Mechanistic Insights on the In Vitro Antibacterial Activity and In Vivo Hepatoprotective Effects of Salvinia auriculata Aubl against Methotrexate-Induced Liver Injury
Abstract
:1. Introduction
2. Results
2.1. LC-ESI-MS/MS Analysis
2.2. Characterization of the Identified Compounds
2.3. In Vitro Antibacterial Activity of SAME
2.4. Mechanism of Action of the Antibacterial Activity of SAME
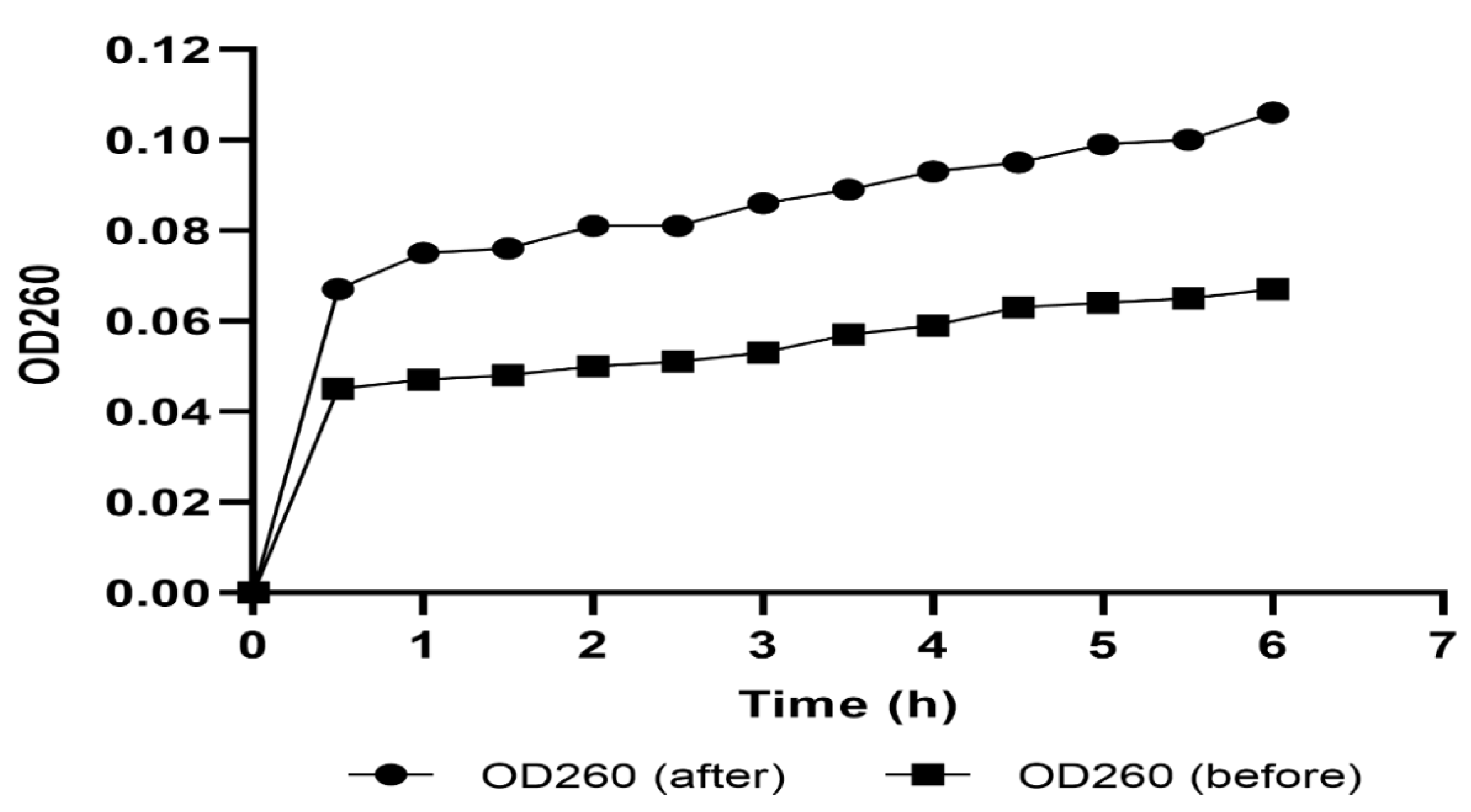
