Comparison of Losartan and Furosemide Interaction with HSA and Their Influence on HSA Antioxidant Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Calorimetric and Spectroscopic Analysis of LOS and FUR Interaction with Human Serum Albumin
2.2. Spectroscopic Analysis of LOS and FUR Influence on Albumin Secondary Structure
2.3. Spectroscopic Analysis of LOS and FUR Influence on Albumin Antioxidant Activity
3. Materials and Methods
3.1. Chemicals
3.2. Methods
3.2.1. Nano Isothermal Titration Calorimetry (nanoITC)
- ΔG—Gibbs free energy change [kcal/mol];
- T—temperature [K];
- ΔS—entropy change [kcal/molK];
- ΔH—enthalpy change [kcal/mol].
3.2.2. UV–VIS Spectrophotometry Measurements
3.2.3. Circular Dichroism (CD) Measurements
- Θ—observed ellipticity for a given wavelength [deg]
- m—the concentration [g/cm3]
- l—the pathlength [cm]
- MRW—a mean residue weight (MRW HSA = 113.7 Da).
3.3. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabbani, G.; Ahn, S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019, 123, 979–990. [Google Scholar] [CrossRef]
- Sharma, G.N.; Gupta, G.; Sharma, P. A Comprehensive Review of Free Radicals, Antioxidants, and Their Relationship with Human Ailments. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India. 2004, 52, 794–804. [Google Scholar]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Infusions and decoctions of mixed herbs used in folk medicine: Synergism in antioxidant potential. Phytother. Res. 2011, 25, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, X.; Yamamoto, K. Bovine serum albumin significantly improves the DPPH free radical scavenging potential of dietary polyphenols and gallic acids. Anticancer. Agents Med. Chem. 2012, 12, 940–948. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Wang, G.; Lu, Y. Probing the interaction of human serum albumin with DPPH in the absence and presence of the eight antioxidants. Spectrochim Acta A Mol. Biomol. Spectrosc. 2015, 137, 1144–1152. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef] [Green Version]
- Simpson, K.L.; McClellan, K.J. Losartan: A review of its use, with special focus on elderly patients. Drugs Aging. 2000, 16, 227–250. [Google Scholar] [CrossRef]
