Abstract
A series of novel benzenesulfonamide derivatives were synthesized bearing para-N β,γ-amino acid or para-N β-amino acid and thiazole moieties and their binding to the human carbonic anhydrase (CA) isozymes determined. These enzymes are involved in various illnesses, such as glaucoma, altitude sickness, epilepsy, obesity, and even cancer. There are numerous compounds that are inhibitors of CA and used as pharmaceuticals. However, most of them bind to most CA isozymes with little selectivity. The design of high affinity and selectivity towards one CA isozyme remains a significant challenge. The beta and gamma amino acid-substituted compound affinities were determined by the fluorescent thermal shift assay and isothermal titration calorimetry for all 12 catalytically active human carbonic anhydrase isozymes, showing the full affinity and selectivity profile. The structures of several compounds were determined by X-ray crystallography, and the binding mode in the active site of CA enzyme was shown.
1. Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are zinc-containing metalloenzymes that catalyze the reversible hydration of CO2 to bicarbonate and maintain the acid–base balance in tissues and blood and, thus, play a crucial physiological role [1,2,3,4,5]. Human CAs belong to the α-family, which consists of 12 catalytically active (CAI, CAII, CAIII, CAIV, CAVA, CAVB, CAVI, CAVII, CAIX, CAXII, CAXIII, CAXIV) and three non-catalytic isozymes (CAVIII, CAX, and CAXI) that are also called carbonic anhydrase-related proteins (CARPs) [6,7]. Due to their different distribution in the body, the functions performed vary widely [8]. Therefore, disruption of normal function results in illnesses associated with only one or several CAs [9]. Thus, CAs are drug targets for a variety of disorders. There is still a significant challenge to design compounds possessing both high affinity and high selectivity for a specific isozyme, due to the structural similarity of the active sites of CAs [10,11,12]. Sulfonamides were discovered in 1940 as CA inhibitors by Mann and Keilin [13], who also reported some side effects of the newly discovered 4-aminobenzenesulfonamide. This was followed by a series of discoveries of a wide variety of compounds [14,15,16,17,18,19,20,21,22,23,24,25], some of which were approved as drugs. However, the desired properties and selectivity for all targeted CAs have not been achieved, and research in this field is ongoing.
The 5-oxopyrrolidine moiety is a part of a variety of compounds of natural or synthetic origin [26,27]. Recently, we have synthesized and examined the binding properties of 3-substituted 1-(4-sulfamoylphenyl)-5-oxopyrrolidine derivatives [28,29,30]. The different halo-substituents at the 4-sulfamoylphenyl moiety improved the binding properties of the compounds and allowed us to obtain several molecules that selectively bind to CAIX. In addition, we also investigated the binding properties of N-sulfamoylphenyl- and N-sulfamoylphenyl-N-thiazolyl-β-alanines and their derivatives [31], which showed better binding results as compared to compounds with a 5-oxopyrrolidine moiety as a linker. With the aim of further investigating the N-thiazolyl-β-alanine residue, we synthesized some analogous thiazole derivatives with the aliphatic substituents at C-4 and C-5 of the thiazole ring and studied their binding to CA. Moreover, to our knowledge, there were no CA inhibition studies of benzenesulfonamides with a β,γ-amino acid moiety as a linker. Therefore, we decided to explore this idea and incorporated the γ-aminobutyric acid moiety into the N-sulfamoylphenyl-β-alanine structure.
2. Results and Discussion
2.1. Organic Synthesis
Pyrrolidinones 1a,b, which were used in this work as precursors, were synthesized according to the methods described in previous works [29,30]. Compounds 4-((2-(hydrazinecarbonyl)-4-hydrazineyl-4-oxobutyl)amino)benzenesulfonamide (2a) and 3-chloro-4-((2-(hydrazinecarbonyl)-4-hydrazineyl-4-oxobutyl)amino)benzenesulfonamide (2b) were synthesized by nucleophilic ring-opening reactions of pyrrolidones 1a,b by heating them under reflux in an excess of hydrazine monohydrate [32] (Scheme 1). The structures of 2a,b and all of the other compounds have been confirmed by FT-IR, 1H and 13C NMR spectroscopy, and mass spectrometry or elemental analysis data. The triplet signals of NH group at 6.39 ppm (2a) and 6.05 ppm (2b) in the 1H NMR spectra of compounds 2a,b confirmed the existence of an open pyrrolidone ring structure. Subsequently, β,γ-amino acid derivatives 2a,b were used as the precursors for the synthesis of a series of hydrazone derivatives. Reactions between compounds 2a,b and ketones (acetone and acetophenone) at reflux temperature provided corresponding hydrazones 3a,b and 5a,b, whereas the ones with corresponding benzaldehydes yielded hydrazones 6a,b–18a,b (Scheme 1). 1H NMR spectra for compounds 3a,b and 5a,b display double sets of resonances for each of the CO-NH group protons, due to the restricted rotation around the amide bond. Similar duplication of CO-NH and N=CH group protons signals was also observed in 1H NMR spectra of compounds 6–18a,b. Since compounds 6–18a,b possess two isomerism centers, existing in each side chain, 10 isomers of these compounds can form. Therefore, CO-NH or N=CH group proton resonances were observed as the sets of multiplets. For instance, in the 1H NMR spectrum for 6a, multiplets at 7.94–8.23 ppm and 11.21–11.65 ppm were assigned to N=CH and CO-NH group protons, respectively. This splitting of the proton resonances indicates that in DMSO-d6 solution hydrazones 3a,b, 5a,b, and 6–18a,b exist as a mixture of Z/E isomers. However, in the attempt to synthesize analogous dihydrazones from compounds 2a,b with methyl ethyl ketone, dioxopyrrolidine derivatives 4a,b were obtained instead of target compounds 4c,d (Scheme 1). The structure of compound 4a was determined by X-ray structural analysis data (Figure 1), thereby confirming the empirical formula—C15H20N4O4S. In addition, in the 1H NMR spectrum of 4a, the multiplet at 2.58–3.14 ppm was assigned to pyrrolidine CH2 group protons, while the multiplet at 3.25–3.59 ppm to CH and NCH2 group protons.
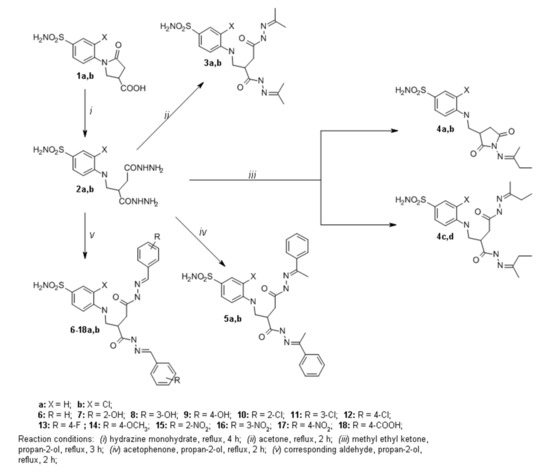
Scheme 1.
Synthesis of compounds 2–18a,b.
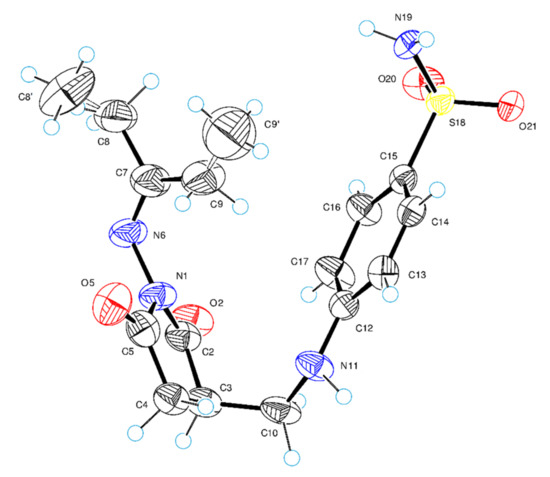
Figure 1.
ORTEP (Oak Ridge Thermal-Ellipsoid Plot Program) molecular structure of compound 4a showing the atom-numbering scheme. The thermal displacement ellipsoids are drawn at the 50% probability level and hydrogen atoms are shown as small spheres of arbitrary radii.
The multiplet at 6.45–6.58 ppm has been ascribed to protons of NH group. Furthermore, the multiplet at 0.82–1.24 ppm and a singlet at 1.70 ppm integrated for three protons of methyl groups, respectively. Similar spectral data were observed in compound 4b 1H and 13C NMR spectra.
Hydrazides 2a,b were used for the synthesis of a series of azole derivatives (Scheme 2). Pyrrole or pyrazole derivatives can be synthesized by the condensation reaction of carboxylic acid hydrazides with aliphatic diketones [33,34]. Pyrroles 19a,b were obtained from hydrazides 2a,b by the reactions with hexane-2,5-dione in propan-2-ol at the reflux temperature of the reaction mixture. For instance, in the 1H NMR spectrum for 19b, singlets at 1.91, 1.94, and 1.98 ppm were assigned to the protons of CH3 groups, and each of the singlets at 5.61 and 5.63 ppm integrated for two protons of CH groups. These proton resonances have confirmed the presence of 2,5-dimethylpyrrole moiety. The singlets at 10.72 and 10.87 ppm have been ascribed to protons of NH groups. Similar signals are found in the compound 19a 1H NMR spectrum as well. Furthermore, there was an attempt to synthesize 3,5-dimethylpyrrazoles 20 and 20a using the same method. Unfortunately, only one of the desired products (compound 20) was obtained. In the 1H NMR spectrum for 20, four singlets integrated for 12 protons of CH3 groups at 2.18, 2.25, 2.35, and 2.43 ppm, and two singlets assigned to the protons in the CH groups at 6.16 and 6.24 ppm have proven the presence of 3,5-dimethylpyrazole moiety in the molecule. Moreover, the triplet at 6.52 ppm assigned to the proton of NH group proved an open 2-pyrrolidone ring structure of compound 20. To obtain compound 20 non-chlorinated analog, previously synthesized pyrazole 21 [30] was isolated from the reaction mixture. The 1H and 13C NMR spectra data for 21 were very similar, as was described in previous work [30], which confirmed a closed 2-pyrrolidone ring structure in 21. We have proposed a hypothesis that, due to the acidic properties of pentane-2,4-dione and high temperature, the 2-pyrrolidone ring closed during the reaction. These conditions were suitable for compound 20 synthesis. We proposed that some steric hindrance, which occurs due to the chlorine substitute at C-2 of the phenyl ring, prevents the closure of the 2-pyrrolidone ring. In order to synthesize the desired product 20a, we have introduced several changes in reaction conditions. When the reaction was carried out at room temperature with NaOAc (to reduce the acidity of pentane-2,4-dione), we failed to detect new products by TLC after 24 h. Then we raised the reaction temperature to 50 °C, and after that we observed compound 21 by TLC.
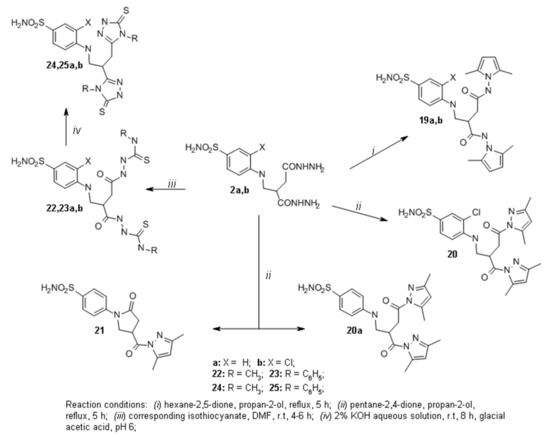
Scheme 2.
Synthesis of compounds 19–25.
Reactions of hydrazides 2a,b with methyl isothiocyanate or its phenyl analog in DMF led to the formation of carbothioamides 22a,b and carbothioamides 23a,b, respectively (Scheme 2). Further condensation reactions of 22a,b and 23a,b in the alkaline medium resulted in the formation of methyl triazolethiones 24a,b and phenyl triazolethiones 25a,b correspondingly. In the 1H NMR spectra for 24a and 24b, singlets at 3.33, 3.39 ppm (24a) and 3.36, 3.40 ppm (24b) have been assigned to the protons of CH3 groups. In the 13C NMR spectrum for 24a, two signals at 166.49 and 166.74 ppm have been attributed to C=S carbon in triazolethione ring. Similar carbon resonances were observed in compounds 24b and 25a,b 13C NMR spectra.
In the next stage of this work, thioureido acid 26 was synthesized by the procedure [31] and used for the synthesis of new functionalized aminothiazoles. Compound 26 was treated with an excess of chloroacetone in water, and sodium acetate was used as a base to obtain thiazole 27 (Scheme 3). In the 1H NMR spectra for 27, singlets at 2.19 and 6.44 ppm have been assigned to the protons of CH3 and CH group, respectively. These proton resonances have confirmed the presence of 4-methylthiazol moiety. Later, ester 28 was synthesized by an esterification reaction of thiazole 27 with an excess of methanol at reflux in the presence of sulfuric acid as a catalyst. Hydrazine hydrate reaction with ester 28 in propan-2-ol at reflux temperature yielded hydrazide 29, which was treated with benzaldehyde in propan-2-ol at reflux to afford hydrazone 30. The 1H NMR spectra 30 display double sets of resonances for the CO-NH and N=CH group protons due to the restricted rotation around the amide bond. The splitting of the proton resonances is also observed for carbon chain protons—two triplets at 2.58 and 3.00 ppm were assigned to the protons of CH2 group, while the multiplet at 4.06–4.36 ppm region to protons of NCH2 group. This proton splitting indicates that in DMSO-d6 solution hydrazone 30 exists as a mixture of Z/E isomers, and, in most cases, the Z isomer predominates [33].
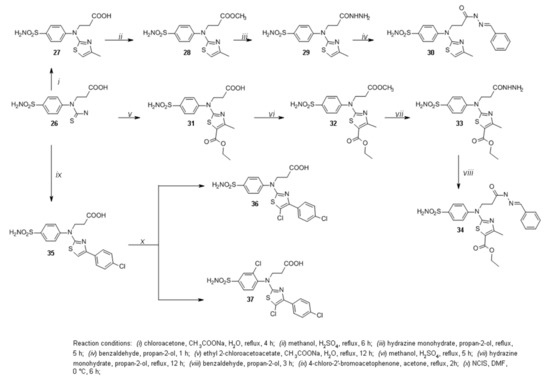
Scheme 3.
Synthesis of compounds 26–37.
The reaction of carboxylic acid 26 with ethyl 2-chloroacetoacetate in water at reflux gave thiazole 31. Sodium acetate was used as a base (Scheme 3). In the 1H NMR spectra for 31, a triplet at 2.19 and a singlet at 2.52 ppm have been assigned to the protons of CH3 groups. In the 13C NMR spectrum for 31, two signals at 14.23 and 17.31 ppm have been attributed to CH3 group carbons and the signal at 60.22 to the carbon of ester CH2 group. These proton resonances have confirmed the formation of desired product 31. Furthermore, thiazole 31 was treated with an excess of methanol in the presence of sulfuric acid as a catalyst to afford ester 32, which was then used in the reaction with hydrazine hydrate to obtain hydrazide 33. The reaction of sulfonamide 33 with benzaldehyde in propan-2-ol at reflux yielded hydrazone 34. Double sets of resonances are also observed in the 1H NMR spectra for 34 similar to those in the spectra for 30. Therefore, this indicates that hydrazone 34 also exists as a mixture of Z/E isomers in DMSO-d6 solution.