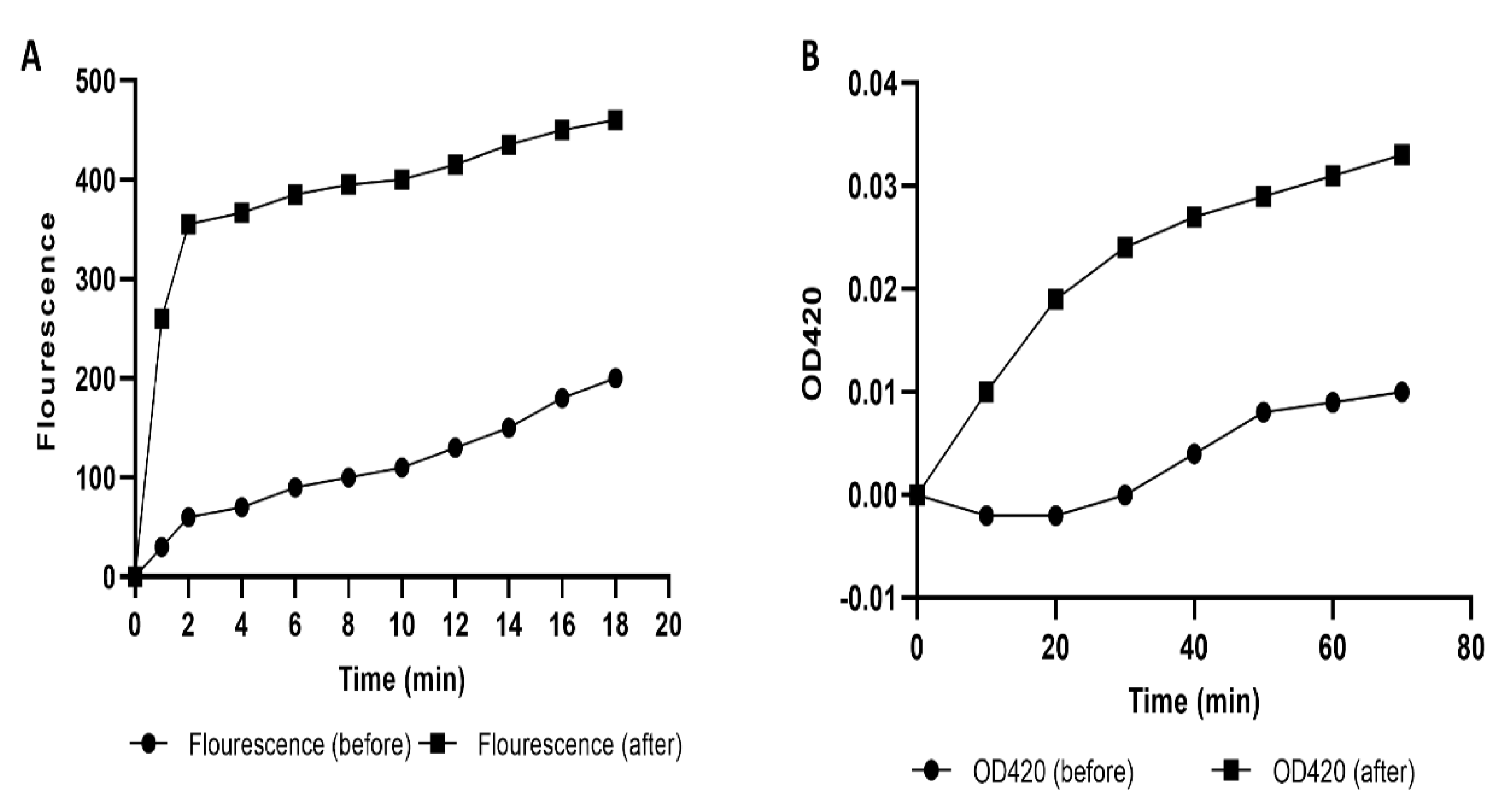
2.4.1. Cell Membrane Integrity
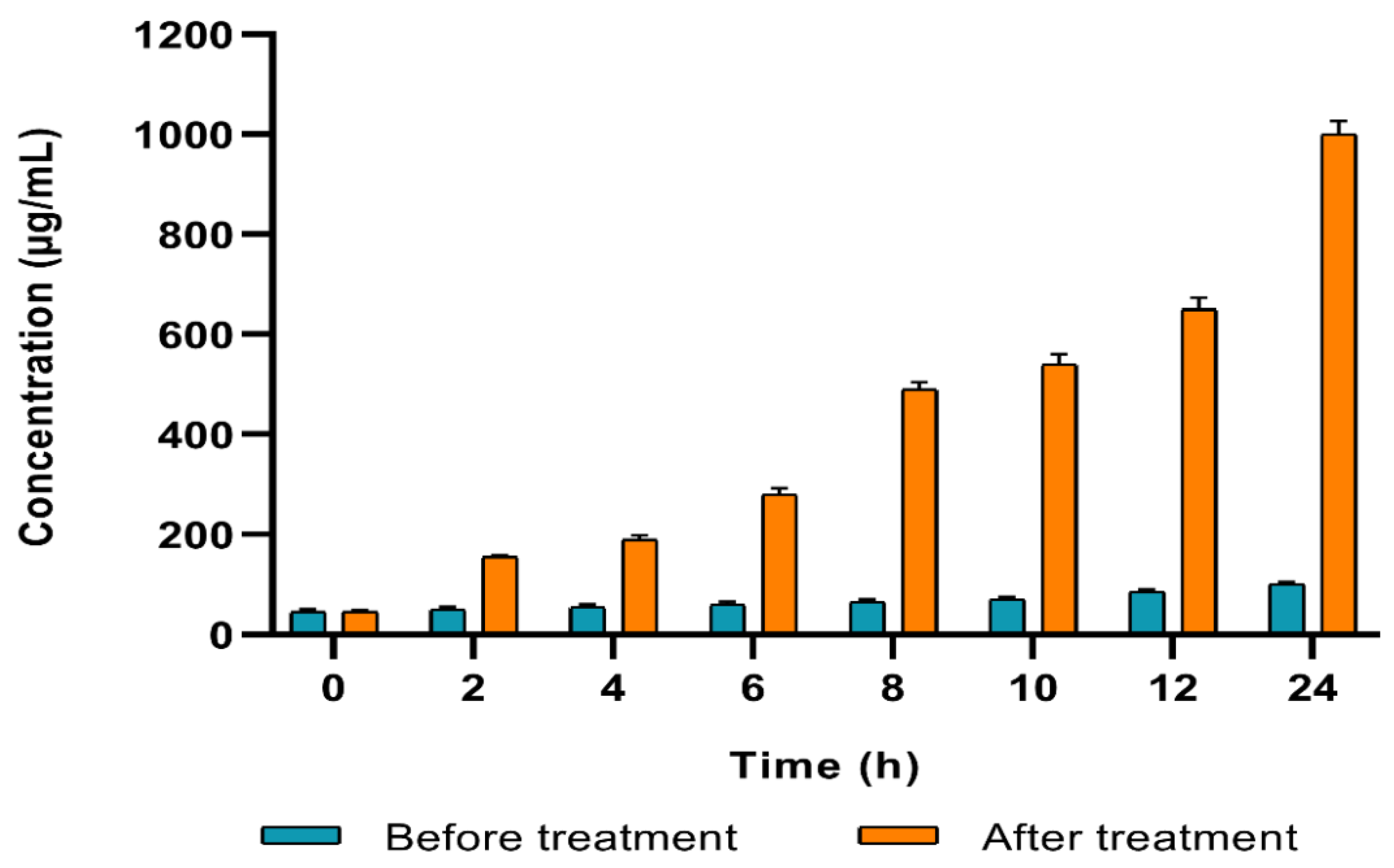
2.4.2. Cell Membrane Permeability
2.4.3. Protein Content
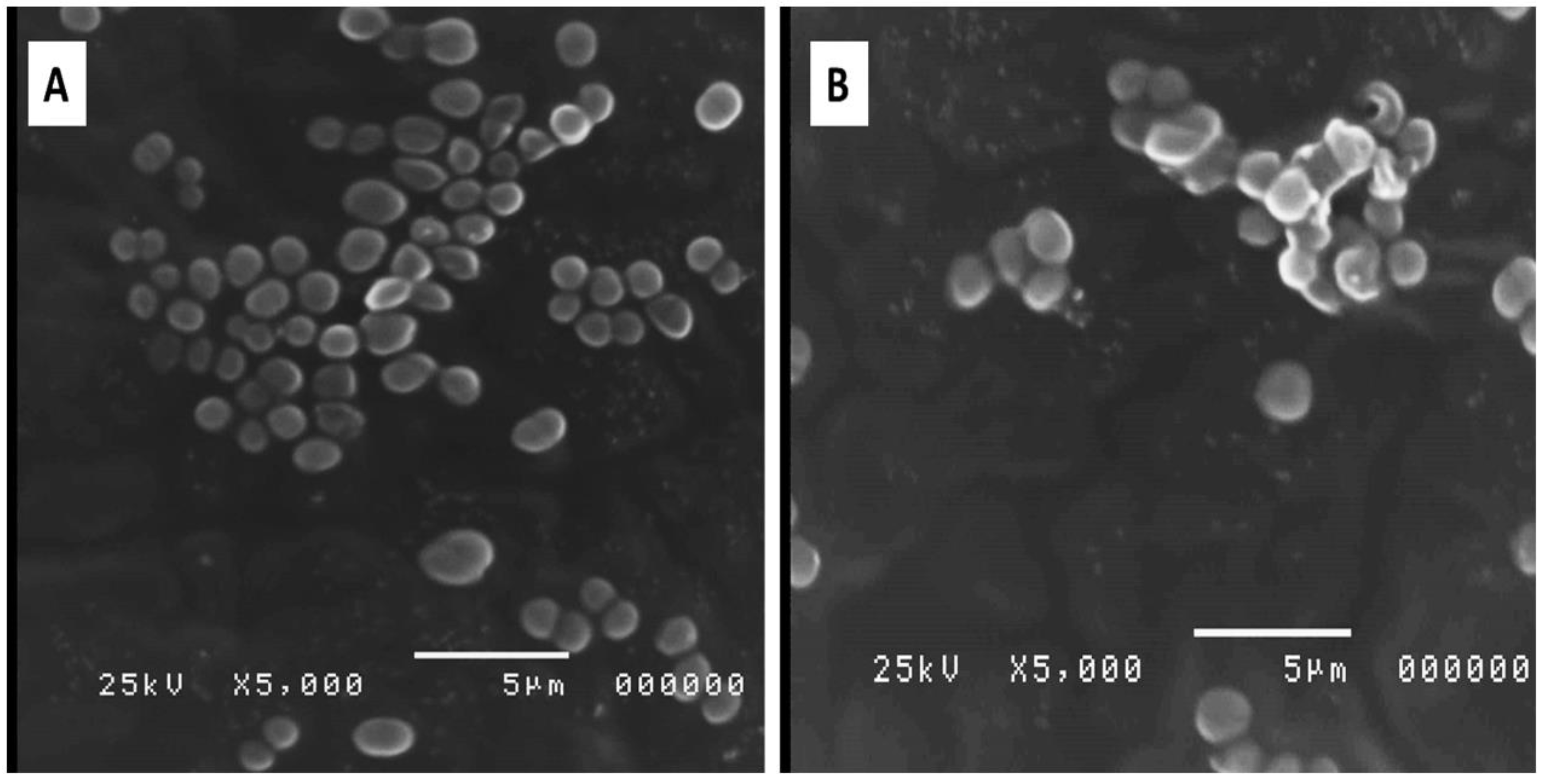
2.4.4. Bacterial Morphology
2.5. In Vivo Hepatoprotecive Activity of SAME
2.5.1. Effects on Serum Indices of Hepatotoxicity
2.5.2. Effects on Hepatic Oxidative Stress Markers
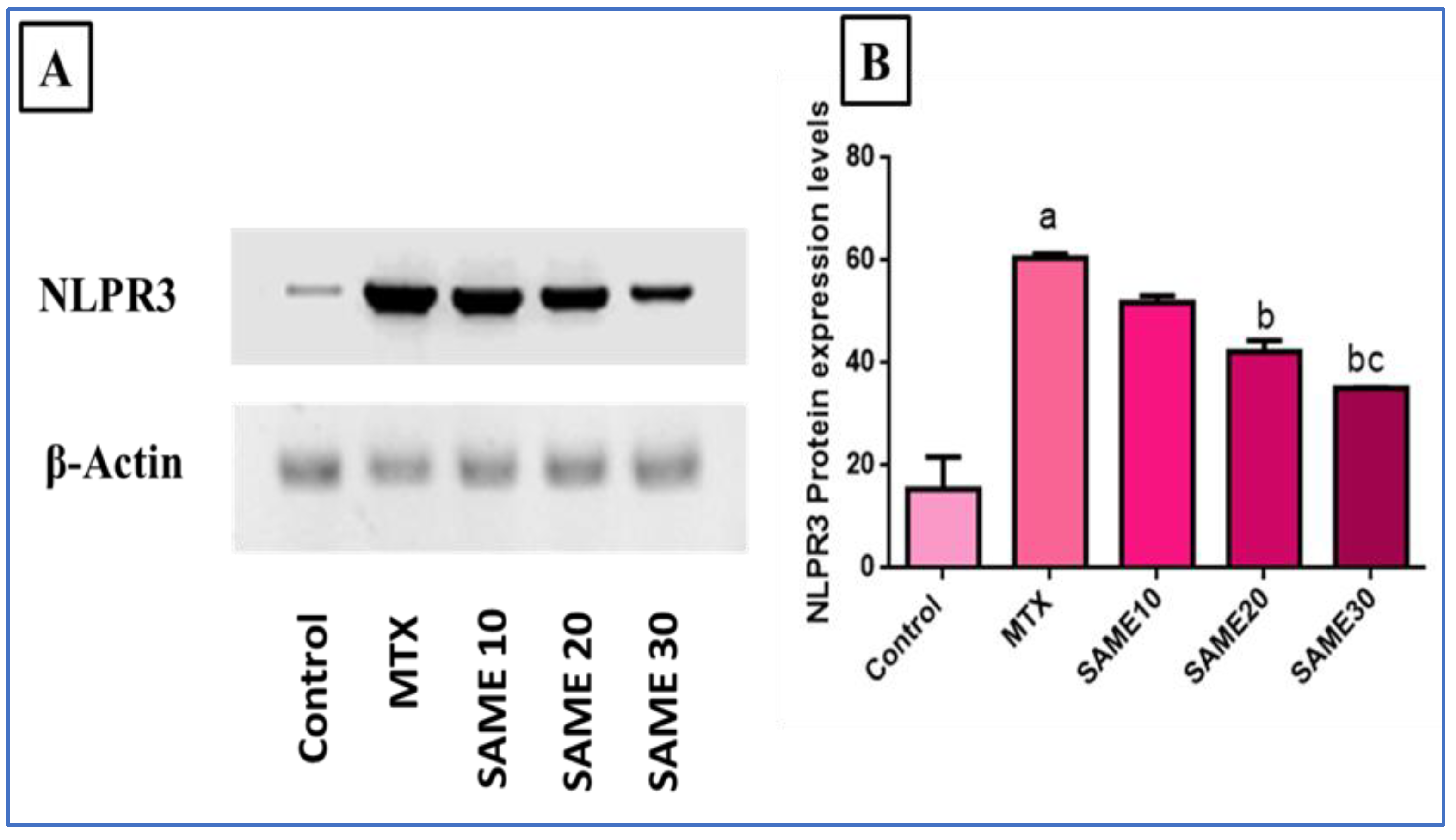
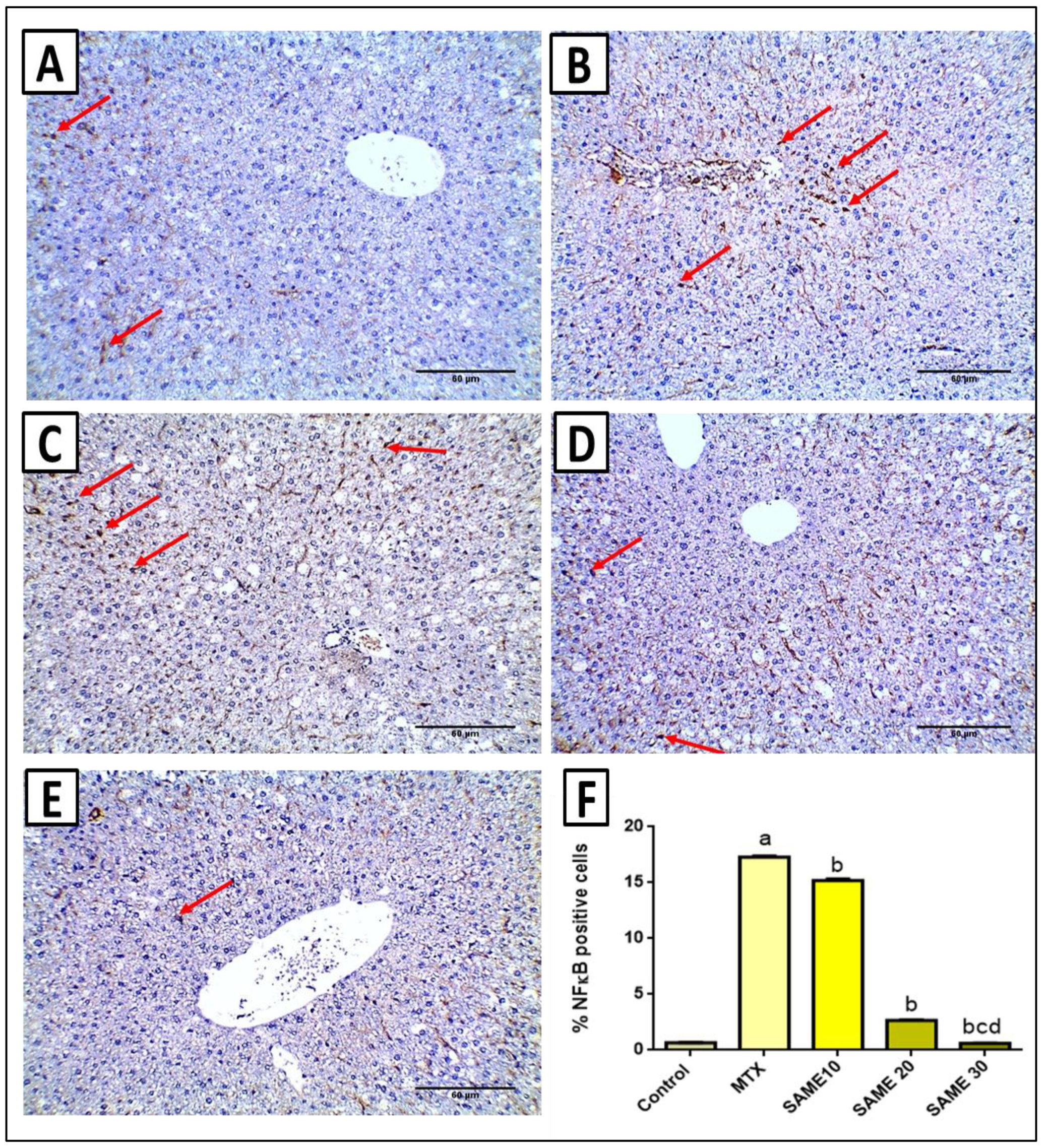
