Cytotoxicity of Thioalkaloid-Enriched Nuphar lutea Extract and Purified 6,6′-Dihydroxythiobinupharidine in Acute Myeloid Leukemia Cells: The Role of Oxidative Stress and Intracellular Calcium
Abstract
:1. Introduction
2. Results
2.1. Thioalkaloid-Enriched Fraction from the N. lutea Leaf Extract (NUP) Inhibits the Growth of AML Cells
2.2. NUP Induces Caspase-Dependent Apoptosis
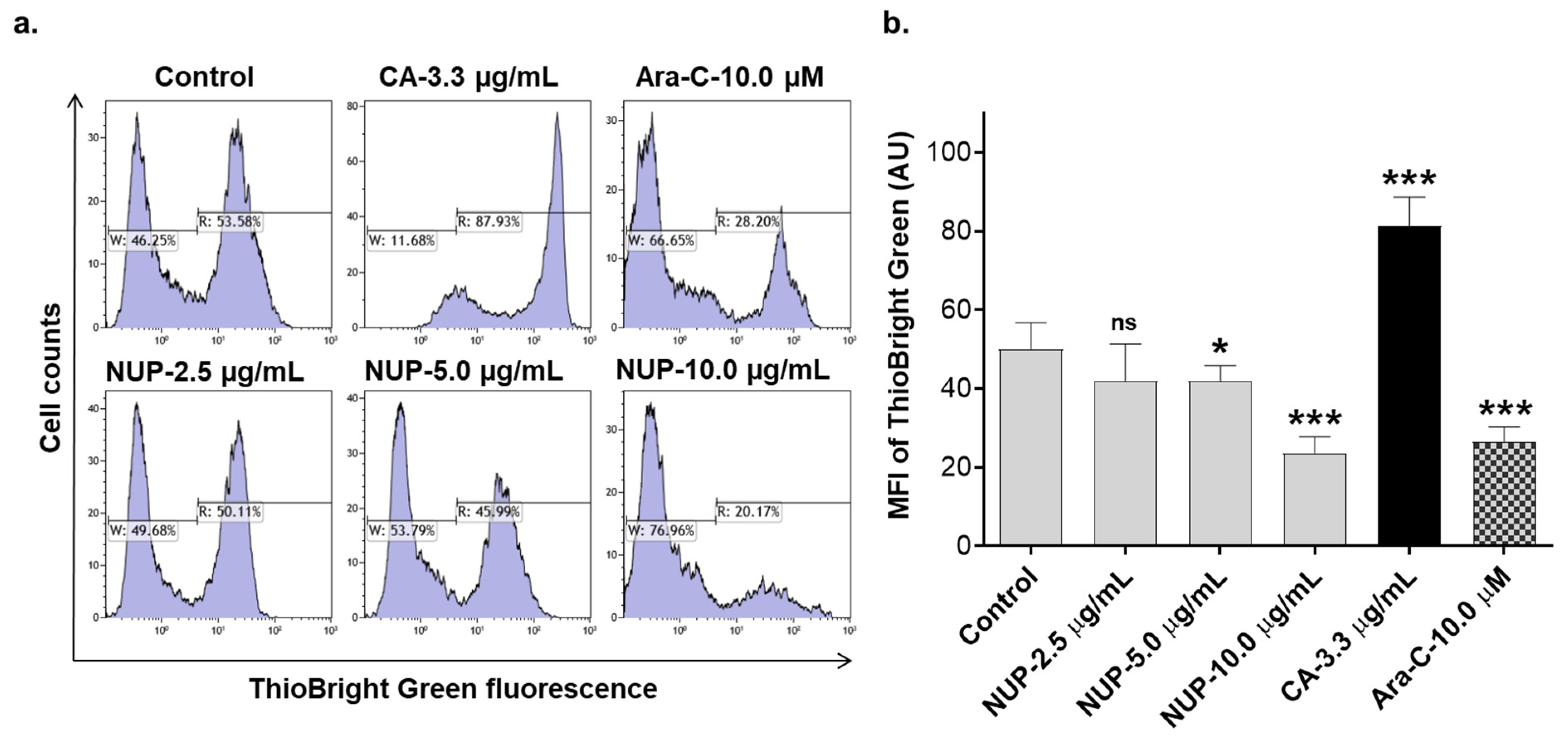
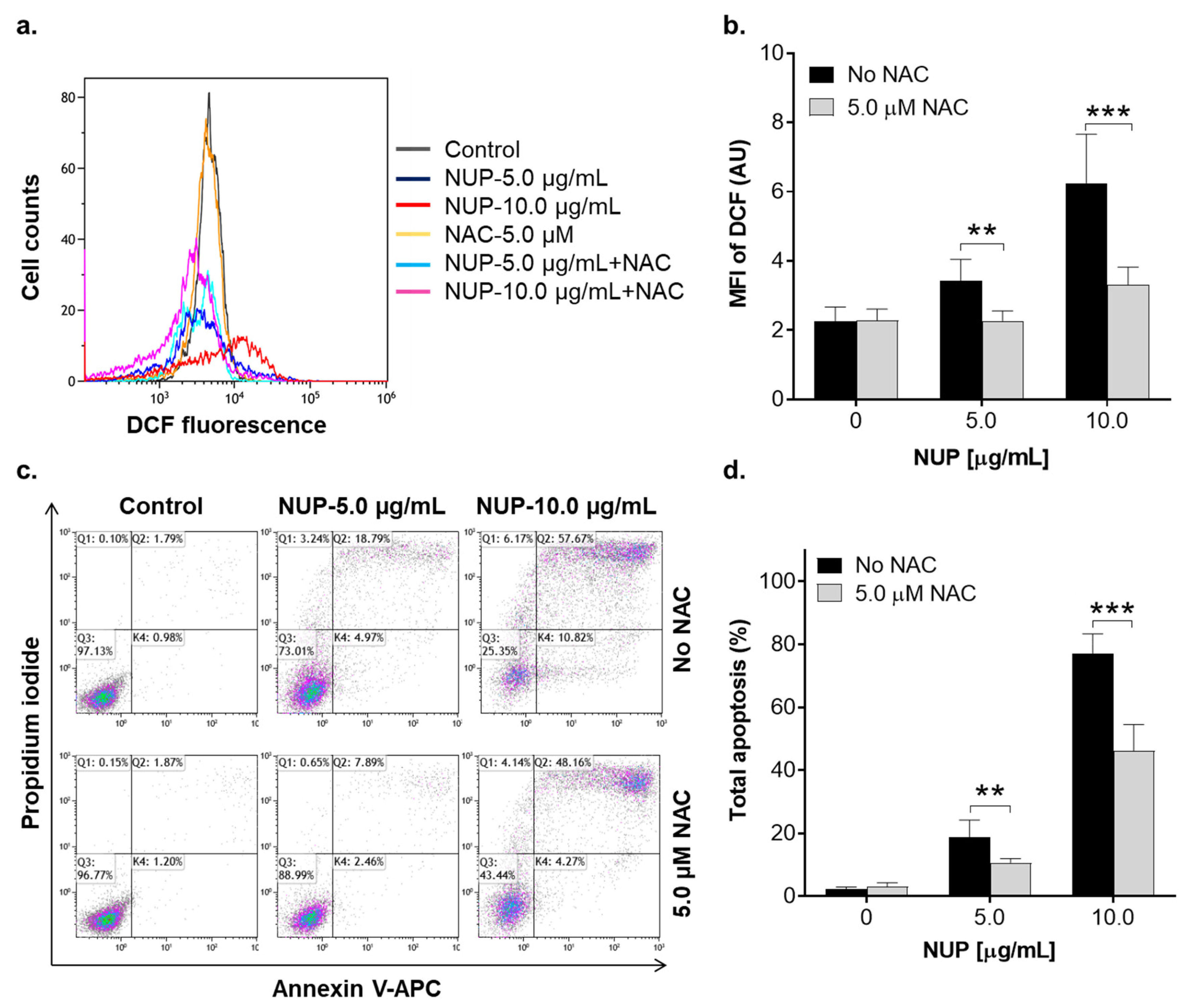
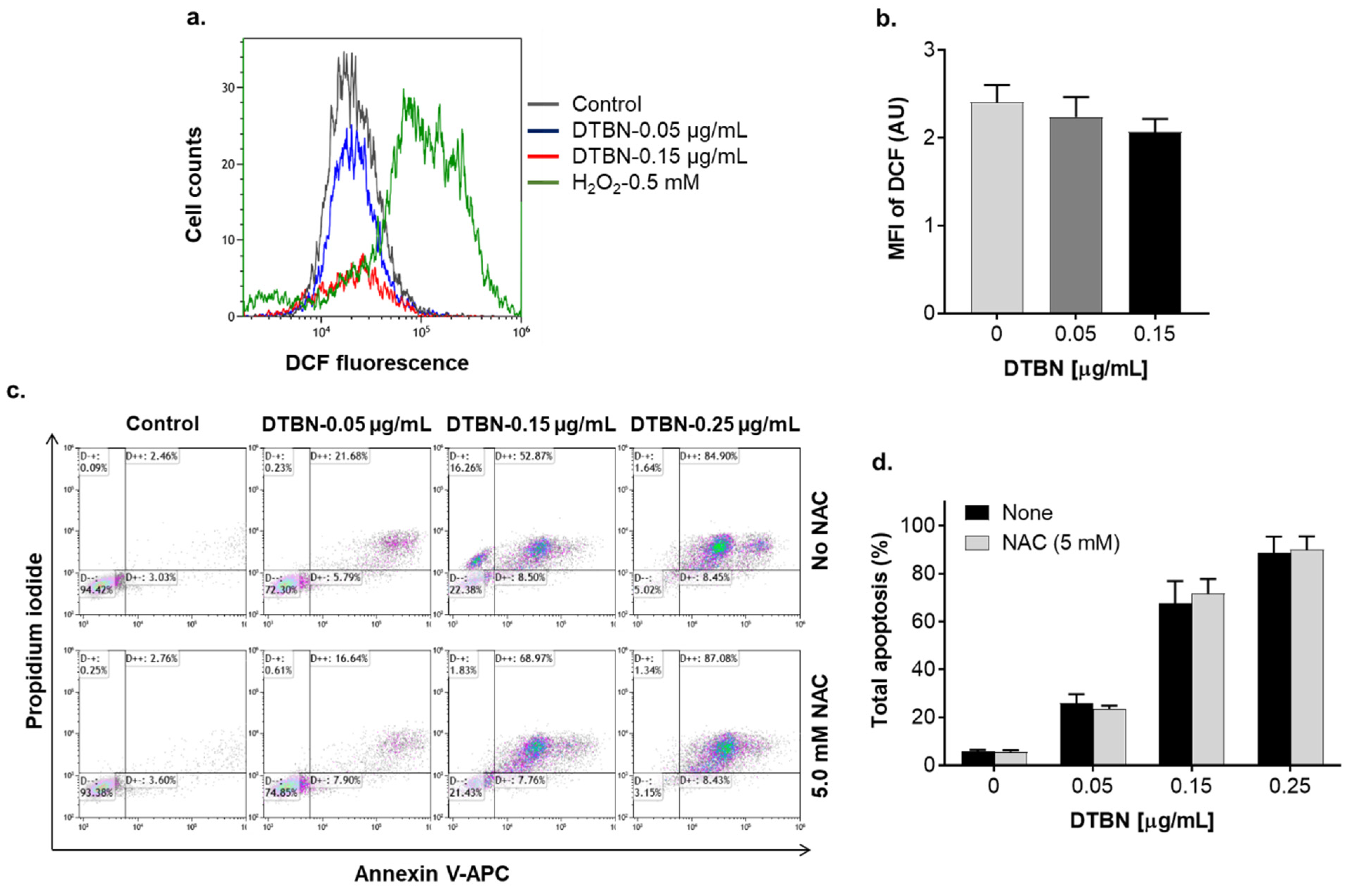
2.3. Involvement of Reactive Oxygen Species in NUP-Induced Apoptosis
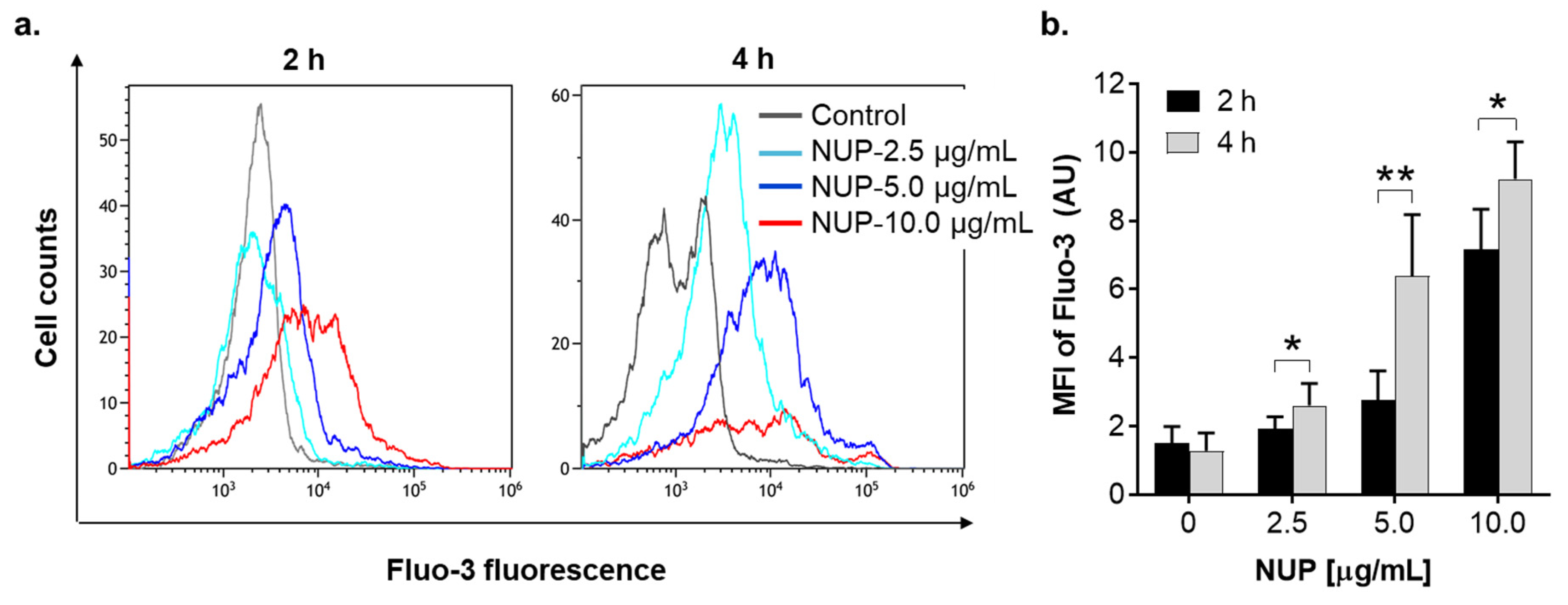
2.4. The Role of Changes in Ca2+cyt Levels in NUP-Induced Apoptosis
2.5. Comparison of the Proapoptotic Effects of NUP and Purified 6,6′-Dihydroxythiobinupharidine
2.6. Effects of NUP and Purified 6,6′-Dihydroxythiobinupharidine on Cell Death and Viability of Peripheral Blood Mononuclear Cells from Healthy Donors
3. Discussion
3.1. Materials
3.2. Preparation of Nuphar lutea Extract and Its Fractions Containing Sesquiterpene Thioalkaloids
3.3. Cell Culture and Enumeration
3.4. Assessment of Apoptosis by Annexin-V and Propidium Iodide Staining
3.5. Preparation of Whole Cell Lysates and Western Blot Analysis
3.6. Determination of Intracellular Levels of Reactive Oxygen Species (ROS)
3.7. Determination of Intracellular Glutathione (GSH) Levels
3.8. Measurements of Steady-State Cytosolic Ca2+ Levels
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Lowenberg, B. Towards precision medicine for AML. Nat. Rev. Clin. Oncol. 2021, 18, 577–590. [Google Scholar] [CrossRef]