Previously synthesized compound 35 [31] was obtained by stirring thioureido acid 26 with 4-chloro-2′-bromoacetophenone in acetone at reflux temperature. Chlorine was introduced into the phenyl ring at the C-3 position of the benzenesulfonamide moiety to determine the halogenation effect on the binding affinity toward carbonic anhydrases. Thiazole 35 was treated with N-chlorosuccinimide in DMF at 0 °C. In the 1H NMR spectra for 36, the doublets at 7.54 and 7.72 ppm were attributed to the protons of the aromatic ring of 4-sulfamoylphenyl moiety. Moreover, there was no signal observed at the 7.38 ppm region as was described in previous work [31], which was assigned to the proton of CH group in the thiazole ring. This data confirmed that electrophilic substitution only occurred in the thiazole ring, which led to the formation of 5-chlorothiazole 36 but not the desired product 37. To synthesize compound 37, some changes were made to the reaction conditions. Performing the reaction at room temperature in DMF with NClS and monitoring the course of the reaction by TLC, after 1 h the formation of compound 36 was observed. It was then decided to raise the temperature to 80 °C [35], and the reaction was reperformed, but after the 20 h the only compound observed by TLC was thiazole 36. Furthermore, chlorination of compound 35 was attempted in 6 M aqueous HCl solution with hydrogen peroxide overnight [29], although the only product isolated from the reaction mixture was 5-chlorothiazole 36.
2.2. Crystal Structure of Compound 4a
The structure of 4a was examined in more detail. Figure 1 shows a perspective view of molecule 4a with thermal ellipsoids and the atom-numbering scheme followed in the text. The newly obtained 2,5-dioxopyrrolidine ring is planar and forms with the CH2 (C10) group a dihedral angle of 104.6(7). In the molecular structure, the 2,5-dioxopyrrolidine is almost perpendicular to both the hydrazone fragment (dihedral angle is 115.0(8)) and the benzenesulfonamide system (dihedral angle is equal to 113.3(8)).
2.3. Compound Binding to Carbonic Anhydrases (CA)
The dissociation constants of all synthesized compounds were measured for five CA isozymes, CAI, CAII, CAIX, CAXII, and CAXIII (Table 1), by the fluorescent thermal shift assay (FTSA, also referred to as TSA and DSF—differential scanning fluorimetry [36,37,38,39]) (Figure 2).

Table 1.
The observed dissociation constants of CAI, CAII, CAIX, CAXII, and CAXIII with tested compounds at pH 7.0 and 37 °C by the fluorescent thermal shift assay. ND–Kd_obs value was not determined. Kd_obs data of compounds 21 [30], 26 [31], and 35 [31] were taken from previous publications, as well as compound laboratory names marked with *. The standard deviation of the FTSA measurements is ±1.6-fold in Kd_obs.
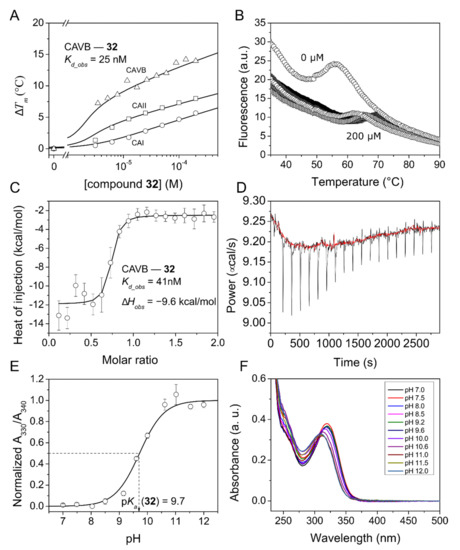
Figure 2.
Fluorescent thermal shift assay (FTSA) data of compound 32 binding to CAI, CAII, and CAVB (panels (A,B), raw data only of CAVB) and isothermal titration calorimetry (ITC) data of compound 32 binding to CAVB (panels (C,D)). Dissociation constant, Kd_obs, values at 37 °C and pH 7.0 in 50 mM sodium phosphate buffer containing 100 mM NaCl are shown near the corresponding graphs in panels (A,C). To calculate the intrinsic dissociation constants, the pKa values of compound sulfonamide group were measured spectrophotometrically at 37 °C (panels (E,F)). The normalized absorbances at selected wavelengths were plotted as a function of pH and fitted to the Henderson–Hasselbalch equation.
The binding affinities of 19 compounds for all 12 catalytically active human CA isozymes were measured to test the selectivity (Table 2 and Figure S1). For clarity we divided our compounds in two groups according to chemical structure: para-N β,γ-amino acid (2a,b, 3a,b, 5a,b–25a,b) and para-N β-amino acid and thiazol (compounds 27–36) bearing benzenesulfonamide derivatives. The binding properties of both groups will be described separately. The affinity of the compounds for five CA isozymes is shown in Figure 3.

Table 2.
The observed dissociation constants of all 12 catalytically active CAs with tested compounds at pH 7.0 and 37 °C by the fluorescent thermal shift assay. The value ≥ 200,000 is the upper limit of Kd_obs detection; ND–Kd_obs value was not determined. The standard deviation of the FTSA measurements is ± 1.6-fold in Kd_obs. The ITC data are shown in the brackets (pH 7.0, 37 °C).
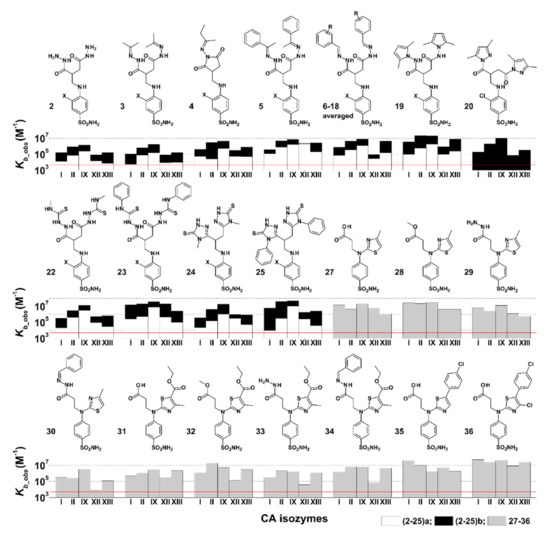
Figure 3.
The observed binding constants (Kb_obs) of compounds 2(a,b)–19(a,b), 20, 22(a,b)–25(a,b), and 27–36 interaction with CAI, CAII, CAIX, CAXII, and CAXIII. Compound chemical structures and numbers are provided above the bar charts showing the Kb_obs. For compounds 2–25, X denotes –H for a compounds (white bars) and –Cl for b compounds (black bars, always higher than the white ones). The red line shows the Kb_obs value of 5000 M−1, the lowest detectable Kb_obs by the FTSA experiment.
For some compounds, the pKa values for the sulfonamide group were measured and used to calculate the intrinsic, pH-independent dissociation constants or changes in Gibbs energy [40] (Figure 4).
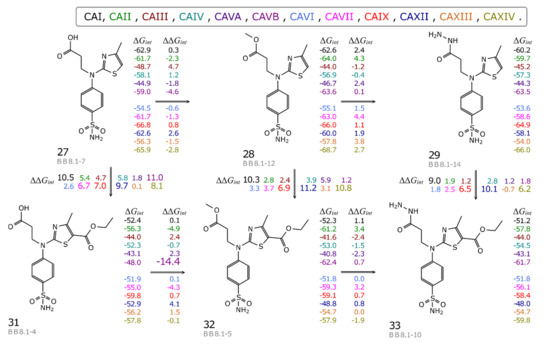
Figure 4.
The map of correlation between compound chemical structures and the changes of the standard intrinsic Gibbs energies of binding (kJ/mol). The energies for compound binding to each CA isozyme (colored according to the list above the map) are listed on the right of each compound, while the differences between the values of neighboring compounds are listed above and below the connecting arrows.
In addition, the binding strength of several compounds was confirmed by isothermal titration calorimetry [41]. The binding data of compounds 28, 31, and 36 to CA II and compound 32 binding to CA VB were in good agreement; the Kd_obs values measured by both methods differed by less than three-fold. Structural characterization of three compounds’ (28, 31, and 36) binding was performed by determining their X-ray crystal structures in complexes with CAII (Figure 5).
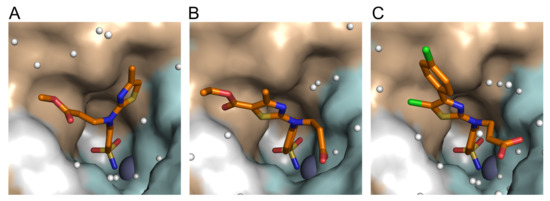
Figure 5.
The X-ray crystallographic structures of (A) compound 28 (BB8.1-12) (PDB ID: 7QGZ); (B) compound 31 (BB8.1-4) (PDB ID: 7QGY); and (C) compound 36 (BB8.3-3) (PDB ID: 7QGX) in the active site of CAII. The zinc ion is shown as a grey sphere and water molecules as small white spheres. The protein surface of CAII is colored brown for hydrophobic residues (A, G, V, I, L, F) and cyan for the residues with polar side chains (R, K, H, E, D, N, Q, T, S, C). The refinement statistics are provided in the Supplementary Materials.
2.3.1. para-N β,γ-Amino Acid-Substituted Benzenesulfonamide Derivatives (2–25a,b) Binding to CA
Tested compounds 2–20a,b and 22–25a,b can be grouped into pairs differing by a chlorine atom at meta- position on the benzene ring: compounds b—chlorinated and compounds a—non-chlorinated. Chlorinated compounds exhibited significantly greater observed affinity towards most CA isozymes (Figure 3 or Table 1), with on average a 40-fold increase in affinity for CAI and CAXIII, 15–20-fold for CAII, CAVII, and CAXIV, and marginally increased affinity toward CAIII, CAIV, CAIX, and CAXII.
Compounds 2a and 2b, which are precursors to compounds 3a,b–25a,b, exhibited weak interaction with CAI, CAII, CAIX, CAXII, and CAXIII (Kd_obs 0.83–130 μM). Compound 2b had a moderately strong interaction with CAIX (Kd_obs = 0.83 μM). The size and hydrophobicity of substituents increases in the direction 3a→6a and 3b→6b, resulting in the increment of affinity toward CAI, CAII, CAIX, CAXII, and CAXIII. However, observed affinity for CAXII of compounds 5a→6a decreased 50 times. Ligand flexibility could also play a role; ligands 5a and 5b having an extra rotatable bond might allow better interaction of the benzene ring with hydrophobic amino acids of CAXII, compared to 6a and 6b. Compound 6b was also tested with all other catalytically active CA isozymes (Table 2); it exhibited weak interaction with CAIII, CAIV, CAVA, and CAXII (Kd_obs ≥ 200,000 nM, 4200 nM, 20,000 nM, and 7900 nM, respectively), moderately strong interaction with CAI and CAVI, and strong interaction with CAII, CAVB, CAVII, CAIX, CAXIII, and CAXIV (Kd_obs in the range of 100–290 nM). Although compounds 4a and 4b contain closed dioxopyrrolidine ring structure (Figure 3), the affinities for CAI, CAII, CAIX, CAXII, and CAXIII did not differ from the other compounds described in this section. No remarkable selectivity toward any CA isozyme was observed for compounds 2a,b, 3a,b, 5a,b, 6a,b, nor even 4a,b, but usually there was an increase in observed affinity (up to 10–100 times) for CAIX compared to other tested CAs.
Ligands 7–18a and 7–18b are structurally similar to 6a and 6b, only differing by symmetric substituents on the phenyl ring. Small substituents such as -OH, -Cl, -F, -OCH3, -COOH, and -NO2 as well as their positions in the phenyl ring did not significantly affect the binding affinity towards CAs. Compounds 7–18a exhibited low affinity for tested CA isozymes, with no significant selectivity for a particular CA. The Kd ranges for CAI were 5.0–36 μM, CAII—1.3–5.0 μM, CAIX—0.5–3.3 μM, CAXII—20–100 μM, and for CAXIII—1.7–23 μM. Chlorinated compounds 7–18b proved to be of more interest, with a generally increased observed affinity toward CAII, CAIX, and CAXIII compared to analogous non-chlorinated compounds 7–18a (Figure 3). Compounds 7–9b, having -OH substituent in, respectively, ortho-, meta-, and para-positions on the benzene ring, interacted similarly with CAI, II, IX, XII, and XIII as compound 6b. Compound 14b, having OCH3 substitute in para-position on the benzene ring, exhibited the strongest interaction with CAII and CAXIII (Kd_obs 59 nM and 66 nM, respectively), although binding was not selective, due to moderately strong interaction with CAI and CAIX (Kd_obs 510 nM and 290 nM, respectively).
Thiourea derivatives 22–25a,b (Figure 3) exhibited similar binding patterns to CAI, CAII, CAIX, CAXII, and CAXIII, showing increases in observed binding affinity going from CAI to CAIX. Compounds 22a–25a had a trend of decreasing affinity toward CA isozymes going from CAIX to CAXIII. A similar trend was not observed for their chlorinated analogs 22–25b, which showed a stronger affinity for CAIX but did not have significant differences in binding strength for CAXII and CAXIII (Table 1). Compounds 22a,b, 23b, and 25b were also tested with all catalytically active CA isozymes, and no selectivity was observed. Compound 22a exhibited weak binding to all tested CA isozymes (micromolar Kd_obs values). This is consistent with a trend of non-chlorinated compounds having weaker observed binding affinity to CAs than chlorinated analogs. On the other hand, 22a interacted with a stronger affinity with CAIX (Kd_obs 330 nM). Compound 22b bound CA isozymes more than three times stronger than 22a and showed the highest affinities for CAIX and CAXIV (Kd_obs values of 100 nM and 130 nM, respectively). The Kd_obs decreased in the direction 22b→23b→25b for most CAs, especially for CAII (400 nM→50 nM→25 nM), CAIX (100 nM→33 nM→25 nM), and CAXIV (from 130 nM→50 nM→25 nM). Compound 25b also had an unusually strong interaction with CAIII (Kd_obs 5000 nM, Table 2), despite having large substituents, which usually contribute to steric hindrance and weak binding to CAIII.
2.3.2. para-N β-Amino Acid and Thiazol Bearing Benzenesulfonamide Derivatives (27–36) Binding to CA
Compounds 27–6 had greater asymmetry than compounds 2–25a,b (Figure 3). Compounds 27–34 and 36 were tested with all catalytically active CA isozymes. In general, compounds from this group exhibited weak interaction with CAIII and CAVA (Table 2), with the Kd_obs values ranging from 17 µM to ≥200 µM (200 µM being the weakest detectable Kd_obs value). Compounds 27–30 differed by substitutes on β-aminoacid carbonyl group, increasing in size (–OH, –OCH3, –NHNH2, –NHN=CPh). Carboxylic acid 27 strongly bound to CAI and CAIX (Kd_obs 67 nM and 48 nM, respectively) but also had moderately strong interactions with CAII, CAVII, CAXII, and CAXIV (Kd_obs in the ranges 170–250 nM). Esterification of thiazole in 27 to compound 28 increased affinity toward CAII, CAVA, CAVB, and CAXIV (Kd_obs values of 45 nM, 50 µM, 46 nM, 29 nM of compound 28, respectively). Of special interest was an increase in affinity toward CAVB in the direction 27→28, with Kd_obs value decreasing from 560 nM to 46 nM (Table 2). The hydrazide 29 interacted with all CAs slightly weaker than 28. Hydrazone 30, having by far the largest, hydrophobic, and inflexible –NHN=CPh group, showed a significantly decreased binding strength to all tested CA isozymes (Kds in the micromolar range). Compounds 31–34 were analogous to compounds 27–30 but had an additional ester substitute on the thiazole ring, which made compounds larger and less flexible than their counterparts (Figure 3). Ligands 31–33 showed similar or significantly weaker interactions with most CA isozymes than their analogous compounds without ethylester group on thiazole ring, 27–29. However, of all tested compounds, the strongest interaction was measured between 32 and CA VB (Kd = 30 nM). Binding affinities of compound 34 towards CAII, CAVB, and CAIX were in the range of 100 nM. Compounds 35 and 36 showed the strongest affinities to CAI (Kd_obs 25 nM (Table 1) and 20 nM (Table 2), respectively). Furthermore, compound 36 interacted strongly with CAII, CAVII, CAIX, and CAXIII (Kd_obs in the range of 23–40 nM), showing no selectivity toward CAs.
