Low Immune Cross-Reactivity between West Nile Virus and a Zika Virus Vaccine Based on Modified Vaccinia Virus Ankara
Abstract
:1. Introduction
2. Results and Discussion
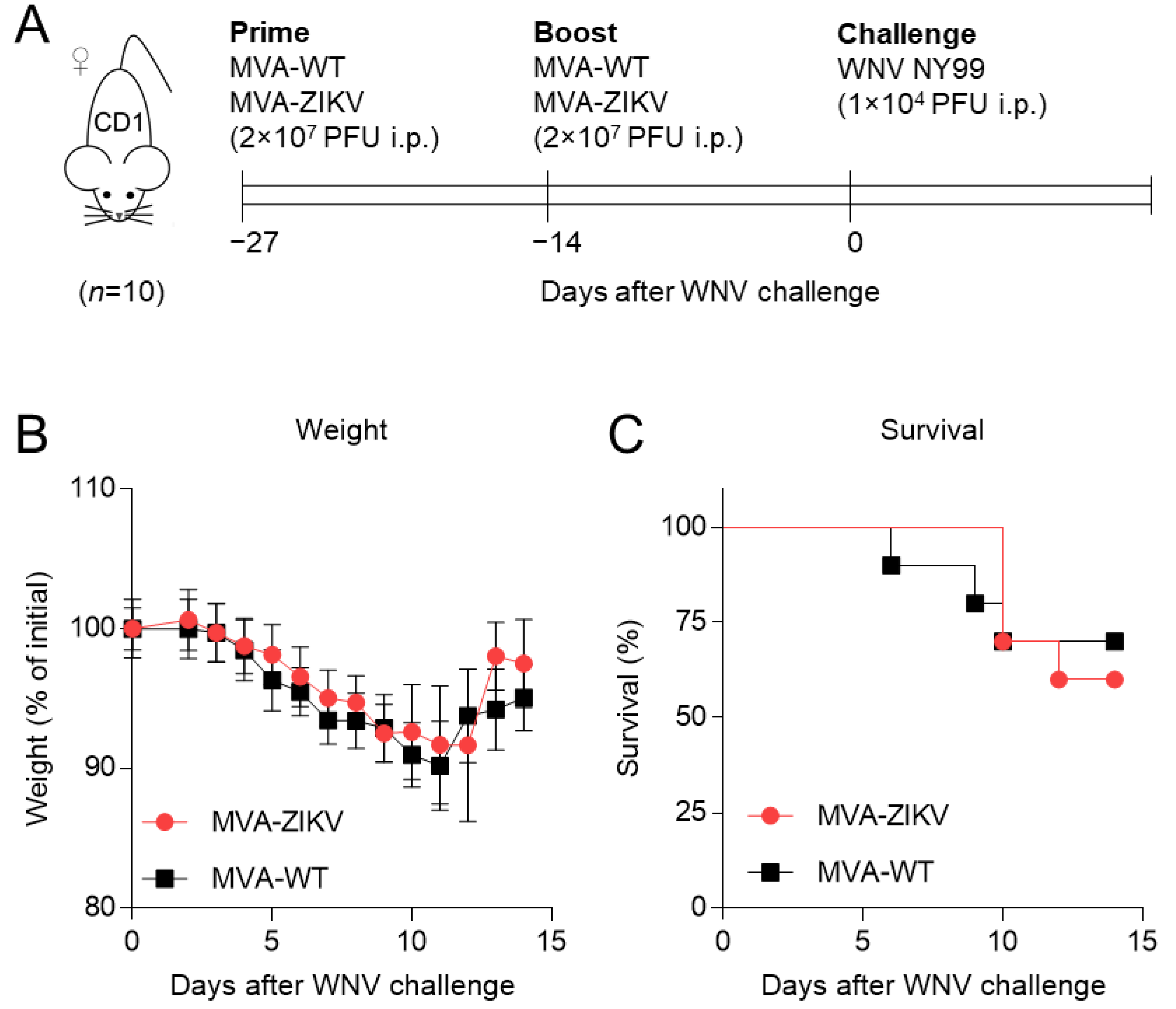
2.1. Immunization with MVA-ZIKV Vaccine Candidate Does Not Affect WNV Infection
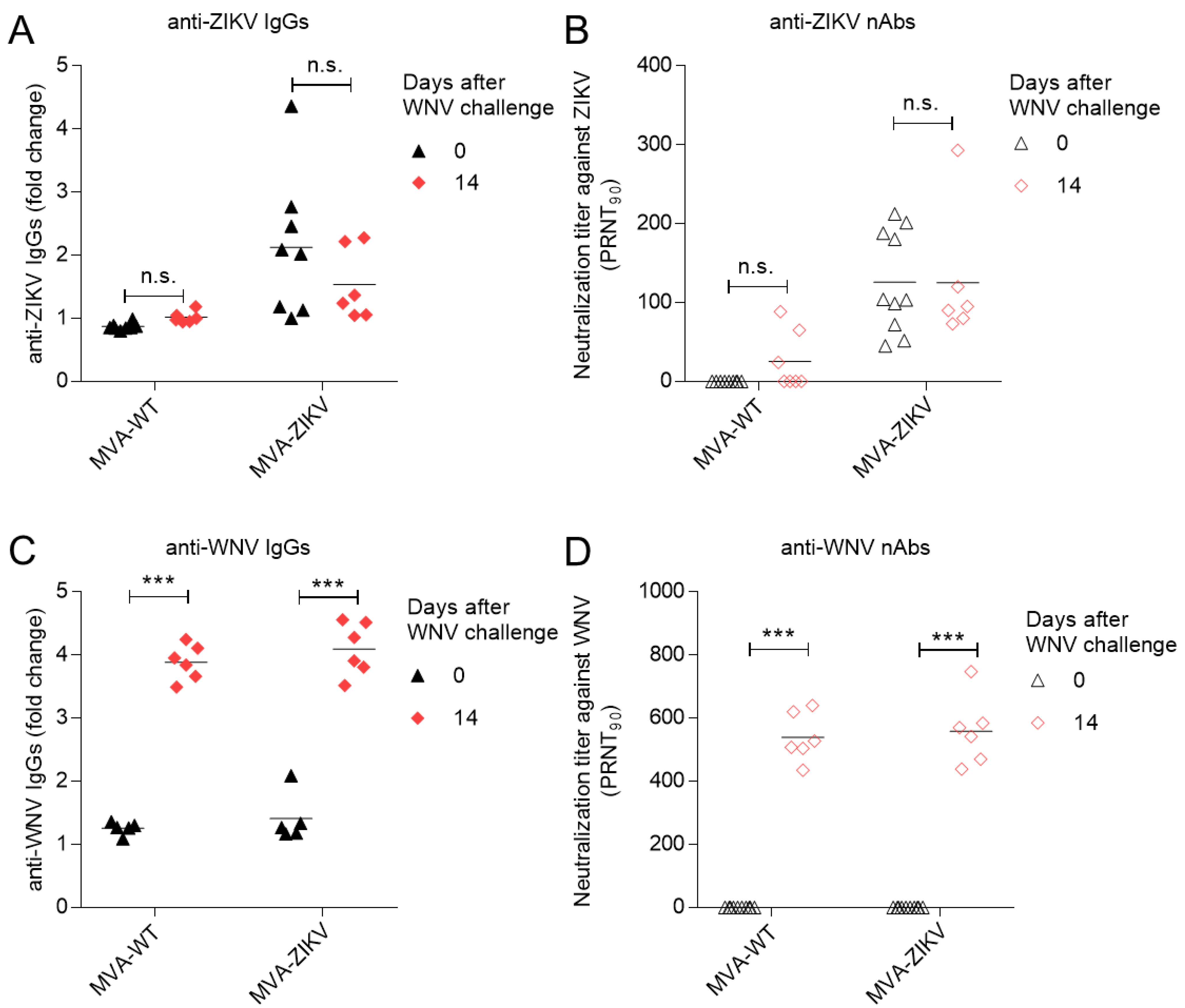
2.2. Vaccination with MVA-ZIKV Does Not Alter the WNV-Specific Humoral Response Elicited by WNV Infection
3. Materials and Methods
3.1. Viruses
3.2. Mice Experiments
3.3. Antibody Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2021, 1, 65. [Google Scholar] [CrossRef] [PubMed]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martinez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barre Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, S.J.; Carlson, C.J.; Tesla, B.; Bonds, M.H.; Ngonghala, C.N.; Mordecai, E.A.; Johnson, L.R.; Murdock, C.C. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob. Chang. Biol. 2021, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Chaurasia, D. The expanding arms of Zika virus: An updated review with recent Indian outbreaks. Rev. Med. Virol. 2021, 31, 1–9. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The first local cases of Zika virus in Europe. Lancet 2019, 394, 1991–1992. [Google Scholar] [CrossRef] [Green Version]
- Pattnaik, A.; Sahoo, B.R.; Pattnaik, A.K. Current Status of Zika Virus Vaccines: Successes and Challenges. Vaccines 2020, 8, 266. [Google Scholar] [CrossRef]
- Vazquez-Calvo, A.; Blazquez, A.B.; Escribano-Romero, E.; Merino-Ramos, T.; Saiz, J.C.; Martin-Acebes, M.A.; Jimenez de Oya, N. Zika virus infection confers protection against West Nile virus challenge in mice. Emerg. Microbes Infect. 2017, 6, e81. [Google Scholar] [CrossRef]
