Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects
Abstract
:1. Introduction
2. Effects of Icaritin on Osteoporosis
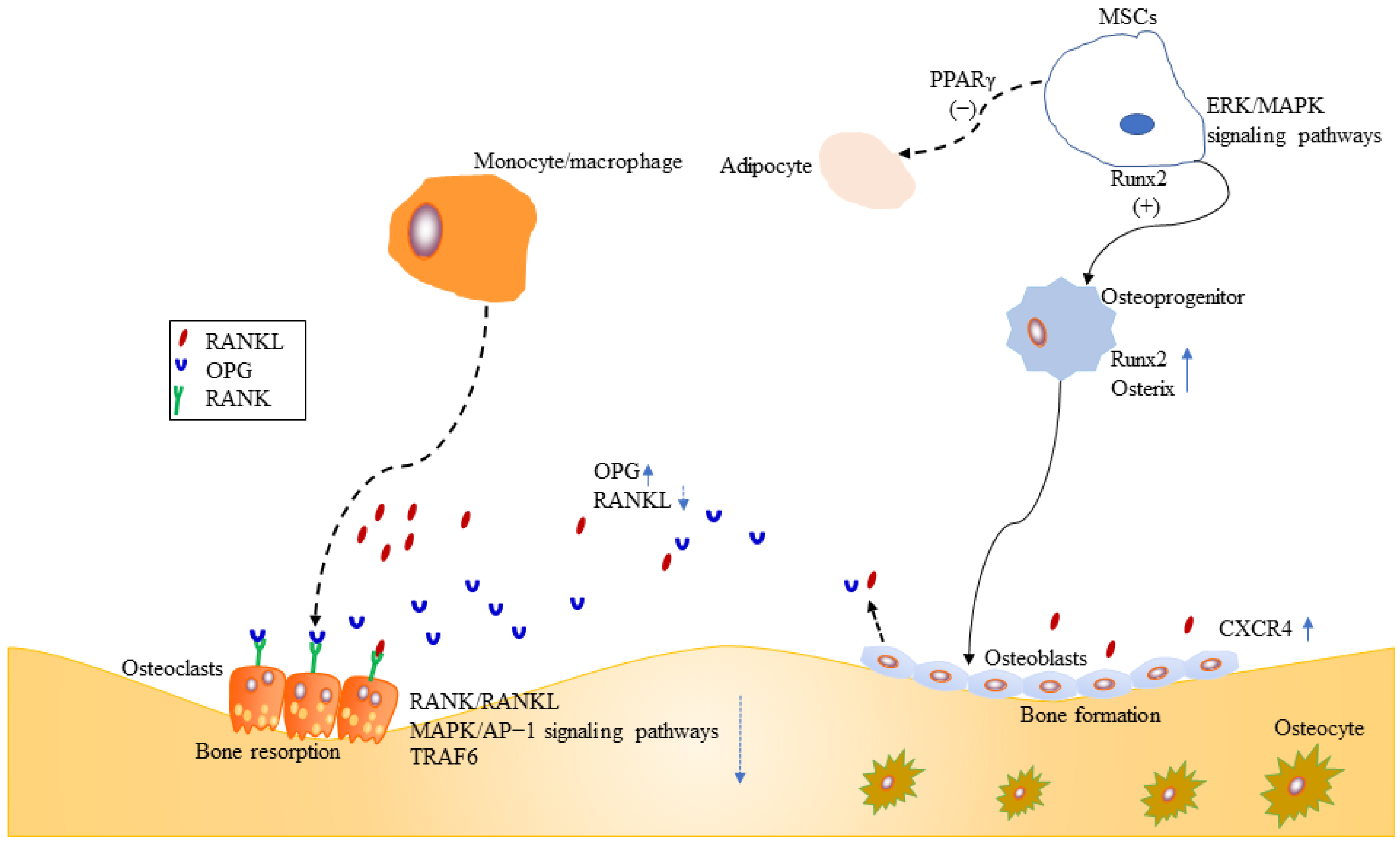
2.1. Mesenchymal Stromal Cells
2.2. Osteoblasts
2.3. Osteoclasts
2.4. Inflammation and Osteoporosis
2.5. Animal Bone Defect Model
3. Pharmacokinetics of Icaritin
4. Drug Delivery Systems
4.1. Liposomes
4.2. Micelles
4.3. Nanoparticles, Nanorods, and Nanocrystals
4.4. Hydrogels
4.5. Bioactive Scaffolds
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| CXCR4 | cysteine-X-C motif chemokine receptor 4 |
| G3 | icaritin-3-glucuronide |
| G5 | icaritin-5-glucuronide |
| G7 | icaritin-7-glucuronide |
| G37 | icaritin-3,7-diglucuronide |
| HCC | hepatocellular carcinoma |
| HSCs | hematopoietic stem cells |
| ICT | Icaritin |
| mPEG | methyl PEG |
| MSCs | mesenchymal stromal cells |
| OPG | osteoprotegerin |
| PEG2000 | polyethylene glycol 2000 |
| PLA | polylactic acid |
| RANK | receptor activator of NF-κB |
| RANKL | receptor activator of nuclear factor-κB ligand |
| SDF-1 | stromal cell-derived factor-1 |
| SDSSD | Ser-Asp-Ser-Ser-Asp |
| TNF | tumor necrosis factor |
| UGT | UDP-glucuronosyltransferase enzymes |
References
- Ordikhani, F.; Zandi, N.; Mazaheri, M.; Luther, G.A.; Ghovvati, M.; Akbarzadeh, A.; Annabi, N. Targeted nanomedicines for the treatment of bone disease and regeneration. Med. Res. Rev. 2021, 41, 1221–1254. [Google Scholar] [CrossRef] [PubMed]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone diseases: Current approach and future perspectives in drug delivery systems for bone targeted therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cornelissen, D.; Silverman, S.; Pinto, D.; Si, L.; Kremer, I.; Bours, S.; de Bot, R.; Boonen, A.; Evers, S.; et al. An Updated Systematic Review of Cost-Effectiveness Analyses of Drugs for Osteoporosis. Pharmacoeconomics 2021, 39, 181–209. [Google Scholar] [CrossRef]
- Zhang, S.Q. Biodistribution evaluation of icaritin in rats by ultra-performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2014, 155, 1382–1387. [Google Scholar] [CrossRef]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Comission, C.P. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science Press: Beijing, China, 2020; Volume I. [Google Scholar]
- Zhang, S.Q. Ultra-high performance liquid chromatography-tandem mass spectrometry for the quantification of icaritin in mouse bone. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 978–979, 24–28. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Zhang, S.Z. Oral absorption, distribution, metabolism, and excretion of icaritin in rats by Q-TOF and UHPLC-MS/MS. Drug Test. Anal. 2017, 9, 1604–1610. [Google Scholar] [CrossRef]
- Sheng, H.; Rui, X.F.; Sheng, C.J.; Li, W.J.; Cheng, X.Y.; Jhummon, N.P.; Yu, Y.C.; Qu, S.; Zhang, G.; Qin, L. A novel semisynthetic molecule icaritin stimulates osteogenic differentiation and inhibits adipogenesis of mesenchymal stem cells. Int. J. Med. Sci. 2013, 10, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, X.; Wang, H.; Qi, L.; Lou, Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience 2007, 145, 911–922. [Google Scholar] [CrossRef]
- Wo, Y.B.; Zhu, D.Y.; Hu, Y.; Wang, Z.Q.; Liu, J.; Lou, Y.J. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J. Cell. Biochem. 2008, 103, 1536–1550. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.C.; Wu, Y.; Gao, X.; Qiao, L.; Yang, Y.; Li, F.; Zou, J.; Wang, Y.H.; Zhang, S.Y.; Li, C.L.; et al. The antitumor effects of icaritin against breast cancer is related to estrogen receptors. Curr. Mol. Med. 2020, 21, 73–85. [Google Scholar] [CrossRef]
- Lai, X.; Ye, Y.; Sun, C.; Huang, X.; Tang, X.; Zeng, X.; Yin, P.; Zeng, Y. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. Int. Immunopharmacol. 2013, 16, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.K.; Li, Q.; Ming Xu, J.; Liang, J.; Cheng, Y.; Fan, Y.; Jiang, J.; Ye, H.; Tao, H.; Li, L.; et al. Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: Immunodynamic biomarkers and overall survival. Cancer Sci. 2020, 111, 4218–4231. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.G.; Zhang, X.; Zhou, Y.P.; Lu, C.; Thu, P.M.; Qian, C.; Zhang, M.; Li, P.; Li, H.J.; Xu, X. Anhydroicaritin, a SREBPs inhibitor, inhibits RANKL-induced osteoclastic differentiation and improves diabetic osteoporosis in STZ-induced mice. Eur. J. Pharmacol. 2017, 809, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Indran, I.R.; Liang, R.L.; Min, T.E.; Yong, E.L. Preclinical studies and clinical evaluation of compounds from the genus Epimedium for osteoporosis and bone health. Pharmacol. Ther. 2016, 162, 188–205. [Google Scholar] [CrossRef]