2.3.3. Intrinsic Thermodynamics of Compounds 27–36 Binding to CAs
We calculated the intrinsic dissociation constants (Kd_int, Table 3) and intrinsic Gibbs energy (ΔGint, Table S1) values of binding between CA isozymes and compounds 27–29, 31–33, and 36 and constructed a compound structure–affinity correlation map (Figure 4) which demonstrates how small changes in functional groups influence compound capability to bind a particular CA isozyme. The intrinsic binding parameters represent the actual binding reaction between the deprotonated sulfonamide and the Zn-bound water form of CA. To calculate the intrinsic binding parameters by Equations (4) and (5), the pKas of tested sulfonamides were measured spectrophotometrically by measuring absorption at different pH values. The average pKa values of compounds 27–29, 31, 32, 35, and 36 were determined to be 10.1. Calculation of the intrinsic values has a significant advantage over the observed values, because the intrinsic values eliminate the pH and buffer effects that may be misleading in the structural origins of the altered affinity [5,42,43].

Table 3.
The intrinsic dissociation constants (Kd_int) for compounds binding to CAs at 37 °C. ND—not determined. *—the pKa value was not determined and is an average of all measured compounds. Experimental data and statistics of pKa values are provided in Supplementary Materials.
All compounds exhibited relatively weak binding to CAIII (Kd_int at limit of detection for compounds 28–34 and Kd_int values of 6 and 2.6 nM, respectively, for compounds 27 and 36) and CAVA (Kd_int at limit of detection for all tested compounds except compound 28, which binds with Kd_int of 13 nM (Table 3)). Figure 4 depicts some compounds in which minor structural changes had a significant effect on affinity for carbonic anhydrases. Esterification of carboxyl group (directions 27→28 and 31→32) (Figure 4) was favorable or had negligible effect on the Gibbs energy for all CAs except for CAXII, which showed a slight decrease in affinity. Addition of methoxy group leads to a large gain in ΔGint for CAVB (ΔΔGint (27→28) = -4.6 and ΔΔGint (31→32) = −14.4 kJ/mol). Further modification of the methoxy with hydrazide group (directions 28→29 and 32→33) decreased the Gibbs energy of binding to all CAs but did not change that to CAVB (ΔΔGint (28→29) = 0.1 and ΔΔGint (32→33) = 0.7 kJ/mol). Interestingly, addition of ethoxycarbonyl group on the thiazole ring (directions 27→31, 28→32, and 29→33) resulted in the decrease of ΔGint or had negligible effect for all CAs, with the largest reduction of affinity for CAXII (ΔΔGint (28→32) = 11.2 kJ/mol). In summary, the intrinsic binding data showed that esterification increased the binding affinity for the majority of CA isozymes (Table 3). Thus, it would be very interesting to investigate the influence of small and hydrophobic substitutes on the carboxyl group with the perspectives of finding possible new lead compounds for use as inhibitors for CAVB while binding weakly to CAVA isozyme and possibly having increased selectivity for CAVB isozyme.
2.3.4. X-ray Crystallography of Human CAII Complexes with Inhibitors
The crystal structures of compounds 28, 31, and 36 in the complex with CAII were determined by X-ray crystallography (Figure 5). The electron density maps of the ligands bound in the active site of CAII are shown in Figure S2 in Supplementary Materials. The data collection and refinement statistics are presented in Table S2. In all three structures, the benzenesulfonamide ring occupies a similar position (Figure 5) due to the interaction with Leu198; only the positions of the substituents differed. The carboxy group of compounds 31 and 36 faces the hydrophilic side (colored cyan in Figure 5) of the active site of CAII and forms hydrogen bonds with Asn62, Asn67, or water molecules, while the methyl ester in compound 28 adheres to a more hydrophobic surface (colored brown in Figure 5). The 2D schemes of the interactions are shown as LigPlot schemes based on X-ray crystallography in Figure S3. The thiazole-based substituent interacts with Phe131 and was found on either side of the Phe131 side chain depending on the other substituents (Figure 5). Thus, the smallest thiazole substituent of compound 28 occupied the “right side” of the hydrophobic pocket, while the larger thiazole-based substituents of compounds 31 and 36 occupied the more spacious “left side” of the pocket, because they would not fit in the pocket on the “right side” without changing the position of the benzenesulfonamide due to steric hindrance.
3. Materials and Methods
3.1. Organic Synthesis
Reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). The melting points were determined on a MEL-TEMP (Electrothermal, Bibby Scientific Company, Burlington, NJ, USA) melting point apparatus and were uncorrected. The IR spectra (ν, cm−1) were recorded on a Perkin–Elmer Spectrum BX FT–IR spectrometer using KBr pellets. The 1H and 13C NMR spectra were recorded in DMSO-d6 medium on Brucker Avance III (400, 101 MHz) spectrometer (see Supplementary Materials, Figures S4–S103). The chemical shifts (δ) were reported in parts per million (ppm), calibrated from TMS (0 ppm) as an internal standard for 1H NMR and DMSO-d6 (39.43 ppm) for 13C NMR. Mass spectra were obtained on a Bruker maXis UHR-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) with ESI ionization. Elemental analysis was performed on a CE-440 elemental analyzer (Exeter Analytical Inc., North Chelmsford, MA, USA). The reaction course and purity of the synthesized compounds were monitored by TLC using aluminium plates pre-coated with the silica gel 60 F254 (MerckKGaA, Darmstadt, Germany). Single crystals of 4a were investigated on a Rigaku, XtaLAB Synergy, Dualflex, HyPix diffractometer. The crystal was kept at 170.0(1) K during data collection. Using Olex2 [44], the structure was solved with the SIR2014 [45] structure solution program using Direct Methods and refined with the olex2.refine [46] refinement package using Gauss–Newton minimization.
3.1.1. General Procedure for the Synthesis of Hydrazides 2a,b
A mixture of carboxylic acid 1a (3.70 g, 13 mmol) or 1b (4.14 g, 13 mmol) and hydrazine monohydrate (6.3 mL, 130 mmol) was heated at reflux for 4 h. The reaction mixture was cooled down, diluted with propan-2-ol, and heated until reflux. Then, the suspension was left to cool down, and the precipitate was filtered off and recrystallized from propan-2-ol and water mixture.
4-((2-(Hydrazinecarbonyl)-4-hydrazineyl-4-oxobutyl)amino)benzenesulfonamide (2a)
White solid, yield 3.58 g (84%); m.p. 164–165 °C; IR (KBr) (v, cm−1): 3399, 3301, 3213, 1607, 1603; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.11–2.37 (m, 2H, CH2), 2.79–2.96 (m, 1H, CH), 2.99–3.33 (m, 2H, NCH2), 4.32 (br. s, 4H, 2NH2), 6.39 (t, 1H, J = 6.0 Hz, NH), 6.60–7.54 (m, 6H, HAr, SO2NH2), 9.01, 9.11 (2s, 2H, 2NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 33.66, 44.40, 110.86, 127.29, 130.16, 151.13, 169.84, 172.00; HRMS (ESI) for C11H18N6O4S + H+, calcd. 330.1110, found 330.1115 [M + H+].
3-Chloro-4-((2-(hydrazinecarbonyl)-4-hydrazineyl-4-oxobutyl)amino)benzenesulfonamide (2b)
White solid, yield 3.81 g (80%); m.p. 183–184 °C; IR (KBr) (v, cm−1): 3312, 3213, 1635, 1616; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.16–2.41 (m, 2H, CH2), 2.90–3.07 (m, 1H, CH), 3.12–3.45 (m, 2H, NCH2), 4.29 (br. s, 4H, 2NH2), 6.05 (t, 1H, J = 5.7 Hz, NH), 6.81–7.72 (m, 5H, HAr, SO2NH2), 9.03, 9.21 (2s, 2H, 2NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 33.46, 44.62, 110.26, 116.83, 126.13, 126.66, 131.25, 146.29, 169.79, 171.89; HRMS (ESI) for C11H11ClN2O5S + H+, calcd. 319.0155, found 319.0150 [M + H+].
3.1.2. General Procedure for the Synthesis of Hydrazones 3a,b
Hydrazide 2a (0.33 g, 1 mmol) or 2b (0.36 g, 1 mmol) was dissolved in acetone (15 mL), and the solution was heated at reflux for 2 h. The reaction mixture was cooled down, and the precipitate was filtered off.
4-((4-Oxo-2-(2-(propan-2-ylidene)hydrazine-1-carbonyl)-4-(2-(propan-2-ylidene)hydrazineyl)butyl)amino)benzenesulfonamide (3a)
White solid, yield 0.28 g (68%); m.p. 79–80 °C; IR (KBr) (v, cm−1): 3391, 3371, 3250, 1687, 1660; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.63–2.10 (m, 12H, 4CH3), 2.52–3.54 (m, 5H, CH2CO, CH, NCH2), 6.38–7.58 (m, 7H, Har, NH, NH2), 9.84–10.19 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 17.02, 17.12, 17.48, 17.60, 24.94, 25.11, 31.94, 33.08, 35.91, 43.51, 44.69, 110.88, 127.26, 130.10, 149.73, 150.31, 150.93, 151.21, 154.96, 156.07, 169.35, 173.19, 174.53; HRMS (ESI) for C17H26N6O4S + H+, calcd. 411.1814, found 411.1809 [M + H+].
3-Chloro-4-((4-oxo-2-(2-(propan-2-ylidene)hydrazine-1-carbonyl)-4-(2-(propan-2-ylidene)hydrazineyl)butyl)amino)benzenesulfonamide (3b)
White solid, yield 0.31 g (70%); m.p. 133–134 °C; IR (KBr) (v, cm−1): 3361, 3227, 1652, 1594; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.67–2.06 (m, 12H, 4CH3), 2.54–3.53 (m, 5H, CH2CO, CH, NCH2), 5.94–6.43 (m, 1H, NH,), 6.84–7.74 (m, 5H, Har, NH2), 9.83–10.25 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 17.04, 17.16, 17.50, 17.61, 24.93, 25.14, 35.26, 36.10, 43.99, 44.80, 110.34, 116.59, 126.10, 126.10, 131.25, 146.21, 149.88, 149.88, 151.10, 163.12, 173.04, 174.54; HRMS (ESI) for C17H25ClN6O4S + H+, calcd. 445.1425, found 445.1419 [M + H+].
3.1.3. General Procedure for the Synthesis of Hydrazones 4a,b
One mmol of hydrazide 2a (0.33 g) or 2b (0.36 g) was dissolved in propan-2-ol (15 mL), and methyl ethyl ketone (0.36 mL, 4 mmol) was added dropwise to the solution. The reaction mixture was heated at reflux for 3 h; then it was cooled down, and the precipitate was filtered off and washed with diethyl ether.
4-(((1-(Butan-2-ylideneamino)-2,5-dioxopyrrolidin-3-yl)methyl)amino)benzenesulfonamide (4a)
White solid, yield 0.25 g (71%); m.p. 117–118 °C; IR (KBr) (v, cm−1): 3332, 3246, 1693, 1597; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 0.82–1.24 (m, 3H, CH3), 1.70 (s, 3H, CH2CH3), 2.01–2.45 (m, 2H, CH2CH3), 2.58–3.14 (m, 2H, CH2pyr), 3.25–3.59 (m, 3H, CH, NCH2), 6.45–6.58 (m, 1H, NH), 6.68 (dd, 2H, J = 8.6, 5.7 Hz, Har), 6.93 (s, 2H, NH2), 7.52 (dd, 2H, J = 8.9, 2.5 Hz, Har); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 10.30, 18.48, 30.81, 31.41, 31.44, 37.79, 42.55, 42.71, 111.04, 111.09, 127.37, 127.40, 130.72, 151.01, 151.06, 171.70, 173.56, 174.33, 176.03, 182.62; HRMS (ESI) for C15H20N4O4S + H+, calcd. 353.1284, found 353.1289 [M + H+].
4-(((1-(Butan-2-ylideneamino)-2,5-dioxopyrrolidin-3-yl)methyl)amino)-3-chlorobenzenesulfonamide (4b)
Brown solid, yield 0.29 g (74%); m.p. 92–93 °C; IR (KBr) (v, cm−1): 3323, 3247, 1694, 1592; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 0.84–1.16 (m, 3H, CH3), 1.69 (s, 3H, CH2CH3), 2.05–2.47 (m, 2H, CH2CH3), 2.53–2.93 (m, 2H, CH2pyr), 3.11–3.79 (m, 3H, CH, NCH2), 6.32 (br. s, 1H, NH), 6.80–7.0 (m, 1H, Har), 7.13 (s, 2H, NH2), 7.48–7.71 (m, 2H, Har); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 10.29, 18.41, 21.90, 24.80, 25.50, 30.77, 31.40, 37.18, 38.14, 43.02, 43.29, 110.24, 110.39, 117.07, 126.14, 126.21, 126.88, 131.63, 146.12, 171.67, 173.62, 174.32, 176.12, 182.71; HRMS (ESI) for C15H19ClN4O4S + H+, calcd. 387.0894, found 387.0890 [M + H+].
3.1.4. General Procedure for the Synthesis of Hydrazones 5a,b
One mmol of hydrazide 2a (0.33 g) or 2b (0.36 g) was dissolved in propan-2-ol (15 mL), and acetophenone (0.35 mL, 3 mmol) was added dropwise to the solution. The reaction mixture was heated at reflux for 2 h; then it was cooled down, and the precipitate was filtered off and washed with diethyl ether and n-hexane.
4-((4-Oxo-2-(2-(1-phenylethylidene)hydrazine-1-carbonyl)-4-(2-(1-phenylethylidene)hydrazineyl)butyl)amino)benzenesulfonamide (5a)
White solid, yield 0.38 g (72%); m.p. 162–163 °C; IR (KBr) (v, cm−1): 3378, 3244, 1652, 1600; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.93–2.38 (m, 6H, 2CH3), 2.67–3.61 (m, 5H, CH2CO, CH, NCH2), 6.42–8.09 (m, 17H, Har, NH, NH2), 10.18–10.90 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 13.57, 13.92, 14.25, 14.76, 26.75, 30.82, 37.81, 42.75, 43.79, 44.72, 110.78, 110.95, 111.07, 118.89, 126.02, 126.13, 126.30, 126.48, 126.50, 127.37, 127.42, 128.17, 128.30, 128.39, 128.46, 128.71, 129.03, 129.79, 130.09, 130.74, 133.22, 138.23, 147.15, 147.82, 151.04, 151.10, 173.81, 174.36, 175.47, 176.06; HRMS (ESI) for C27H30N6O4S + H+, calcd. 535.2127, found 535.2123 [M + H+].
3-Chloro-4-((4-oxo-2-(2-(1-phenylethylidene)hydrazine-1-carbonyl)-4-(2-(1-phenylethylidene)hydrazineyl)butyl)amino)benzenesulfonamide (5b)
White solid, yield 0.43 g (75%); m.p. 158–159 °C; IR (KBr) (v, cm−1): 3345, 3248, 1700, 1592; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.10–2.32 (m, 6H, 2CH3), 2.54–3.24 (m, 5H, CH2CO, CH, NCH2), 6.23–6.42 (m, 1H, NH), 6.85–7.99 (m, 15H, Har, NH2), 10.26–10.83 (m, 2H, 2CONH); HRMS (ESI) for C27H29ClN6O4S + H+, calcd. 569.1738, found 569.1735 [M + H+].