2.5.3. Effects on Nod-Like Receptor-3 (NLPR3) Inflammasome Signaling Axis
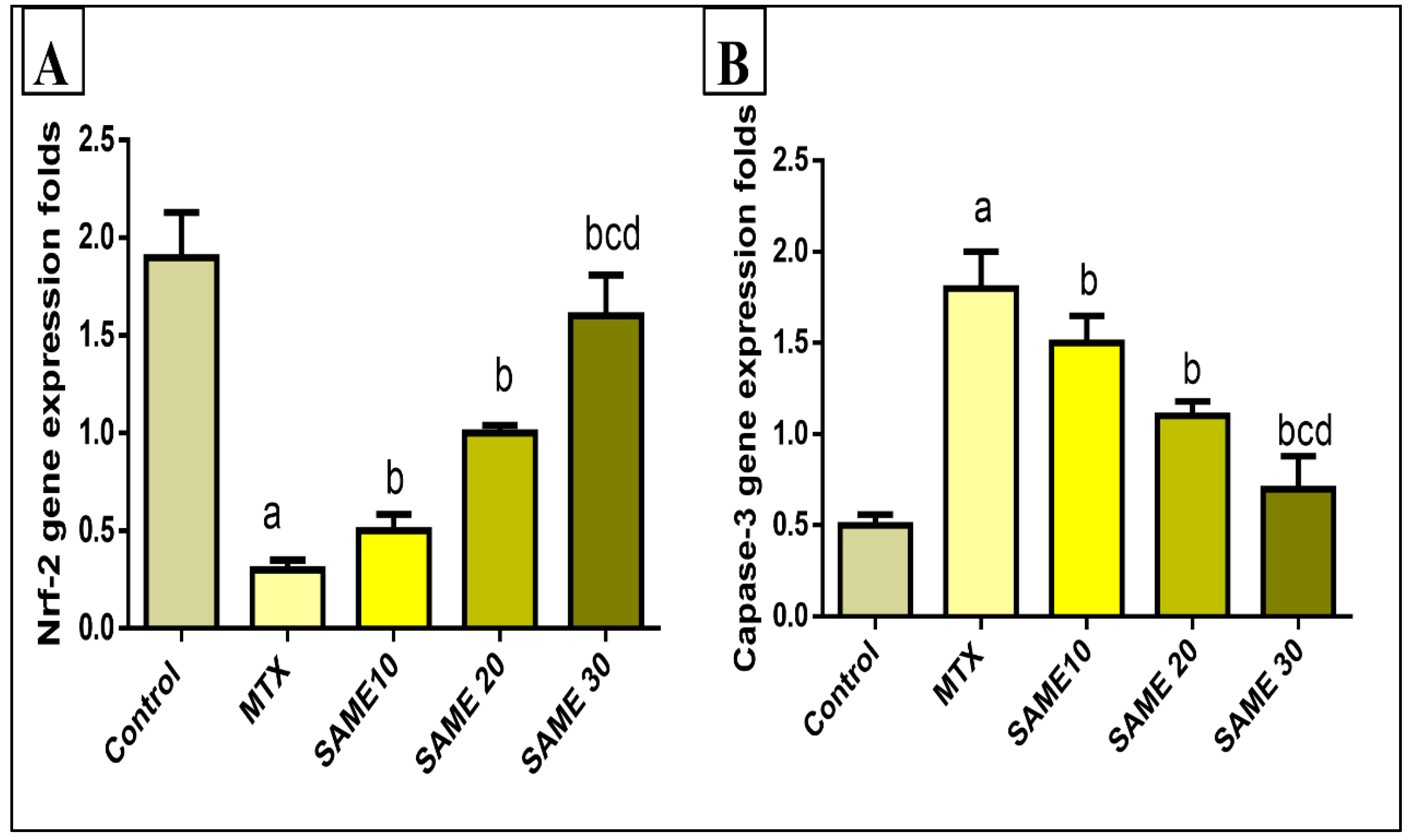
2.5.4. Effects on Hepatic Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Gene Expression
2.5.5. Effects on the Hepatic Caspase-3 Gene Expression
2.6. Immunohistochemical Studies
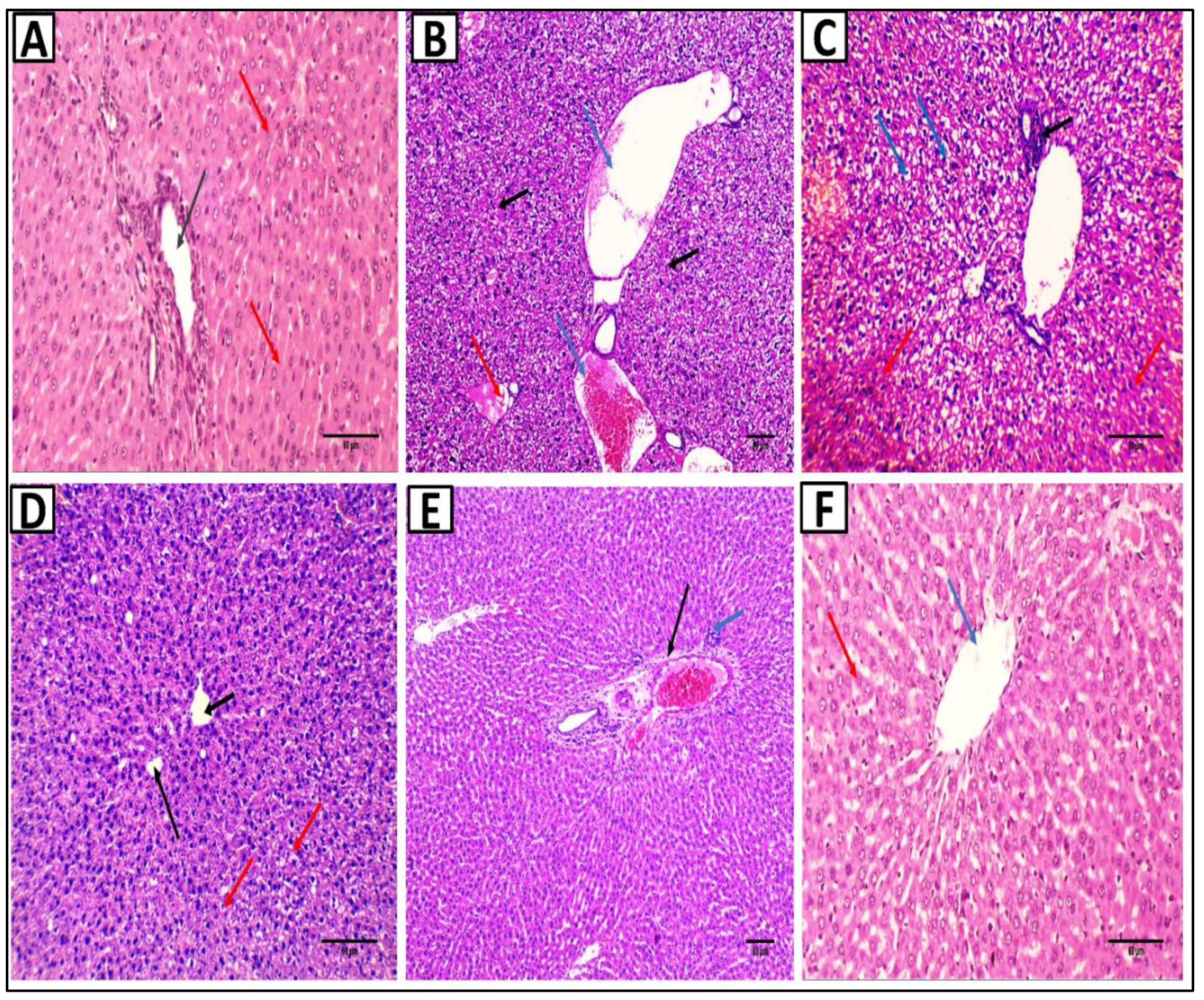
2.7. Histopathological Studies
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Plant Materials and Extract Preparation
4.3. Drugs and Chemicals
4.4. Bacterial Isolates
4.5. LC-ESI-MS/MS for Metabolite Profiling
4.6. In Vitro Antibacterial Activity
4.6.1. Antibiotic Susceptibility Testing
4.6.2. Screening of the Antibacterial Activity of SAME
4.6.3. MIC
4.6.4. Mode of Action of the Antibacterial Activity
- Loss of cellular content
- b.
- Effect on membrane permeability
- c.
- Estimation of the protein content
- d.