- Sica, D.A.; Gehr, T.W.; Ghosh, S. Clinical pharmacokinetics of losartan. Clin. Pharmacokinet. 2005, 44, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.R.; Assiri, E.; Khalil, N.Y.; Abdel-Aziz, H.A. Losartan: Comprehensive Profile. Profiles Drug. Subst. Excip. Relat. Methodol. 2015, 40, 159–194. [Google Scholar] [PubMed]
- Puskarich, M.A.; Cummins, N.W.; Ingraham, N.E.; Wacker, D.A.; Reilkoff, R.A.; Driver, B.E.; Biros, M.H.; Bellolio, F.; Chipman, J.G.; Nelson, A.C.; et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine 2021, 37, 100957. [Google Scholar] [CrossRef] [PubMed]
- Ponto, L.L.; Schoenwald, R.D. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I). Clin. Pharmacokinet. 1990, 18, 381–408. [Google Scholar]
- Joannidis, M.; Klein, S.J.; Ostermann, M. 10 myths about frusemide. Intensive Care Med. 2019, 45, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Maillard, J.O.; Descombes, E.; Fellay, G.; Regamey, C. Repeated transient anuria following losartan administration in a patient with a solitary kidney. Ren Fail. 2001, 23, 143–147. [Google Scholar] [CrossRef]
- Arias, S.C.; Souza, R.A.; Malheiros, D.M.; Fanelli, C.; Fujihara, C.K.; Zatz, R. An association of losartan-hydrochlorothiazide, but not losartan-furosemide, completely arrests progressive injury in the remnant kidney. Am. J. Physiol. Renal. Physiol. 2016, 310, F135–F143. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, K.; Kohno, M.; Yukiiri, K.; Masugata, H.; Wada, Y.; Takagi, Y.; Ohmori, K. Influence of the angiotensin II receptor antagonist losartan on diuretic-induced metabolic effects in elderly hypertensive patients: Comparison with a calcium channel blocker. Int. J. Clin. Pharmacol. Ther. 2001, 39, 417–422. [Google Scholar] [CrossRef]
- Bojko, B.; Sułkowska, A.; Maciążek-Jurczyk, M.; Równicka, J.; Pentak, D.; Sułkowski, W.W. Alterations of furosemide binding to serum albumin induced by increased level of fatty acid. J. Pharm. Biomed. Anal. 2010, 51, 273–277. [Google Scholar] [CrossRef]
- Moeinpour, F.; Mohseni-Shahri, F.S.; Malaekeh-Nikouei, B.; Nassirli, H. Investigation into the interaction of losartan with human serum albumin and glycated human serum albumin by spectroscopic and molecular dynamics simulation techniques: A comparison study. Chem. Biol. Interact. 2016, 257, 4–13. [Google Scholar] [CrossRef]
- Yasseen, Z.J.; Ghossain, M.E. Studies on Binding of Widely used Drugs with Human Serum Albumin at Different Temperatures and PHs. J. Biomedical. Sci. 2016, 5, 3. [Google Scholar] [CrossRef]