- Estey, E.; Karp, J.E.; Emadi, A.; Othus, M.; Gale, R.P. Recent drug approvals for newly diagnosed acute myeloid leukemia: Gifts or a Trojan horse? Leukemia 2020, 34, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.M.; Still, P.C.; Perez, L.B.; Grever, M.R.; Kinghorn, A.D. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr. Drug Targets 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [Green Version]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Busselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaikwad, S.; Srivastava, S.K. Role of Phytochemicals in Perturbation of Redox Homeostasis in Cancer. Antioxidants 2021, 10, 83. [Google Scholar] [CrossRef]
- Zhamanbayeva, G.T.; Aralbayeva, A.N.; Murzakhmetova, M.K.; Tuleukhanov, S.T.; Danilenko, M. Cooperative antiproliferative and differentiation-enhancing activity of medicinal plant extracts in acute myeloid leukemia cells. Biomed. Pharmacother. = Biomed. Pharmacother. 2016, 82, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ossikbayeva, S.; Khanin, M.; Sharoni, Y.; Trachtenberg, A.; Tuleukhanov, S.; Sensenig, R.; Rom, S.; Danilenko, M.; Orynbayeva, Z. Curcumin and Carnosic Acid Cooperate to Inhibit Proliferation and Alter Mitochondrial Function of Metastatic Prostate Cancer Cells. Antioxidants 2021, 10, 1591. [Google Scholar] [CrossRef]
- Pesakhov, S.; Khanin, M.; Studzinski, G.P.; Danilenko, M. Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutr. Cancer 2010, 62, 811–824. [Google Scholar] [CrossRef]
- Vue, B.; Zhang, S.; Chen, Q.H. Synergistic Effects of Dietary Natural Products as Anti-Prostate Cancer Agents. Nat. Prod. Commun. 2015, 10, 2179–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini-Zare, M.S.; Sarhadi, M.; Zarei, M.; Thilagavathi, R.; Selvam, C. Synergistic effects of curcumin and its analogs with other bioactive compounds: A comprehensive review. Eur. J. Med. Chem. 2021, 210, 113072. [Google Scholar] [CrossRef] [PubMed]