3.1.5. General Procedure for the Synthesis of Hydrazones 6a,b–18a,b
The mixture of hydrazide 2a (0.17 g, 0.5 mmol) or 2b (0.18 g, 0.5 mmol) with corresponding aldehyde (1.5 mmol) and propan-2-ol (15 mL) was heated at reflux for 2 h. Then, the reaction mixture was cooled down, and precipitate was filtered off and recrystallized from 1,4-dioxane.
4-((2-(2-(Benzylidene)hydrazine-1-carbonyl)-4-(2-(benzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (6a)
White solid, yield 0.21 g (84%); m.p. 208–209 °C; IR (KBr) (v, cm−1): 3364, 3239, 1655, 1601; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.79–3.59 (m, 5H, CH2CO, CH, NCH2), 6.57–7.75 (m, 17H, Har, NH, NH2), 7.94–8.23 (m, 2H, NCH), 11.21–11.65 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.17, 32.68, 33.64, 36.07, 43.81, 44.70, 110.81, 110.90, 126.68, 126.71, 126.74, 126.92, 126.96, 127.33, 127.40, 128.78, 128.82, 129.69, 129.77, 130.23, 134.19, 134.26, 134.35, 142.68, 142.95, 143.23, 145.80, 146.26, 151.01, 151.12, 167.33, 169.60, 172.67, 172.96, 174.45, 174.62; HRMS (ESI) for C25H26N6O4S + H+, calcd. 507.1814, found 507.1809 [M + H+].
4-((2-(2-(Benzylidene)hydrazine-1-carbonyl)-4-(2-(benzylidene)hydrazineyl)-4-oxobutyl)amino)-3-chlorobenzenesulfonamide (6b)
White solid, yield 0.24 g (89%); m.p. 212–213 °C; IR (KBr) (v, cm−1): 3406, 3243, 1662, 1598; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.63–3.63 (m, 5H, CH2CO, CH, NCH2), 6.13–6.40 (m, 1H, NH), 6.92–7.74 (m, 15H, Har, NH2), 7.91–8.27 (m, 2H, NCH), 11.22–11.67 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.23, 33.65, 35.91, 44.27, 109.98, 116.89, 126.06, 126.07, 126.07, 126.67, 126.70, 126.98, 128.78, 129.69, 131.19, 134.14, 134.26, 142.72, 142.97, 143.43, 143.63, 146.22, 153.77, 172.88, 174.68; HRMS (ESI) for C25H25ClN6O4S + H+, calcd. 541.1425, found 541.1419 [M + H+].
4-((2-(2-(2-Hydroxybenzylidene)hydrazine-1-carbonyl)-4-(2-(2-hydroxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (7a)
White solid, yield 0.20 g (74%); m.p. 177–178 °C; IR (KBr) (v, cm−1): 3290, 3252, 3163, 1668, 1602; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.56–3.65 (m, 5H, CH2CO, CH, NCH2), 6.57–7.78 (m, 15H, Har, NH, NH2), 8.20–8.47 (m, 2H, NCH), 9.81–10.36 (m, 1H, OH), 11.06–11.40 (m, 2H, 2CONH), 11.61–12.01 (m, 1H, OH); HRMS (ESI) for C25H26N6O6S + H+, calcd. 539.1713, found 539.1709 [M + H+].
3-Chloro-4-((2-(2-(2-hydroxybenzylidene)hydrazine-1-carbonyl)-4-(2-(2-hydroxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (7b)
White solid, yield 0.22 g (76%); m.p. 204–205 °C; IR (KBr) (v, cm−1): 3294, 3247, 3190, 1667, 1593; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.52–3.75 (m, 5H, CH2CO, CH, NCH2), 6.17–6.47 (m, 1H, NH), 6.57–8.47(m, 15H, Har, NCH, NH2), 9.95–10.19 (m, 1H, OH), 11.04–11.43 (m, 2H, 2CONH), 11.66–11.94 (m, 1H, OH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 31.41, 33.82, 35.36, 44.03, 44.60, 116.12, 116.33, 116.54, 116.99, 118.20, 118.57, 118.60, 119.32, 119.62, 120.09, 120.39, 126.10, 126.80, 129.35, 130.85, 131.36, 131.45, 133.25, 141.01, 146.21, 146.64, 147.19, 156.35, 157.28, 157.32, 158.65, 162.81, 166.77, 167.18, 169.03, 169.37, 172.22, 172.49, 174.01, 174.34; HRMS (ESI) for C25H25ClN6O6S + H+, calcd. 573.1323, found 573.1322 [M + H+].
4-((2-(2-(3-Hydroxybenzylidene)hydrazine-1-carbonyl)-4-(2-(3-hydroxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (8a)
White solid, yield 0.22 g (81%); m.p. 192–193 °C; IR (KBr) (v, cm−1): 3444, 3373, 3283, 3172, 1657, 1597; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.57–3.60 (m, 5H, CH2CO, CH, NCH2), 6.58–7.59 (m, 15H, Har, NH, NH2), 7.85–8.15 (m, 2H, NCH), 9.62 (br. s,, 2H, OH), 11.16–11.62 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 30.98, 31.87, 32.66, 32.67, 34.33, 36.15, 43.71, 44.71, 47.87, 110.93, 112.56, 112.63, 117.00, 117.29, 118.13, 118.37, 118.68, 127.35, 127.47, 129.83, 130.27, 135.54, 135.62, 142.98, 143.20, 146.43, 151.05, 151.14, 157.64, 157.72, 166.92, 169.31, 169.63, 172.56, 172.89, 174.52; HRMS (ESI) for C25H26N6O6S + H+, calcd. 539.1713, found 539.1707 [M + H+].
3-Chloro-4-((2-(2-(3-hydroxybenzylidene)hydrazine-1-carbonyl)-4-(2-(3-hydroxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (8b)
White solid, yield 0.21 g (72%); m.p. 119–120 °C; IR (KBr) (v, cm−1): 3412, 3319, 3231, 3067, 1643, 1597; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.61–3.62 (m, 5H, CH2CO, CH, NCH2), 6.09–6.32 (m, 1H, NH), 6.68–7.67 (m, 13H, Har, NH2), 7.82–8.15 (m, 2H, NCH), 9.46–9.67 (m, 2H, OH), 11.12–11.63 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.00, 32.58, 35.36, 36.18, 44.32, 44.84, 110.01, 110.46, 112.53, 112.74, 113.57, 116.88, 117.02, 117.55, 118.12, 118.74, 126.12, 126.22, 126.79, 126.87, 129.85, 131.31, 131.37, 135.43, 135.46, 135.56, 143.06, 143.27, 143.93, 146.20, 146.59, 157.64, 157.67, 166.93, 167.22, 169.22, 169.55, 172.58, 172.81, 174.29, 174.63; HRMS (ESI) for C25H25ClN6O6S + H+, calcd. 573.1323, found 573.1317 [M + H+].
4-((2-(2-(4-Hydroxybenzylidene)hydrazine-1-carbonyl)-4-(2-(4-hydroxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (9a)
White solid, yield 0.25 g (93%); m.p. 186–187 °C; IR (KBr) (v, cm−1): 3442, 3356, 3288, 3190, 1657, 1602; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.60–3.28 (m, 5H, CH2CO, CH, NCH2), 6.51–7.61 (m, 15H, Har, NH, NH2), 7.83–8.12 (m, 2H, NCH), 9.90 (br. s, 2H, OH), 10.99–11.43 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.38, 33.13, 36.47, 37.27, 44.15, 111.31, 116.12, 116.22, 125.66, 125.70, 125.78, 127.79, 127.86, 128.83, 128.88, 129.14, 129.21, 130.61, 130.65, 143.42, 143.72, 144.12, 146.56, 146.87, 147.02, 147.28, 151.48, 151.59, 151.61, 159.50, 159.57, 159.62, 159.71, 159.76, 167.47, 169.75, 172.77, 173.12, 174.44, 174.66; HRMS (ESI) for C25H26N6O6S + H+, calcd. 539.1713, found 539.1708 [M + H+].
3-Chloro-4-((2-(2-(4-hydroxybenzylidene)hydrazine-1-carbonyl)-4-(2-(4-hydroxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (9b)
White solid, yield 0.24 g (83%); m.p. 226–227 °C; IR (KBr) (v, cm−1): 3434, 3327, 3236, 3079, 1633, 1593; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.65–3.62 (m, 5H, CH2CO, CH, NCH2), 6.11–6.41 (m, 1H, NH), 6.72–7.75 (m, 13H, Har, NH2), 7.80–8.14 (m, 2H, NCH), 9.74–9.99 (m, 2H, OH), 10.99–11.45 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.06, 32.57, 35.31, 36.01, 44.28, 44.86, 109.99, 110.24, 115.67, 115.73, 116.88, 125.17, 125.34, 126.08, 126.77, 126.88, 128.38, 128.47, 128.67, 128.75, 131.18, 143.02, 143.94, 146.25, 159.06, 159.12, 159.21, 159.31, 169.20, 172.33, 172.58, 174.27; HRMS (ESI) for C25H25ClN6O6S + H+, calcd. 573.1323, found 573.1319 [M + H+].
4-((2-(2-(2-Chlorobenzylidene)hydrazine-1-carbonyl)-4-(2-(2-chlorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (10a)
White solid, yield 0.25 g (86%); m.p. 174–175 °C; IR (KBr) (v, cm−1): 3452, 3328, 3081, 1694, 1598; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.57–3.61 (m, 5H, CH2CO, CH, NCH2), 6.54–7.76 (m, 15H, Har, NH, NH2), 8.31–8.68 (m, 2H, NCH), 11.43–11.91 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.37, 35.98, 43.92, 110.81, 110.92, 126.58, 126.69, 127.39, 127.59, 127.77, 128.22, 129.89, 130.20, 130.24, 130.32, 130.52, 131.17, 131.42, 131.47, 131.49, 132.86, 133.06, 133.20, 134.69, 138.82, 139.04, 139.25, 142.21, 151.02, 151.10, 158.28, 169.75, 172.79, 173.05, 174.90; HRMS (ESI) for C25H24Cl2N6O4S + H+, calcd. 575.1035, found 575.1031 [M + H+].
3-Chloro-4-((2-(2-(2-chlorobenzylidene)hydrazine-1-carbonyl)-4-(2-(2-chlorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (10b)
White solid, yield 0.26 g (85%); m.p. 203–204 °C; IR (KBr) (v, cm−1): 3438, 3337, 3077, 1688, 1602; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.64–3.67 (m, 5H, CH2CO, CH, NCH2), 6.17–6.43 (m, 1H, NH), 6.98–8.00 (m, 13H, Har, NH2), 8.29–8.64 (m, 2H, NCH), 11.44–11.92 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.38, 32.53, 35.27, 35.84, 44.28, 110.01, 110.41, 116.85, 126.03, 126.42, 126.55, 126.79, 126.86, 127.41, 127.52, 127.76, 128.21, 129.85, 129.89, 130.19, 131.18, 131.37, 131.47, 132.85, 133.00, 133.07, 138.86, 139.40, 139.64, 142.35, 146.22, 158.26, 167.40, 169.64, 172.80, 172.99, 174.95; HRMS (ESI) for C25H23Cl3N6O4S + H+, calcd. 609.0645, found 609.0642 [M + H+].
4-((2-(2-(3-Chlorobenzylidene)hydrazine-1-carbonyl)-4-(2-(3-chlorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (11a)
White solid, yield 0.24 g (83%); m.p. 190–191 °C; IR (KBr) (v, cm−1): 3356, 3240, 3193, 1638, 1600; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.59–3.64 (m, 5H, CH2CO, CH, NCH2), 6.56–8.25 (m, 17H, Har, NH, NCH, NH2), 11.33–11.83 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.09, 32.61, 36.16, 36.87, 43.77, 44.64, 110.74, 110.93, 125.22, 125.45, 125.57, 125.93, 126.00, 126.16, 126.93, 127.34, 127.43, 127.87, 129.41, 130.26, 130.30, 130.65, 130.88, 131.17, 133.59, 133.65, 133.72, 135.78, 136.44, 136.61, 141.19, 141.41, 141.71, 144.10, 144.56, 151.02, 151.08, 160.61, 167.18, 167.49, 169.80, 172.81, 173.08, 174.78; HRMS (ESI) C25H24Cl2N6O4S + H+, calcd. 575.1035, found 575.1033 [M + H+].
3-Chloro-4-((2-(2-(3-chlorobenzylidene)hydrazine-1-carbonyl)-4-(2-(3-chlorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (11b)
Light-brown solid, yield 0.24 g (79%); m.p. 175–176 °C; IR (KBr) (v, cm−1): 3347, 3244, 3192, 1642, 1593; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.61–3.75 (m, 5H, CH2CO, CH, NCH2), 6.15–6.44 (m, 1H, NH), 6.90–8.06 (m, 15H, Har, NH2, NCH), 11.23–11.86 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 31.33, 35.41, 37.52, 43.21, 44.22, 109.85, 110.24, 116.86, 117.14, 125.20, 125.55, 125.85, 126.08, 126.20, 126.28, 126.78, 126.92, 127.40, 127.86, 129.35, 130.65, 130.87, 131.04, 131.16, 131.24, 131.66, 131.79, 133.58, 133.66, 133.71, 133.87, 135.11, 135.78, 136.48, 141.21, 141.90, 146.11, 146.20, 160.22, 160.61, 172.60, 172.82, 173.00, 174.46, 174.90; HRMS (ESI) for C25H23Cl3N6O4S + H+, calcd. 609.0645, found 609.0641 [M + H+].
4-((2-(2-(4-Chlorobenzylidene)hydrazine-1-carbonyl)-4-(2-(4-chlorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (12a)
Light-yellow solid, yield 0.25 g (86%); m.p. 179–180 °C; IR (KBr) (v, cm−1): 3401, 3243, 3073, 1663, 1600; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.58–3.57 (m, 5H, CH2CO, CH, NCH2), 6.58–7.73 (m, 15H, Har, NH, NH2), 7.86–8.04 (m, 2H, NCH), 11.14–11.76 (m, 2H, 2CONH); HRMS (ESI) C25H24Cl2N6O4S + H+, calcd. 575.1035, found 575.1030 [M + H+].
3-Chloro-4-((2-(2-(4-chlorobenzylidene)hydrazine-1-carbonyl)-4-(2-(4-chlorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (12b)
White solid, yield 0.25 g (83%); m.p. 211–212 °C; IR (KBr) (v, cm−1): 3404, 3242, 3071, 1661, 1595; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.62–3.66 (m, 5H, CH2CO, CH, NCH2), 6.09–6.38 (m, 1H, NH), 6.92–7.76 (m, 13H, Har, NH2), 7.87–8.28 (m, 2H, NCH), 11.20–11.83 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.36, 32.50, 35.19, 35.79, 44.34, 44.72, 110.01, 110.43, 116.85, 126.06, 126.85, 128.24, 128.28, 128.35, 128.54, 128.61, 128.80, 128.86, 131.19, 131.36, 133.05, 133.13, 133.18, 133.29, 134.13, 134.19, 134.26, 134.37, 141.50, 141.73, 142.07, 142.29, 144.55, 145.09, 146.25, 167.04, 167.31, 169.60, 172.72, 172.87, 174.62, 174.87; HRMS (ESI) for C25H23Cl3N6O4S + H+, calcd. 609.0645, found 609.0640 [M + H+].