- Szkudlarek, A.; Pentak, D.; Ploch, A.; Pożycka, J.; Maciążek-Jurczyk, M. In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States. Molecules 2017, 22, 2249. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, N.; Ahmad, E.; Rehan, M.; Rabbani, G.; Ajmal, M.R.; Zaidi, Y.; Subbarao, N.; Khan, R.H. Biophysical insight into furosemide binding to human serum albumin: A study to unveil its impaired albumin binding in uremia. J. Phys. Chem. B 2013, 117, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Subramanian, S. Thermodynamics of Protein Association Reactions: Forces Contributing to Stability. Biochemistry 1981, 20, 3096. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, Y.; Liu, J.; Tang, P.; Sun, Q.; Xiong, X.; Tang, B.; He, J.; Li, H. Binding modes of environmental endocrine disruptors to human serum albumin: Insights from STD-NMR, ITC, spectroscopic and molecular docking studies. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marković, O.S.; Cvijetić, I.N.; Zlatović, M.V.; Opsenica, I.M.; Konstantinović, J.M.; Terzić Jovanović, N.V.; Šolaja, B.A.; Verbić, T.Ž. Human serum albumin binding of certain antimalarials. Spectrochimm Acta A Mol. Biomol. Spectrosc. 2018, 192, 128–139. [Google Scholar] [CrossRef]
- Bou-Abdallah, F.; Sprague, S.E.; Smith, B.M.; Giffune, T.R. Binding thermodynamics of Diclofenac and Naproxen with human and bovine serum albumins: A calorimetric and spectroscopic study. J. Chem. Thermodyn. 2016, 103, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, E.S.; Tazikeh-Lemeskia, E.; Sabouryb, A.A. Isothermal Titration Calorimetry and Molecular Dynamics Simulation Studies on the Binding of Indometacin with Human Serum Albumin. Biomacromol. J. 2016, 2, 34–43. [Google Scholar]
- Lelis, C.A.; Hudson, E.A.; Ferreira, G.M.D.; Ferreira, G.M.D.; da Silva, L.H.M.; da Silva, M.D.C.H.; Pinto, M.S.; Pires, A.C.D.S. Binding thermodynamics of synthetic dye Allura Red with bovine serum albumin. Food Chem. 2017, 217, 52–58. [Google Scholar] [CrossRef]
- Momeni, L.; Shareghi, B.; Saboury, A.A.; Farhadian, S. Comparative Studies on the Interaction of Spermidine with Bovine Trypsin by Multispectroscopic and Docking Methods. J. Phys. Chem. B 2016, 120, 9632–9641. [Google Scholar] [CrossRef]