- Jimi, E.; Hirata, S.; Osawa, K.; Terashita, M.; Kitamura, C.; Fukushima, H. The current and future therapies of bone regeneration to repair bone defects. Int. J. Dent. 2012, 2012, 148261. [Google Scholar]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in bone erosive diseases: Perspectives in osteoporosis treatment. Trends Endocrinol. Metab. 2021, 32, 76–94. [Google Scholar]
- Tao, H.; Ma, D.D. Evidence for transdifferentiation of human bone marrow-derived stem cells: Recent progress and controversies. Pathology 2003, 35, 6–13. [Google Scholar]
- Ugurlu, B.; Karaoz, E. Comparison of similar cells: Mesenchymal stromal cells and fibroblasts. Acta Histochem. 2020, 122, 151634. [Google Scholar]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [PubMed] [Green Version]
- Pino, A.M.; Rosen, C.J.; Rodriguez, J.P. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol. Res. 2012, 45, 279–287. [Google Scholar] [PubMed] [Green Version]
- Hardy, R.; Cooper, M.S. Glucocorticoid-induced osteoporosis-a disorder of mesenchymal stromal cells? Front. Endocrinol. 2011, 2, 24. [Google Scholar] [PubMed] [Green Version]
- Farge, D.; Loisel, S.; Lansiaux, P.; Tarte, K. Mesenchymal stromal cells for systemic sclerosis treatment. Autoimmun. Rev. 2021, 20, 102755. [Google Scholar] [PubMed]
- Teli, P.; Kale, V.; Vaidya, A. Extracellular vesicles isolated from mesenchymal stromal cells primed with neurotrophic factors and signaling modifiers as potential therapeutics for neurodegenerative diseases. Curr. Res. Transl. Med. 2021, 69, 103286. [Google Scholar]
- Hu, C.; Wu, Z.; Li, L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int. J. Biol. Sci. 2020, 16, 893–903. [Google Scholar]
- Wang, L.; Li, S.; Wang, H.Y.; Zeng, J.; Zhang, Z.Z.; Lv, D.Y.; Kuang, W.H. In a rat model of acute liver failure, icaritin improved the therapeutic effect of mesenchymal stem cells by activation of the hepatocyte growth factor/c-Met pathway. Evid. Based Complement. Alternat. Med. 2019, 2019, 4253846. [Google Scholar]
- Ruiz, M.; Toupet, K.; Maumus, M.; Rozier, P.; Jorgensen, C.; Noel, D. TGFBI secreted by mesenchymal stromal cells ameliorates osteoarthritis and is detected in extracellular vesicles. Biomaterials 2020, 226, 119544. [Google Scholar]
- Elgaz, S.; Bonig, H.; Bader, P. Mesenchymal stromal cells for osteonecrosis. J. Transl. Med. 2020, 18, 399. [Google Scholar]
- Mathew, S.A.; Naik, C.; Cahill, P.A.; Bhonde, R.R. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol. Life Sci. 2020, 77, 253–265. [Google Scholar]
- Borlongan, C.V.; Glover, L.E.; Tajiri, N.; Kaneko, Y.; Freeman, T.B. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog. Neurobiol. 2011, 95, 213–228. [Google Scholar] [PubMed] [Green Version]
- Yamada, A.; Iwata, T.; Yamato, M.; Okano, T.; Izumi, Y. Diverse functions of secreted frizzled-related proteins in the osteoblastogenesis of human multipotent mesenchymal stromal cells. Biomaterials 2013, 34, 3270–3278. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Sohn, J.; Tuan, R.S. Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res. Ther. 2015, 6, 238. [Google Scholar] [CrossRef] [Green Version]
- Iwata, T.; Kawamoto, T.; Sasabe, E.; Miyazaki, K.; Fujimoto, K.; Noshiro, M.; Kurihara, H.; Kato, Y. Effects of overexpression of basic helix-loop-helix transcription factor Dec1 on osteogenic and adipogenic differentiation of mesenchymal stem cells. Eur. J. Cell Biol. 2006, 85, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westendorf, J.J. Transcriptional co-repressors of Runx2. J. Cell. Biochem. 2006, 98, 54–64. [Google Scholar]
- Bae, J.S.; Gutierrez, S.; Narla, R.; Pratap, J.; Devados, R.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Javed, A. Reconstitution of Runx2/Cbfa1-null cells identifies a requirement for BMP2 signaling through a Runx2 functional domain during osteoblast differentiation. J. Cell. Biochem. 2007, 100, 434–449. [Google Scholar]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar]
