In Vitro and In Vivo Evaluation of a Cyclic LyP-1-Modified Nanosystem for Targeted Endostatin Delivery in a KYSE-30 Cell Xenograft Athymic Nude Mice Model
Abstract
1. Introduction
2. Results and Discussion
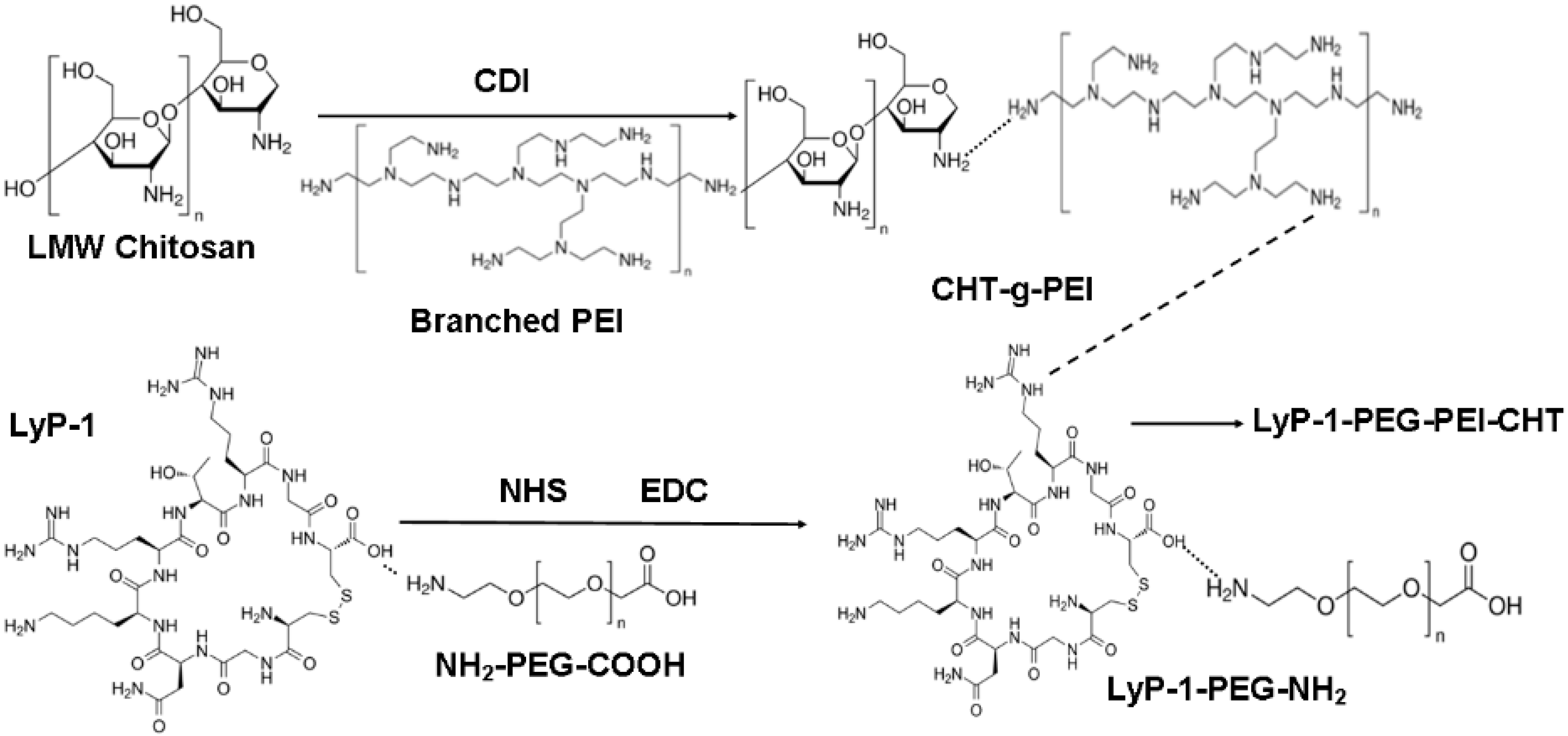
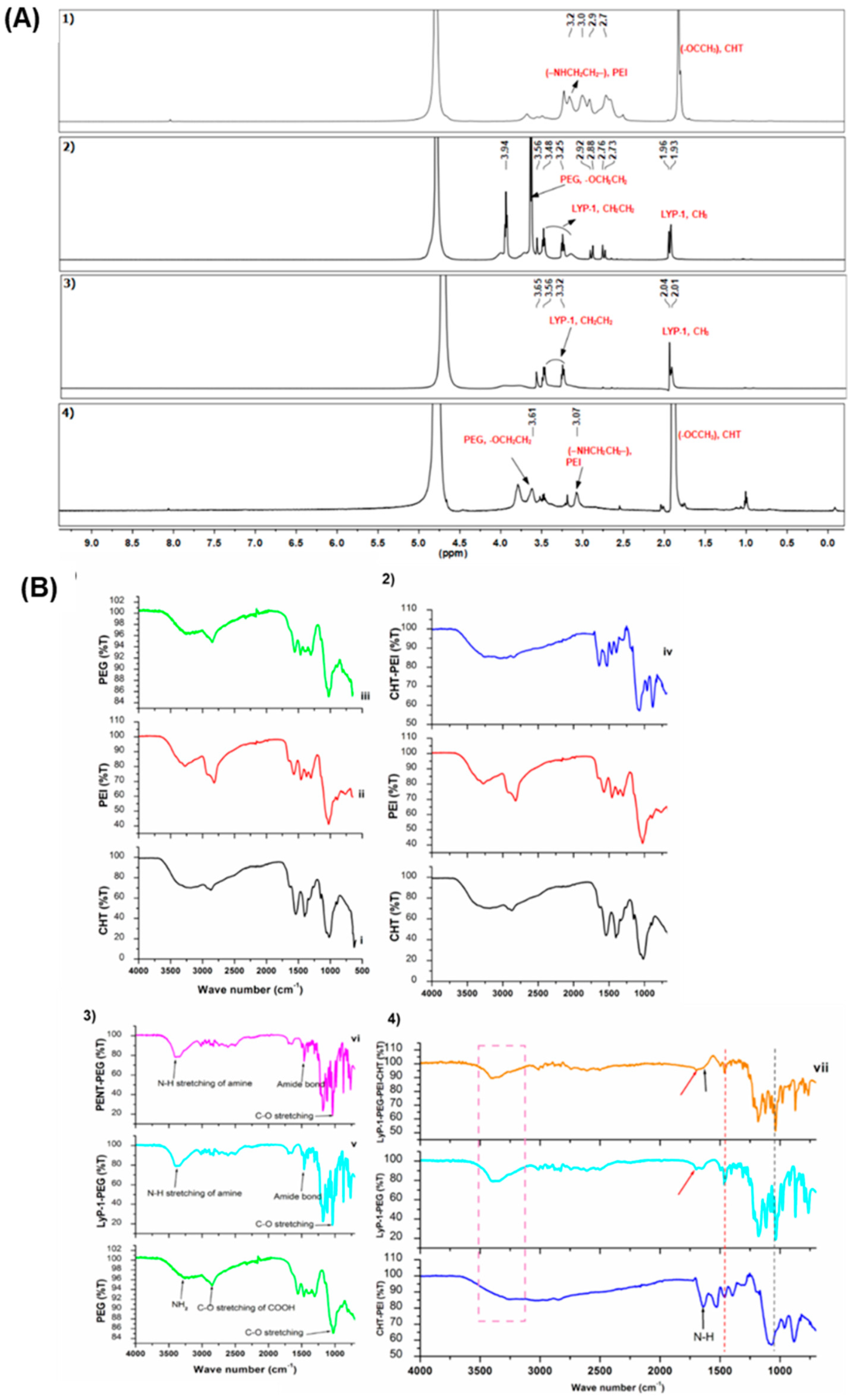
2.1. Structure and Function Modification for the LyP-1 Functionalized Nanosystem Synthesized
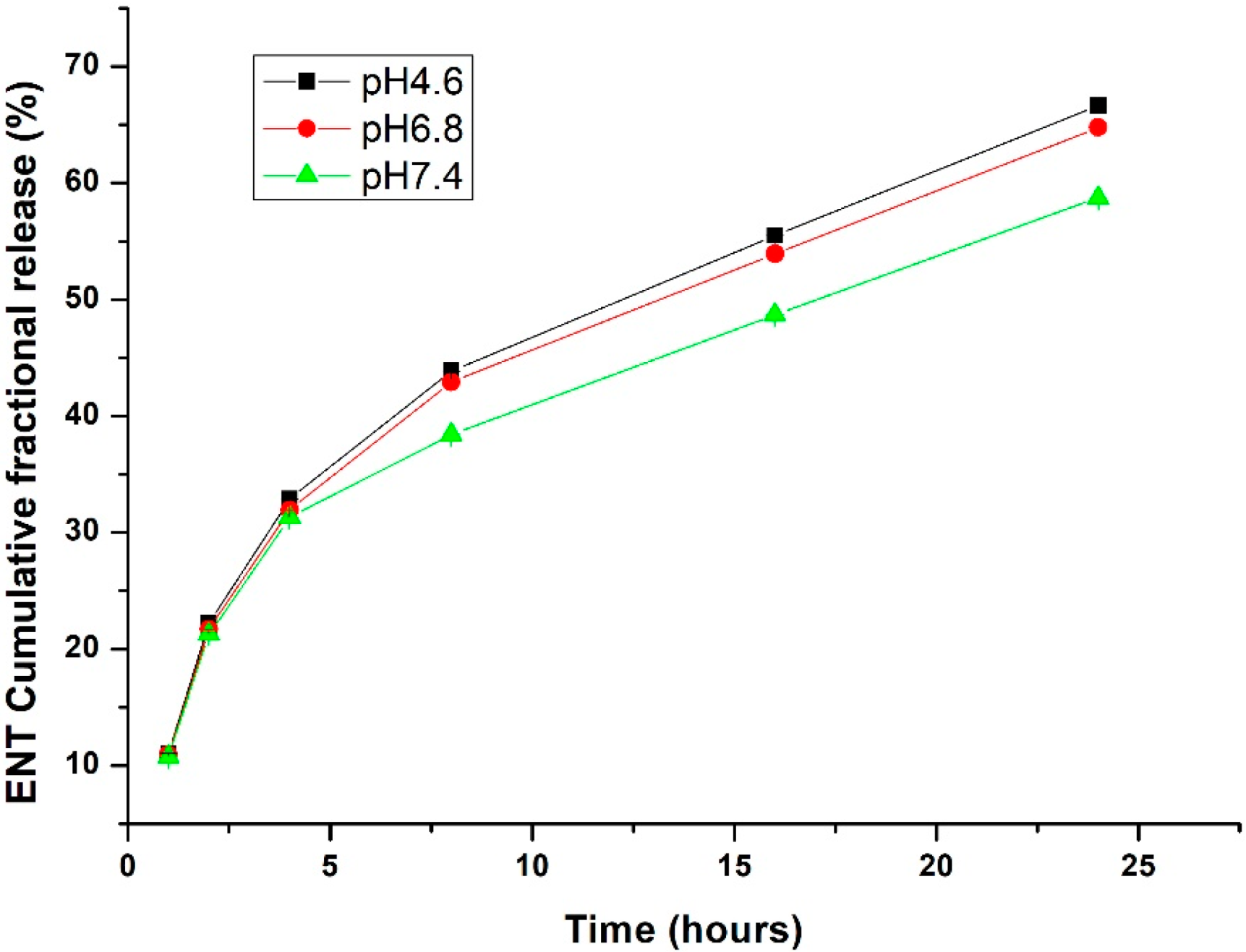
In Vitro ENT Release from the LyP-1-Functionalized Nanoparticles
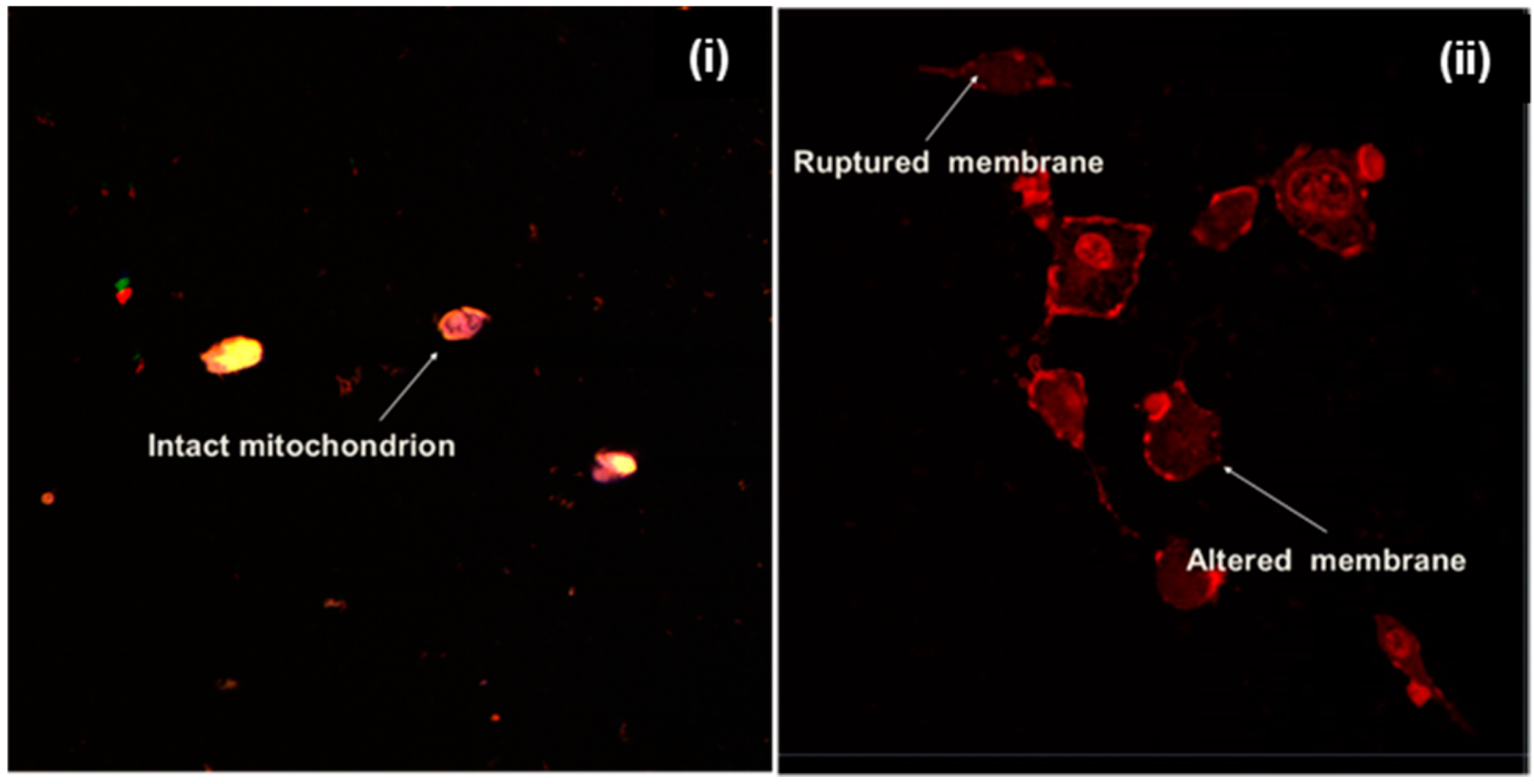
2.2. Influence of the Physicochemical Properties of the ENT-Loaded LyP-1-Modified Nanosystem on Cellular Uptake, Internalization and Co-Localization
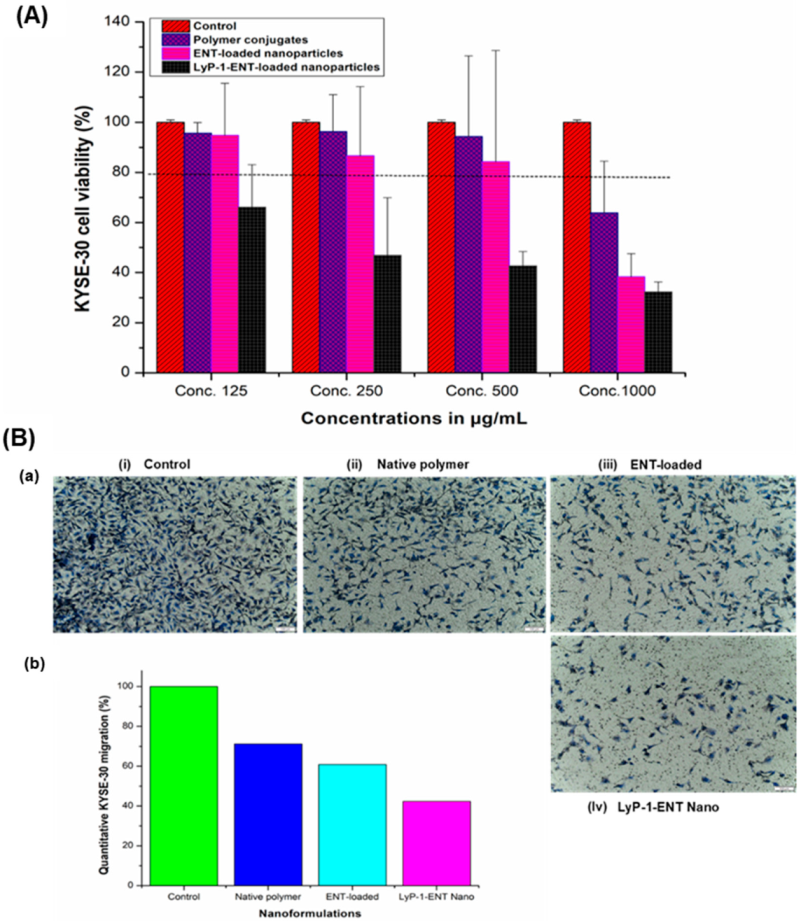
2.3. Binding Specificity and Cytotoxic Effects of the ENT-Loaded LyP-1-Modified Nanosystem to KYSE-30 Cells
2.4. Assessment of Anti-Proliferative Effects and Cell Migration
2.5. Anti-Angiogenic Measurement Using VEGF-C and MMP2 Assays
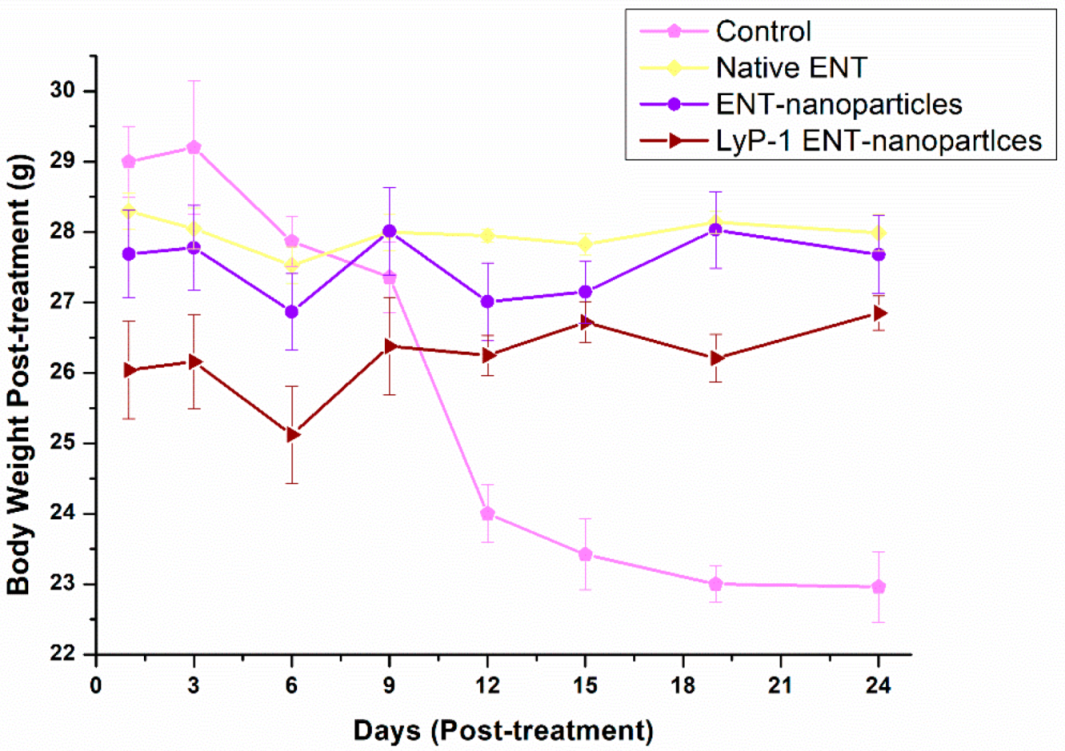
2.6. In Vivo Assessment of the ENT-Loaded LyP-1 Modified Nanosystem in a Xenograft
Effects on Tumor Volume and Mouse Weight
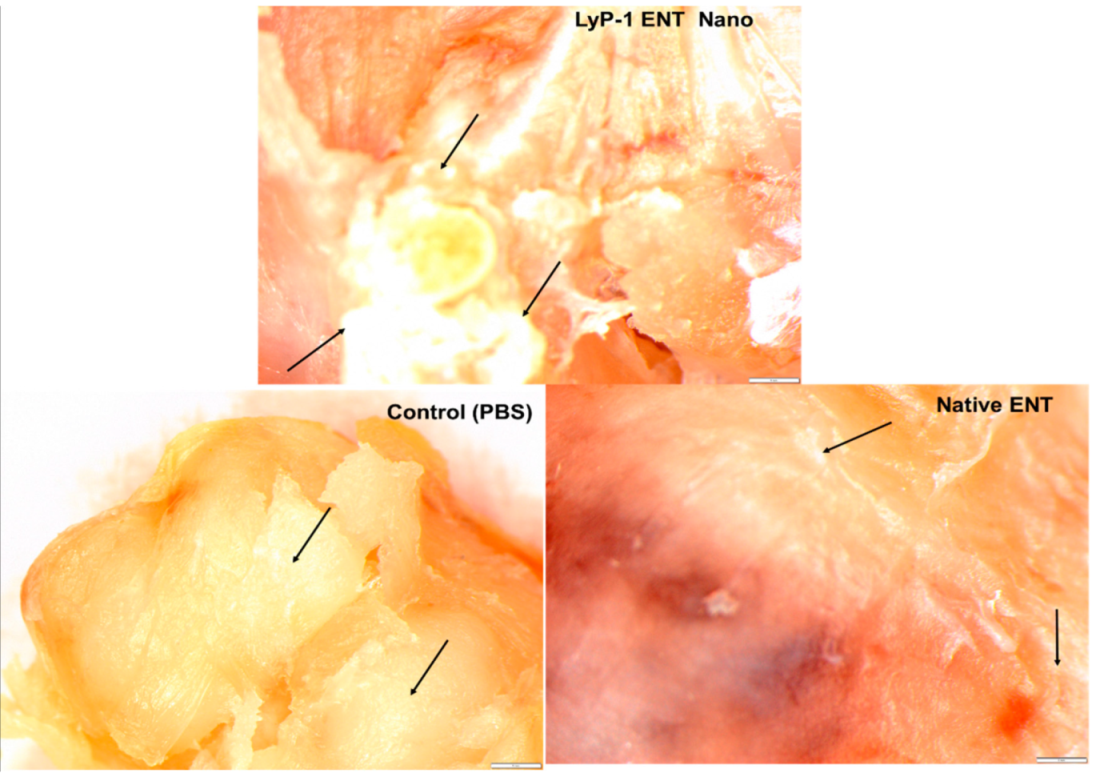
2.7. Tumor Necrosis on KYSE-30 Xenograft
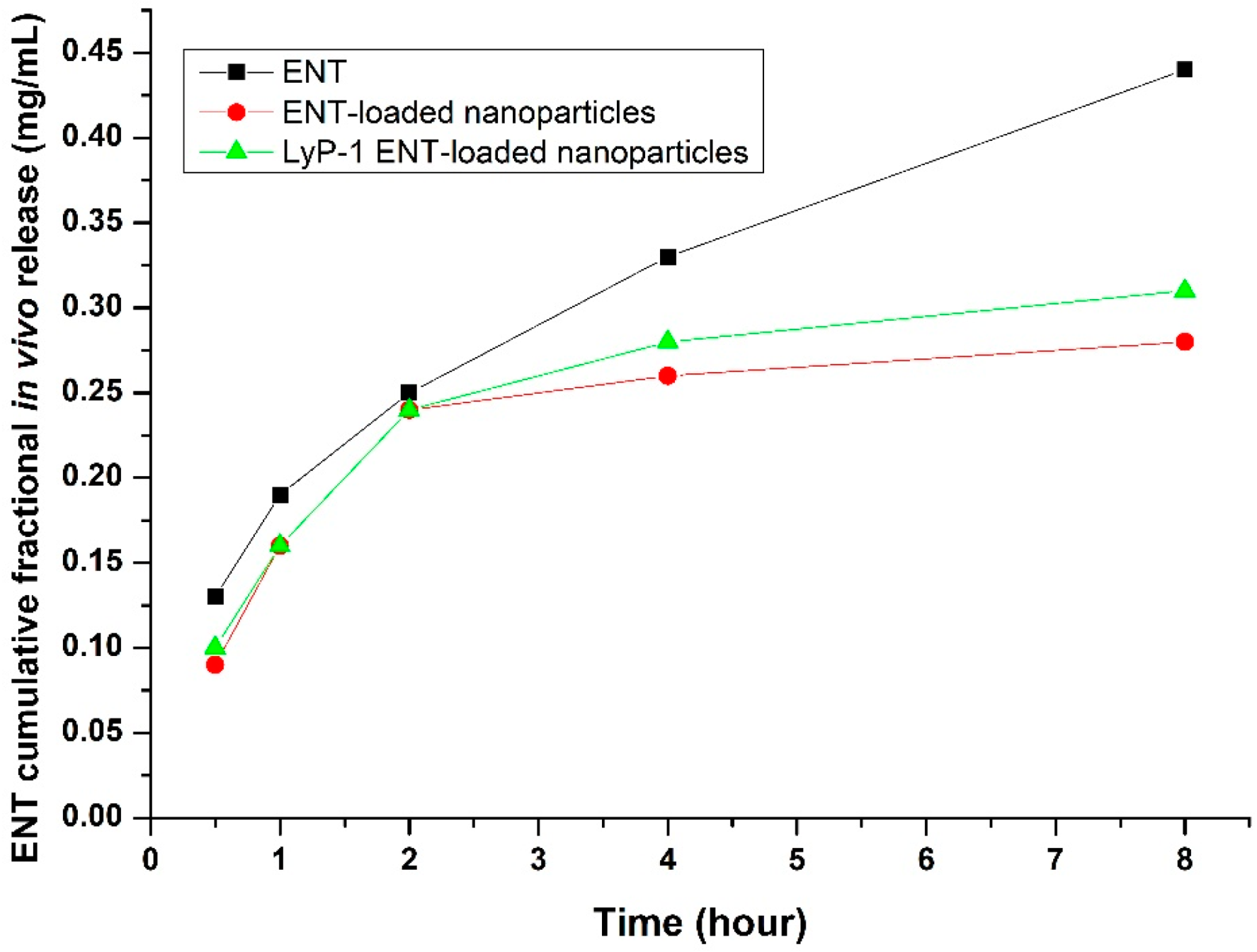
2.8. In Vivo Measurement of ENT Release from the LyP-1-Modified Nanosystem in the KYSE-30 Cell Xenograft Mice Model
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of the Grafted Chitosan-Based Nanosystem
3.2.2. Phase 1: Conjugation of LyP-1 onto PEG-NH2
3.2.3. Phase 2: Conjugation of Synthesized LyP-1-PEG-NH2 onto CHT-PEI
3.2.4. Preparation of the ENT-Loaded Nanosystem Using the Synthesized LyP-1-PEG-NH2-CHT-PEI Polymeric Platform
3.2.5. Physicochemical Property Evaluation of the LyP-1-Modified Nanosystem
3.2.6. Preparation of KYSE-30 Cell Culture
3.2.7. Determination of the Cytocompatibility of the Nanosystem Components and Cell Proliferation Assay
3.2.8. Assessment of Nanosystem Uptake, Internalization, Co-Localization and Sub-Cellular Biodistribution
3.2.9. In Vitro Cell Migration Measurement Using Boyden Chambers
3.2.10. Anti-Angiogenic Evaluation of VEGF and MMP2