- Ren, G.; Sun, H.; Guo, J.; Fan, J.; Li, G.; Xu, S. Molecular mechanism of the interaction between resveratrol and trypsin via spectroscopy and molecular docking. Food Funct. 2019, 10, 3291–3302. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.L.; Miles, A.J.; Whitmore, L.; Wallace, B.A. Distinct circular dichroism spectroscopic signatures of polyproline II and unordered secondary structures: Applications in secondary structure analyses. Protein Sci. 2014, 23, 1765–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciążek-Jurczyk, M.; Morak-Młodawska, B.; Jeleń, M.; Kopeć, W.; Szkudlarek, A.; Owczarzy, A.; Kulig, K.; Rogóż, W.; Pożycka, J. The Influence of Oxidative Stress on Serum Albumin Structure as a Carrier of Selected Diazaphenothiazine with Potential Anticancer Activity. Pharmaceuticals 2021, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Owczarzy, A.; Zięba, A.; Pożycka, J.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Spectroscopic Studies of Quinobenzothiazine Derivative in Terms of the In Vitro Interaction with Selected Human Plasma Proteins. Part 1. Molecules 2021, 26, 4776. [Google Scholar] [CrossRef] [PubMed]
- Venyaminov, S.Y.; Vassilenko, K.S. Determination of protein tertiary structure class from circular dichroism spectra. Anal. Biochem. 1994, 222, 176–184. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Ranjbar, S.; Ghobadi, S.; Khodarahmi, R.; Nemati, H. Spectroscopic characterization of furosemide binding to human carbonic anhydrase II. Int. J. Biol. Macromol. 2012, 50, 910–917. [Google Scholar] [CrossRef]
- Gheitasi, I.; Moosavi, S.M. Combination Therapy with Losartan and α-Tocopherol in Acute Ureteral Obstruction-Induced Renal Excretory Dysfunction and Acidification Defect. Iran J. Med. Sci. 2014, 39, 357–366. [Google Scholar]
- Zal, F.; Taheri, R.; Khademi, F.; Keshavarz, E.; Rajabi, S.; Mostafavi-Pour, Z. The combined effect of furosemide and propranolol on GSH homeostasis in ACHN renal cells. Toxicol. Mech. Methods 2014, 24, 412–416. [Google Scholar] [CrossRef]
- Ivanov, M.; Mihailović-Stanojević, N.; Grujić Milanović, J.; Jovović, Đ.; Marković-Lipkovski, J.; Ćirović, S.; Miloradović, Z. Losartan improved antioxidant defense, renal function and structure of postischemic hypertensive kidney. PLoS ONE 2014, 9, e96353. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Yang, H.; Xue, Q.L.; Chuang, Y.F.; Roy, C.N.; Abadir, P.; Walston, J.D. Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp. Gerontol. 