- Ge, C.; Cawthorn, W.P.; Li, Y.; Zhao, G.; Macdougald, O.A.; Franceschi, R.T. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARgamma transcription factors. J. Cell. Physiol. 2016, 231, 587–596. [Google Scholar]
- Li, Y.; Ge, C.; Franceschi, R.T. MAP Kinase-dependent RUNX2 phosphorylation is necessary for epigenetic modification of chromatin during osteoblast differentiation. J. Cell. Physiol. 2017, 232, 2427–2435. [Google Scholar]
- Luo, G.; Xu, B.; Wang, W.; Wu, Y.; Li, M. Study of the osteogenesis effect of icariside II and icaritin on canine bone marrow mesenchymal stem cells. J. Bone Miner. Metab. 2018, 36, 668–678. [Google Scholar]
- Herberg, S.; Kondrikova, G.; Hussein, K.A.; Johnson, M.H.; Elsalanty, M.E.; Shi, X.; Hamrick, M.W.; Isales, C.M.; Hill, W.D. Mesenchymal stem cell expression of stromal cell-derived factor-1beta augments bone formation in a model of local regenerative therapy. J. Orthop. Res. 2015, 33, 174–184. [Google Scholar] [PubMed]
- Herberg, S.; Shi, X.; Johnson, M.H.; Hamrick, M.W.; Isales, C.M.; Hill, W.D. Stromal cell-derived factor-1beta mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS ONE 2013, 8, e58207. [Google Scholar]
- Lim, R.Z.L.; Li, L.; Yong, E.L.; Chew, N. STAT-3 regulation of CXCR4 is necessary for the prenylflavonoid Icaritin to enhance mesenchymal stem cell proliferation, migration and osteogenic differentiation. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1680–1692. [Google Scholar]
- Dirckx, N.; Van Hul, M.; Maes, C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 170–191. [Google Scholar]
- He, L.; Lee, J.; Jang, J.H.; Sakchaisri, K.; Hwang, J.; Cha-Molstad, H.J.; Kim, K.A.; Ryoo, I.J.; Lee, H.G.; Kim, S.O.; et al. Osteoporosis regulation by salubrinal through eIF2alpha mediated differentiation of osteoclast and osteoblast. Cell Signal. 2013, 25, 552–560. [Google Scholar]
- Adhami, M.D.; Rashid, H.; Chen, H.; Clarke, J.C.; Yang, Y.; Javed, A. Loss of Runx2 in committed osteoblasts impairs postnatal skeletogenesis. J. Bone Miner. Res. 2015, 30, 71–82. [Google Scholar]
- Franceschi, R.T.; Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Yang, S.; Reith, E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect. Tissue Res. 2003, 44 (Suppl. 1), 109–116. [Google Scholar]
- Pratap, J.; Galindo, M.; Zaidi, S.K.; Vradii, D.; Bhat, B.M.; Robinson, J.A.; Choi, J.Y.; Komori, T.; Stein, J.L.; Lian, J.B.; et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003, 63, 5357–5362. [Google Scholar]
- Artigas, N.; Urena, C.; Rodriguez-Carballo, E.; Rosa, J.L.; Ventura, F. Mitogen-activated protein kinase (MAPK)-regulated interactions between Osterix and Runx2 are critical for the transcriptional osteogenic program. J. Biol. Chem. 2014, 289, 27105–27117. [Google Scholar]
- Wu, Z.; Ou, L.; Wang, C.; Yang, L.; Wang, P.; Liu, H.; Xiong, Y.; Sun, K.; Zhang, R.; Zhu, X. Icaritin induces MC3T3-E1 subclone14 cell differentiation through estrogen receptor-mediated ERK1/2 and p38 signaling activation. Biomed. Pharmacother. 2017, 94, 1–9. [Google Scholar]
- Wei, Z.; Shi, W.; Chen, K.; Zhou, J.; Wang, M. Icaritin promotes maturation and mineralization of mouse osteoblast MC3T3-E1 cells through CXCR4/SDF-1 signal pathway. Zhejiang Da Xue Xue Bao Yi Xue Ban 2017, 46, 571–577. [Google Scholar] [PubMed]
- Lim, R.Z.L.; Li, L.; Chew, N.; Yong, E.L. The prenylflavonoid Icaritin enhances osteoblast proliferation and function by signal transducer and activator of transcription factor 3 (STAT-3) regulation of C-X-C chemokine receptor type 4 (CXCR4) expression. Bone 2017, 105, 122–133. [Google Scholar] [PubMed]
- Peng, S.; Zhang, G.; Zhang, B.-T.; Guo, B.; He, Y.; Bakker, A.J.; Pan, X.; Zhen, W.; Hung, L.; Qin, L.; et al. The beneficial effect of Icaritin on osteoporotic bone is dependent on the treatment initiation timing in adult ovariectomized rats. Bone 2013, 55, 230–240. [Google Scholar] [PubMed]
- Kohli, S.S.; Kohli, V.S. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J. Endocrinol. Metab. 2011, 15, 175–181. [Google Scholar] [CrossRef]