3.2.11. Development of Tumor Xenografts and Evaluation of Anti-Necrotic Function of the ENT-Loaded LyP-1-Modified Nanosystem
3.2.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.M.S.A.; Oon, C.E. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina 2018, 54, 8. [Google Scholar] [CrossRef]
- Folkman, J. What Is the Evidence That Tumors Are Angiogenesis Dependent? J. Natl. Cancer Inst. 1990, 82, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Staton, C.A.; Reed, M.W.R.; Brown, N.J. A Critical Analysis of Current in vitro and in vivo Angiogenesis Assays. Int. J. Exp. Pathol. 2009, 90, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Ji, M.; Song, X.; Zhu, Y.; Wang, Z.; Zhang, X.; Wu, S.; Chen, H.; Mei, L.; Zheng, Y. Co-Delivery of Docetaxel and Endostatin by a Biodegradable Nanoparticle for the Synergistic Treatment of Cervical Cancer. Nanoscale Res. Lett. 2012, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Eden, H.S.; Chen, X. Peptides in Cancer Nanomedicine: Drug Carriers, Targeting Ligands and Protease Substrates. J. Control. Release 2012, 159, 2–13. [Google Scholar] [CrossRef]
- Sznol, M.; Davis, T. Tumor antigens as targets for anticancer drug development. In Anticancer Drug Development; Kerr, B.C.B.J., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 157–170. ISBN 978-0-12-072651-6. [Google Scholar]
- Wigle, J.T.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.D.; Jackson, D.G.; Oliver, G. An Essential Role for Prox1 in the Induction of the Lymphatic Endothelial Cell Phenotype. EMBO J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef]
- Adeyemi, S.A.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. Design and Characterization of Endostatin-Loaded Nanoparticles for In vitro Antiangiogenesis in Squamous Cell Carcinoma. J. Nanomater. 2017, 2017, 2539065. [Google Scholar] [CrossRef]
- Adeyemi, S.A.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. Synthesis and in vitro Characterization of a PH-Responsive Chitosan- Polyethylenimine Nanosystem for the Delivery of Therapeutic Proteins. J. Drug Deliv. Sci. Technol. 2017, 39, 266–276. [Google Scholar] [CrossRef]
