Gunn, R.N.; Searle, G.E.; Howes, O.; Rabiner, E.A.; Kapur, S. Phosphodiesterase 10A in Schizophrenia: A PET Study Using [11C]IMA107. Am. J. Psychiatry 2016, 173, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Lepage, M.; Habib, R.; Tulving, E. Hippocampal PET Activations of Memory Encoding and Retrieval: The HIPER Model. Hippocampus 1998, 8, 313–322. [Google Scholar] [CrossRef]
- Schacter, D.L.; Wagner, A.D. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 1999, 9, 7–24. [Google Scholar] [CrossRef] [Green Version]
- Strange, B.A.; Fletcher, P.C.; Henson, R.N.A.; Friston, K.J.; Dolan, R.J. Segregating the Functions of Human Hippocampus. Proc. Natl. Acad. Sci. USA 1999, 96, 4034–4039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siuciak, J.A. The Role of Phosphodiesterases in Schizophrenia: Therapeutic Implications. CNS Drugs 2008, 22, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; McCarthy, S.A.; Chapin, D.S.; Fujiwara, R.A.; James, L.C.; Williams, R.D.; Stock, J.L.; McNeish, J.D.; Strick, C.A.; Menniti, F.S.; et al. Genetic Deletion of the Striatum-Enriched Phosphodiesterase PDE10A: Evidence for Altered Striatal Function. Neuropharmacology 2006, 51, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.J.; Chapin, D.S.; Cianfrogna, J.; Corman, M.L.; Hajos, M.; Harms, J.F.; Hoffman, W.E.; Lebel, L.A.; McCarthy, S.A.; Nelson, F.R.; et al. Preclinical Characterization of Selective Phosphodiesterase 10A Inhibitors: A New Therapeutic Approach to the Treatment of Schizophrenia. J. Pharmacol. Exp. Ther. 2008, 325, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Grauer, S.M.; Pulito, V.L.; Navarra, R.L.; Kelly, M.P.; Kelley, C.; Graf, R.; Langen, B.; Logue, S.; Brennan, J.; Jiang, L.; et al. Phosphodiesterase 10A Inhibitor Activity in Preclinical Models of the Positive, Cognitive, and Negative Symptoms of Schizophrenia. J. Pharmacol. Exp. Ther. 2009, 331, 574–590. [Google Scholar] [CrossRef] [Green Version]
- Kehler, J.; Nielsen, J. PDE10A Inhibitors: Novel Therapeutic Drugs for Schizophrenia. Curr. Pharm. Des. 2011, 17, 137–150. [Google Scholar] [CrossRef]
- Uthayathas, S.; Masilamoni, G.J.; Shaffer, C.L.; Schmidt, C.J.; Menniti, F.S.; Papa, S.M. Phosphodiesterase 10A Inhibitor MP-10 Effects in Primates: Comparison with Risperidone and Mechanistic Implications. Neuropharmacology 2014, 77, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Piccart, E.; De Backer, J.-F.; Gall, D.; Lambot, L.; Raes, A.; Vanhoof, G.; Schiffmann, S.; D’Hooge, R. Genetic Deletion of PDE10A Selectively Impairs Incentive Salience Attribution and Decreases Medium Spiny Neuron Excitability. Behav. Brain Res. 2014, 268, 48–54. [Google Scholar] [CrossRef]
- Sano, H.; Nagai, Y.; Miyakawa, T.; Shigemoto, R.; Yokoi, M. Increased Social Interaction in Mice Deficient of the Striatal Medium Spiny Neuron-Specific Phosphodiesterase 10A2. J. Neurochem. 2008, 105, 546–556. [Google Scholar] [CrossRef]
- Kortte, K.B.; Horner, M.D.; Windham, W.K. The Trail Making Test, Part B: Cognitive Flexibility or Ability to Maintain Set? Appl. Neuropsychol. 2002, 9, 106–109. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale; Psychological Corporation: San Antonio, TX, USA, 1997; ISBN 978-0-15-898103-1. [Google Scholar]
- Harth, S.; Müller, S.V.; Aschenbrenner, S.; Tucha, O.; Lange, K.W. Regensburger Wortflüssigkeits-Test (RWT). Z. Neuropsychol. 2004, 15, 315–321. [Google Scholar] [CrossRef]