4-((2-(2-(4-Fluorobenzylidene)hydrazine-1-carbonyl)-4-(2-(4-fluorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (13a)
White solid, yield 0.20 g (74%); m.p. 153–154 °C; IR (KBr) (v, cm−1): 3297, 3214, 3071, 1639, 1601; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.08–2.34 (m, 2H, CH2CO), 2.79–3.30 (m, 3H, CH, NCH2), 6.39 (t, 1H, J = 6.0 Hz, NH), 6.61–7.76 (m, 14H, Har, NH2), 7.90–8.21 (m, 2H, NCH), 11.10–11.69 (m, 2H, 2CONH); HRMS (ESI) C25H24F2N6O4S + H+, calcd. 543.1626, found 543.1621 [M + H+].
3-Chloro-4-((2-(2-(4-fluorobenzylidene)hydrazine-1-carbonyl)-4-(2-(4-fluorobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (13b)
White solid, yield 0.23 g (79%); m.p. 207–208 °C; IR (KBr) (v, cm−1): 3410, 3244, 3076, 1662, 1597; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.62–3.67 (m, 5H, CH2CO, CH, NCH2), 6.15–6.36 (m, 1H, NH), 6.90–7.80 (m, 13H, Har, NH2), 7.88–8.26 (m, 2H, NCH), 11.24–11.70 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.37, 33.74, 35.19, 44.35, 44.75, 110.02, 110.42, 115.72, 115.94, 116.84, 116.91, 126.06, 126.77, 126.85, 128.73, 128.81, 129.02, 129.10, 129.18, 130.89, 131.16, 141.61, 141.85, 142.16, 142.39, 144.74, 145.30, 146.27, 161.64, 161.80, 164.09, 167.25, 169.52, 172.54, 172.67, 172.83, 174.56, 174.80; HRMS (ESI) for C25H23ClF2N6O4S + H+, calcd. 577.1236, found 577.1231 [M + H+].
4-((2-(2-(4-Methoxybenzylidene)hydrazine-1-carbonyl)-4-(2-(4-methoxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (14a)
White solid, yield 0.21 g (75%); m.p. 168–169 °C; IR (KBr) (v, cm−1): 3344, 3188, 3076, 1651, 1603; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.77–3.52 (m, 5H, CH2CO, CH, NCH2), 3.79 (s, 6H, 2CH3), 6.58–7.67 (m, 15H, Har, NH, NH2), 7.89–8.20 (m, 2H, NCH), 11.02–11.57 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.24, 33.65, 35.98, 43.88, 44.39, 55.24, 55.29, 55.29, 110.86, 114.28, 126.81, 126.90, 127.29, 127.43, 128.25, 128.52, 130.17, 142.54, 143.00, 151.05, 151.12, 160.54, 160.73, 166.70, 169.83, 172.00, 172.36, 172.72, 174.41; HRMS (ESI) C27H30N6O6S + H+, calcd. 567.2026, found 567.2021 [M + H+].
3-Chloro-4-((2-(2-(4-methoxybenzylidene)hydrazine-1-carbonyl)-4-(2-(4-methoxybenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (14b)
Light-yellow solid, yield 0.22 g (73%); m.p. 198–199 °C; IR (KBr) (v, cm−1): 3355, 3243, 3072, 1660, 1595; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.60–3.61 (m, 5H, CH2CO, CH, NCH2), 3.79, 3.82 (2s, 6H, 2CH3), 6.09–6.44 (m, 1H, NH), 6.88–8.20 (m, 15H, Har, NCH, NH2), 11.01–11.55 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.30, 32.57, 35.22, 44.33, 55.21, 55.28, 55.39, 109.97, 114.27, 114.39, 126.11, 126.57, 126.71, 126.87, 128.24, 128.49, 128.57, 129.97, 131.12, 131.31, 142.60, 143.22, 146.28, 160.49, 160.72, 161.66, 172.42, 172.64, 174.23, 174.45; HRMS (ESI) for C27H29ClN6O6S + H+, calcd. 601.1636, found 601.1631 [M + H+].
4-((2-(2-(2-Nitrobenzylidene)hydrazine-1-carbonyl)-4-(2-(2-nitrobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (15a)
Yellow solid, yield 0.24 g (80%); m.p. 197–198 °C; IR (KBr) (v, cm−1): 3361, 3194, 3058, 1641, 1598; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.60–3.57 (m, 5H, CH2CO, CH, NCH2), 6.59–8.22 (m, 15H, Har, NH, NH2), 8.33–8.66 (m, 2H, NCH), 11.50–12.07 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.38, 32.63, 33.81, 35.88, 44.03, 44.65, 110.82, 110.94, 124.62, 124.70, 124.79, 127.36, 127.58, 127.67, 127.75, 127.82, 127.91, 128.45, 128.50, 128.60, 128.74, 128.81, 129.43, 130.22, 130.34, 130.52, 132.16, 133.57, 133.69, 133.93, 138.23, 138.45, 138.60, 141.29, 141.57, 141.93, 147.97, 148.00, 148.02, 148.14, 148.88, 151.06, 151.10, 158.67, 167.37, 167.60, 169.93, 172.96, 173.16, 174.96, 175.15; HRMS (ESI) C25H24N8O8S + H+, calcd. 597.1516, found 597.1513 [M + H+].
3-Chloro-4-((2-(2-(2-nitrobenzylidene)hydrazine-1-carbonyl)-4-(2-(2-nitrobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (15b)
Yellow solid, yield 0.29 g (94%); m.p. 178–179 °C; IR (KBr) (v, cm−1): 3358, 3243, 3073, 1646, 1591; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.68–3.69 (m, 5H, CH2CO, CH, NCH2), 6.17–6.39 (m, 1H, NH), 6.92–7.76 (m, 13H, Har, NH2), 8.32–8.68 (m, 2H, NCH), 11.59–12.03 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.38, 33.78, 35.26, 35.83, 44.31, 44.70, 110.07, 116.84, 116.95, 124.62, 124.69, 124.78, 126.03, 126.80, 127.43, 127.82, 127.88, 128.45, 128.48, 129.43, 130.35, 131.17, 131.40, 132.16, 133.53, 133.71, 133.92, 138.27, 138.67, 138.98, 146.23, 147.96, 148.00, 148.14, 148.88, 158.67, 167.58, 169.84, 172.95, 173.11, 174.90, 175.16; HRMS (ESI) for C25H23ClN8O8S + H+, calcd. 631.1126, found 631.1121 [M + H+].
4-((2-(2-(3-Nitrobenzylidene)hydrazine-1-carbonyl)-4-(2-(3-nitrobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (16a)
Light-yellow solid, yield 0.26 g (87%); m.p. 218–219 °C; IR (KBr) (v, cm−1): 3352, 3260, 3074, 1667, 1595; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.61–3.58 (m, 5H, CH2CO, CH, NCH2), 6.58–8.59 (m, 17H, Har, NH, NCH, NH2), 11.29–11.99 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.40, 32.63, 34.03, 34.30, 36.10, 36.78, 36.82, 43.96, 44.62, 110.73, 110.95, 120.89, 121.06, 121.34, 122.66, 124.00, 125.83, 127.34, 130.17, 130.37, 130.66, 132.10, 132.68, 133.18, 133.25, 134.44, 135.24, 136.03, 136.23, 140.64, 140.85, 143.84, 148.16, 148.21, 151.11, 160.50, 167.36, 167.60, 169.76, 169.98, 172.88, 173.10, 174.85, 175.09; HRMS (ESI) C25H24N8O8S + H+, calcd. 597.1516, found 597.1514 [M + H+].
3-Chloro-4-((2-(2-(3-nitrobenzylidene)hydrazine-1-carbonyl)-4-(2-(3-nitrobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (16b)
White solid, yield 0.28 g (90%); m.p. 199–200 °C; IR (KBr) (v, cm−1): 3368, 3242, 3071, 1659, 1598; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.66–3.69 (m, 5H, CH2CO, CH, NCH2), 6.14–6.33 (m, 1H, NH), 6.83–8.61 (m, 15H, Har, NCH, NH2), 11.48–12.02 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.44, 35.31, 35.92, 44.33, 44.66, 109.93, 116.76, 116.97, 120.83, 120.93, 121.23, 121.37, 123.93, 124.14, 126.07, 126.76, 127.35, 130.11, 130.36, 131.11, 131.40, 131.47, 132.12, 132.72, 133.20, 135.90, 136.06, 136.18, 140.69, 141.00, 143.44, 143.73, 144.01, 146.24, 148.08, 148.17, 167.35, 167.57, 169.92, 172.89, 173.03, 174.93, 175.21; HRMS (ESI) for C25H23ClN8O8S + H+, calcd. 631.1126, found 631.1122 [M + H+].
4-((2-(2-(4-Nitrobenzylidene)hydrazine-1-carbonyl)-4-(2-(4-nitrobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (17a)
Yellow solid, yield 0.28 g (93%); m.p. 256–257 °C; IR (KBr) (v, cm−1): 3413, 3239, 3066, 1665, 1601; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.60–3.60 (m, 5H, CH2CO, CH, NCH2), 6.56–8.36 (m, 17H, Har, NH, NCH, NH2), 11.51–12.00 (m, 2H, 2CONH); HRMS (ESI) C25H24N8O8S + H+, calcd. 597.1516, found 597.1511 [M + H+].
3-Chloro-4-((2-(2-(4-nitrobenzylidene)hydrazine-1-carbonyl)-4-(2-(4-nitrobenzylidene)hydrazineyl)-4-oxobutyl)amino)benzenesulfonamide (17b)
Yellow solid, yield 0.25 g (81%); m.p. 220–221 °C; IR (KBr) (v, cm−1): 3390, 3247, 3072, 1662, 1596; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.66–3.67 (m, 5H, CH2CO, CH, NCH2), 6.17–6.37 (m, 1H, NH), 6.92–8.37 (m, 15H, Har, NCH, NH2), 11.45–12.14 (m, 2H, 2CONH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.49, 35.15, 35.75, 44.38, 44.65, 110.01, 110.45, 116.80, 116.96, 123.87, 124.01, 124.01, 126.06, 126.77, 127.34, 127.57, 127.64, 127.81, 127.90, 131.18, 140.32, 140.52, 140.57, 140.88, 141.11, 143.44, 143.97, 146.24, 147.59, 147.77, 167.64, 173.04, 173.16, 175.37; HRMS (ESI) for C25H23ClN8O8S + H+, calcd. 631.1126, found 631.1121 [M + H+].
4,4′-(((2-(((4-Sulfamoylphenyl)amino)methyl)succinyl)bis(hydrazin-2-yl-1-ylidene))bis(methaneylylidene))dibenzoic acid (18a)
Light-yellow solid, yield 0.27 g (91%); m.p. 308–309 °C; IR (KBr) (v, cm−1): 3319, 3191, 3074, 1667, 1626, 1601; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.70–3.54 (m, 5H, CH2CO, CH, NCH2), 6.45–8.51 (m, 17H, Har, NH, NCH, NH2), 11.32–11.94 (m, 2H, 2CONH), 13.03 (br. s, 2H, 2OH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.76, 35.07, 35.75, 49.98, 118.91, 126.49, 126.71, 126.98, 127.13, 127.39, 128.53, 129.55, 129.78, 129.82, 130.32, 131.64, 133.15, 137.48, 138.23, 139.01, 141.87, 142.73, 161.01, 166.86, 166.94, 172.79, 173.65; HRMS (ESI) C27H26N6O8S + H+, calcd. 595.1611, found 595.1606 [M + H+].
4,4′-(((2-(((2-Chloro-4-sulfamoylphenyl)amino)methyl)succinyl)bis(hydrazin-2-yl-1-ylidene))bis(methaneylylidene))dibenzoic acid (18b)
Light-brown solid, yield 0.28 g (90%); m.p. 294–295 °C; IR (KBr) (v, cm−1): 3348, 3245, 3194, 3064, 1671, 1643, 1597; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.65–3.73 (m, 5H, CH2CO, CH, NCH2), 6.13–6.40 (m, 1H, NH), 6.84–8.33 (m, 15H, Har, NCH, NH2), 11.36–11.87 (m, 2H, 2CONH), 13.09 (br. s, 2H, 2OH); HRMS (ESI) for C27H25ClN6O8S + H+, calcd. 629.1221, found 629.1216 [M + H+].
3.1.6. General Procedure for the Synthesis of Pyrroles 19a,b
A mixture of hydrazide 2a (0.46 g, 1.4 mmol) or 2b (0.51 g, 1.4 mmol), hexane-2,5-dione (0.8 mL, 7 mmol), and 30 mL of propan-2-ol was heated under reflux for 5h. Then, the reaction mixture was filtered, and the liquid fractions were evaporated under reduced pressure. The residue was diluted with water (20 mL), and the precipitate was filtered off, washed with water, dried, and recrystallized from propan-2-ol.
N1,N4-bis(2,5-Dimethyl-1H-pyrrol-1-yl)-2-(((4-sulfamoylphenyl)amino)methyl)succinamide (19a)
Light-brown solid, yield 0.51 g (75%); m.p. 195–196 °C; IR (KBr) (v, cm−1): 3399, 3362, 3248, 1667, 1598; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.86–2.02 (m, 12H, 4CH3), 2.55–2.82 (m, 2H, CH2), 3.15–3.31 (m, 2H, NCH2), 3.39–3.56 (m, 1H, CH), 5.60, 5.63 (2s, 4H, 4CHpyr), 6.61–6.77 (m, 3H, Har, NH), 6.95 (br. s, 2H, NH2), 7.48–7.57 (m, 2H, Har), 10.70, 10.81 (2s, 2H, 2NNH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 10.88, 10.92, 10.94, 11.04, 32.83, 44.55, 102.79, 102.89, 102.95, 110.88, 126.62, 126.85, 126.99, 130.56, 150.86, 169.93, 172.10; HRMS (ESI) for C23H30N6O4S + H+, calcd. 487.2127, found 487.2122 [M + H+].
2-(((2-Chloro-4-sulfamoylphenyl)amino)methyl)-N1,N4-bis(2,5-dimethyl-1H-pyrrol-1-yl)succinamide (19b)
Light-brown solid, yield 0.59 g (81%); m.p. 232–233 °C; IR (KBr) (v, cm−1): 3401, 3356, 3244, 1660, 1594; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.91, 1.94, 1.98 (3s, 12H, 4CH3), 2.58–2.84 (m, 2H, CH2), 3.36–3.43 (m, 2H, NCH2), 3.58–3.68 (m, 1H, CH), 5.61, 5.63 (2s, 4H, 4CHpyr), 6.24 (t, 1H, J = 5.5 Hz, NH), 7.00 (d, J = 8.6 Hz, 1H, Har), 7.16 (br. s, 2H, NH2), 7.52–7.71 (m, 2H, Har), 10.72, 10.87 (2s, 2H, 2NNH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 10.89, 10.93, 11.00, 11.04, 32.76, 44.69, 102.84, 102.90, 102.96, 110.33, 117.03, 126.15, 126.63, 126.79, 126.84, 126.97, 131.62, 146.12, 169.91, 172.04; HRMS (ESI) for C23H29ClN6O4S + H+, calcd. 521.1738, found 521.1732 [M + H+].