- Laakkonen, P.; Åkerman, M.E.; Biliran, H.; Yang, M.; Ferrer, F.; Karpanen, T.; Hoffman, R.M.; Ruoslahti, E. Antitumor Activity of a Homing Peptide That Targets Tumor Lymphatics and Tumor Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 9381–9386. [Google Scholar] [CrossRef]
- Gazaille, C.; Sicot, M.; Akiki, M.; Lautram, N.; Dupont, A.; Saulnier, P.; Eyer, J.; Bastiat, G. Characterization of Biological Material Adsorption to the Surface of Nanoparticles without a Prior Separation Step: A Case Study of Glioblastoma-Targeting Peptide and Lipid Nanocapsules. Pharm. Res. 2021, 38, 681–691. [Google Scholar] [CrossRef]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Yu, X.; Jin, C.; Yang, F.; Fu, D.; Long, J.; Xu, J.; Zhan, C.; Lu, W. LyP-1-Conjugated Nanoparticles for Targeting Drug Delivery to Lymphatic Metastatic Tumors. Int. J. Pharm. 2010, 385, 150–156. [Google Scholar] [CrossRef]
- Hauert, S.; Bhatia, S.N. Mechanisms of Cooperation in Cancer Nanomedicine: Towards Systems Nanotechnology. Trends Biotechnol. 2014, 32, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic Extracellular Microenvironment and Cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor Microenvironment Sensitive Doxorubicin Delivery and Release to Glioma Using Angiopep-2 Decorated Gold Nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and Characteristics Governing Cellular Internalization and Trans-Barrier Trafficking of Nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size Matters: Gold Nanoparticles in Targeted Cancer Drug Delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef]
- Shayan, R.; Achen, M.G.; Stacker, S.A. Lymphatic Vessels in Cancer Metastasis: Bridging the Gaps. Carcinogenesis 2006, 27, 1729–1738. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Park, J.; Nam, J.; Won, N.; Jin, H.; Jung, S.; Jung, S.; Cho, S.-H.; Kim, S. Compact and Stable Quantum Dots with Positive, Negative, or Zwitterionic Surface: Specific Cell Interactions and Non-Specific Adsorptions by the Surface Charges. Adv. Funct. Mater. 2011, 21, 1558–1566. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Kanapathipillai, M.; Brock, A.; Ingber, D.E. Nanoparticle Targeting of Anti-Cancer Drugs That Alter Intracellular Signaling or Influence the Tumor Microenvironment. Adv. Drug Deliv. Rev. 2014, 79–80, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Pearce, T.R.; Shroff, K.; Kokkoli, E. Peptide Targeted Lipid Nanoparticles for Anticancer Drug Delivery. Adv. Mater. 2012, 24, 3803–3822. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of Drugs and Nanoparticles to Tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef]
- Conibear, A.C.; Chaousis, S.; Durek, T.; Rosengren, K.J.; Craik, D.J.; Schroeder, C.I. Approaches to the Stabilization of Bioactive Epitopes by Grafting and Peptide Cyclization. Biopolymers 2016, 106, 89–100. [Google Scholar] [CrossRef]
- Christian, S.; Pilch, J.; Akerman, M.E.; Porkka, K.; Laakkonen, P.; Ruoslahti, E. Nucleolin Expressed at the Cell Surface Is a Marker of Endothelial Cells in Angiogenic Blood Vessels. J. Cell Biol. 2003, 163, 871–878. [Google Scholar] [CrossRef]
- Marasini, R.; Thanh Nguyen, T.D.; Rayamajhi, S.; Aryal, S. Synthesis and Characterization of a Tumor-Seeking LyP-1 Peptide Integrated Lipid–Polymer Composite Nanoparticle. Mater. Adv. 2020, 1, 469–480. [Google Scholar] [CrossRef]
- Leist, M.; Jäättelä, M. Four Deaths and a Funeral: From Caspases to Alternative Mechanisms. Nat. Rev. Mol. Cell Biol. 2001, 2, 589–598. [Google Scholar] [CrossRef]
- Lovrić, J.; Cho, S.J.; Winnik, F.M.; Maysinger, D. Unmodified Cadmium Telluride Quantum Dots Induce Reactive Oxygen Species Formation Leading to Multiple Organelle Damage and Cell Death. Chem. Biol. 2005, 12, 1227–1234. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Song, H.; Canup, B.S.B.; Gou, S.; She, Z.; Dai, F.; Ke, B.; Xiao, B. Inhibition of Growth and Lung Metastasis of Breast Cancer by Tumor-Homing Triple-Bioresponsive Nanotherapeutics. J. Control. Release 2020, 328, 454–469. [Google Scholar] [CrossRef]
- ShiDu Yan, S. Disrupting Cancer Cell Function by Targeting Mitochondria. Integr. Cancer Sci. Ther. 2014, 1, 17–25. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pustylnikov, S.; Costabile, F.; Beghi, S.; Facciabene, A. Targeting Mitochondria in Cancer: Current Concepts and Immunotherapy Approaches. Transl. Res. J. Lab. Clin. Med. 2018, 202, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Fogal, V.; Zhang, L.; Krajewski, S.; Ruoslahti, E. Mitochondrial/Cell-Surface Protein P32/GC1qR as a Molecular Target in Tumor Cells and Tumor Stroma. Cancer Res. 2008, 68, 7210–7218. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Walsh, M.L.; Chen, L.B. Localization of Mitochondria in Living Cells with Rhodamine 123. Proc. Natl. Acad. Sci. USA 1980, 77, 990–994. [Google Scholar] [CrossRef]
- Goodwin, A.M. In vitro Assays of Angiogenesis for Assessment of Angiogenic and Anti-Angiogenic Agents. Microvasc. Res. 2007, 74, 172–183. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the AlamarBlue Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095489. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The Use of Alamar Blue Assay for Quantitative Analysis of Viability, Migration and Invasion of Choriocarcinoma Cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef]
- Mavuso, S.; Choonara, Y.E.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Pillay, V. A Dual PH/Redox Responsive Copper-Ligand Nanoliposome Bioactive Complex for the Treatment of Chronic Inflammation. Int. J. Pharm. 2016, 509, 348–359. [Google Scholar] [CrossRef]
- Agemy, L.; Kotamraju, V.R.; Friedmann-Morvinski, D.; Sharma, S.; Sugahara, K.N.; Ruoslahti, E. Proapoptotic Peptide-Mediated Cancer Therapy Targeted to Cell Surface P32. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro Cell Migration and Invasion Assays. Mutat. Res. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Antiangiogenesis in Cancer Therapy--Endostatin and Its Mechanisms of Action. Exp. Cell Res. 2006, 312, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, M.; Chen, J.; He, L.; Zhao, N.; Chen, K. Effect of VEGF-C SiRNA and Endostatin on Ring Formation and Proliferation of Esophageal Squamous Cell Carcinoma Lymphatic Endothelial Cells. Onco Targets Ther. 2016, 9, 6727–6732. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jang, J.W.; Lee, O.H.; Yeon, J.; Choi, E.Y.; Kim, K.W.; Lee, S.T.; Kwon, Y.G. Endostatin Inhibits Endothelial and Tumor Cellular Invasion by Blocking the Activation and Catalytic Activity of Matrix Metalloproteinase. Cancer Res. 2000, 60, 5410–5413. [Google Scholar]