- Gold, J.M.; Carpenter, C.; Randolph, C.; Goldberg, T.E.; Weinberger, D.R. Auditory Working Memory and Wisconsin Card Sorting Test Performance in Schizophrenia. Arch. Gen. Psychiatry 1997, 54, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Nye, J.A.; Purselle, D.; Plisson, C.; Voll, R.J.; Stehouwer, J.S.; Votaw, J.R.; Kilts, C.D.; Goodman, M.M.; Nemeroff, C.B. Decreased Brainstem and Putamen Sert Binding Potential in Depressed Suicide Attempters Using [11c]-Zient Pet Imaging. Depress. Anxiety 2013, 30, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Chergui, K.; Rachleff, I.; Flajolet, M.; Zhang, X.; Yacoubi, M.E.; Vaugeois, J.-M.; Nomikos, G.G.; Greengard, P. Alterations in 5-HT1B Receptor Function by P11 in Depression-Like States. Science 2006, 311, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Tatarczynska, E.; Klodzinska, A.; Stachowicz, K.; Chojnacka-Wójcik, E. Effects of a Selective 5-HT1B Receptor Agonist and Antagonists in Animal Models of Anxiety and Depression. Behav. Pharmacol. 2004, 15, 523–534. [Google Scholar] [CrossRef]
- Chenu, F.; David, D.J.P.; Leroux-Nicollet, I.; Maïtre, E.L.; Gardier, A.M.; Bourin, M. Serotonin1B Heteroreceptor Activation Induces an Antidepressant-like Effect in Mice with an Alteration of the Serotonergic System. J. Psychiatry Neurosci. 2008, 33, 541–550. [Google Scholar]
- Marchand, W.R.; Lee, J.N.; Johnson, S.; Thatcher, J.; Gale, P.; Wood, N.; Jeong, E.-K. Striatal and Cortical Midline Circuits in Major Depression: Implications for Suicide and Symptom Expression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 290–299. [Google Scholar] [CrossRef]
- Tanaka, S.C.; Doya, K.; Okada, G.; Ueda, K.; Okamoto, Y.; Yamawaki, S. Prediction of Immediate and Future Rewards Differentially Recruits Cortico-Basal Ganglia Loops. Nat. Neurosci. 2004, 7, 887–893. [Google Scholar] [CrossRef]
- Takahashi, H.; Rissling, A.J.; Pascual-Marqui, R.; Kirihara, K.; Pela, M.; Sprock, J.; Braff, D.L.; Light, G.A. Neural Substrates of Normal and Impaired Preattentive Sensory Discrimination in Large Cohorts of Nonpsychiatric Subjects and Schizophrenia Patients as Indexed by MMN and P3a Change Detection Responses. NeuroImage 2013, 66, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, A.; Ruhé, H.G.; Munkholm, K. The Relationship between Dose and Serotonin Transporter Occupancy of Antidepressants—A Systematic Review. Mol. Psychiatry 2022, 27, 192–201. [Google Scholar] [CrossRef]
- Cumming, P.; Abi-Dargham, A.; Gründer, G. Molecular Imaging of Schizophrenia: Neurochemical Findings in a Heterogeneous and Evolving Disorder. Behav. Brain Res. 2021, 398, 113004. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.J.; Chohan, M.O.; Slifstein, M.; Kegeles, L.S.; Moore, H.; Abi-Dargham, A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol. Psychiatry 2017, 81, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauscher, J.; Kielbasa, W.; Iyengar, S.; Vandenhende, F.; Peng, X.; Mozley, D.; Gehlert, D.R.; Marek, G. Development of the 2nd Generation Neurokinin-1 Receptor Antagonist LY686017 for Social Anxiety Disorder. Eur. Neuropsychopharmacol. 2010, 20, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Uchida, H.; Bies, R.R.; Caravaggio, F.; Suzuki, T.; Plitman, E.; Mar, W.; Gerretsen, P.; Pollock, B.G.; Mulsant, B.H.; et al. Dopamine D2/3 Receptor Occupancy Following Dose Reduction Is Predictable With Minimal Plasma Antipsychotic Concentrations: An Open-Label Clinical Trial. Schizophr. Bull. 2016, 42, 212–219. [Google Scholar] [CrossRef] [Green Version]

| Tracer target | Radiotracer | Name: Synonyms | Study |
|---|---|---|---|