- Pivonka, P.; Zimak, J.; Smith, D.W.; Gardiner, B.S.; Dunstan, C.R.; Sims, N.A.; Martin, T.J.; Mundy, G.R. Theoretical investigation of the role of the RANK-RANKL-OPG system in bone remodeling. J. Theor. Biol. 2010, 262, 306–316. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Fouque-Aubert, A.; Chapurlat, R. Influence of RANKL inhibition on immune system in the treatment of bone diseases. Jt. Bone Spine 2008, 75, 5–10. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, L.; Zhang, S.; Jiang, T.; Yang, Y.; Li, R. Effects of icariin on the regulation of the OPG-RANKL-RANK system are mediated through the MAPK pathways in IL-1beta-stimulated human SW1353 chondrosarcoma cells. Int. J. Mol. Med. 2014, 34, 1720–1726. [Google Scholar]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar]
- Huang, J.; Yuan, L.; Wang, X.; Zhang, T.L.; Wang, K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007, 81, 832–840. [Google Scholar]
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.L.; Elliott, G.; Kelley, M.J.; Sarosi, I.; et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545. [Google Scholar] [PubMed] [Green Version]
- Wang, Y.; Hou, J.F.; Zhou, Z.L. Chicken receptor activator of nuclear factor-kappaB ligand induces formation of chicken osteoclasts from bone marrow cells and also directly activates mature osteoclasts. Poult. Sci. 2008, 87, 2344–2349. [Google Scholar] [PubMed]
- Mangashetti, L.S.; Khapli, S.M.; Wani, M.R. IL-4 inhibits bone-resorbing activity of mature osteoclasts by affecting NF-kappa B and Ca2+ signaling. J. Immunol. 2005, 175, 917–925. [Google Scholar] [PubMed] [Green Version]
- Moreno, J.L.; Kaczmarek, M.; Keegan, A.D.; Tondravi, M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: Irreversible inhibition of the differentiation program activated by RANKL. Blood 2003, 102, 1078–1086. [Google Scholar] [PubMed] [Green Version]
- Hakeda, Y.; Kobayashi, Y.; Yamaguchi, K.; Yasuda, H.; Tsuda, E.; Higashio, K.; Miyata, T.; Kumegawa, M. Osteoclastogenesis inhibitory factor (OCIF) directly inhibits bone-resorbing activity of isolated mature osteoclasts. Biochem. Biophys. Res. Commun. 1998, 251, 796–801. [Google Scholar]
- Burgess, T.L.; Qian, Y.; Kaufman, S.; Ring, B.D.; Van, G.; Capparelli, C.; Kelley, M.; Hsu, H.; Boyle, W.J.; Dunstan, C.R.; et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J. Cell. Biol. 1999, 145, 527–538. [Google Scholar]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar]
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999, 13, 1015–1024. [Google Scholar]
- Naito, A.; Azuma, S.; Tanaka, S.; Miyazaki, T.; Takaki, S.; Takatsu, K.; Nakao, K.; Nakamura, K.; Katsuki, M.; Yamamoto, T.; et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 1999, 4, 353–362. [Google Scholar]
- Weisz, H.M.; Basel-Vanagaite, L.; Krauss, A.; Konen, O.; Levy, Y.; Garty, B.Z.; Smirin-Yosef, P.; Maya, I.; Lagovsky, I.; Taub, E.; et al. Homozygous deletion of RAG1, RAG2 and 5′ region TRAF6 causes severe immune suppression and atypical osteopetrosis. Clin. Genet. 2017, 91, 902–907. [Google Scholar]
- Liu, Y.-Q.; Yang, Q.-X.; Cheng, M.-C.; Xiao, H.-B. Synergistic inhibitory effect of Icariside II with Icaritin from Herba Epimedii on pre-osteoclastic RAW264.7 cell growth. Phytomedicine 2014, 21, 1633–1637. [Google Scholar] [PubMed]
- Tan, E.M.; Li, L.; Indran, I.R.; Chew, N.; Yong, E.L. TRAF6 Mediates Suppression of Osteoclastogenesis and Prevention of Ovariectomy-Induced Bone Loss by a Novel Prenylflavonoid. J. Bone Miner. Res. 2017, 32, 846–860. [Google Scholar] [PubMed] [Green Version]
- Yong, E.L.; Cheong, W.F.; Huang, Z.; Thu, W.P.P.; Cazenave-Gassiot, A.; Seng, K.Y.; Logan, S. Randomized, double-blind, placebo-controlled trial to examine the safety, pharmacokinetics and effects of Epimedium prenylflavonoids, on bone specific alkaline phosphatase and the osteoclast adaptor protein TRAF6 in post-menopausal women. Phytomedicine 2021, 91, 153680. [Google Scholar] [PubMed]
- Xu, B.; He, Y.; Lu, Y.; Ren, W.; Shen, J.; Wu, K.; Xu, K.; Wu, J.; Hu, Y. Glucagon like peptide 2 has a positive impact on osteoporosis in ovariectomized rats. Life Sci. 2019, 226, 47–56. [Google Scholar] [PubMed]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar]
- Mundy, G.R. Osteoporosis and inflammation. Nutr. Rev. 2007, 65, S147–S151. [Google Scholar]