- Lai, S.-L.; Cheah, S.-C.; Wong, P.-F.; Noor, S.M.; Mustafa, M.R. In vitro and In vivo Anti-Angiogenic Activities of Panduratin A. PLoS ONE 2012, 7, e38103. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Hwang, S.; Kim, Y.-M.; Pyun, B.-J.; Kim, T.-Y.; Lee, S.-T.; Gho, Y.S.; Kwon, Y.-G. Endostatin Blocks Vascular Endothelial Growth Factor-Mediated Signaling via Direct Interaction with KDR/Flk-1. J. Biol. Chem. 2002, 277, 27872–27879. [Google Scholar] [CrossRef]
- Sun, J.; Hemler, M.E. Regulation of MMP-1 and MMP-2 Production through CD147/Extracellular Matrix Metalloproteinase Inducer Interactions. Cancer Res. 2001, 61, 2276–2281. [Google Scholar]
- Oguntade, A.S.; Al-Amodi, F.; Alrumayh, A.; Alobaida, M.; Bwalya, M. Anti-Angiogenesis in Cancer Therapeutics: The Magic Bullet. J. Egypt. Natl. Cancer Inst. 2021, 33, 15. [Google Scholar] [CrossRef]
- Ma, Y.; Su, X.; Li, X.; Zhi, X.; Jiang, K.; Xia, J.; Li, H.; Yan, C.; Zhou, L. Combined Detection of Peripheral Blood VEGF and Inflammation Biomarkers to Evaluate the Clinical Response and Prognostic Prediction of Non-Operative ESCC. Sci. Rep. 2021, 11, 15305. [Google Scholar] [CrossRef]
- Ghasemali, S.; Farajnia, S.; Barzegar, A.; Rahmati-Yamchi, M.; Baghban, R.; Rahbarnia, L.; Nodeh, H.R.Y. New Developments in Anti-Angiogenic Therapy of Cancer, Review and Update. Anti-Cancer Agents Med. Chem. 2021, 21, 3–19. [Google Scholar] [CrossRef]
- Chuai, Y.; Rizzuto, I.; Zhang, X.; Li, Y.; Dai, G.; Otter, S.J.; Bharathan, R.; Stewart, A.; Wang, A. Vascular Endothelial Growth Factor (VEGF) Targeting Therapy for Persistent, Recurrent, or Metastatic Cervical Cancer. Cochrane Database Syst. Rev. 2021, 3, CD013348. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.H.; Ozawa, S.; Ando, N.; Ueda, M.; Kitajima, M. Vascular Endothelial Growth Factor Expression Predicts Outcome and Lymph Node Metastasis in Squamous Cell Carcinoma of the Esophagus. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 1161–1168. [Google Scholar]