- Pesakhov, S.; Nachliely, M.; Barvish, Z.; Aqaqe, N.; Schwartzman, B.; Voronov, E.; Sharoni, Y.; Studzinski, G.P.; Fishman, D.; Danilenko, M. Cancer-selective cytotoxic Ca2+ overload in acute myeloid leukemia cells and attenuation of disease progression in mice by synergistically acting polyphenols curcumin and carnosic acid. Oncotarget 2016, 7, 31847–31861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachtenberg, A.; Muduli, S.; Sidoryk, K.; Cybulski, M.; Danilenko, M. Synergistic Cytotoxicity of Methyl 4-Hydroxycinnamate and Carnosic Acid to Acute Myeloid Leukemia Cells via Calcium-Dependent Apoptosis Induction. Front. Pharmacol. 2019, 10, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; O’Brien, S.; Cortes, J. Homoharringtonine/omacetaxine mepesuccinate: The long and winding road to food and drug administration approval. Clin. Lymphoma Myeloma Leuk. 2013, 13, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Norsworthy, K.J.; By, K.; Subramaniam, S.; Zhuang, L.; Del Valle, P.L.; Przepiorka, D.; Shen, Y.L.; Sheth, C.M.; Liu, C.; Leong, R.; et al. FDA Approval Summary: Glasdegib for Newly Diagnosed Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 6021–6025. [Google Scholar] [CrossRef] [Green Version]
- Ozer, J.; Eisner, N.; Ostrozhenkova, E.; Bacher, A.; Eisenreich, W.; Benharroch, D.; Golan-Goldhirsh, A.; Gopas, J. Nuphar lutea thioalkaloids inhibit the nuclear factor kappaB pathway, potentiate apoptosis and are synergistic with cisplatin and etoposide. Cancer Biol. Ther. 2009, 8, 1860–1868. [Google Scholar] [CrossRef] [Green Version]