- Briot, K.; Geusens, P.; Em, B.I.; Lems, W.F.; Roux, C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017, 28, 3301–3314. [Google Scholar]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and its metabolites as potential protective phytochemicals against Alzheimer’s disease. Front. Pharmacol. 2019, 10, 271. [Google Scholar] [CrossRef]
- Hwang, E.; Lin, P.; Ngo, H.T.T.; Gao, W.; Wang, Y.S.; Yu, H.S.; Yi, T.H. Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-kappaB signaling pathways: A comparative study on UVB-irradiated human keratinocytes. Photochem. Photobiol. Sci. 2018, 17, 1396–1408. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Mei, Q.; Lu, T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015, 126, 57–68. [Google Scholar] [CrossRef]
- Chen, S.H.; Lei, M.; Xie, X.H.; Zheng, L.Z.; Yao, D.; Wang, X.L.; Li, W.; Zhao, Z.; Kong, A.; Xiao, D.M.; et al. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013, 9, 6711–6722. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Zheng, L.-Z.; Xie, X.H.; Wang, X.L.; Lai, Y.X.; Chen, S.K.; Zhang, M.; Wang, Y.X.; Griffith, J.F.; Qin, L. Comparative study of poly (lactic-co-glycolic acid)/tricalcium phosphate scaffolds incorporated or coated with osteogenic growth factors for enhancement of bone regeneration. J. Orthop. Transl. 2014, 2, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.S.; Li, Y.Y.; Luo, Y.P.; Jin, J.F.; Sun, Y.X.; Zheng, L.Z.; Lai, Y.X.; Li, L.; Fu, G.H.; Qin, L.; et al. Bioactive PLGA/tricalcium phosphate scaffolds incorporating phytomolecule icaritin developed for calvarial defect repair in rat model. J. Orthop. Transl. 2020, 24, 112–120. [Google Scholar] [CrossRef]
- Shen, P.; Wong, S.P.; Yong, E.L. Sensitive and rapid method to quantify icaritin and desmethylicaritin in human serum using gas chromatography–mass spectrometry. J. Chromatogr. B 2007, 857, 47–52. [Google Scholar]
- Shen, P.; Wong, S.P.; Li, J.; Yong, E.L. Simple and sensitive liquid chromatography–tandem mass spectrometry assay for simultaneous measurement of five Epimedium prenylflavonoids in rat sera. J. Chromatogr. B 2009, 877, 71–78. [Google Scholar]
- Zhou, J.; Chen, Y.; Wang, Y.; Gao, X.; Qu, D.; Liu, C. A comparative study on the metabolism of Epimedium koreanum Nakai-prenylated flavonoids in rats by an intestinal enzyme (lactase phlorizin hydrolase) and intestinal flora. Molecules 2014, 19, 177–203. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, G.N.; Li, Y.; Zhang, L.; You, C.; Zheng, Y. Oral absorption and excretion of icaritin, an aglycone and also active metabolite of prenylflavonoids from the Chinese medicine Herba Epimedii in rats. Phytomedicine 2012, 19, 1024–1028. [Google Scholar]
- Rong, Y.; Meng, Z.; Li, J.; Zhu, X.; Gan, H.; Gu, R.; Wu, Z.; Sun, W.; Liu, T.; Zheng, Y.; et al. Application of ultra high-performance liquid chromatography tandem mass spectrometry to investigate the regioselective glucuronidation of icaritin in vitro. J. Pharm. Biomed. Anal. 2018, 154, 444–453. [Google Scholar]
- Cao, Y.F.; He, R.R.; Cao, J.; Chen, J.X.; Huang, T.; Liu, Y. Drug-drug interactions potential of icariin and its intestinal metabolites via inhibition of intestinal udp-glucuronosyltransferases. Evid. Based. Complement Alternat. Med. 2012, 2012, 395912. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.L.; Zheng, S.L. Effects of icaritin on cytochrome P450 enzymes in rats. Pharmazie 2014, 69, 301–305. [Google Scholar]
- Wang, L.; Hong, X.; Yao, Z.; Dai, Y.; Zhao, G.; Qin, Z.; Wu, B.; Gonzalez, F.J.; Yao, X. Glucuronidation of icaritin by human liver microsomes, human intestine microsomes and expressed UDP-glucuronosyltransferase enzymes: Identification of UGT1A3, 1A9 and 2B7 as the main contributing enzymes. Xenobiotica 2018, 48, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Tu, Y.; Yin, T.; Meng, Z.; Dou, G.; Hu, M. Rapid intestinal glucuronidation and hepatic glucuronide recycling contributes significantly to the enterohepatic circulation of icaritin and its glucuronides in vivo. Arch. Toxicol. 2020, 94, 3737–3749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q. Dynamic biodistribution of icaritin and its Phase-II metabolite in rat tissues by ultra-high performance liquid chromatography-tandem mass spectrometry. Anal. Sci. 2016, 32, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.W.; Yang, Y.X.; Huang, L.H.; Zhang, S.Q. Pharmacokinetics and metabolism of icaritin in rats by UPLC-MS/MS. Food Sci. Nutr. 2019, 7, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Alex, D.; Siu, S.O.; Chu, I.K.; Renn, J.; Winkler, C.; Lou, S.; Tsui, S.K.; Zhao, H.Y.; Yan, W.R.; et al. Combined in vivo imaging and omics approaches reveal metabolism of icaritin and its glycosides in zebrafish larvae. Mol. Biosyst. 2011, 7, 2128–2138. [Google Scholar] [CrossRef]