- He, X.; Lang, X.; Yu, J.; Zhu, L.; Qin, Z.; Liu, X.; Chen, P.; Dai, C.; Chen, T.; Li, X.; et al. The effects of Japanese encephalitis virus antibodies on Zika virus infection. Med. Microbiol. Immunol. 2020, 209, 177–188. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Monzon, A.M.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; De Jesus-Gonzalez, L.A.; Reyes-Ruiz, J.M.; Del Angel, R.M. The role of anti-flavivirus humoral immune response in protection and pathogenesis. Rev. Med. Virol. 2020, 30, e2100. [Google Scholar] [CrossRef] [PubMed]
- Duehr, J.; Lee, S.; Singh, G.; Foster, G.A.; Krysztof, D.; Stramer, S.L.; Bermudez Gonzalez, M.C.; Menichetti, E.; Geretschlager, R.; Gabriel, C.; et al. Tick-Borne Encephalitis Virus Vaccine-Induced Human Antibodies Mediate Negligible Enhancement of Zika Virus Infection InVitro and in a Mouse Model. mSphere 2018, 3, e00011-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.; Paul, A.M.; Sun, H.; He, J.; Yang, M.; Bai, F.; Chen, Q. A plant-produced vaccine protects mice against lethal West Nile virus infection without enhancing Zika or dengue virus infectivity. Vaccine 2018, 36, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Beesetti, H.; Brown, J.A.; Ahuja, R.; Ramasamy, V.; Shanmugam, R.K.; Poddar, A.; Batra, G.; Krammer, F.; Lim, J.K.; et al. Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models. EBioMedicine 2020, 60, 102991. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Fernandez de Marco, M.; Giovannini, A.; Ippoliti, C.; Danzetta, M.L.; Svartz, G.; Erster, O.; Groschup, M.H.; Ziegler, U.; Mirazimi, A.; et al. Emerging Mosquito-Borne Threats and the Response from European and Eastern Mediterranean Countries. Int. J. Environ. Res. Public Health 2018, 15, 2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camp, J.V.; Nowotny, N. The knowns and unknowns of West Nile virus in Europe: What did we learn from the 2018 outbreak? Expert Rev. Anti Infect. Ther. 2020, 18, 145–154. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Suleyman, O.M.; Ortega-Prieto, A.M.; Skelton, J.K.; Bonnesoeur, P.; Blohm, A.; Carregaro, V.; Silva, J.S.; James, E.A.; Maillere, B.; et al. T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci. Rep. 2018, 8, 672. [Google Scholar] [CrossRef]