- Ozer, J.; Fishman, D.; Eilam, B.; Golan-Goldhirsh, A.; Gopas, J. Anti-Metastatic Effect of Semi-Purified Nuphar Lutea Leaf Extracts. J. Cancer 2017, 8, 1433–1440. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Yoshida, K.; Miyagawa, K.; Nemoto, Y.; Asao, Y.; Yoshikawa, M. Nuphar alkaloids with immediately apoptosis-inducing activity from Nuphar pumilum and their structural requirements for the activity. Bioorg. Med. Chem. Lett. 2006, 16, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, M.; Nakamura, S.; Hegazy, M.E.F.; Sugimoto, Y.; Hayashi, N.; Nakashima, S.; Yoshikawa, M.; Efferth, T.; Matsuda, H. Cytotoxicity of sesquiterpene alkaloids from Nuphar plants toward sensitive and drug-resistant cell lines. Food Funct. 2018, 9, 6279–6286. [Google Scholar] [CrossRef] [PubMed]
- Mallick, D.J.; Korotkov, A.; Li, H.; Wu, J.; Eastman, A. Nuphar alkaloids induce very rapid apoptosis through a novel caspase-dependent but BAX/BAK-independent pathway. Cell Biol. Toxicol. 2019, 35, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.H.; Chapple, I.L.C.; Shapira, L.; Golan-Goldhirsh, A.; Gopas, J.; Polak, D. Nupharidine enhances Aggregatibacter actinomycetemcomitans clearance by priming neutrophils and augmenting their effector functions. J. Clin. Periodontol. 2019, 46, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.M. Gitksan medicinal plants--cultural choice and efficacy. J. Ethnobiol. Ethnomed. 2006, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- El Beyrouthy, M.; Arnold, N.; Delelis-Dusollier, A.; Dupont, F. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar] [CrossRef]
- Nakae, H.; Yokoi, A.; Kodama, H.; Horikawa, A. Comparison of the Effects on Rib Fracture between the Traditional Japanese Medicine Jidabokuippo and Nonsteroidal Anti-Inflammatory Drugs: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 837958. [Google Scholar] [CrossRef] [Green Version]
- Uprety, Y.; Lacasse, A.; Asselin, H. Traditional Uses of Medicinal Plants from the Canadian Boreal Forest for the Management of Chronic Pain Syndromes. Pain Pract. 2016, 16, 459–466. [Google Scholar] [CrossRef]