2014, 58, 174–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodovici, M.; Bigagli, E.; Tarantini, F.; Di Serio, C.; Raimondi, L. Losartan reduces oxidative damage to renal DNA and conserves plasma antioxidant capacity in diabetic rats. Exp. Biol. Med. 2015, 240, 1500–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayabasi, H.; Yilmaz, Z.; Sit, D.; Kadiroglu, A.K.; Yilmaz, E. The effects of Losartan on oxidative stress and inflammation in non-diabetic patients undergoing chronic hemodialysis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 235–242. [Google Scholar] [PubMed]
- Karanovic, D.; Grujic-Milanovic, J.; Miloradovic, Z.; Ivanov, M.; Jovovic, D.; Vajic, U.J.; Zivotic, M.; Markovic-Lipkovski, J.; Mihailovic-Stanojevic, N. Effects of Single and Combined Losartan and Tempol Treatments on Oxidative Stress, Kidney Structure and Function in Spontaneously Hypertensive Rats with Early Course of Proteinuric Nephropathy. PLoS ONE 2016, 11, e0161706. [Google Scholar] [CrossRef]
- Teixeira, J.A.; Siqueira, A.B. Thermal and spectroscopic characterization, antioxidant evaluation and pyrolysis of losartan with some bivalent metals. J. Anal. Appl. Pyrolysis 2016, 117, 17–24. [Google Scholar] [CrossRef]
- Lahet, J.J.; Lenfant, F.; Courderot-Masuyer, C.; Ecarnot-Laubriet, E.; Vergely, C.; Durnet-Archeray, M.J.; Freysz, M.; Rochette, L. In vivo and in vitro antioxidant properties of furosemide. Life Sci. 2003, 73, 1075–1082. [Google Scholar] [CrossRef]
- Ihara, H.; Hashizume, N.; Hasegawa, T.; Yoshida, M. Antioxidant capacities of ascorbic acid, uric acid, alpha-tocopherol, and bilirubin can be measured in the presence of another antioxidant, serum albumin. J. Clin. Lab. Anal. 2004, 18, 45–49. [Google Scholar] [CrossRef]
- Soriani, M.; Pietraforte, D.; Minetti, M. Antioxidant potential of anaerobic human plasma: Role of serum albumin and thiols as scavengers of carbon radicals. Arch. Biochem. Biophys. 1994, 312, 180–188. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Bocedi, A.; Cattani, G.; Stella, L.; Massoud, R.; Ricci, G. Thiol disulfide exchange reactions in human serum albumin: The apparent paradox of the redox transitions of Cys34. FEBS J. 2018, 285, 3225–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdon, E.; Loreau, N.; Lagrost, L.; Blache, D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic. Res. 2005, 39, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Albumin-drug interaction and its clinical implication. Biochim Biophys Acta 2013, 1830, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Rogóż, W.; Pożycka, J.; Kulig, K.; Owczarzy, A.; Szkudlarek, A.; Maciążek-Jurczyk, M. New look at the metabolism of nonsteroidal anti-inflammatory drugs: Influence on human serum albumin antioxidant activity. J. Biomol. Struct Dyn. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siegei, S.; Frost, P.; Porto, F. Effects of Indoleacetic Acid and Other Oxidation Regulators on in Vitro Peroxidation and Experimental Conversion of Eugenol to Lignin. Plant Physiol. 1960, 35, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, P. The use of the stable free radical diphenlpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 2, 211–219. [Google Scholar]
- Sreerama, N.; Woody, R.W. Estimation of Protein Secondary Structure from Circular Dichroism Spectra: Comparison of CONTIN, SELCON, and CDSSTR Methods with an Expanded Reference Set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]

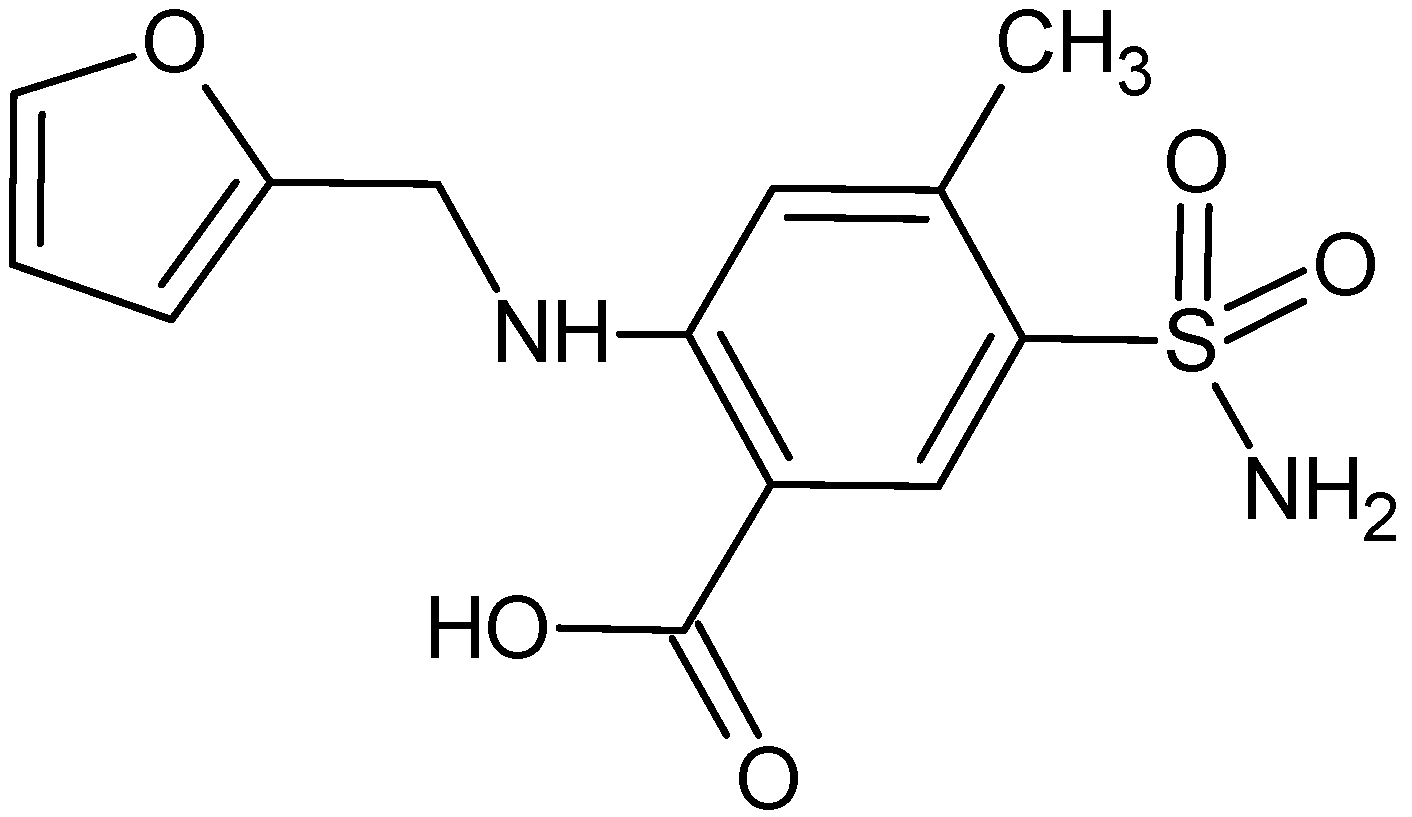

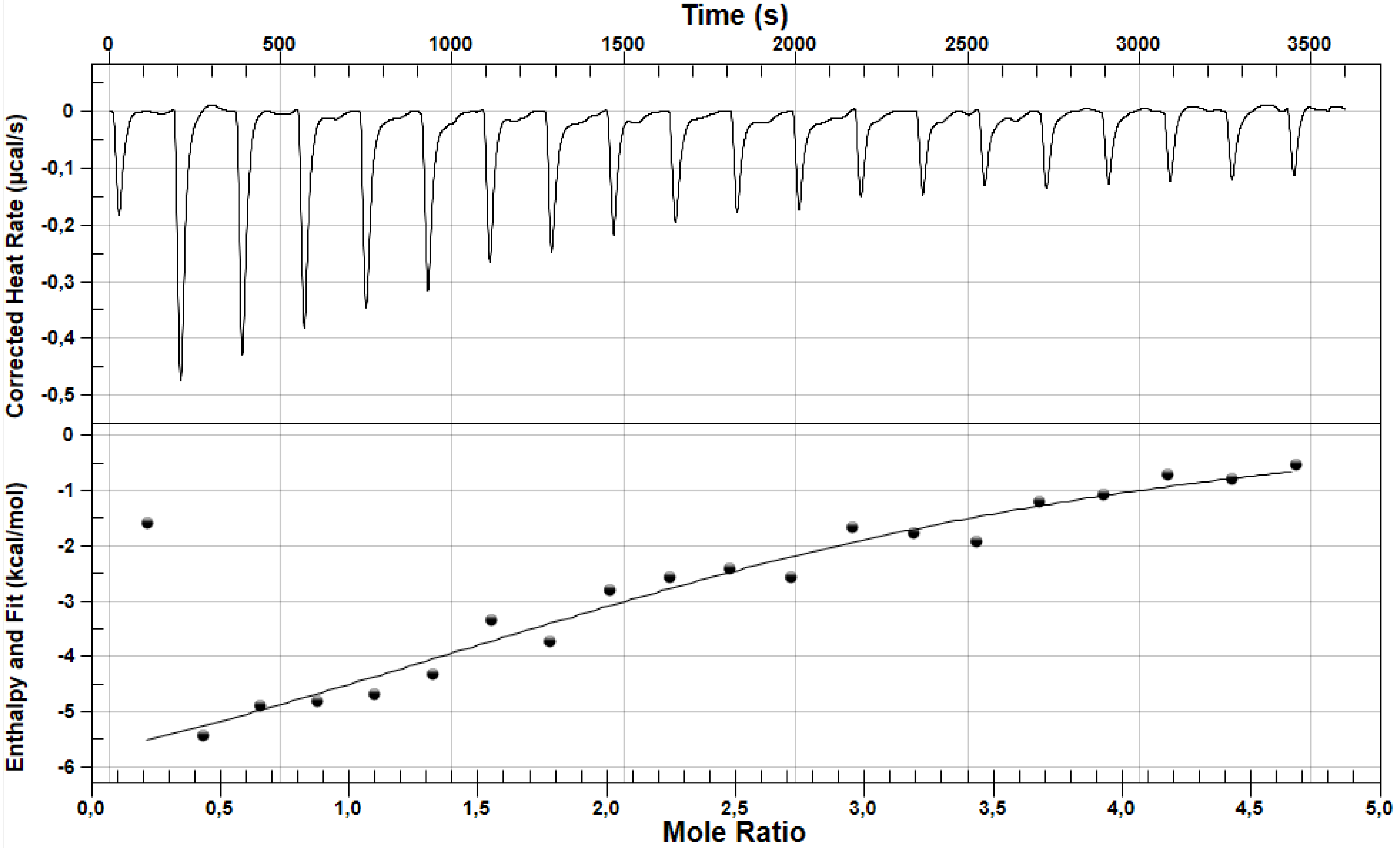
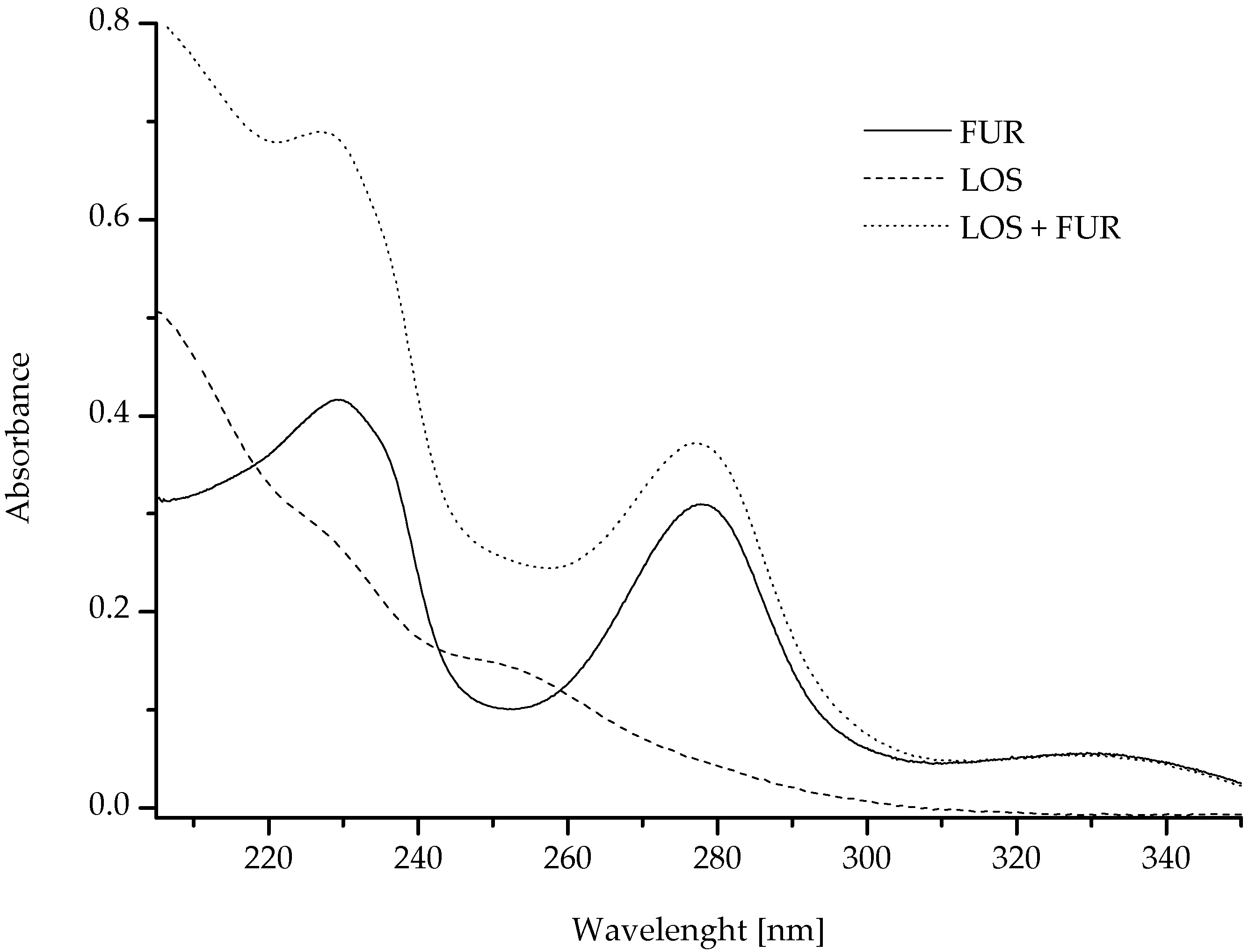
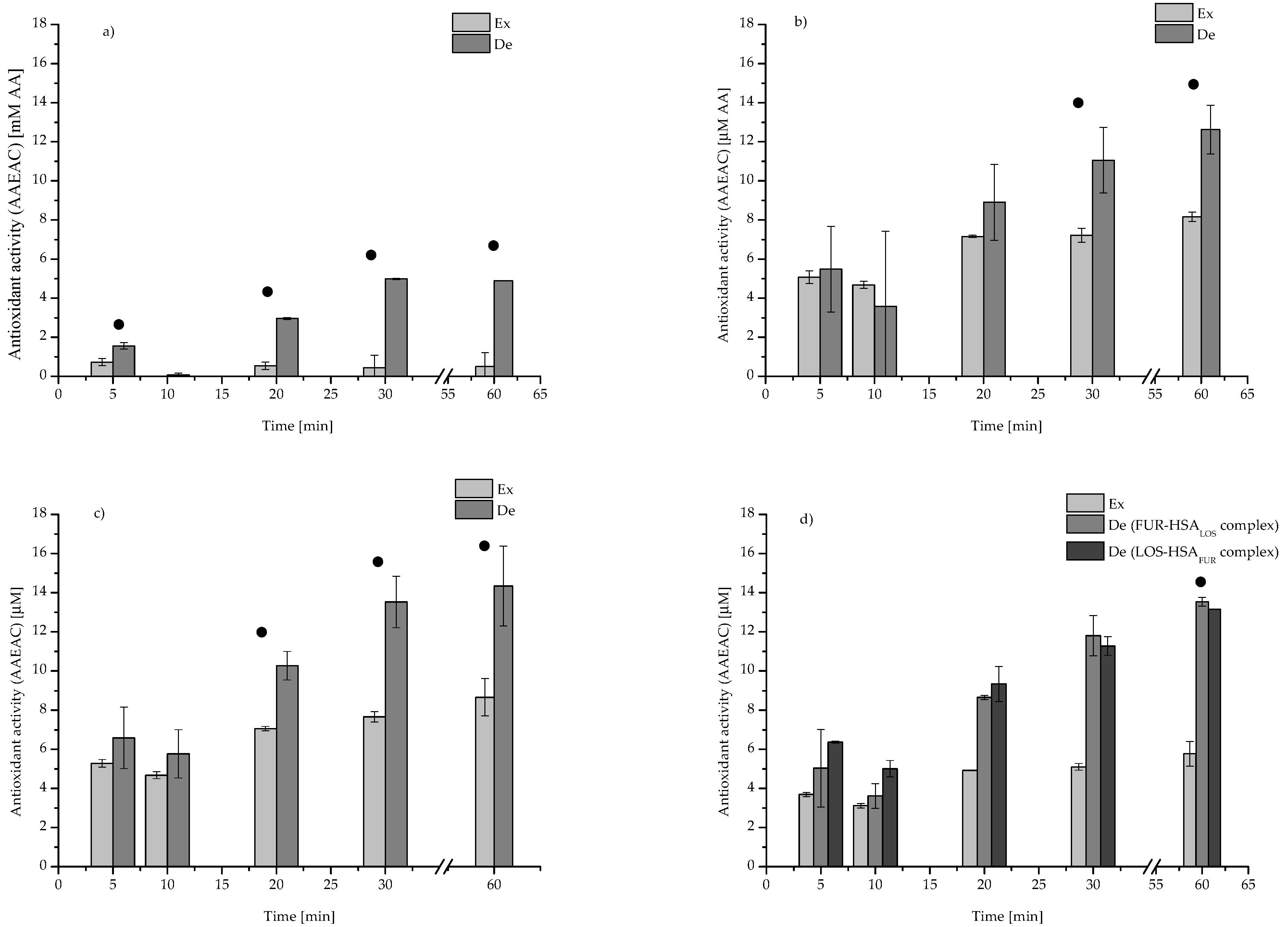
| Parameters | LOS | FUR |
|---|---|---|
| Ka ± SD * × 104 [M−1] | 6.22 ± 1.96 | 4.11 ± 1.22 |
| n ± SD * | 6.16 ± 0.33 | 1.73 ± 1.18 |
| ΔH ± SD * [kcal/mol] | −1.10 ± 0.01 | −11.54 ± 3.77 |
| ΔS ± SD * [cal/molK] | 18.21 ± 0.67 | −17.65 ± 12.06 |
| ΔG ± SD * [kcal/mol] | −6.52 ± 0.19 | −6.28 ± 0.18 |
| λmax [nm] | Absorbance ± SD * | Mathematic Sum of LOS and FUR Absorbance ± SD * | Effect ** (I/NI) | ||
|---|---|---|---|---|---|
| LOS | FUR | LOS + FUR | |||
| 207 | 0.4938 ± 0.0009 | 0.3155 ± 0.0017 | 0.7436 ± 0.0027 | 0.8092 ± 0.0027 | I |
| 218 | 0.3514 ± 0.0004 | 0.3523 ± 0.0041 | 0.6709 ± 0.0045 | 0.7038 ± 0.0045 | I |
| 230 | 0.2626 ± 0.0002 | 0.4165 ± 0.0010 | 0.6576 ± 0.0012 | 0.6791 ± 0.0012 | I |
| 278 | 0.0475 ± 0.0005 | 0.3021 ± 0.0103 | 0.3187 ± 0.0110 | ± 0.0108 | I |
 | ||
|---|---|---|
| Sample | λmin [nm] | [Θ]MRW ± SD * [deg × cm2 × dmol−1] |
| HSA (3.0 × 10−6 M) | 209 | −22,867.66 ± 218.75 |
| 221 | −21,543.93 ± 201.70 | |
| LOS-HSA (molar ratio 4.44:1) | 209 | −21,882.95 ± 316.43 |
| 221 | −20,584.20 ± 363.67 | |
| FUR-HSA (molar ratio 4.44:1) | 209 | −21,969.26 ± 51.14 |