3.1.7. 3-Chloro-4-((4-(3,5-dimethyl-1H-pyrazol-1-yl)-2-(3,5-dimethyl-1H-pyrazole-1-carbonyl)-4-oxobutyl)amino)benzenesulfonamide (20)
A mixture of hydrazide 2b (0.51 g, 1.4 mmol), pentane-2,4-dione (0.6 mL, 6 mmol) and 20 mL of propan-2-ol was heated at reflux for 5 h. Then it was cooled down and the precipitate was filtered off, washed with diethyl ether and recrystallized from propan-2-ol to afford light-brown solid, yield 0.54 g (78%); m.p. 201–202 °C; IR (KBr) (v, cm−1): 3371, 1723, 1595; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.18, 2.25, 2.35, 2.43 (4s, 12H, 4CH3), 3.34–3.44 (m, 3H, CH, CH2), 3.61–3.75 (m, 2H, NCH2), 6.16, 6.24 (2s, 2H, 2CHpyr), 6.52 (t, 1H, J = 5.9 Hz, NH), 7.13 (br. s, 2H, NH2), 7.25 (d, 1H, J = 8.7 Hz, Har), 7.56–7.65 (m, 2H, Har); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 13.56, 13.70, 14.07, 14.20, 35.39, 44.32, 110.76, 111.40, 111.71, 116.99, 126.04, 126.85, 131.50, 143.51, 143.81, 146.19, 151.93, 152.24, 172.07, 173.76; HRMS (ESI) for C21H25ClN6O4S + H+, calcd. 493.1425, found 493.1419 [M + H+].
3.1.8. General Procedure for the Synthesis of Compounds 22a,b and 23a,b
Hydrazide 2a (1 g, 3 mmol) or 2b (1.10 g, 3 mmol) was dissolved in dimethylformamide (5 mL), and methyl isothiocyanate (0.58 g, 8 mmol) or phenyl isothiocyanate (0.9 mL, 7.5 mmol) was added dropwise, respectively. Reaction mixtures were stirred at room temperature for 4–6 h and then diluted with water (20 mL). The precipitate was filtered off, washed with water, dried, and recrystallized from 1,4-dioxane.
2,2′-(2-(((4-Sulfamoylphenyl)amino)methyl)succinyl)bis(N-methylhydrazine-1-carbothioamide) (22a)
White solid, yield 1.13 g (79%); m.p. 192–193 °C; IR (KBr) (v, cm−1): 3381, 3299, 3196, 1657, 1601; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.81–2.89 (m, 6H, 2CH3), 2.91–3.32 (m, 5H, CH, NCH2, CH2), 6.44 (t, 1H, J = 6.0 Hz, ArNH), 6.67 (d, 2H, J = 8.5 Hz, Har,), 6.94 (br. s, 2H, NH2), 7.52 (d, 2H, J = 8.5 Hz, Har,), 7.62–7.97 (m, 2H, 2NH), 9.19 (br. s, 2H, 2NH), 9.91, 10.05 (2s, 2H, 2NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 30.87, 33.44, 35.83, 44.32, 111.07, 127.37, 130.64, 150.98, 171.19, 172.71, 182.17; HRMS (ESI) for C15H24N8O4S3 + H+, calcd. 477.1161, found 477.1155 [M + H+].
2,2′-(2-(((2-Chloro-4-sulfamoylphenyl)amino)methyl)succinyl)bis(N-methylhydrazine-1-carbothioamide (22b)
Light-brown solid, yield 1.19 g (78%); m.p. 212–213 °C; IR (KBr) (v, cm−1): 3386, 3300, 3175, 1660, 1594; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.84, 2.85 (2s, 6H, 2CH3), 2.93–3.51 (m, 5H, CH, CH2, NCH2), 6.17 (t, 1H, J = 5.8 Hz, ArNH), 6.90 (d, 1H, J = 8.8 Hz, Har), 7.14 (br. s, 2H, NH2), 7.57 (d, 1H, J = 8.6 Hz, Har), 7.67–7.97 (m, 2H, 2NH), 9.09–9.38 (m, 2H, 2NH), 9.94, 10.10 (2s, 2H, NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 30.88, 33.22, 44.24, 110.38, 117.05, 126.13, 126.77, 131.50, 146.24, 171.29, 172.69, 182.04, 182.17, 182.26; HRMS (ESI) for C15H23ClN8O4S3+H+, calcd. 511.0771, found 511.0773 [M + H+].
2,2′-(2-(((4-Sulfamoylphenyl)amino)methyl)succinyl)bis(N-phenylhydrazine-1-carbothioamide) (23a)
White solid, yield 1.64 g (91%); m.p. 136–137 °C; IR (KBr) (v, cm−1): 3212, 1660, 1597; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.54–3.27 (m, 5H, NCH2, CH, CH2), 6.35–7.85 (m, 17H, NH, NH2, Har), 9.32–10.50 (m, 6H, 6NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 30.80, 33.35, 35.81, 44.28, 111.09, 116.79, 121.03, 123.66, 123.98, 124.45, 125.96, 127.38, 128.14, 128.45, 128.98, 129.93, 130.66, 139.00, 139.03, 141.19, 150.94, 162.32; HRMS (ESI) for C25H28N8O4S3 + H+, calcd. 601.1474, found 601.1468 [M + H+].
2,2′-(2-(((2-Chloro-4-sulfamoylphenyl)amino)methyl)succinyl)bis(N-phenylhydrazine-1-carbothioamide) (23b)
White solid, yield 1.71 g (90%); m.p. 158–159 °C; IR (KBr) (v, cm−1): 3244, 1683, 1593; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.40–4.00 (m, 5H, CH2, CH, NCH2), 6.17 (br. s, 1H, NH), 6.83–7.87 (m, 15H, NH2, Har), 9.38–10.55 (m, 6H, 6NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 30.79, 30.97, 33.17, 35.80, 44.17, 110.34, 116.80, 117.05, 123.82, 124.40, 126.13, 126.24, 126.77, 128.11, 128.51, 131.09, 131.53, 139.02, 146.19, 158.23, 162.31; HRMS (ESI) for C25H27ClN8O4S3 + H+, calcd. 635.1084, found 635.1079 [M + H+].
3.1.9. General Procedure for the Synthesis of Compounds 24a,b and 25a,b
A mixture of thiosemicarbazide 22a,b (0.5 mmol) or 23a,b (0.5 mmol) and 2% aqueous KOH solution (20 mL) was stirred at room temperature for 8 h. Afterwards, it was acidified with glacial acetic acid to pH 6. The formed precipitate was filtered off, washed with water, and recrystallized from propan-2-ol.
4-((2,3-bis(4-Methyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)propyl)amino)benzenesulfonamide (24a)
Brown solid, yield 0.16 g (73%); m.p. 170–171 °C; IR (KBr) (v, cm−1): 3332, 3215, 3087; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.13–3.23 (m, 2H, CH2), 3.33, 3.39 (2s, 6H, 2CH3), 3.49–3.61 (m, 2H, NCH2), 3.61–3.71 (m, 1H, CH), 6.57–6.73 (m, 3H, ArNH, Har), 6.95 (br. s, 2H, NH2), 7.52 (d, J = 8.4 Hz, 2H, Har), 13.52, 13.64 (2s, 2H, NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 26.50, 29.73, 29.99, 32.58, 45.84, 110.92, 127.52, 130.77, 150.71, 150.84, 153.36, 166.49, 166.74; HRMS (ESI) for C15H20N8O2S3 + H+, calcd. 441.0950, found 441.0944 [M + H+].
4-((2,3-bis(4-Methyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)propyl)amino)-3-chlorobenzenesulfonamide (24b)
Light-brown solid, yield 0.17 g (71%); m.p. 151–152 °C; IR (KBr) (v, cm−1): 3308, 3241, 3078; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.15–3.22 (m, 2H, CH2), 3.36, 3.40 (2s, 6H, 2CH3), 3.57–3.67 (m, 2H, NCH2), 3.71–3.83 (m, 1H, CH), 6.39 (t, 1H, J = 6.1 Hz, ArNH), 6.95 (d, J = 8.7 Hz, 1H, Har), 7.13 (br. s, 2H, NH2), 7.54 (d, J = 8.7 Hz, 1H, Har), 7.65 (s, 1H, Har), 13.51, 13.63 (2s, 2H, NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 26.32, 29.73, 30.00, 32.36, 45.69, 110.20, 116.94, 126.17, 126.98, 131.61, 146.13, 150.68, 153.15, 166.52, 166.74; HRMS (ESI) for C15H19ClN8O2S3+H+, calcd. 475.0560, found 475.0557 [M + H+].
4-((2,3-bis(4-Phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)propyl)amino)benzenesulfonamide (25a)
White solid, yield 0,19 g (68%); m.p. 258–259 °C; IR (KBr) (v, cm−1): 3381, 3201, 3074; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.79–2.87 (m, 2H, CH2), 3.03–3.21 (m, 2H, NCH2), 3.25–3.38 (m, 1H, CH), 6.09 (d, J = 8.4 Hz, 2H, Har), 6.59 (t, 1H, J = 6.2 Hz, ArNH), 6.86–7.68 (m, 14H, NH2, Har), 13.44 (br. s, 2H, 2NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 26.94, 32.43, 45.31, 110.49, 127.20, 127.94, 129.42, 129.56, 130.63, 133.14, 133.17, 149.96, 149.98, 152.45, 167.62, 167.76; HRMS (ESI) for C25H24N8O2S3+H+, calcd. 565.1263, found 565.1257 [M + H+].
4-((2,3-bis(4-Phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)propyl)amino)-3-chlorobenzenesulfonamide (25b)
White solid, yield 0,22 g (73%); m.p. 175–176 °C; IR (KBr) (v, cm−1): 3351, 3192, 3065; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.76–3.79 (m, 5H, CH2, CH, NCH2,), 5.90 (d, J = 8.8 Hz, H, Har), 6.34 (t, 1H, J = 6.2 Hz, ArNH), 6.92–7.75 (m, 15H, NH2, Har), 12.82 (br. s, 2H, 2NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 26.98, 31.92, 45.22, 109.03, 116.84, 117.01, 125.60, 126.86, 127.77, 128.07, 129.06, 129.23, 129.44, 129.49, 131.39, 133.04, 133.27, 145.15, 150.01, 152.32, 167.52, 167.78; HRMS (ESI) for C25H23ClN8O2S3+H+, calcd. 599.0873, found 599.0867 [M + H+].
3.1.10. 3-((4-Methylthiazol-2-yl)(4-sulfamoylphenyl)amino)propanoic acid (27)
Carboxylic acid 26 (0.45 g, 1.5 mmol) and NaOAc (0.20 g, 2.5 mmol) were dissolved into 10 mL of hot water. Then, chloroacetone (0.20 mL, 2.5 mmol) was added, and reaction mixture was heated at reflux for 4h. Then, it was cooled down, and the precipitate was filtered off and washed with diethyl ether and n-hexane to afford light-pinkish solid, yield 0.50 g (98%); m.p. 162–163 °C; IR (KBr) (v, cm−1): 3300, 1680, 1497; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.19 (s, 3H, CH3), 2.63 (t, 2H, J = 7.2 Hz, CH2), 4.14 (t, 2H, J = 7.3 Hz, CH2), 6.44 (s, 1H, CH), 7.39 (s, 2H, NH2), 7.62 (d, 2H, J = 8.3 Hz, Har), 7.85 (d, 2H, J = 8.3 Hz, Har), 12.36 (br. s, 1H, OH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 17.39, 32.25, 48.53, 103.51, 125.15, 127.33, 140.99, 147.39, 148.45, 167.23, 172.48; Anal. Calcd. for C13H15N3O4S2: C 45.74; H 4.43; N 12.31%. Found: C 45.82; H 4.48; N 12.30%.
3.1.11. Methyl 3-((4-Methylthiazol-2-yl)(4-sulfamoylphenyl)amino)propanoate (28)
A mixture of thiazole 27 (0.41 g, 1.2 mmol), H2SO4 (0.1 mL), and 15 mL of methanol were heated under reflux for 6 h. Then, the reaction mixture was filtered, and the liquid fractions were evaporated under reduced pressure. The residue was diluted with 5% aqueous Na2CO3 solution (20 mL), and the precipitate was filtered off, washed with water, dried, and recrystallized from propan-2-ol to afford white solid, yield 0,32 g (74%); m.p. 72–73 °C; IR (KBr) (v, cm−1): 1679, 1507; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.19 (s, 3H, CH3), 2.72 (t, 2H, J = 7.0 Hz, CH2), 3.51 (s, 3H, CH3), 4.18 (t, 2H, J = 7.0 Hz, CH2), 6.43 (s, 1H, CH), 7.40 (s, 2H, NH2), 7.61 (d, 2H, J = 8.3 Hz, Har), 7.85 (d, 2H, J = 8.3 Hz, Har); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 17.37, 32.12, 48.48, 51.43, 103.52, 125.35, 127.36, 141.17, 147.31, 148.44, 167.22, 171.39; Anal. Calcd. for C14H17N3O4S2: C 47.31; H 4.82; N 11.82%. Found: C 47.56; H 4.83; N 11.81%.
3.1.12. 4-((3-Hydrazineyl-3-oxopropyl)(4-methylthiazol-2-yl)amino)benzenesulfonamide (29)
Ester 28 (0.53 g, 1.5 mmol) and hydrazine monohydrate (0.15 g, 3 mmol) were dissolved into 5 mL of propan-2-ol, and the reaction mixture was heated under reflux for 5 h. Then, it was cooled down, and the precipitate was filtered off, washed with diethyl ether, and n-hexane to afford white solid, yield 0.47 g (89%); m.p. 167–168 °C; IR (KBr) (v, cm−1): 3382, 3260, 1644, 1498; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.19 (s, 3H, CH3), 2.44 (t, 2H, J = 7.3 Hz, CH2), 3.92–4.72 (m, 4H, NH2, CH2), 6.45 (s, 1H, CH), 7.44–7.96 (m, 6H, NH2, Har), 9.06 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 17.40, 31.77, 49.21, 103.58, 124.93, 127.22, 127.26, 140.77, 147.41, 148.43, 167.18, 169.14; Anal. Calcd. for C13H17N5O3S2: C 43.93; H 4.82; N 19.70%. Found: C 44.04; H 4.75; N 19.50%.
3.1.13. 4-((3-(2-Benzylidenehydrazineyl)-3-oxopropyl)(4-methylthiazol-2-yl)amino)benzenesulfonamide (30)
Hydrazide 29 (0.53 g, 1.5 mmol), benzaldehyde (0.19 g, 1.8 mmol), and 10 mL of propan-2-ol were mixed under reflux for 1 h. Then, the reaction mixture was cooled down, and the precipitate was filtered off, washed with diethyl ether, and recrystallized from propan-2-ol to afford white solid, yield 0.58 g (87%); m.p. 170–171 °C; IR (KBr) (v, cm−1): 3247, 1671, 1595, 1499; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.08 (s, 3H, CH3), 2.58 (t, 0.70H, J = 7.2 Hz, CH2), 3.00 (t, 1.30H, J = 7.4 Hz, CH2) 4.06–4.36 (m, 2H, NCH2), 7.13–8.14 (m, 13H, NH2, CH, N=CH, HAr), 11.35, 11.38, 11.41, 11.44 (4s, 1H, NH); Anal. Calcd. for C20H21N5O3S2: C 54.16; H 4.77; N 15.79%. Found: C 54.36; H 4.65; N 15.90%.
3.1.14. 3-((5-(Ethoxycarbonyl)-4-methylthiazol-2-yl)(4-sulfamoylphenyl)amino)propanoic acid (31)
Carboxylic acid 26 (1.00 g, 3 mmol) and NaOAc (0.30 g, 3.6 mmol) were dissolved into 10 mL of hot water. Then, ethyl 2-chloroacetoacetate (0.70 g, 4.25 mmol) was added, and the reaction mixture was heated at reflux for 12h. Then, it was cooled down, and the precipitate was filtered off and washed with water and n-hexane to afford white solid, yield 1.05 g (85%); m.p. 221–222 °C; IR (KBr) (v, cm−1): 3546, 1708, 1677, 1509; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.20 (t, 3H, J = 7.1 Hz, CH3), 2.52 (s, 3H, CH3), 2.65 (t, 2H, J = 7.1 Hz, CH2), 4.08–4.28 (m, 4H, 2CH2), 7.48 (s, 2H, NH2), 7.70 (d, 2H, J = 8.4 Hz, Har), 7.95 (d, 2H, J = 8.4 Hz, Har), 12.38 (s, 1H, OH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 14.23, 17.31, 32.14, 48.34, 60.22, 109.47, 127.19, 127.69, 143.05, 146.02, 159.13, 161.56, 169.25, 172.23; Anal. Calcd. for C16H19N3O6S2: C 46.48; H 4.63; N 10.16%. Found: C 46.29; H 4.56; N 10.10%.