| Serotonin 5-HT1A receptor | [11C]WAY100635 | N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide trihydrochloride | Anisman et al. [56] |
| Serotonin 5-HT1B receptor | [11C]AZ10419369 | 5-methyl-8-(4-[11C]methyl-piperazin-1-yl)-4-oxo-4H-chromene-2-carboxylic acid (4-morpholin-4-yl-phenyl)-amide | Tiger et al. [57] |
| [11C]P943 | R-1-[4-(2-methoxy-isopropyl)-phenyl]-3-[2-(4-methyl-piperazin-1-yl) benzyl]-pyrrolidin-2-one | Murrough et al. [58] | |
| Serotonin 5-HT6 receptor | [11C]GSK215083 | [11C]-[N-methyl]3-[(3-fluorophenyl)sulfonyl]-8-(4-methyl-1-piperazi nyl)quinoline | Radhakrishnan et al. [59] |
| SERT | [11C]DASB | [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)- benzonitrile | Kim et al. [60] |
| 4-[18F]-ADAM | N,N-dimethyl-2-(2-amino-4-[18F]fluorophenylthio)benzylamine | Yeh et al. [61] | |
| DA D2 receptor | [11C]raclopride | 3,5-dichloro-N-[[(2S)-1-ethylpyrrolidin-2-yl]methyl]-2-hydroxy-6-[11C]methoxy-benzamide | Hamilton et al. [62] |
| DA D2/3 receptor | [11C]PHNO | [11C]-(+)-4-propyl-9-hydroxynaphthoxazine | Caravaggio et al. [63] |
| [18F]fallypride | 5-(3-[18F]fluoropropyl)-2,3-dimethoxy-N-[(2S)-1-prop-2-enylpyrrolidin-2-yl]methyl]benzamide | Veselinović et al. [64] | |
| DA transporter (DAT) | [11C]-altropane | 2β-carbomethoxy-3β-(4-fluorophenyl)-N-((E)-3-iodo-prop-2-enyl)tropane | Pizzagalli et al. [65] |
| [18F]FE-PE2I | N-(3-iodoprop-2E-enyl)-2β-carbo-[18F]fluoroethoxy-3β-[4-methylphenyl]-nortropane | Moriya et al. [66] | |
| [11C]PE2l | [11C]N-(3-iodoprop-2E-enyl)-2β-carbomethoxy-3β-(4-methylphenyl)nortropane | Artiges et al. [67] | |
| DA synthesis capacity | [18F]-FDOPA | [18F]-6-L-fluoro-L-3,4-dihydroxyphenylalanine | Guerra et al. [68] |
| Metabotropic glutamate receptor 5 | [11C]ABP688 | 3-(6-methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-[11C]-methyl-oxime | Guerra et al. [68] |
| Norepinephrine transporter (NET) | (S,S)-[18F]FMeNER-D2 | (S,S)-2-(α-(2-[18F]fluoro[2 H2]methoxyphenoxy) benzyl)morpholine | Moriguchi et al. [69] Arakawa et al. [70] |
| Phosphodiesterase 10A (PDE10A) | [11C]IMA107 | 5-[(3R)-3-fluoropyrrolidin-1-yl]-N-[11C]methyl-2-(3-methylquinoxalin-2-yl)-N-tetrahydropyran-4-yl-pyrazolo[1,5-α]pyrimidin-7-amine | Marques et al. [71] |
| Glucose metabolism | [18F]FDG | 2-deoxy-2-[18F]fluoro-D-glucose | Kim et al. [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.-D.; Kim, Y.-B.; Kim, J.-H.; Kim, J.-H.; Kwon, D.-H.; Lee, H.; Cho, Z.-H. Future Prospects of Positron Emission Tomography–Magnetic Resonance Imaging Hybrid Systems and Applications in Psychiatric Disorders. Pharmaceuticals 2022, 15, 583. https://doi.org/10.3390/ph15050583
Son Y-D, Kim Y-B, Kim J-H, Kim J-H, Kwon D-H, Lee H, Cho Z-H. Future Prospects of Positron Emission Tomography–Magnetic Resonance Imaging Hybrid Systems and Applications in Psychiatric Disorders. Pharmaceuticals. 2022; 15(5):583. https://doi.org/10.3390/ph15050583
Chicago/Turabian StyleSon, Young-Don, Young-Bo Kim, Jong-Hoon Kim, Jeong-Hee Kim, Dae-Hyuk Kwon, Haigun Lee, and Zang-Hee Cho. 2022. "Future Prospects of Positron Emission Tomography–Magnetic Resonance Imaging Hybrid Systems and Applications in Psychiatric Disorders" Pharmaceuticals 15, no. 5: 583. https://doi.org/10.3390/ph15050583
APA StyleSon, Y.-D., Kim, Y.-B., Kim, J.-H., Kim, J.-H., Kwon, D.-H., Lee, H., & Cho, Z.-H. (2022). Future Prospects of Positron Emission Tomography–Magnetic Resonance Imaging Hybrid Systems and Applications in Psychiatric Disorders. Pharmaceuticals, 15(5), 583. https://doi.org/10.3390/ph15050583