- Crescenzi, M.; Persano, L.; Esposito, G.; Zulato, E.; Borsi, L.; Balza, E.; Ruol, A.; Ancona, E.; Indraccolo, S.; Amadori, A. Vandetanib Improves Anti-Tumor Effects of L19mTNFalpha in Xenograft Models of Esophageal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Kordt, M.; Palme, R.; Grambow, E.; Vollmar, B.; Zechner, D. Diagnostic Ability of Methods Depicting Distress of Tumor-Bearing Mice. Animals 2021, 11, 2155. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Shin, J.-Y.; Kim, J.-O.; Son, K.-H.; Kim, Y.S.; Jung, C.K.; Kang, J.-H. Anti-Tumor Efficacy of CKD-516 in Combination with Radiation in Xenograft Mouse Model of Lung Squamous Cell Carcinoma. BMC Cancer 2020, 20, 1057. [Google Scholar] [CrossRef]
- Murray, S.; Schell, K.; McCarthy, D.O.; Albertini, M.R. Tumor Growth, Weight Loss and Cytokines in SCID Mice. Cancer Lett. 1997, 111, 111–115. [Google Scholar] [CrossRef]
- Bredholt, G.; Mannelqvist, M.; Stefansson, I.M.; Birkeland, E.; Bø, T.H.; Øyan, A.M.; Trovik, J.; Kalland, K.-H.; Jonassen, I.; Salvesen, H.B.; et al. Tumor Necrosis Is an Important Hallmark of Aggressive Endometrial Cancer and Associates with Hypoxia, Angiogenesis and Inflammation Responses. Oncotarget 2015, 6, 39676–39691. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Natali, L.; Nisi, M.; De Leo, M.; Daniele, S.; Costa, B.; Graziani, F.; Gabriele, M.; Braca, A.; Trincavelli, M.L.; et al. Negative Effects of a High Tumour Necrosis Factor-α Concentration on Human Gingival Mesenchymal Stem Cell Trophism: The Use of Natural Compounds as Modulatory Agents. Stem Cell Res. Ther. 2018, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Samaratunga, H.; Delahunt, B.; Srigley, J.R.; Berney, D.M.; Cheng, L.; Evans, A.; Furusato, B.; Leite, K.R.M.; MacLennan, G.T.; Martignoni, G.; et al. Granular Necrosis: A Distinctive Form of Cell Death in Malignant Tumours. Pathology 2020, 52, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor Necrosis Factor and Cancer, Buddies or Foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Rossi, C.R.; Pilati, P.; Nitti, D. Tumor Necrosis Factor, Cancer and Anticancer Therapy. Cytokine Growth Factor Rev. 2005, 16, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Pei, Y.; Uzunalli, G.; Hyun, H.; Lyle, L.T.; Yeo, Y. Surface Modification of Polymeric Nanoparticles with M2pep Peptide for Drug Delivery to Tumor-Associated Macrophages. Pharm. Res. 2019, 36, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Neerooa, B.N.H.M.; Ooi, L.