- Scanning electron microscopy (SEM)
4.7. In Vivo Hepatoprotective Activity
4.7.1. Experimental Design
4.7.2. Sample Collection
4.7.3. Serum Indices of Hepatotoxicity
4.7.4. Assessment of Lipid Peroxidation
4.7.5. Measurement of Liver Nitric Oxide Levels
4.7.6. Assessment of SOD Activity
4.7.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7.8. Western Blot Analysis for NLPR3 Inflammasome
4.7.9. Histopathological Examination of Liver Sections
4.7.10. Immunohistochemical Staining of NF-κB
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.S.; Fan, J.G.; Zhang, Z.; Gao, B.; Wang, H.Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Dalaklioglu, S.; Genc, G.; Aksoy, N.; Akcit, F.; Gumuslu, S. Resveratrol ameliorates methotrexate-induced hepatotoxicity in rats via inhibition of lipid peroxidation. Hum. Exp. Toxicol. 2013, 32, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.; Amaral, M.E.C. Does the structural complexity of aquatic macrophytes explain the diversity of associated spider assemblages? J. Arachnol. 2001, 29, 227–237. [Google Scholar] [CrossRef]
- Heneidy, S.Z.; Halmy, M.W.A.; Fakhry, A.M.; El-Makawy, A.M. The status and potential distribution of Hydrocotyle umbellata L. and Salvinia auriculata Aubl. under climate change scenarios. Aquat. Ecol. 2019, 53, 509–528. [Google Scholar] [CrossRef]
- Espinoza-Quiñones, F.R.; Módenes, A.; Thomé, L.; Palácio, S.; Trigueros, D.; Oliveira, A.; Szymanski, N. Study of the bioaccumulation kinetic of lead by living aquatic macrophyte Salvinia auriculata. Chem. Eng. J. 2009, 150, 316–322. [Google Scholar] [CrossRef]
- Espinoza-Quiñones, F.R.; Dall’Oglio, I.C.; de Pauli, A.R.; Romani, M.; Módenes, A.N.; Trigueros, D.E.G. Insights into brewery wastewater treatment by the electro-Fenton hybrid process: How to get a significant decrease in organic matter and toxicity. Chemosphere 2021, 263, 128367. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Mikulyuk, A. Salvinia auriculata (Giant salvinia). In Invasive Species Compendium; CABI: Wallingford, UK, 2018. [Google Scholar]
- da Silva, A.A.; de Oliveira, J.A.; de Campos, F.V.; Ribeiro, C.; dos Santos Farnese, F.; Costa, A.C. Phytoremediation potential of Salvinia molesta for arsenite contaminated water: Role of antioxidant enzymes. Theor. Exp. Plant. Physiol. 2018, 30, 275–286. [Google Scholar] [CrossRef]
- Naheed, N.; Maher, S.; Saleem, F.; Khan, A.; Wadood, A.; Rasheed, S.; Choudhary, M.I.; Froeyen, M.; Abdullah, I.; Mirza, M.U. New isolate from Salvinia molesta with antioxidant and urease inhibitory activity. Drug Dev. Res. 2021, 82, 1169–1181. [Google Scholar] [CrossRef]
- Gini, T.; Jeya Jothi, G. Column chromatography and HPLC analysis of phenolic compounds in the fractions of Salvinia molesta mitchell. Egypt. J. Basic Appl. Sci. 2018, 5, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [Green Version]
- Farshadzadeh, Z.; Pourhajibagher, M.; Taheri, B.; Ekrami, A.; Modarressi, M.H.; Azimzadeh, M.; Bahador, A. Antimicrobial and anti-biofilm potencies of dermcidin-derived peptide DCD-1L against Acinetobacter baumannii: An in vivo wound healing model. BMC Microbiol. 2022, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Al-Kadmy, I.M.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Abd Ellah, N.H.; Ahmed, E.A.; Abd-Ellatief, R.B.; El-Masry, E.A. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef] [PubMed]
- Abo-Haded, H.M.; Elkablawy, M.A.; Al-Johani, Z.; Al-Ahmadi, O.; El-Agamy, D.S. Hepatoprotective effect of sitagliptin against methotrexate induced liver toxicity. PLoS ONE 2017, 12, e0174295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Ghosh, S.; Choudhury, S.; Adhikary, A.; Manna, K.; Dey, S.; Sa, G.; Das, T.; Chattopadhyay, S. Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways. J. Nutr. Biochem. 2013, 24, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Jahovic, N.; Çevik, H.; Şehirli, A.Ö.; Yeğen, B.Ç.; Şener, G. Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J. Pineal Res. 2003, 34, 282–287. [Google Scholar] [CrossRef]
- Xiao, T.; Cui, Y.; Ji, H.; Yan, L.; Pei, D.; Qu, S. Baicalein attenuates acute liver injury by blocking NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2021, 534, 212–218. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.-W.; Evankovich, J.; Yan, W.; Rosborough, B.R.; Nace, G.W.; Ding, Q.; Loughran, P.; Beer-Stolz, D.; Billiar, T.R. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J. Immunol. 2013, 191, 2665–2679. [Google Scholar] [CrossRef]
- Wree, A.; Eguchi, A.; McGeough, M.D.; Pena, C.A.; Johnson, C.D.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014, 59, 898–910. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.-Y.; Ge, M.; Mennone, A.; Hoque, R.; Ouyang, X.; Boyer, J.L. Inflammasome Is Activated in the Liver of Cholestatic Patients and Aggravates Hepatic Injury in Bile Duct–Ligated Mouse. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Frissen, M.; Liao, L.; Schneider, K.M.; Djudjaj, S.; Haybaeck, J.; Wree, A.; Rolle-Kampczyk, U.; von Bergen, M.; Latz, E.; Boor, P. Bidirectional role of NLRP3 during acute and chronic cholestatic liver injury. Hepatology 2021, 73, 1836–1854. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-G.; Yan, L.; Mai, F.-Y.; Shi, Z.-J.; Xu, L.-H.; Jing, Y.-Y.; Zha, Q.-B.; Ouyang, D.-Y.; He, X.-H. Baicalin inhibits NOD-like receptor family, pyrin containing domain 3 inflammasome activation in murine macrophages by augmenting protein kinase A signaling. Front. Immunol. 2017, 8, 1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Wang, Z.; Yuan, Z.; Lv, S.; Su, Q. Baicalin ameliorates atherosclerosis by inhibiting NLRP3 inflammasome in apolipoprotein E-deficient mice. Diabetes Vasc. Dis. Res. 2020, 17, 1479164120977441. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, Z.; Deng, C.; Zhang, J.; Ren, G.; Sun, A.; Mani, S.; Wang, Z.; Dou, W. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci. Rep. 2017, 7, 16374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [Green Version]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Abd El-Twab, S.M.; Hozayen, W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019, 10, 4593–4607. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. WJG 2014, 20, 8082. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Zhang, Q.; Pi, J.; Woods, C.G.; Andersen, M.E. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol. Appl. Pharmacol. 2010, 244, 84–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Hellerbrand, C.; Koehler, U.A.; Bugnon, P.; Kan, Y.-W.; Werner, S.; Beyer, T.A. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab. Investig. 2008, 88, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- El-Maati, M.F.A.; Mahgoub, S.A.; Labib, S.M.; Al-Gaby, A.M.; Ramadan, M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016, 8, 494–504. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. Biofactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The protective effect of chlorogenic acid on vascular senescence via the Nrf2/HO-1 pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef]
- Wang, D.; Hou, J.; Wan, J.; Yang, Y.; Liu, S.; Li, X.; Li, W.; Dai, X.; Zhou, P.; Liu, W. Dietary chlorogenic acid ameliorates oxidative stress and improves endothelial function in diabetic mice via Nrf2 activation. J. Int. Med. Res. 2021, 49, 0300060520985363. [Google Scholar] [CrossRef]
- Han, D.; Gu, X.; Gao, J.; Wang, Z.; Liu, G.; Barkema, H.W.; Han, B. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21Waf1/Cip1 to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radic. Biol. Med. 2019, 137, 1–12. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Morsy, M.A.; Abdalla, A.M.; Hamouda, A.H.; Alhaider, I.A. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediat. Inflamm. 2015, 2015, 859383. [Google Scholar] [CrossRef] [Green Version]
- Soukhtanloo, M.; Mohtashami, E.; Maghrouni, A.; Mollazadeh, H.; Mousavi, S.H.; Roshan, M.K.; Tabatabaeizadeh, S.-A.; Hosseini, A.; Vahedi, M.M.; Jalili-Nik, M. Natural products as promising targets in glioblastoma multiforme: A focus on NF-κB signaling pathway. Pharmacol. Rep. 2020, 72, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-Y.; Yuan, M.; Song, K.; Yu, J.-L.; Li, J.-H.; Huang, J.-N.; Yi, P.-F.; Fu, B.-D.; Shen, H.-Q. Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-κB pathway activation. Int. Immunopharmacol. 2019, 72, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Elekhnawy, E.A.; Sonbol, F.I.; Elbanna, T.E.; Abdelaziz, A.A. Evaluation of the impact of adaptation of Klebsiella pneumoniae clinical isolates to benzalkonium chloride on biofilm formation. Egypt. J. Med. Hum. Genet. 2021, 22, 51. [Google Scholar] [CrossRef]
- Yadav, S.K.; Bhujel, R.; Hamal, P.; Mishra, S.K.; Sharma, S.; Sherchand, J.B. Burden of multidrug-resistant acinetobacter baumannii infection in hospitalized patients in a tertiary care hospital of Nepal. Infect. Drug Resist. 2020, 13, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, R.; Dahal, R.; Mishra, S.; Parajuli, K.; Rijal, B.; Sherchand, J.; Kirikae, T.; Ohara, H.; Pokhrel, B. Ventilator Associated Pneumonia in Tertiary Care Hospital, Maharajgunj, Kathmandu, Nepal. J. Inst. Med. 2013, 35, 21–28. [Google Scholar]
- Liu, C.-P.; Chiang, T.-T.; Liu, Y.-M.; Kuo, S.-C.; Yang, Y.-S.; Lee, Y.-T.; Chen, T.-L.; Shih, S.-C.; Chang, Y. A multicenter study on clinical characteristics of Acinetobacter bacteremia in patients with liver cirrhosis. J. Microbiol. Immunol. Infect. 2019, 52, 956–965. [Google Scholar] [CrossRef]
- Simões, E.R.B.; Santos, E.A.; de Abreu, M.C.; do Nascimento Silva, J.; Nunes, N.M.F.; da Costa, M.P.; Pessoa, O.D.L.; Pessoa, C.; Ferreira, P.M.P. Biomedical properties and potentiality of Lippia microphylla Cham. and its essential oils. J. Intercult. Ethnopharmacol. 2015, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.; Lee, C.-R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef]
- Dholvitayakhun, A.; Trachoo, N.; Narkkong, N.-a.; Cushnie, T.T. Using scanning and transmission electron microscopy to investigate the antibacterial mechanism of action of the medicinal plant Annona squamosa Linn. J. Herb. Med. 2017, 7, 31–36. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. The ultrastructural damage caused by Eugenia zeyheri and Syzygium legatii acetone leaf extracts on pathogenic Escherichia coli. BMC Vet. Res. 2020, 16, 326. [Google Scholar] [CrossRef]
- Huxley, A. The New Royal Horticultural Society Dictionary of Gardening; Nature Pub Group: London, UK, 1992. [Google Scholar]
- Heneidy, S.Z. Plant. Atlas: The Botanic Garden (Alex), Faculty of Science, Alexandria University; Alexandria University: Alexandria Governorate, Egypt, 2010. [Google Scholar]
- MacFaddin, J. Biochemical Tests for Identification of Medical Bacteria, Williams and Wilkins. Phila. PA 2000, 3, 113. [Google Scholar]