- Waidha, K.; Zurgil, U.; Ben-Zeev, E.; Gopas, J.; Rajendran, S.; Golan-Goldhirsh, A. Inhibition of Cysteine Proteases by 6,6′-Dihydroxythiobinupharidine (DTBN) from Nuphar lutea. Molecules 2021, 26, 4743. [Google Scholar] [CrossRef]
- Yildirim, A.B.; Karakas, F.P.; Turker, A.U. In vitro antibacterial and antitumor activities of some medicinal plant extracts, growing in Turkey. Asian Pac. J. Trop. Med. 2013, 6, 616–624. [Google Scholar] [CrossRef]
- Okamura, S.; Nishiyama, E.; Yamazaki, T.; Otsuka, N.; Taniguchi, S.; Ogawa, W.; Hatano, T.; Tsuchiya, T.; Kuroda, T. Action mechanism of 6,6′-dihydroxythiobinupharidine from Nuphar japonicum, which showed anti-MRSA and anti-VRE activities. Biochim. Biophys Acta 2015, 1850, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Winer, H.; Ozer, J.; Shemer, Y.; Reichenstein, I.; Eilam-Frenkel, B.; Benharroch, D.; Golan-Goldhirsh, A.; Gopas, J. Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus. Molecules 2020, 25, 1657. [Google Scholar] [CrossRef] [Green Version]
- Cullen, W.P.; LaLonde, R.T.; Wang, C.J.; Wong, C.F. Isolation and in vitro antifungal activity of 6,6′-dihydroxythiobinupharidine. J. Pharm. Sci. 1973, 62, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jacob, M.; Walker, L.; Tekwani, B. Screening North American plant extracts in vitro against Trypanosoma brucei for discovery of new antitrypanosomal drug leads. BMC Complement. Altern. Med. 2016, 16, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalvie, E.D.; Gopas, J.; Golan-Goldhirsh, A.; Osheroff, N. 6,6′-Dihydroxythiobinupharidine as a poison of human type II topoisomerases. Bioorg. Med. Chem. Lett. 2019, 29, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Akhtar, S.; Mateo, J.M.; Merhi, M.; Taha, R.; Omri, H.E.; Mraiche, F.; et al. Sanguinarine suppresses growth and induces apoptosis in childhood acute lymphoblastic leukemia. Leuk. Lymphoma 2019, 60, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Swamy, S.M.; Huat, B.T. Intracellular glutathione depletion and reactive oxygen species generation are important in alpha-hederin-induced apoptosis of P388 cells. Mol. Cell. Biochem. 2003, 245, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Thastrup, O.; Cullen, P.J.; Drobak, B.K.; Hanley, M.R.; Dawson, A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 1990, 87, 2466–2470. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, D.; Ye, B.; Zhong, F.; Chen, G. Curcumin induces the apoptosis of non-small cell lung cancer cells through a calcium signaling pathway. Int. J. Mol. Med. 2015, 35, 1610–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, S.; Fas, S.C.; Giaisi, M.; Muller, W.W.; Merling, A.; Gulow, K.; Edler, L.; Krammer, P.H.; Li-Weber, M. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis. Blood 2008, 111, 2354–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.J.; Kim, E.H.; Kwon, T.K.; Park, S.A.; Choi, K.S. Simultaneous mitochondrial Ca2+ overload and proteasomal inhibition are responsible for the induction of paraptosis in malignant breast cancer cells. Cancer Lett. 2012, 324, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Doughty, H.P.; Eells, A.J.; Johnson, T.A.; Hastings, J.P.; Crowther, C.M.; Andrus, M.B.; Kenealey, J.D. The Effects of 4′-Esterified Resveratrol Derivatives on Calcium Dynamics in Breast Cancer Cells. Molecules 2017, 22, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachtenberg, A.; Sidoryk, K.; Alreate, S.; Muduli, S.; Les, A.; Cybulski, M.; Danilenko, M. Structure-Activity Relationship of Hydroxycinnamic Acid Derivatives for Cooperating with Carnosic Acid and Calcitriol in Acute Myeloid Leukemia Cells. Biomedicines 2021, 9, 1517. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, W.C.; Wong, K.L.; Cheng, K.S.; Leung, Y.M.; Yang, S.E. Gossypol stimulates opening of a Ca2+- and Na+-permeable but Ni2+- and Co2+-impermeable pore in bEND.3 endothelial cells. Clin. Exp. Pharmacol. Physiol. 2018, 45, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Vancauwenberghe, E.; Noyer, L.; Derouiche, S.; Lemonnier, L.; Gosset, P.; Sadofsky, L.R.; Mariot, P.; Warnier, M.; Bokhobza, A.; Slomianny, C.; et al. Activation of mutated TRPA1 ion channel by resveratrol in human prostate cancer associated fibroblasts (CAF). Mol. Carcinog. 2017, 56, 1851–1867. [Google Scholar] [CrossRef]
- Baguley, B.C.; Drummond, C.J.; Chen, Y.Y.; Finlay, G.J. DNA-Binding Anticancer Drugs: One Target, Two Actions. Molecules 2021, 26, 552. [Google Scholar] [CrossRef] [PubMed]
- Waidha, K.; Anto, N.P.; Jayaram, D.R.; Golan-Goldhirsh, A.; Rajendran, S.; Livneh, E.; Gopas, J. 6,6′-Dihydroxythiobinupharidine (DTBN) Purified from Nuphar lutea Leaves Is an Inhibitor of Protein Kinase C Catalytic Activity. Molecules 2021, 26, 2785. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Kabir, N.N.; Ronnstrand, L. Protein kinase C (PKC) as a drug target in chronic lymphocytic leukemia. Med. Oncol. 2013, 30, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiong, L.; Yu, G.; Li, D.; Peng, T.; Luo, D.; Xu, J. Cathepsin S silencing induces apoptosis of human hepatocellular carcinoma cells. Am. J. Transl. Res. 2015, 7, 100–110. [Google Scholar]