3.1.15. Ethyl 2-((3-Methoxy-3-oxopropyl)(4-sulfamoylphenyl)amino)-4-methylthiazole-5-carboxylate (32)
A mixture of thiazole 31 (0.42 g, 1 mmol), H2SO4 (0.1 mL) and 15 mL of methanol were heated under reflux for 5 h. Then, the reaction mixture was filtered, and the liquid fractions were evaporated under reduced pressure. The residue was diluted with 5% aqueous Na2CO3 solution (20 mL); the precipitate was filtered off, washed with water, dried, and recrystallized from propan-2-ol to afford white solid, yield 0.32 g (74%); m.p. 140–141 °C; IR (KBr) (v, cm−1): 3371, 1732, 1678, 1520; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.19 (t, 3H, J = 7.1 Hz, CH3), 2.48 (s, 3H, CH3), 2.72 (t, 2H, J = 6.9 Hz, CH2), 3.51 (s, 3H, CH3), 4.13 (q, 2H, J = 7.1 Hz, CH2), 4.21 (t, 2H, J = 6.9 Hz, CH2), 7.47 (s, 2H, NH2), 7.68 (d, 2H, J = 8.3 Hz, Har), 7.94 (d, 2H, J = 8.2 Hz, Har); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 14.21, 17.28, 32.01, 48.28, 51.48, 60.22, 109.54, 127.24, 127.70, 143.14, 145.94, 159.08, 161.53, 169.21, 171.15; Anal. Calcd. for C17H21N3O6S2: C 47.76; H 4.95; N 9.83%. Found: C 47.77; H 4.94; N 9.99%.
3.1.16. Ethyl 2-((3-(Hydrazineyloxy)-3-oxopropyl)(4-sulfamoylphenyl)amino)-4-methylthiazole-5-carboxylate (33)
A mixture of ester 32 (0.43 g, 1 mmol), hydrazine monohydrate (0.15 g, 3 mmol), and 15 mL of propan-2-ol were heated under reflux for 12 h. Then, the reaction mixture was cooled down, and the precipitate was filtered off and recrystallized from propan-2-ol to afford white solid, yield 0.38 g (88%); m.p. 206–207 °C; IR (KBr) (v, cm−1): 3325, 3223, 1712, 1679, 1507; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.19 (t, 3H, J = 7.1 Hz, CH3), 2.43 (t, 2H, J = 7.1 Hz, CH2), 2.48 (s, 3H, CH3), 4.06–4.24 (m, 6H, NH2, 2CH2), 7.47 (s, 2H, SO2NH2), 7.67 (d, 2H, J = 8.1 Hz, Har), 7.93 (d, 2H, J = 8.2 Hz, Har), 9.07 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 14.25, 17.33, 31.71, 49.20, 60.21, 109.45, 127.14, 127.63, 142.92, 146.11, 159.17, 161.58, 168.86, 169.21; Anal. Calcd. for C16H21N5O5S2: C 44.95; H 4.95; N 16.38%. Found: C 45.20; H 4.90; N 16.23%.
3.1.17. Ethyl 2-((3-(2-Benzylidenehydrazineyl)-3-oxopropyl)(4-sulfamoylphenyl)amino)-4-methylthiazole-5-carboxylate (34)
A mixture of hydrazide 33 (0.43 g, 1 mmol), benzaldehyde (0.13 g, 1.2 mmol), and 15 mL of propan-2-ol were heated under reflux for 3 h. Then, the reaction mixture was cooled down and diluted with diethyl ether. The precipitate was filtered off to afford white solid, yield 0.44 g (85%); m.p. 195–196 °C; IR (KBr) (v, cm−1): 3269, 1678, 1655, 1553 1513; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.11–1.27 (m, 3H, CH3), 2.51 (s, 3H, CH3), 2.64 (t, 0.75H, J = 7.1 Hz, CH2), 3.07 (t, 1.25H, J = 7.3 Hz, CH2), 4.01–4.50 (m, 4H, 2CH2), 7.15–8.15 (m, 12H, Har, CH, NH2), 11.39 (s, 0.7H, NH), 11.45 (s, 0.3H, NH); Anal. Calcd. for C23H25N5O5S2: C 53.58; H 4.89; N 13.58%. Found: C 53.23; H 4.67; N 13.34%.
3.1.18. 3-((5-Chloro-4-(4-chlorophenyl)thiazol-2-yl)(4-sulfamoylphenyl)amino)propanoic acid (36)
Thiazole 35 (0.70 g, 1.6 mmol) and N-chlorosuccinimide (0.60 g, 4.5 mmol) were dissolved into 3 mL of DMF, and the reaction mixture was stirred at 0 °C for 6 h. Then, it was diluted with cold water, and the precipitate was filtered off and dissolved into 20 mL of 5% aqueous NaOH solution. The mixture was acidified with glacial acetic acid to pH 6 to afford light-yellowish solid, yield 0.53 g (71%); m.p. 109–110 °C; IR (KBr) (v, cm−1): 3353, 1727, 1510; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.67 (t, 2H, J = 7.1 Hz, CH2), 4.20 (t, 2H, J = 7.1 Hz, CH2), 7.46 (s, 2H, NH2), 7.54 (d, 2H, J = 8.4 Hz, Har), 7.72 (d, 2H, J = 8.4 Hz, Har), 7.91 (d, 4H, J = 8.4 Hz, Har), 12.32 (br. s, 1H, OH); 13C NMR (101 MHz, DMSO-d6) (δ, ppm): 32.30, 48.13, 107.25, 126.10, 127.65, 128.60, 129.44, 131.42, 132.97, 142.31, 144.05, 146.30, 163.41, 172.46; Anal. Calcd. for C19H16Cl2N2O4S2: C 48.41; H 3.42; N 5.94%. Found: C 48.29; H 3.35; N 5.99%.
3.2. Determination of Compounds Binding to Human CA Isozymes
3.2.1. Protein Preparation
Human CA isozymes were expressed in E. coli (CAI, II, III, IV, VA, VB, VII, XII, XIII, XIV) or mammalian cells (CAVI and CAIX), chromatographically purified following the published protocols [47], and stored at −80 °C. The protein concentration was determined by UV absorption at 280 nm using Beer–Lambert law. The CAII used for crystallization was additionally purified by affinity chromatography.
3.2.2. Fluorescent Thermal Shift Assay (FTSA)
The fluorescent thermal shift assay was performed to obtain the observed binding constants (inversely proportional to the observed dissociation constants (Kd_obss)) between CA isozymes and synthesized compounds. The QIAGEN Rotor-Gene Q was used to carry out the experiments, with a blue channel used for excitation (365 nm) and detection (460 nm). FTSA in this case relies on an extrinsic probe, 8-anilino-1-naphthalenesulfonate (ANS), to detect the fluorescence increase during protein denaturation, from which protein melting temperature (Tm) can be determined. Since the binding compounds usually thermally stabilize a protein, an increase in Tm is observed when increasing the compound concentration. The observed affinity of ligands for CAs was determined by plotting the Tm as a function of ligand concentration and then fitting a curve as described previously [48]. Samples consisted of constant concentration of CAs (5 µM for all proteins except 7.5 or 10 µM of CAIV) and varied concentrations of ligand ((0–200) µM) in 50 mM sodium phosphate buffer at pH 7.0 containing 100 mM NaCl, 50 µM ANS, and 2% (v/v) DMSO. Samples were heated from 25 °C to 99 °C at the speed of 1 °C/min. An example of an experiment is given in Figure 2.
3.2.3. Isothermal Titration Calorimetry (ITC)
ITC determined the observed standard enthalpy changes upon binding as well as the Kd_obs values [41] that were compared to ones obtained by FTSA. The ITC experiments were performed by adding the proteins to the calorimeter cell and the compound solutions to the syringe at 37 ºC. The CAII binding with compounds 28, 31, and 36 and CAVB with compound 32 (Figure S104) were determined using a MicroCal PEAQ-ITC calorimeter (Northampton, MA, USA). Experiments consisted of 19 injections, the first injection of 0.4 μL over 0.8 s, with the remaining injections of 2 μL over 4 s with spacing of 150 s between injections, at temperature 37 °C, reference power 10 μcal/s, and stirring speed of 750 rpm. The protein concentrations for CAII and CAVB were 10 μM and 8 μM, respectively; the compound concentration was 10 times higher than the protein (100 μM for compounds tested with CAII, 80 μM for compound tested with CAVB). Proteins and compounds were diluted in sodium phosphate 50 mM buffer at pH 7.0, containing 100 mM NaCl and 2% (v/v) DMSO. Data were analyzed using NITPIC (version 1.2.7) [49,50] and Sedphat (v. 14.0) [51] software; the heat of dilution was subtracted from the titration curves.
3.2.4. Determination of Compound Sulfonamide Group pKa
To calculate the intrinsic thermodynamic parameters of compound binding to CA isozymes, the pKa values of the sulfonamide group and the water molecule bound to the Zn(II) ion at the active site of CA proteins had to be determined. The pKa values for CA isozyme-bound water molecules were taken from [40], CAI—8.1; CAII—6.9; CAIII—6.5; CAIV—6.6; CAVA—6.7; CAVB—7.0; CAVI—6.0; CAVII—6.8; CAIX—6.6; CAXII—6.8; CAXIII—8.0; CAXIV—6.1. Compound pKa values were measured by obtaining UV-Vis spectra at 37 °C at different pH values (from 7.0 to 12.0 with 0.5 pH increment) using BMG Labtech CLARIOstarPlus plate reading spectrophotometer. Compounds were diluted to a constant concentration of 50–200 µM (concentration was chosen depending on compound solubility and absorbance peak) in a universal buffer, consisting of 50 mM sodium phosphate, 50 mM sodium acetate, 25 mM sodium borate, and 50 mM sodium chloride, and also contained 2% (v/v) DMSO. The pKa values were calculated as described previously in [28,52] by normalizing the absorbance, plotting it as a function of pH, then fitting to the Henderson–Hasselbalch equation using the least square method (Figure S105).
The pKas of compounds 27, 28, 29, 31, 32, 35, and 36 were measured, with an average pKa value of 10.1 for all compounds. Since the pKa values were only able to be measured for compound group 26–36, due to universal buffer pH limitations, intrinsic thermodynamic parameters were not calculated for compounds 2(a,b)–25(a,b), only for compounds 26–36 using experimentally obtained pKa values when possible and using the average pKa value mentioned above for compounds not measured.
3.2.5. Calculation of the Intrinsic Thermodynamic Parameters
The intrinsic thermodynamic parameters were calculated as described in [40]. The intrinsic reaction of inhibitor binding to CA is shown in Equation (1). The observed reaction includes the associated protonation–deprotonation reactions of protein, ligand, and buffer and thus has to be accounted for in order to make correct conclusions about compound binding affinities. It is known that CA proteins bind sulfonamides only when water molecules are coordinated to the Zn(II) ion, and sulfonamides only bind to CA proteins when their amino group is deprotonated (confirmed by neutron crystallography [53,54,55]):
When calculating Kd_int values, these protein and ligand fractions from Equation (1) have to be accounted for (Equations (2) and (3)). The intrinsic dissociation constant is calculated by multiplying Kd_obs value by the corresponding fractions of protein and ligand (Equation (4)).
The intrinsic standard Gibbs energy changes upon binding were calculated using Equation (5). Here, R—universal ideal gas constant (8.3144 J/mol·K), T—absolute temperature (310.15 K).
3.2.6. X-ray Crystallography: Crystallization, Data Collection, and Structure Determination
CAII was concentrated by ultrafiltration to 31 mg/mL and mixed with the crystallization solution (0.1 M sodium bicine (pH 9.0), 0.2 M ammonium sulfate, and 2 M sodium malonate (pH 7.0)) at a ratio 1:1 and crystallized by the sitting drop method. One μL of 50 mM compound solution in DMSO was combined with 50 μL of reservoir solution for crystals soaking.
X-ray diffraction data were collected at the EMBL beamline P13 at PETRA III ring of DESY synchrotron in Hamburg. Crystals were flash cooled to 100 K without additional cryo-protection. The data were processed and scaled using XDS [56], MOSFLM [57], and SCALA [58]. MOLREP [59] was used for molecular replacement, with an initial model of 4HT0. The model was refined with REFMAC [60] and fitted in the electron density map using COOT [61]. The 3D models of compounds were constructed by the AVOGADRO [62] program, while ligand parameter files were created using LIBCHECK [63,64]. The PDB IDs, data collection, and refinement statistics are listed in Table S2 in Supplementary Materials. The 2D schemes based on X-ray crystallography are shown as LigPlot+ [65] schemes in Figure S3.
4. Conclusions
A series of benzenesulfonamides bearing para-N β,γ-amino acid or para-N β-amino acid and thiazole moieties were synthesized, and the binding affinities for the entire family of 12 catalytically active human CA isozymes were determined by the fluorescent thermal shift assay. Selected compound affinities were also determined by isothermal titration calorimetry, while X-ray crystallography helped to determine the structural arrangement of the inhibitors in the active site of CAII. The intrinsic affinities that are independent of protonation reactions were determined according to the described model. The analysis showed that the chlorine atom in the meta-position of para-N β-amino acid bearing benzenesulfonamide derivatives increases the observed affinity for CA over non-chlorinated compounds, while the substituents located further away from this group of benzenesulfonamides have lower influence on the affinity. The most potent compound, 36, bearing para-N β-amino acid and thiazole moieties bound CAI and CAIX with 20 nM Kd_obs. Correlation of the chemical structures of the compounds with the intrinsic affinities showed that small changes in the chemical structure of the compound can have significant effects on the affinity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15040477/s1, Table S1: Intrinsic standard Gibbs energy change values for compounds binding to CAs; Table S2: X-ray crystallography data collection and refinement statistics; Figure S1: Determination of compound 32 binding to human CAs by the fluorescent thermal shift assay; Figure S2: The electron densities of the ligands in the active site of CAII; Figure S3: 2D schemes of interaction of compound 28 (PDB ID: 7QGZ), compound 31 (PDB ID: 7QGY), and compound 36 (PDB ID: 7QGX) with the active site of CAII; Figures S4–S103: 1H or 13C NMR of compounds at 400 MHz (DMSO-d6) or at 101 MHz (DMSO-d6), respectively; Figure S104: Isothermal titration calorimetry data of CAII binding with compounds 31, 28 and 36; Figure S105: pKa of sulfonamide amino group.
Author Contributions
Conceptualization, B.B., A.Z., D.M. and V.M. (Vytautas Mickevičius); methodology, B.B., T.Š., V.P.-L., V.M. (Vilma Michailovienė), A.M. and S.B.; software, S.G.; validation, V.M. (Vytautas Mickevičius), D.M.; Formal Analysis, B.B., T.Š., V.P.-L. and S.B.; Investigation, B.B., T.Š., V.P.-L., V.M. (Vilma Michailovienė), A.M. and S.B.; resources, V.M. (Vytautas Mickevičius) and D.M.; data curation, E.M. and V.K.; writing, B.B., T.Š. and V.P.-L.; writing—review and editing, E.M., A.Z., V.M. (Vytautas Mickevičius) and D.M.; visualization, B.B., T.Š. and V.P.-L.; supervision, V.M. (Vytautas Mickevičius) and D.M.; project administration, V.M. (Vytautas Mickevičius), A.Z., and D.M.; funding acquisition, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union Structural Funds co-financed project No. 01.2.2-CPVA-K-703-03-0006 implemented under measure of the European Union Funds Investment Operational Program for 2014–2020 No. 01.2.2-CPVA-K-703 “Promotion of centers of excellence and innovation and technology transfer centers” while implementing Priority axis 1, ”Strengthening research and development and innovation”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
Access to the EMBL beamline P13 at PETRA III (DESY) has been supported by iNEXT-Discovery, project number 871037, funded by the Horizon 2020 program of the European Commission.