| 221 | −20,580.90 ± 92.13 | |
| FUR-HSALOS (molar ratio 4.44:1:4.44) | 209 | −19,931.44 ± 131.10 |
| 221 | −18,647.65 ± 180.95 | |
| LOS-HSAFUR (molar ratio 4.44:1:4.44) | 209 | −19,713.56 ± 41.71 |
| 221 | −18,498.56 ± 51.83 | |
| Sample | % α-Helix ± SD * | % β-Sheet ± SD * | % Turn ± SD * | % Other ± SD * |
|---|---|---|---|---|
| HSA (3.0 × 10−6 M) | 36.55 ± 1.06 | 11.95 ± 0.64 | 20.75 ± 0.07 | 30.70 ± 0.42 |
| LOS-HSA (molar ratio 4.44:1) | 35.70 ± 0.14 | 12.60 ± 0.14 | 20.70 ± 0.00 | 31.05 ± 0.07 |
| FUR-HSA (molar ratio 4.44:1) | 35.90 ± 0.42 | 13.20 ± 0.00 | 20.40 ± 0.28 | 30.45 ± 0.21 |
| FUR-HSALOS (molar ratio 4.44:1:4.44) | 35.70 ± 0.42 | 12.95 ± 0.49 | 20.75 ± 0.21 | 30.60 ± 0.14 |
| LOS-HSAFUR (molar ratio 4.44:1:4.44) | 35.20 ± 0.00 | 13.35 ± 0.21 | 20.40 ± 0.00 | 31.10 ± 0.14 |
| Antioxidant Activity (AAEAC) ± SD * [µM AA] | |||||
|---|---|---|---|---|---|
| Sample | Time [min] | ||||
| 5 | 10 | 20 | 30 | 60 | |
| HSA (2 × 10−4 M) | 9.63 ± 0.05 | 9.35 ± 0.36 | 13.68 ± 0.36 | 14.43 ± 0.72 | 16.32 ± 0.48 |
| LOS (4 × 10−4 M) | 0.50 ± 0.71 | 0.00 ± 0.00 | 0.63 ± 0.25 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| FUR (4 × 10−4 M) | 0.94 ± 0.34 | 0.00 ± 0.00 | 0.44 ± 0.14 | 0.88 ± 1.25 | 1.00 ± 1.41 |
| LOS-FUR (molar ratio 1:1) | 1.55 ± 0.17 | 0.07 ± 0.09 | 2.96 ± 0.05 | 4.98 ± 0.03 | 4.89 ± 0.01 |
| LOS:HSA (molar ratio 2:1) | 5.48 ± 2.19 | 3.58 ± 3.86 | 8.91 ± 1.94 | 11.05 ± 1.68 | 12.62 ± 1.25 |
| FUR:HSA (molar ratio 2:1) | 6.59 ± 1.57 | 5.77 ± 1.24 | 10.27 ± 0.73 | 13.52 ± 1.32 | 14.34 ± 2.04 |
| FUR-HSALOS (molar ratio 2:1:2) | 5.04 ± 1.98 | 3.62 ± 0.63 | 8.65 ± 0.11 | 11.80 ± 1.03 | 13.53 ± 0.23 |
| LOS-HSAFUR (molar ratio 2:1:2) | 6.37 ± 0.05 | 5.01 ± 0.42 | 9.33 ± 0.89 | 11.27 ± 0.48 | 13.15 ± 0.00 |
| Antioxidant Activity (AAEAC) ± SD * [µM AA] | |||||
|---|---|---|---|---|---|
| Sample | Time [min] | ||||
| ABTS Assay | |||||
| 5 | 10 | 20 | 30 | 60 | |
| LOS (1 × 10−4 M) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| FUR (1 × 10−4 M) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.44 ± 2.86 |
| LOS:FUR (molar ratio 1:1) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| LOS (4 × 10−4 M) | 0.00 ± 0.00 | 0.46 ± 0.37 | 1.53 ± 0.54 | 2.19 ± 0.59 | 2.85 ± 0.61 |
| FUR (4 × 10−4 M) | 2.61 ± 0.42 | 5.48 ± 0.55 | 7.73 ± 0.01 | 9.67 ± 1.13 | 12.18 ± 2.81 |