- Attallah, N.G.; El-Kadem, A.H.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Alanazi, A.S.; Al-Hamoud, G.A.; Ragab, A.E. Promising Antiviral Activity of Agrimonia pilosa Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 Supported with In Vivo Mice Study. Pharmaceuticals 2021, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.M.; Negm, W.A.; Elekhnawy, E.; Elmongy, E.I.; Altwaijry, N.; El-Haroun, H.; El-Masry, T.A.; El-Sherbeni, S.A. Elucidation of Phytochemical Content of Cupressus macrocarpa Leaves: In Vitro and In Vivo Antibacterial Effect against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Antibiotics 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.; Patel, J.; Bobenchik, A.; Campeau, S.; Cullen, S.; Galas, M.; Gold, H.; Humphries, R.; Kirn, T.; Lewis Ii, J. M100 Performance Standards for Antimicrobial Susceptibility Testing A CLSI supplement for global application. In Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2020. [Google Scholar]
- Elmongy, E.I.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Attallah, N.G.; Altwaijry, N.; Batiha, G.E.-S.; El-Sherbeni, S.A. Antidiarrheal and Antibacterial Activities of Monterey Cypress Phytochemicals: In Vivo and In Vitro Approach. Molecules 2022, 27, 346. [Google Scholar] [CrossRef] [PubMed]
- Elekhnawy, E.; Sonbol, F.; Abdelaziz, A.; Elbanna, T. An investigation of the impact of triclosan adaptation on Proteus mirabilis clinical isolates from an Egyptian university hospital. Braz. J. Microbiol. 2021, 52, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Elekhnawy, E. Investigation of the Antibacterial Activity and Efflux Pump Inhibitory Effect of Cycas thouarsii R. Br. Extract against Klebsiella pneumoniae Clinical Isolates. Pharmaceuticals 2021, 14, 756. [Google Scholar] [CrossRef]
- Alotaibi, B.; Mokhtar, F.A.; El-Masry, T.A.; Elekhnawy, E.; Mostafa, S.A.; Abdelkader, D.H.; Elharty, M.E.; Saleh, A.; Negm, W.A. Antimicrobial Activity of Brassica rapa L. Flowers Extract on Gastrointestinal Tract Infections and Antiulcer Potential Against Indomethacin-Induced Gastric Ulcer in Rats Supported by Metabolomics Profiling. J. Inflamm. Res. 2021, 14, 7411. [Google Scholar] [CrossRef]
- Ramasamy, S.P.; Rajendran, A.; Pallikondaperumal, M.; Sundararajan, P.; Husain, F.M.; Khan, A.; Hakeem, M.J.; Alyousef, A.A.; Albalawi, T.; Alam, P. Broad-Spectrum Antimicrobial, Antioxidant, and Anticancer Studies of Leaf Extract of Simarouba glauca DC In Vitro. Antibiotics 2022, 11, 59. [Google Scholar] [CrossRef]
- Attallah, N.G.; Negm, W.A.; Elekhnawy, E.; Altwaijry, N.; Elmongy, E.I.; El-Masry, T.A.; Alturki, E.A.; Yousef, D.A.; Shoukheba, M.Y. Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen. Antibiotics 2021, 10, 859. [Google Scholar] [CrossRef]
- Srilaxmi, P.; Sareddy, G.R.; Setty, O.H.; Babu, P.P. Protective efficacy of natansnin, a dibenzoyl glycoside from Salvinia natans against CCl 4 induced oxidative stress and cellular degeneration in rat liver. BMC Pharmacol. 2010, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.R.; Elkablawy, M.A.; Sheik, B.Y.; Baraka, H.N. Antioxidant and tissue-protective studies on Ajwa extract: Dates from al-Madinah al-Monwarah, Saudia Arabia. J. Environ. Anal. Toxicol 2013, 3, 2161-0525. [Google Scholar] [CrossRef]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Suyasunanont, D.; Klaikeaw, N. Curcumin decreased oxidative stress, inhibited NF-B activation, and improved liver pathology in ethanol-induced liver injury in rats. J. Biomed. Biotechnol. 2009, 2009, 981963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
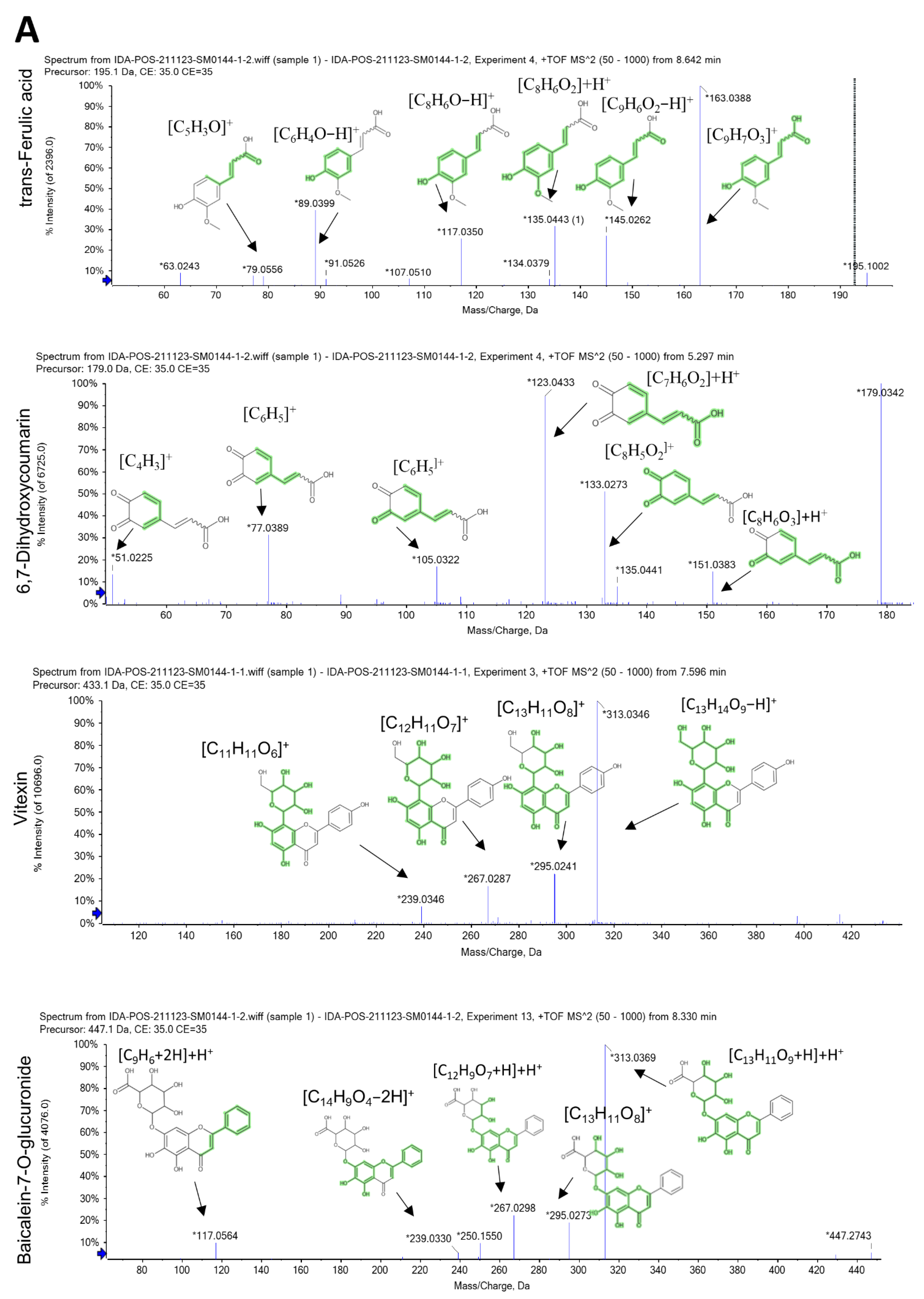
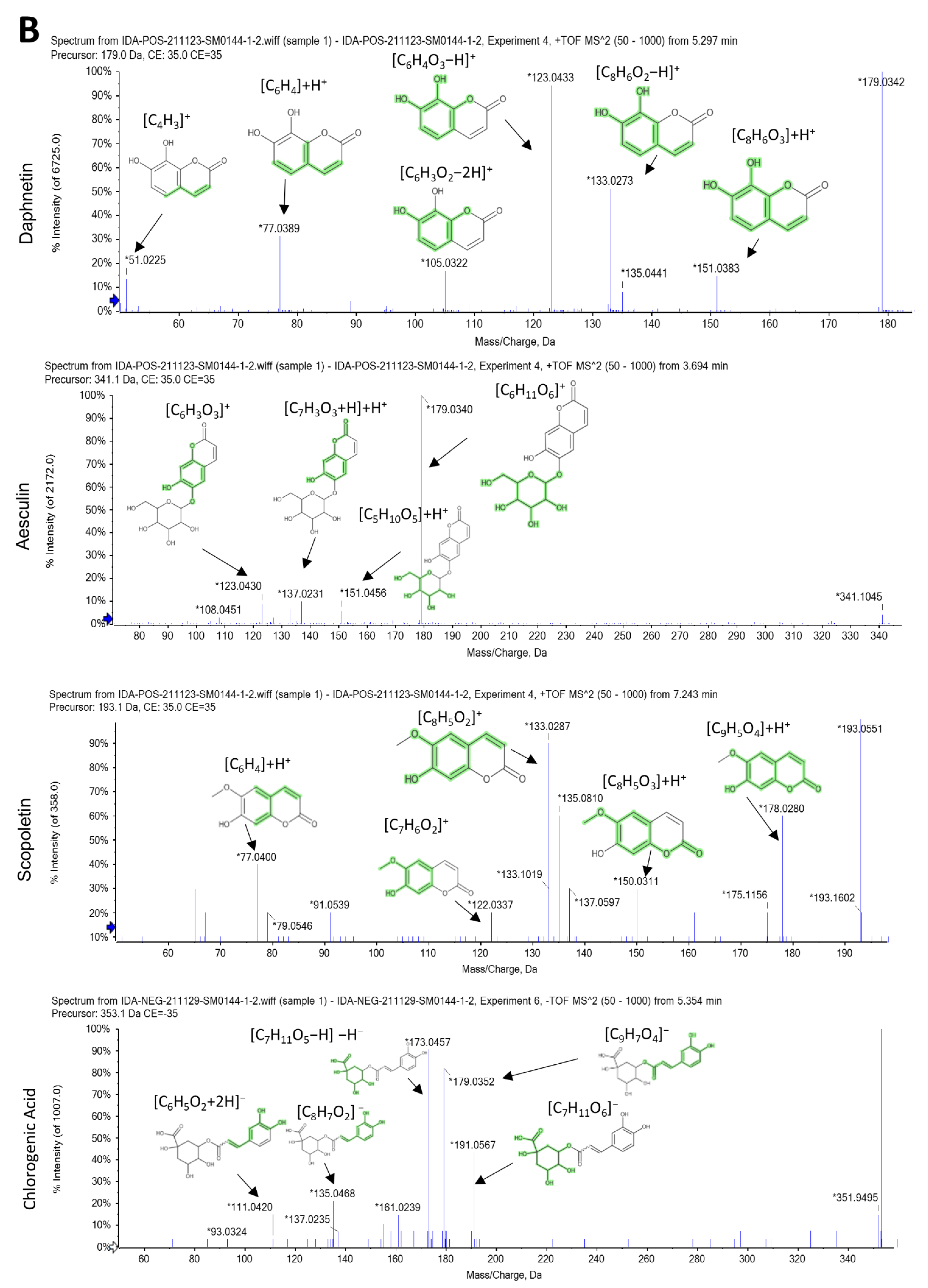

| No. | Rt (min) | Precursor m/z | Error ppm | Metabolite Name | Formula | Adduct | MS/MS Spectrum | Class |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.36 | 179.034 | 0.5 | Caffeic acid | C9H8O4 | [M-H]− | 107.050 [C7H5O+2H]−, 117.034 [C8H6O]−H−, 135.045 [C8H7O2]− | Hydroxy cinnamic acids |
| 2 | 1.42 | 181.049 | 0.4 | Caffeic acid | C9H8O4 | [M+H]+ | 53.002 [C3H2O−H]+, 122.036 [C7H6O2]+, 137.059 [C8H7O2+H]+H+, 163.038 [C9H7O3]+ | Hydroxy cinnamic acids |
| 3 | 2.14 | 169.097 | 0.1 | Pyridoxamine | C8H12N2O2 | [M+H]+ | 70.065 [C4H6N+H]+H+, 86.060 [C4H7NO]+H+, 124.063 [C6H6N2O+H]+H+ | Pyridoxamine 5’-phosphates |
| 4 | 3.76 | 341.086 | 5.3 | Esculin | C15H16O9 | [M+H]+ | 108.02 [C6H3O2]+H+, 123.007 [C6H3O3]+, 137.023 [C7H3O3+H]+H+, 151.060 [C5H10O5]+H+, 179.055 [C6H11O6]+ | Coumarin glycosides |
| 5 | 4.22 | 147.045 | 0.1 | Trans- Cinnamate | C9H8O2 | [M-H]− | 77.039 [C6H5]−, 103.055 [C8H7]−, 119.050 [C8H6O+H]− | Cinnamic acids |
| 6 | 4.747 | 195.087 | −0.8 | Caffeine | C8H10N4O2 | [M+H]+ | 110.071 [C5H7N3]+H+, 138.066 [C6H7N3O]+H+, 151.037 [C6H4N3O2]+H+ | Xanthines |
| 7 | 5.01 | 154.049 | −0.8 | 3-hydroxy anthranilic acid | C7H7NO3 | [M+H]+ | 94.02 [C6H4O+H]+H+, 112.03 [C5H5NO2]+H+, 122.02 [C7H4O2+H]+H+, 140.03 [C6H6NO3]+ | Hydroxy benzoic acid derivatives |
| 8 | 5.07 | 177.019 | −0.6 | 6,7-dihydroxy coumarin | C9H6O4 | [M-H]− | 77.039 [C6H4+H]−, 105.034 [C7H4O+H]−, 133.029 [C8H6O2]−H−, 149.024 [C8H6O3]−H− | 6,7-dihydroxy coumarins |
| 9 | 5.198 | 355.102 | −0.9 | Chlorogenic acid (3-caffeoylquinic acid) | C16H18O9 | [M+H]+ | 89.02 [C3H4O3]+H+, 117.018 [C4H6O4−H]+, 135.044 [C8H7O2]+, 145.049 [C6H10O4−H]+, 163.038 [C9H7O3]+, 337.091 [C16H17O8]+ | Quinic acids and derivatives |
| 10 | 5.296 | 179.033 | 0.6 | 6,7-dihydroxycoumarin (esculetin) | C9H6O4 | [M+H]+ | 51.02 [C4H3]+, 77.03 [C6H5]+, 105.03 [C7H5O]+, 123.04 [C7H6O2]+H+, 133.02 [C8H5O2]+, 135.02 [C8H5O2+H]+H+, 151.0 [C8H6O3]+H+ | 6,7-dihydroxy coumarins |
| 11 | 5.3 | 179.033 | 0.6 | Daphnetin | C9H6O4 | [M+H]+ | 77.038 [C6H4]+H+, 104.997 [C6H3O2−2H]+, 123.007 [C6H4O3−H]+, 133.028 [C8H6O2−H]+, 135.044 [C8H6O2]+H+, 151.038 [C8H6O3]+H+ | 7,8-dihydroxy coumarins |
| 12 | 5.352 | 353.087 | −0.4 | Chlorogenic acid | C16H18O9 | [M-H]− | 111.045 [C6H5O2+2H]−, 135.045 [C8H7O2]−, 161.024 [C9H7O3−H]−H−, 173.045 [C7H11O5−H]−H−, 179.035 [C9H7O4]−, 191.0561 [C7H11O6]− | Quinic acids and derivatives |