Conflicts of Interest
D.M., A.Z. declare that they have several patents and patent applications on carbonic anhydrase inhibitors. Other authors declare that they have no conflicts of interest, including no financial, personal, or other relationships with other people or organizations.
References
- Meldrum, N.U.; Roughton, F.J.W. Carbonic anhydrase. Its preparation and properties. J. Physiol. 1933, 80, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, S.; Coleman, J.E. The catalytic mechanism of carbonic anhydrase. Proc. Natl. Acad. Sci. USA 1973, 70, 2505–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodgson, S.J. The Carbonic Anhydrases: Overview of Their Importance in Cellular Physiology and in Molecular Genetics. In The Carbonic Anhydrases: Cellular Physiology and Molecular Genetics; Dodgson, S.J., Tashian, R.E., Gros, G., Carter, N.D., Eds.; Springer: Boston, MA, USA, 1991; pp. 3–9. [Google Scholar]
- Parkkila, S. An overview of the distribution and function of carbonic anhydrase isozymes in mammals. In The Carbonic Anhydrases: New Horizons; Chegwidden, W.R., Carter, N.D., Edwards, Y.H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2000; pp. 79–93. [Google Scholar]
- Krishnamurthy, V.M.; Kaufman, G.K.; Urbach, A.R.; Gitlin, I.; Gudiksen, K.L.; Weibel, D.B.; Whitesides, G.M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein−Ligand Binding. Chem. Rev. 2008, 108, 946–1051. [Google Scholar] [CrossRef] [Green Version]
- McKenna, R.; Frost, S.C. Overview of the carbonic anhydrase family. Sub-Cell. Biochem. 2014, 75, 3–5. [Google Scholar] [CrossRef]
- Aspatwar, A.; Tolvanen, M.E.E.; Ortutay, C.; Parkkila, S. Carbonic anhydrase related proteins: Molecular biology and evolution. Sub-Cell. Biochem. 2014, 75, 135–156. [Google Scholar]
- Frost, S.C. Physiological functions of the alpha class of carbonic anhydrases. Sub-Cell. Biochem. 2014, 75, 9–30. [Google Scholar] [CrossRef]
- Chegwidden, W.R. The Carbonic anhydrases in Health and Disease. In The Carbonic Anhydrases: Current and Emerging Therapeutic Targets; Chegwidden, W.R., Carter, N.D., Eds.; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–12. [Google Scholar]
- Aggarwal, M.; Boone, C.D.; Kondeti, B.; McKenna, R. Structural annotation of human carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2013, 28, 267–277. [Google Scholar] [CrossRef]
- Pinard, M.A.; Mahon, B.; McKenna, R. Probing the Surface of Human Carbonic Anhydrase for Clues towards the Design of Isoform Specific Inhibitors. BioMed Res. Int. 2015, 2015, 453543. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, M.; Kondeti, B.; McKenna, R. Insights towards sulfonamide drug specificity in a-carbonic anhydrases. Bioorg. Med. Chem. 2013, 21, 1526–1533. [Google Scholar] [CrossRef] [Green Version]
- Mann, T.; Keilin, D. Sulphanilamide as a Specific Inhibitor of Carbonic Anhydrase. Nature 1940, 146, 164–165. [Google Scholar] [CrossRef]
- Poulsen, S.-A. Carbonic anhydrase inhibition as a cancer therapy: A review of patent literature, 2007–2009. Expert Opin. Ther. Pat. 2010, 20, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, M.; McKenna, R. Update on carbonic anhydrase inhibitors: A patent review (2008–2011). Expert Opin. Ther. Pat. 2012, 22, 903–915. [Google Scholar] [CrossRef]
- Carta, F.; Scozzafava, A.; Supuran, C.T. Sulfonamides: A patent review (2008–2012). Expert Opin. Ther. Pat. 2012, 22, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Novel therapies for glaucoma: A patent review 2007–2011. Expert Opin. Ther. Pat. 2012, 22, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T. Diuretics with carbonic anhydrase inhibitory action: A patent and literature review (2005–2013). Expert Opin. Ther. Pat. 2013, 23, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Monti, S.M.; Supuran, C.T.; De Simone, G. Anticancer carbonic anhydrase inhibitors: A patent review (2008–2013). Expert Opin. Ther. Pat. 2013, 23, 737–749. [Google Scholar] [CrossRef]
- Lomelino, C.; McKenna, R. Carbonic anhydrase inhibitors: A review on the progress of patent literature (2011–2016). Expert Opin. Ther. Pat. 2016, 26, 947–956. [Google Scholar] [CrossRef]
- Gulçin, İ.; Taslimi, P. Sulfonamide inhibitors: A patent review 2013-present. Expert Opin. Ther. Pat. 2018, 28, 541–549. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008–2018). Expert Opin. Ther. Pat. 2018, 28, 729–740. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Adrenergic agonists and antagonists as antiglaucoma agents: A literature and patent review (2013–2019). Expert Opin. Ther. Pat. 2019, 29, 805–815. [Google Scholar] [CrossRef]
- Supuran, C.T.; Altamimi, A.S.A.; Carta, F. Carbonic anhydrase inhibition and the management of glaucoma: A literature and patent review 2013–2019. Expert Opin. Ther. Pat. 2019, 29, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, P.; Carradori, S.; Campestre, C.; Poce, G. Novel therapies for glaucoma: A patent review (2013–2019). Expert Opin. Ther. Pat. 2019, 29, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Quan, J.; Zhao, Y.; Yang, D.; Zhao, Q.; Liu, P.; Cheng, M.; Ma, C. Design, synthesis and biological evaluation of 1-benzyl-5-oxopyrrolidine-2-carboximidamide derivatives as novel neuroprotective agents. Eur. J. Med. Chem. 2019, 182, 111654. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, L.W.; Abuel-Maaty, S.M.; Mohammed, W.A.; Galal, M.A. Synthesis and biological evaluation of new oxopyrrolidine derivatives as inhibitors of acetyl cholinesterase and β amyloid protein as anti—Alzheimer’s agents. Bioorg. Chem. 2018, 76, 210–217. [Google Scholar] [CrossRef]
- Vaškevičienė, I.; Paketurytė, V.; Pajanok, N.; Žukauskas, Š.; Sapijanskaitė, B.; Kantminienė, K.; Mickevičius, V.; Zubrienė, A.; Matulis, D. Pyrrolidinone-bearing methylated and halogenated benzenesulfonamides as inhibitors of carbonic anhydrases. Bioorg. Med. Chem. 2019, 27, 322–337. [Google Scholar] [CrossRef]
- Balandis, B.; Ivanauskaitė, G.; Smirnovienė, J.; Kantminienė, K.; Matulis, D.; Mickevičius, V.; Zubrienė, A. Synthesis and structure–affinity relationship of chlorinated pyrrolidinone-bearing benzenesulfonamides as human carbonic anhydrase inhibitors. Bioorg. Chem. 2020, 97, 103658. [Google Scholar] [CrossRef]
- Rutkauskas, K.; Zubrienė, A.; Tumosienė, I.; Kantminienė, K.; Mickevičius, V.; Matulis, D. Benzenesulfonamides bearing pyrrolidinone moiety as inhibitors of carbonic anhydrase IX: Synthesis and binding studies. Med. Chem. Res. 2017, 26, 235–246. [Google Scholar] [CrossRef]
- Vaškevičienė, I.; Paketurytė, V.; Zubrienė, A.; Kantminienė, K.; Mickevičius, V.; Matulis, D. N-Sulfamoylphenyl- and N-sulfamoylphenyl-N-thiazolyl-β-alanines and their derivatives as inhibitors of human carbonic anhydrases. Bioorg. Chem. 2017, 75, 16–29. [Google Scholar] [CrossRef]
- Peleckis, A.; Anusevičius, K.; Šiugždaitė, J.; Mickevičius, V. Nucleophilic Ring Opening Of 1,4-Disubstituted 2-Pyrrolidones with Hydrazine. Synthesis of Azoles with a High Antibacterial Activity. Chemija 2018, 29, 135–144. Available online: http://lmaleidykla.lt/ojs/index.php/chemija/article/view/3717 (accessed on 10 June 2021). [CrossRef]
- Parašotas, I.; Kantminienė, K.; Urbonavičiūtė, E.; Anusevičius, K.; Tumosienė, I.; Jonuškienė, I.; Vaickelionienė, R.; Mickevičius, V. Synthesis and Biological Evaluation of Novel Di- and Trisubstituted Thiazole Derivatives. Heterocycles 2017, 94, 1074. Available online: http://www.heterocycles.jp/newlibrary/libraries/abst/25320 (accessed on 10 June 2021). [CrossRef]
- Tumosienė, I.; Peleckis, A.; Jonuškienė, I.; Vaickelionienė, R.; Kantminienė, K.; Šiugždaitė, J.; Beresnevičius, Z.J.; Mickevičius, V. Synthesis of Novel 1,2- and 2-Substituted Benzimidazoles with High Antibacterial and Antioxidant Activity. Mon. Für Chem.-Chem. Mon. 2018, 149, 577–594. Available online: http://link.springer.com/10.1007/s00706-017-2066-x (accessed on 10 June 2021). [CrossRef]
- Gudmundsson, K.S.; Tidwell, J.; Lippa, N.; Koszalka, G.W.; van Draanen, N.; Ptak, R.G.; Drach, J.C.; Townsend, L.B. Synthesis and Antiviral Evaluation of Halogenated β-d- and -l-Erythrofuranosylbenzimidazoles. J. Med. Chem. 2000, 43, 2464–2472. Available online: https://pubs.acs.org/doi/10.1021/jm990195p (accessed on 10 June 2021). [CrossRef] [PubMed]
- Pantoliano, M.W.; Petrella, E.C.; Kwasnoski, J.D.; Lobanov, V.S.; Myslik, J.; Graf, E.; Carver, T.; Asel, E.; Springer, B.A.; Lane, P.; et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen. 2001, 6, 429–440. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, P.A.; Yanchunas, J.; Newitt, J.A.; Tao, L.; Kiefer, S.E.; Ortega, M.; Kut, S.; Burford, N.; Goldfarb, V.; Duke, G.J.; et al. Assessing compound binding to the Eg5 motor domain using a thermal shift assay. Anal. Biochem. 2009, 392, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Oerlemans, R.; Groves, M.R. Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophys. Rev. 2020, 12, 85–104. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.E.; Spry, C.; Abell, C. Differential Scanning Fluorimetry as Part of a Biophysical Screening Cascade. In Methods and Principles in Medicinal Chemistry; Erlanson, D.A., Jahnke, W., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2016; pp. 139–172. [Google Scholar] [CrossRef]
- Zubrienė, A.; Matulis, D. Observed Versus Intrinsic Thermodynamics of Inhibitor Binding to Carbonic Anhydrases. In Carbonic Anhydrase as Drug Target: Thermodynamics and Structure of Inhibitor Binding; Matulis, D., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 107–123. [Google Scholar] [CrossRef]
- Leavitt, S.; Freire, E. Direct Measurement of Protein Binding Energetics by Isothermal Titration Calorimetry. Curr. Opin. Struct. Biol. 2001, 11, 560–566. [Google Scholar] [CrossRef]
- Taylor, P.W.; King, R.W.; Burgen, A.S.V. Influence of pH on the kinetics of complex formation between aromatic sulfonamides and human carbonic anhydrase. Biochemistry 1970, 9, 3894–3902. [Google Scholar] [CrossRef]
- Engberg, P.; Lindskog, S. Effects of pH and inhibitors on the absorption spectrum of cobalt(II)-substituted carbonic anhydrase III from bovine skeletal muscle. FEBS Lett. 1984, 170, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickevičiūtė, A.; Juozapaitienė, V.; Michailovienė, V.; Jachno, J.; Matulienė, J.; Matulis, D. Recombinant Production of 12 Catalytically Active Human CA Isoforms. In Carbonic Anhydrase as Drug Target: Thermodynamics and Structure of Inhibitor Binding; Matulis, D., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 15–37. [Google Scholar] [CrossRef]
- Cimmperman, P.; Baranauskienė, L.; Jachimovičiūtė, S.; Jachno, J.; Torresan, J.; Michailovienė, V.; Matulienė, J.; Sereikaitė, J.; Bumelis, V.; Matulis, D. A Quantitative Model of Thermal Stabilization and Destabilization of Proteins by Ligands. Biophys. J. 2008, 95, 3222–3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, S.; Vargas, C.; Zhao, H.; Piszczek, G.; Brautigam, C.A.; Schuck, P. High-Precision Isothermal Titration Calorimetry with Automated Peak Shape Analysis. Anal. Chem. 2012, 84, 5066–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brautigam, C.A.; Zhao, H.; Vargas, C.; Keller, S.; Schuck, P. Integration and global analysis of isothermal titration calorimetry data for studying macromolecular interactions. Nat. Protocols. 2016, 11, 882–894. [Google Scholar] [CrossRef]
- Zhao, H.; Piszczek, G.; Schuck, P. SEDPHAT—A platform for global ITC analysis and global multi-method analysis of molecular interactions. Methods 2015, 76, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Snyder, P.W.; Mecinović, J.; Moustakas, D.T.; Thomas, S.W.; Harder, M.; Mack, E.T.; Lockett, M.R.; Héroux, A.; Sherman, W.; Whitesides, G.M. Mechanism of the hydrophobic effect in the biomolecular recognition of arylsulfonamides by carbonic anhydrase. Proc. Natl. Acad. Sci. USA 2011, 108, 17889–17894. [Google Scholar] [CrossRef] [Green Version]
- Fisher, S.Z.; Aggarwal, M.; Kovalevsky, A.Y.; Silverman, D.N.; McKenna, R. Neutron Diffraction of Acetazolamide-Bound Human Carbonic Anhydrase II Reveals Atomic Details of Drug Binding. J. Am. Chem. Soc. 2012, 134, 14726–14729. [Google Scholar] [CrossRef] [Green Version]
- Kovalevsky, A.; Aggarwal, M.; Velazquez, H.; Cuneo, M.J.; Blakeley, M.P.; Weiss, K.L.; Smith, J.C.; Fisher, S.Z.; McKenna, R. “To Be or Not to Be” Protonated: Atomic Details of Human Carbonic Anhydrase-Clinical Drug Complexes by Neutron Crystallography and Simulation. Structure 2018, 26, 383–390.e3. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, M.; Kovalevsky, A.Y.; Velazquez, H.; Fisher, S.Z.; Smith, J.C.; McKenna, R. Neutron structure of human carbonic anhydrase II in complex with methazolamide: Mapping the solvent and hydrogen-bonding patterns of an effective clinical drug. IUCrJ. 2016, 3, 319–325. [Google Scholar] [CrossRef]
- Kabsch, W. XDS . Acta Crystallogr. Sect. D 2010, D66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Vagin, A.; Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of ıt Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Lebedev, A.A.; Young, P.; Isupov, M.N.; Moroz, O.V.; Vagin, A.A.; Murshudov, G.N. JLigand: A graphical tool for the CCP4 template-restraint library. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Vagin, A.A.; Steiner, R.A.; Lebedev, A.A.; Potterton, L.; McNicholas, S.; Long, F.; Murshudov, G.N. REFMAC5 dictionary: Organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2184–2195. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).