-T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.-Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels 2021, 7, 60. [Google Scholar] [CrossRef]
- Adeyemi, S.A.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Folate-Decorated, Endostatin-Loaded, Nanoparticles for Anti-Proliferative Chemotherapy in Esophaegeal Squamous Cell Carcinoma. Biomed. Pharmacother. 2019, 119, 109450. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Lee, D.; Li, P. Intracellular Uptake and Release of Poly(Ethyleneimine)-Co-Poly(Methyl Methacrylate) Nanoparticle/PDNA Complexes for Gene Delivery. Int. J. Pharm. 2006, 311, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cui, S.; Zhao, Y.; Zhang, C.; Zhang, S.; Peng, X. Grafting Chitosan with Polyethylenimine in an Ionic Liquid for Efficient Gene Delivery. PLoS ONE 2015, 10, e0121817. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, S.A.; Choonara, Y.E. In Vitro and In Vivo Evaluation of a Cyclic LyP-1-Modified Nanosystem for Targeted Endostatin Delivery in a KYSE-30 Cell Xenograft Athymic Nude Mice Model. Pharmaceuticals 2022, 15, 353. https://doi.org/10.3390/ph15030353
Adeyemi SA, Choonara YE. In Vitro and In Vivo Evaluation of a Cyclic LyP-1-Modified Nanosystem for Targeted Endostatin Delivery in a KYSE-30 Cell Xenograft Athymic Nude Mice Model. Pharmaceuticals. 2022; 15(3):353. https://doi.org/10.3390/ph15030353
Chicago/Turabian StyleAdeyemi, Samson A., and Yahya E. Choonara. 2022. "In Vitro and In Vivo Evaluation of a Cyclic LyP-1-Modified Nanosystem for Targeted Endostatin Delivery in a KYSE-30 Cell Xenograft Athymic Nude Mice Model" Pharmaceuticals 15, no. 3: 353. https://doi.org/10.3390/ph15030353
APA StyleAdeyemi, S. A., & Choonara, Y. E. (2022). In Vitro and In Vivo Evaluation of a Cyclic LyP-1-Modified Nanosystem for Targeted Endostatin Delivery in a KYSE-30 Cell Xenograft Athymic Nude Mice Model. Pharmaceuticals, 15(3), 353. https://doi.org/10.3390/ph15030353