| 13 | 6.172 | 255.065 | −0.8 | Daidzein | C15H10O4 | [M+H]+ | 91.017 [C6H4O−H]+, 131.049 [C9H5O+H]+H+, 181.028 [C12H6O2−H]+, 182.036 [C12H6O2]+, 199.038 [C12H7O3]+, 219.044 [C15H8O2−H]+, 237.054 [C15H9O3]+ | Isoflavones |
| 14 | 7.16 | 193.049 | −0.1 | Scopoletin | C10H8O4 | [M+H]+ | 77.038 [C6H4]+H+, 122.036 [C7H6O2]+, 133.028 [C8H5O2]+, 137.059 [C8H7O2+H]+H+, 150.031 [C8H5O3]+H+, 178.026 [C9H5O4]+H+ | 7-hydroxy coumarins |
| 15 | 7.65 | 433.112 | 4.5 | Apigenin 8-C-glucoside (vitexin) | C21H20O10 | [M+H]+ | 239.0346 [C11H11O6]+, 267.0287 [C12H11O7]+, 295.0241 [C13H11O8]+, 313.0346 [C13H14O9−H]+ | Flavonoid 8-C-glycosides |
| 16 | 8.32 | 447.092 | 1.4 | Baicalein-7-O-glucuronide | C21H18O11 | [M+H]+ | 117.069 [C9H6+2H]+H+, 239.033 [C14H9O4−2H]+, 267.049 [C12H9O7+H]+H+, 295.044 [C13H11O8]+, 313.055 [C13H11O9+H]+H+ | Flavonoid-7-O-glucuronides |
| 17 | 8.41 | 463.088 | 0.7 | 6-hydroxy kaempferol 3-glucoside | C21H20O12 | [M-H]− | 301.035 [C15H9O7]−, 315.051 [C16H10O7+H]−, 343.045 [C17H12O8]−H−, 403.067 [C19H16O10]−H− | Flavonoid-3-O-glycosides |
| 18 | 8.68 | 195.065 | −0.2 | Trans-ferulic acid | C10H10O4 | [M+H]+ | 63.02 [C5H4−H]+, 79.05 [C5H3O]+, 117.03 [C8H6O−H]+, 135.04 [C8H6O2]+H+, 145.02 [C9H6O2−H]+, 163.03 [C9H7O3]+ | Hydroxy cinnamic acids |
| 19 | 8.76 | 449.108 | 0.3 | Cyanidin 4″-glucoside | C21H21O11 | [M] + | 147.065 [C6H10O4]+H+, 252.062 [C12H14O6−2H]+, 273.096 [C12H15O7+H]+H+ | Anthocyanidin -3-O-glycosides |
| 20 | 9.742 | 287.055 | 0.9 | Luteolin | C15H10O6 | [M+H]+ | 135.044 [C8H6O2]+H+, 153.018 [C7H4O4]+H+, 215.070 [C13H8O3+2H]+H+, 255.065 [C15H8O4+2H]+H+, 269.044 [C15H9O5]+ | Flavones |
| 21 | 10.52 | 269.045 | 0.6 | Apigenin | C15H10O5 | [M-H]− | 117.034 [C8H6O]−H−, 149.024 [C8H5O3]−, 183.045 [C12H6O2+H]−, 225.055 [C14H9O3]−, 227.035 [C13H9O4−H]-H−, 254.058 [C15H9O4+H]− | Flavones |
| 22 | 10.76 | 211.132 | −0.3 | (+-)-Jasmonic acid | C12H18O3 | [M+H]+ | 81.1 [C6H10−H]+, 123.1 [C8H10O]+H+, 147.03 [C11H17−2H]+, 157.1 [C8H11O3+H]+H+, 165.1 [C11H17O]+ | Jasmonic acids |
| 23 | 10.88 | 271.060 | 0.9 | Apigenin | C15H10O5 | [M+H]+ | 119.08 [C8H6O]+H+, 153.02 [C7H4O4]+H+, 253.14 [C15H9O4]+ | Flavones |
| 24 | 10.89 | 271.060 | 0.9 | Genistein | C15H10O5 | [M+H]+ | 119.049 [C8H6O]+H+, 153.018 [C7H4O4]+H+, 243.065 [C12H10O4]+, 253.049 [C15H9O4]+ | Isoflavones |
| Isolate Number | Resistance Profile * | Isolate Number | Resistance Profile * |
|---|---|---|---|
| A1 | CEF- SXT-GEN-AMK | A15 | CEF-SXT-CIP |
| A2 | CEF-CXM-TOB-CIP | A16 | CXM-TET-CHL-CIP |
| A3 | CXM-GEN-CHL | A17 | CEF-SXT-GEN |
| A4 | AZM-TET | A18 | SXT-GEN-AMK-TOB |
| A5 | TET-CHL | A19 | CEF-CXM-CAZ-SXT-GEN-CIP-CHL |
| A6 | CEF-CXM-CHL-CIP-LVX | A20 | AMK-CHL |
| A7 | CAZ-SXT-GEN-TET | A21 | CEF-CXM-CAZ-GEN |
| A8 | SXT-TET-CHL | A21 | GEN-AMK-TOB-TET-CHL |
| A9 | CEF-CXM-SXT | A22 | CEF |
| A10 | TET | A23 | CXM-AZM-TET |
| A11 | AZM-CHL | A24 | CAZ-GEN-AZM-CIP-LVX |
| A12 | SXT-GEN-AMK | A25 | CXM-TET-CHL |
| A13 | AMK-AZM-CHL | A26 | CEF |
| A14 | CEF-CXM-SXT-CHL-CIP-IPM | A27 | CXM-AZM-CHL |
| Alanine Amino Transferase (ALT) (U/mL) | Aspartate Amino Transferase (AST) (U/mL) | Lactate Dehydrogenase (LDH) (pg/mL) | |
|---|---|---|---|
| Control | 41.77 ± 4.8 | 69.08 ± 10.2 | 66.82 ± 8.6 |
| MTX | 53.98 ± 6.25 a | 153.87 ± 12.5 a | 131.98 ± 9.6 a |
| SAME 10 | 51.26 ± 3.8 | 141.78 ± 8.35 | 118.87 ± 10.7 |
| SAME 20 | 46.92 ± 8.1 | 132.53 ± 7.36 b | 88.25 ± 5.9 b |
| SAME 30 | 42.67 ± 4.5 bc | 78.33 ± 8.31 bcd | 72.78 ± 3.6 bcd |
| Hepatic MDA Content (nm/gm Tissue) | Hepatic NO Content (nmol/g Tissue) | Hepatic SOD Activity (U/mg Tissue) | |
|---|---|---|---|
| Control | 148 ± 12.2 | 12.7 ± 1.35 | 2.95 ± 0.18 |
| MTX | 230 ± 13.95 a | 33.2 ± 3.1 a | 2.1 ± 0.28 a |
| SAME 10 | 210 ± 7.34 | 27.1 ± 2.6 | 2.35 ± 0.31 |
| SAME 20 | 185 ± 10.4 b | 21.6 ± 1.92 b | 2.7 ± 0.21 b |
| SAME 30 | 158 ± 6.51 bc | 14.2 ± 0.98 bcd | 3.05 ± 0.41 bcd |
| Histological Parameters | Normal Control | MTX | SAME 10 | SAME 20 | SAME 30 |
|---|---|---|---|---|---|
| Hepatoportal and sinusoidal congestion | - | +++ | + | + | - |
| Hydropic degeneration | - | +++ | ++ | + | - |
| Cellular necrosis | - | +++ | ++ | + | - |
| Apoptosis | - | +++ | + | + | - |
| Inflammatory cellular infiltrate | - | +++ | ++ | + | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attallah, N.G.M.; Mokhtar, F.A.; Elekhnawy, E.; Heneidy, S.Z.; Ahmed, E.; Magdeldin, S.; Negm, W.A.; El-Kadem, A.H. Mechanistic Insights on the In Vitro Antibacterial Activity and In Vivo Hepatoprotective Effects of Salvinia auriculata Aubl against Methotrexate-Induced Liver Injury. Pharmaceuticals 2022, 15, 549. https://doi.org/10.3390/ph15050549
Attallah NGM, Mokhtar FA, Elekhnawy E, Heneidy SZ, Ahmed E, Magdeldin S, Negm WA, El-Kadem AH. Mechanistic Insights on the In Vitro Antibacterial Activity and In Vivo Hepatoprotective Effects of Salvinia auriculata Aubl against Methotrexate-Induced Liver Injury. Pharmaceuticals. 2022; 15(5):549. https://doi.org/10.3390/ph15050549
Chicago/Turabian StyleAttallah, Nashwah G. M., Fatma Alzahraa Mokhtar, Engy Elekhnawy, Selim Z. Heneidy, Eman Ahmed, Sameh Magdeldin, Walaa A. Negm, and Aya H. El-Kadem. 2022. "Mechanistic Insights on the In Vitro Antibacterial Activity and In Vivo Hepatoprotective Effects of Salvinia auriculata Aubl against Methotrexate-Induced Liver Injury" Pharmaceuticals 15, no. 5: 549. https://doi.org/10.3390/ph15050549
APA StyleAttallah, N. G. M., Mokhtar, F. A., Elekhnawy, E., Heneidy, S. Z., Ahmed, E., Magdeldin, S., Negm, W. A., & El-Kadem, A. H. (2022). Mechanistic Insights on the In Vitro Antibacterial Activity and In Vivo Hepatoprotective Effects of Salvinia auriculata Aubl against Methotrexate-Induced Liver Injury. Pharmaceuticals, 15(5), 549. https://doi.org/10.3390/ph15050549