- Oliveira, R.A.; de Oliveira-Filho, E.F.; Fernandes, A.I.; Brito, C.A.; Marques, E.T.; Tenorio, M.C.; Gil, L.H. Previous dengue or Zika virus exposure can drive to infection enhancement or neutralisation of other flaviviruses. Mem. Inst. Oswaldo Cruz. 2019, 114, e190098. [Google Scholar] [CrossRef] [Green Version]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Garg, H.; Yeh, R.; Watts, D.M.; Mehmetoglu-Gurbuz, T.; Resendes, R.; Parsons, B.; Gonzales, F.; Joshi, A. Enhancement of Zika virus infection by antibodies from West Nile virus seropositive individuals with no history of clinical infection. BMC Immunol. 2021, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Acklin, J.A.; Cattle, J.D.; Moss, A.S.; Brown, J.A.; Foster, G.A.; Krysztof, D.; Stramer, S.L.; Lim, J.K. Evaluating the Safety of West Nile Virus Immunity During Congenital Zika Virus Infection in Mice. Front. Immunol. 2021, 12, 686411. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.; Marín, Q.M.; Lazaro-Frias, A.; Jimenez de Oya, N.; Blazquez, A.B.; Escribano-Romero, E.; CO, S.S.; Ortego, J.; Saiz, J.C.; Esteban, M.; et al. A Vaccine Based on a Modified Vaccinia Virus Ankara Vector Expressing Zika Virus Structural Proteins Controls Zika Virus Replication in Mice. Sci. Rep. 2018, 8, 17385. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.; Martin-Acebes, M.A.; Poderoso, T.; Lazaro-Frias, A.; Saiz, J.C.; Sorzano, C.O.S.; Esteban, M.; Garcia-Arriaza, J. The combined vaccination protocol of DNA/MVA expressing Zika virus structural proteins as efficient inducer of T and B cell immune responses. Emerg. Microbes Infect. 2021, 10, 1441–1456. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Elong Ngono, A.