- Wang, T.; Feng, X.C.; Ding, L.Q.; Wang, K.; Shi, X.L.; Chai, L.W.; Li, Y.; Qiu, F. Metabolic profiling of icaritin in rats using UHPLC-Q/TOF-MS. Chin. Herb. Med. 2019, 11, 185–191. [Google Scholar]
- Zhang, B.; Chen, X.; Zhang, R.; Zheng, F.; Du, S.; Zhang, X. Metabolite profiling, pharmacokinetics, and in vitro glucuronidation of icaritin in rats by ultra-performance liquid chromatography coupled with mass spectrometry. J. Anal. Methods Chem. 2017, 2017, 1073607. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Ma, Y.; Hu, X.; Peng, Y.; Zheng, J. Identification of quinone methide intermediate resulting from metabolic activation of icaritin in vitro and in vivo. Chem. Res. Toxicol. 2019, 32, 969–973. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Cai, X.; Huang, Q.; Xiao, J.; Cheng, Y. Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials 2021, 265, 120404. [Google Scholar]
- Zhang, G.; Guo, B.; Wu, H.; Tang, T.; Zhang, B.T.; Zheng, L.; He, Y.; Yang, Z.; Pan, X.; Chow, H.; et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012, 18, 307–314. [Google Scholar] [CrossRef]
- Nirwan, N.; Nikita; Sultana, Y.; Vohora, D. Liposomes as multifaceted delivery system in the treatment of osteoporosis. Expert Opin. Drug Deliv. 2021, 18, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Zreiqat, H. Nanoparticles: A promising new therapeutic platform for bone regeneration? Nanomedicine 2017, 12, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Liu, X. Bone site-specific delivery of siRNA. J. Biomed. Res. 2016, 30, 264–271. [Google Scholar] [CrossRef]
- Luhmann, T.; Germershaus, O.; Groll, J.; Meinel, L. Bone targeting for the treatment of osteoporosis. J. Control. Release 2012, 161, 198–213. [Google Scholar] [CrossRef]
- Stapleton, M.; Sawamoto, K.; Almeciga-Diaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of bone targeting drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef]
- Dang, L.; Liu, J.; Li, F.; Wang, L.; Li, D.; Guo, B.; He, X.; Jiang, F.; Liang, C.; Liu, B.; et al. Targeted delivery systems for molecular therapy in skeletal disorders. Int. J. Mol. Sci. 2016, 17, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsumi, H.; Yamashita, S.; Morishita, M.; Yamamoto, A. Bone-targeted drug delivery systems and strategies for treatment of bone metastasis. Chem. Pharm. Bull. 2020, 68, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, C.; Huang, H.; Huang, J.; Deng, A.; Zou, P.; Tan, X. Bone-targeted delivery of simvastatin-loaded PEG-PLGA micelles conjugated with tetracycline for osteoporosis treatment. Drug Deliv. Transl. Res. 2018, 8, 1090–1102. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Cai, L.; Liu, T.; Wang, X.; Zhang, Y.; Zhou, Z.; Li, T.; Liu, M.; Lai, R.; et al. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int. J. Nanomed. 2020, 15, 7967–7977. [Google Scholar] [CrossRef]
- Yamashita, S.; Katsumi, H.; Hibino, N.; Isobe, Y.; Yagi, Y.; Kusamori, K.; Sakane, T.; Yamamoto, A. Development of PEGylated carboxylic acid-modified polyamidoamine dendrimers as bone-targeting carriers for the treatment of bone diseases. J. Control. Release 2017, 262, 10–17. [Google Scholar] [CrossRef]
- Addison, W.N.; Miller, S.J.; Ramaswamy, J.; Mansouri, A.; Kohn, D.H.; McKee, M.D. Phosphorylation-dependent mineral-type specificity for apatite-binding peptide sequences. Biomaterials 2010, 31, 9422–9430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekido, T.; Sakura, N.; Higashi, Y.; Miya, K.; Nitta, Y.; Nomura, M.; Sawanishi, H.; Morito, K.; Masamune, Y.; Kasugai, S.; et al. Novel drug delivery system to bone using acidic oligopeptide: Pharmacokinetic characteristics and pharmacological potential. J. Drug Target. 