| LOS:FUR (molar ratio 1:1) | 0.16 ± 0.23 | 2.08 ± 0.16 | 4.51 ± 0.26 | 6.95 ± 0.42 | 8.83 ± 1.41 |
| Antioxidant Activity (AAEAC) ± SD * [µM AA] | ||||||
|---|---|---|---|---|---|---|
| Sample | Time [min] | |||||
| ABTS Assay | ||||||
| 5 | 10 | 20 | 30 | 60 | ||
| HSA (5 × 10−5 M) | De | 23.85 ± 0.24 | 25.57 ± 0.43 | 27.36 ± 0.35 | 27.09 ± 0.63 | 26.01 ± 0.12 |
| LOS-HSA (molar ratio 2:1) | De | 17.07 ± 0.51 | 22.31 ± 0.56 | 25.50 ± 0.50 | 26.00 ± 0.37 | 25.72 ± 0.30 |
| Ex | 11.93 ± 0.12 | 12.78 ± 0.22 | 13.68 ± 0.17 | 13.55 ± 0.31 | 13.01 ± 0.06 | |
| Effect | s | s | s ** | s ** | s ** | |
| FUR-HSA (molar ratio 2:1) | De | 19.19 ± 0.02 | 24.02 ± 0.16 | 26.60 ± 0.12 | 25.75 ± 1.61 | 26.00 ± 0.20 |
| Ex | 11.93 ± 0.12 | 12.78 ± 0.22 | 13.68 ± 0.17 | 13.55 ± 0.31 | 13.12 ± 0.09 | |
| Effect | s | s | s ** | s ** | s ** | |
| FUR-HSALOS (molar ratio 2:1:2) | De | 12.41 ± 0.45 | 18.32 ± 0.11 | 21.72 ± 0.67 | 21.58 ± 1.81 | 24.37 ± 1.96 |
| Ex | 7.95 ± 0.08 | 8.52 ± 0.14 | 9.12 ± 0.12 | 9.03 ± 0.21 | 8.74 ± 0.14 | |
| Effect | s | s | s ** | s ** | s ** | |
| LOS-HSAFUR (molar ratio 2:1:2) | De | 12.43 ± 0.64 | 17.82 ± 0.33 | 22.11 ± 0.42 | 24.37 ± 1.96 | 24.59 ± 0.45 |
| Ex | 7.95 ± 0.08 | 8.52 ± 0.14 | 9.12 ± 0.12 | 9.03 ± 0.21 | 8.74 ± 0.14 | |
| Effect | s | s | s ** | s ** | s ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogóż, W.; Pożycka, J.; Owczarzy, A.; Kulig, K.; Maciążek-Jurczyk, M. Comparison of Losartan and Furosemide Interaction with HSA and Their Influence on HSA Antioxidant Potential. Pharmaceuticals 2022, 15, 499. https://doi.org/10.3390/ph15050499
Rogóż W, Pożycka J, Owczarzy A, Kulig K, Maciążek-Jurczyk M. Comparison of Losartan and Furosemide Interaction with HSA and Their Influence on HSA Antioxidant Potential. Pharmaceuticals. 2022; 15(5):499. https://doi.org/10.3390/ph15050499
Chicago/Turabian StyleRogóż, Wojciech, Jadwiga Pożycka, Aleksandra Owczarzy, Karolina Kulig, and Małgorzata Maciążek-Jurczyk. 2022. "Comparison of Losartan and Furosemide Interaction with HSA and Their Influence on HSA Antioxidant Potential" Pharmaceuticals 15, no. 5: 499. https://doi.org/10.3390/ph15050499
APA StyleRogóż, W., Pożycka, J., Owczarzy, A., Kulig, K., & Maciążek-Jurczyk, M. (2022). Comparison of Losartan and Furosemide Interaction with HSA and Their Influence on HSA Antioxidant Potential. Pharmaceuticals, 15(5), 499. https://doi.org/10.3390/ph15050499