- Fei, M.; Zhang, L.; Wang, H.; Zhu, Y.; Niu, W.; Tang, T.; Han, Y. Inhibition of Cathepsin S Induces Mitochondrial Apoptosis in Glioblastoma Cell Lines Through Mitochondrial Stress and Autophagosome Accumulation. Front. Oncol. 2020, 10, 516746. [Google Scholar] [CrossRef] [PubMed]








| Cell Line | 24 h | 48 h | 72 h |
|---|---|---|---|
| KG-1a | 4.06 ± 0.33 | 1.21 ± 0.15 | 0.90 ± 0.58 |
| HL60 | 2.40 ± 0.14 | 1.03 ± 0.14 | 1.09 ± 0.13 |
| U937 | 1.65 ± 0.15 | 0.97 ± 0.18 | 0.89 ± 0.05 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muduli, S.; Golan-Goldhirsh, A.; Gopas, J.; Danilenko, M. Cytotoxicity of Thioalkaloid-Enriched Nuphar lutea Extract and Purified 6,6′-Dihydroxythiobinupharidine in Acute Myeloid Leukemia Cells: The Role of Oxidative Stress and Intracellular Calcium. Pharmaceuticals 2022, 15, 410. https://doi.org/10.3390/ph15040410
Muduli S, Golan-Goldhirsh A, Gopas J, Danilenko M. Cytotoxicity of Thioalkaloid-Enriched Nuphar lutea Extract and Purified 6,6′-Dihydroxythiobinupharidine in Acute Myeloid Leukemia Cells: The Role of Oxidative Stress and Intracellular Calcium. Pharmaceuticals. 2022; 15(4):410. https://doi.org/10.3390/ph15040410
Chicago/Turabian StyleMuduli, Suchismita, Avi Golan-Goldhirsh, Jacob Gopas, and Michael Danilenko. 2022. "Cytotoxicity of Thioalkaloid-Enriched Nuphar lutea Extract and Purified 6,6′-Dihydroxythiobinupharidine in Acute Myeloid Leukemia Cells: The Role of Oxidative Stress and Intracellular Calcium" Pharmaceuticals 15, no. 4: 410. https://doi.org/10.3390/ph15040410
APA StyleMuduli, S., Golan-Goldhirsh, A., Gopas, J., & Danilenko, M. (2022). Cytotoxicity of Thioalkaloid-Enriched Nuphar lutea Extract and Purified 6,6′-Dihydroxythiobinupharidine in Acute Myeloid Leukemia Cells: The Role of Oxidative Stress and Intracellular Calcium. Pharmaceuticals, 15(4), 410. https://doi.org/10.3390/ph15040410