2001, 9, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, K.; Miya, K.; Sekido, T.; Higashi, Y.; Nomura, M.; Fujisawa, R.; Morito, K.; Masamune, Y.; Waki, Y.; Kasugai, S.; et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology 2001, 142, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, L.; Zhang, J.; Wu, H.; Wang, N.; Tong, W.; Xu, J.; Huang, L.; Zhang, Y.; Yang, Z.; et al. A novel bone targeting delivery system carrying phytomolecule icaritin for prevention of steroid-associated osteonecrosis in rats. Bone 2018, 106, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Yang, L.; Zhang, S.; Liu, J.; Sun, Y.; Wang, X. A bone-resorption surface-targeting nanoparticle to deliver anti-miR214 for osteoporosis therapy. Int. J. Nanomed. 2017, 12, 7469–7482. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Park, C.W.; Choi, Y.; Lin, J.; Seo, D.H.; Kim, H.S.; Lee, S.Y.; Kang, I.C. A novel small-molecule PPI inhibitor targeting integrin alphavbeta3-osteopontin interface blocks bone resorption in vitro and prevents bone loss in mice. Biomaterials 2016, 98, 131–142. [Google Scholar] [CrossRef]
- Sun, Y.; Ye, X.; Cai, M.; Liu, X.; Xiao, J.; Zhang, C.; Wang, Y.; Yang, L.; Liu, J.; Li, S.; et al. Osteoblast-targeting-peptide modified nanoparticle for siRNA/microRNA delivery. ACS Nano 2016, 10, 5759–5768. [Google Scholar] [CrossRef]
- Liang, C.; Guo, B.; Wu, H.; Shao, N.; Li, D.; Liu, J.; Dang, L.; Wang, C.; Li, H.; Li, S.; et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 2015, 21, 288–294. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Cao, H.; Li, L.; Chow, D.H.; Tian, L.; Wu, H.; Zhang, J.; Wang, N.; Zheng, L.; et al. A bone-targeting delivery system carrying osteogenic phytomolecule icaritin prevents osteoporosis in mice. Biomaterials 2018, 182, 58–71. [Google Scholar] [CrossRef]
- Guo, J.; Zeng, H.; Liu, Y.; Shi, X.; Liu, Y.; Liu, C.; Chen, Y. Multicomponent thermosensitive lipid complexes enhance desmoplastic tumor therapy through boosting anti-angiogenesis and synergistic strategy. Int. J. Pharm. 2021, 601, 120533. [Google Scholar] [CrossRef]
- Yang, J.G.; Zhang, J.; Chen, X.J.; Zhou, G. Stable loading and delivery of icaritin using PEG-PCL micelles for effective treatment of oral squamous cell carcinoma. Curr. Drug Deliv. 2020, 18, 975–983. [Google Scholar] [CrossRef]
- Shan, S.; Yao, J.; Xu, L.; Han, S.; Cao, J.; Zhang, G.; Sun, Y. Preparation of Icaritin-loaded mPEG-PLA micelles and evaluation on ischemic brain injury. J. Biomed. Nanotechnol. 2019, 15, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, X.; Yao, H.; Yin, H.; Ma, X.; Jin, M.; Lu, X.; Wang, Q.; Meng, K.; Yuan, Q. Enhanced oral absorption of icaritin by using mixed polymeric micelles prepared with a creative acid-base shift method. Molecules 2021, 26, 3450. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Guo, J.; Hu, M.; Gao, Y.; Huang, L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano 2020, 14, 4816–4828. [Google Scholar] [CrossRef]
- Tang, C.; Meng, K.; Chen, X.; Yao, H.; Kong, J.; Li, F.; Yin, H.; Jin, M.; Liang, H.; Yuan, Q. Preparation, characterization, and in vivo evaluation of amorphous icaritin nanoparticles prepared by a reactive precipitation technique. Molecules 2021, 26, 2913. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, T.; Li, H.; Fu, J.; Ao, H.; Lu, L.; Han, M.; Guo, Y.; Yue, F.; Wang, X. Hydrous icaritin nanorods with excellent stability improves the in vitro and in vivo activity against breast cancer. Drug Deliv. 2020, 27, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, S.; Chang, Q.; Zhang, L.; Wang, G.; Chen, W.; Miao, X.; Zheng, Y. A strategy for the improvement of the bioavailability and antiosteoporosis activity of BCS IV flavonoid glycosides through the formulation of their lipophilic aglycone into nanocrystals. Mol. Pharm. 2013, 10, 2534–2542. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, J.; Zhang, K.; Yao, H.; Zheng, N.; Zheng, L.; Wang, J.; Wei, K.; Xiao, X.; Qin, L.; et al. Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. ACS Cent. Sci. 2019, 5, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.H.; Wang, X.L.; Zhang, G.; He, Y.X.; Leng, Y.; Tang, T.T.; Pan, X.; Qin, L. Biofabrication of a PLGA-TCP-based porous bioactive bone substitute with sustained release of icaritin. J. Tissue Eng. Regen. Med. 2015, 9, 961–972. [Google Scholar] [CrossRef]