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017, 8, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Camacho, C.; Kim, Y.C.; Abbink, P.; Larocca, R.A.; Huiskonen, J.T.; Barouch, D.H.; Reyes-Sandoval, A. Assessment of Immunogenicity and Efficacy of a Zika Vaccine Using Modified Vaccinia Ankara Virus as Carriers. Pathogens 2019, 8, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brault, A.C.; Domi, A.; McDonald, E.M.; Talmi-Frank, D.; McCurley, N.; Basu, R.; Robinson, H.L.; Hellerstein, M.; Duggal, N.K.; Bowen, R.A.; et al. A Zika Vaccine Targeting NS1 Protein Protects Immunocompetent Adult Mice in a Lethal Challenge Model. Sci. Rep. 2017, 7, 14769. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St John, A.L. Cross-Reactive Immunity among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez de Oya, N.; Pérez, P.; Blázquez, A.-B.; Escribano-Romero, E.; Esteban, M.; Saiz, J.-C.; García-Arriaza, J.; Martín-Acebes, M.A. Low Immune Cross-Reactivity between West Nile Virus and a Zika Virus Vaccine Based on Modified Vaccinia Virus Ankara. Pharmaceuticals 2022, 15, 354. https://doi.org/10.3390/ph15030354
Jiménez de Oya N, Pérez P, Blázquez A-B, Escribano-Romero E, Esteban M, Saiz J-C, García-Arriaza J, Martín-Acebes MA. Low Immune Cross-Reactivity between West Nile Virus and a Zika Virus Vaccine Based on Modified Vaccinia Virus Ankara. Pharmaceuticals. 2022; 15(3):354. https://doi.org/10.3390/ph15030354
Chicago/Turabian StyleJiménez de Oya, Nereida, Patricia Pérez, Ana-Belén Blázquez, Estela Escribano-Romero, Mariano Esteban, Juan-Carlos Saiz, Juan García-Arriaza, and Miguel A. Martín-Acebes. 2022. "Low Immune Cross-Reactivity between West Nile Virus and a Zika Virus Vaccine Based on Modified Vaccinia Virus Ankara" Pharmaceuticals 15, no. 3: 354. https://doi.org/10.3390/ph15030354
APA StyleJiménez de Oya, N., Pérez, P., Blázquez, A.-B., Escribano-Romero, E., Esteban, M., Saiz, J.-C., García-Arriaza, J., & Martín-Acebes, M. A. (2022). Low Immune Cross-Reactivity between West Nile Virus and a Zika Virus Vaccine Based on Modified Vaccinia Virus Ankara. Pharmaceuticals, 15(3), 354. https://doi.org/10.3390/ph15030354