- Wang, X.L.; Xie, X.H.; Zhang, G.; Chen, S.H.; Yao, D.; He, K.; Wang, X.H.; Yao, X.S.; Leng, Y.; Fung, K.P.; et al. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J. Orthop. Res. 2013, 31, 164–172. [Google Scholar] [CrossRef]
- Qin, L.; Yao, D.; Zheng, L.; Liu, W.C.; Liu, Z.; Lei, M.; Huang, L.; Xie, X.; Wang, X.; Chen, Y.; et al. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: Proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials 2015, 59, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Wang, X.L.; Xie, X.H.; Zheng, L.Z.; Yao, D.; Wang, D.P.; Leng, Y.; Zhang, G.; Qin, L. Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: An in vitro efficacy study. Acta Biomater. 2012, 8, 3128–3137. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.H.; Wang, X.L.; Zhang, G.; He, Y.X.; Wang, X.H.; Liu, Z.; He, K.; Peng, J.; Leng, Y.; Qin, L. Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular icaritin. Biomed. Mater. 2010, 5, 054109. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.C.B.; Kumar, N.S.; Banerjee, P.; Reddy, B.V.S. A review on the origin of nanofibers/nanorods structures and applications. J. Mater. Sci. Mater. Med. 2021, 32, 68. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Y.; Ao, H.; Fu, J.; Guo, Y.; Han, M.; Yan, X.; Chen, X.; Wang, X. A comparative study on the in vitro and in vivo antitumor efficacy of icaritin and hydrous icaritin nanorods. Drug Deliv. 2020, 27, 1176–1187. [Google Scholar] [CrossRef]
- Junghanns, J.U.; Muller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]

| Animal | Dosage | Biological Sample | Quantification Method | Pharmacokinetic Properties | Reference |
|---|---|---|---|---|---|
| Male SD rats | 5 mg/kg by single i.v. | Plasma | HPLC-UV | C0 (μg/mL) 7.20 ± 1.67 t1/2,λz (h) 0.43 ± 0.11 AUC0–2h (h·μg/mL) 1.42 ± 0.28 AUC0–∞ (h·μg/mL) 1.46 ± 0.30 Cl (mL/h/kg) 2.12 ± 0.42 V (L/kg) 3.54 ± 0.64 | [88] |
| Female SD rats | 20, 40, 60 mg/kg/day by i.p. for 7 days | Plasma | UPLC-MS/MS | 20 40 60 mg/kg/day tmax (h) 1 0.5 1 AUC0–8h (h·ng/mL) 1963 7450 15,885 | [5] |
| FVB/NCrlVr mice | 10 mg/kg by single i.p. | Spine | UHPLC-MS/MS | t1/2,λz (h) 10.68 AUC0–120h (h·ng/g) 642 Cl (g/h/kg) 15,486 V (g/kg) 238,683 MRT (h) 45.02 | [8] |
| SD rats | 10 mg/kg by single i.p. | Liver, spleen, kidney, heart, lung, muscle, adipose, and brain | UHPLC-MS/MS | t1/2,λz, tmax, AUC0–72h, AUC0–∞, Cl, V, MRT0–72h, and MRT0–∞ of various tissues were obtained, see ref [94] for details | [94] |
| Male SD rats | 2 and 40 mg/kg by single i.v. and i.g., respectively | Plasma | UHPLC-MS/MS | i.v. i.g. t1/2,λz (h) 1.72 ± 0.37 7.37 ± 1.32 AUC0–t (h·ng/mL) 699 ± 143 561 ± 97 AUC0–∞ (h·ng/mL) 700 ± 154 607 ± 89 Cl (L/h/kg) 2.86 ± 0.47 65.92 ± 11.38 V (L/kg) 7.10 ± 1.69 700.84 ± 107.95 MRT0–t (h) 0.43 ± 0.12 6.02 ± 0.98 MRT0–∞ (h) 0.45 ± 0.14 8.18 ± 1.27 | [9] |
| Male SD rats | 40 mg/kg single i.g. | liver, spleen, kidney, heart, lung, muscle, and brain | UHPLC-MS/MS | t1/2,λz, tmax, AUC0–24h, AUC0–∞, Cl, V, MRT0–24h, and MRT0–∞ of various tissues were obtained, see ref [9] for details | [9] |
| Male SD rats | 100 mg/kg by single i.g. | Plasma | UPLC-MS/MS | tmax (h) 5.3 ± 1.1 Cmax (ng/mL) 294.5 ± 22.7 t1/2 (h) 8.3 ± 1.0 AUC0–48h (h·ng/mL) 3048.5 ± 289.0 AUC0–∞ (h·ng/mL) 3145.0 ± 302.3 MRT0–48h (h) 9.6 ± 1.1 MRT0–∞ (h) 10.9 ± 1.3 | [98] |
| Female SD rats | 40 mg/kg by single i.p. | Plasma | UPLC-MS/MS | t1/2,λz (h) 3.14 ± 0.34 AUC0–24h (h·ng/g) 7937 ± 442 Cl (g/h/kg) 5.43 ± 0.85 V (g/kg) 22.79 ± 3.01 MRT0–24h (h) 7.55 ± 0.97 | [95] |
| Male Wistar rats | 5 mg/kg by single i.g. | Plasma | UPLC-MS/MS | Cmax (nmol/L) 7.12 ± 1.19 AUC0–48h (h·nmol/L) 37.00 ± 12.98 | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Zhang, S.-Q. Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects. Pharmaceuticals 2022, 15, 397. https://doi.org/10.3390/ph15040397
Gao L, Zhang S-Q. Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects. Pharmaceuticals. 2022; 15(4):397. https://doi.org/10.3390/ph15040397
Chicago/Turabian StyleGao, Lifang, and Shuang-Qing Zhang. 2022. "Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects" Pharmaceuticals 15, no. 4: 397. https://doi.org/10.3390/ph15040397
APA StyleGao, L., & Zhang, S.-Q. (2022). Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects. Pharmaceuticals, 15(4), 397. https://doi.org/10.3390/ph15040397






