Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4
Abstract
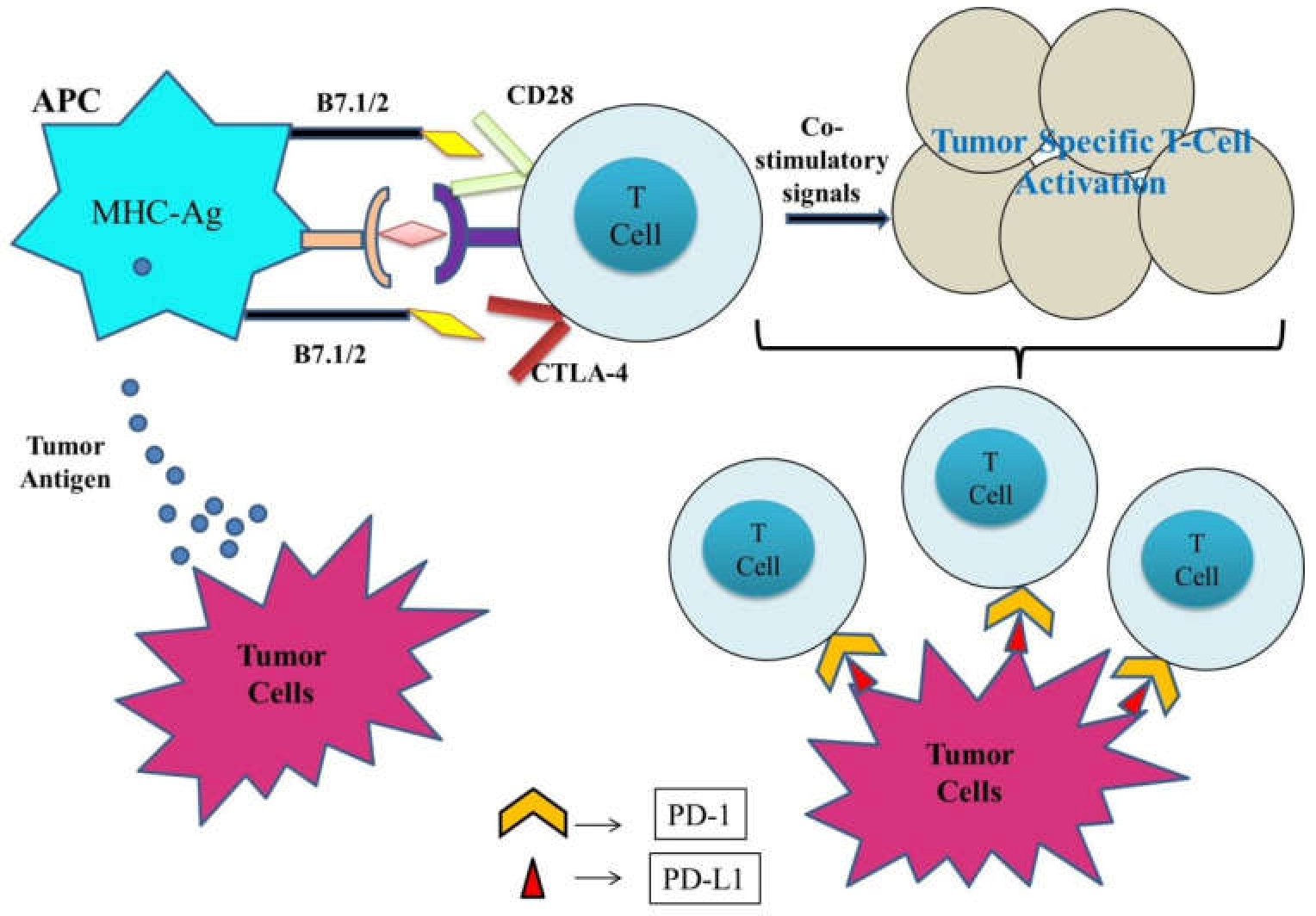
:1. Introduction
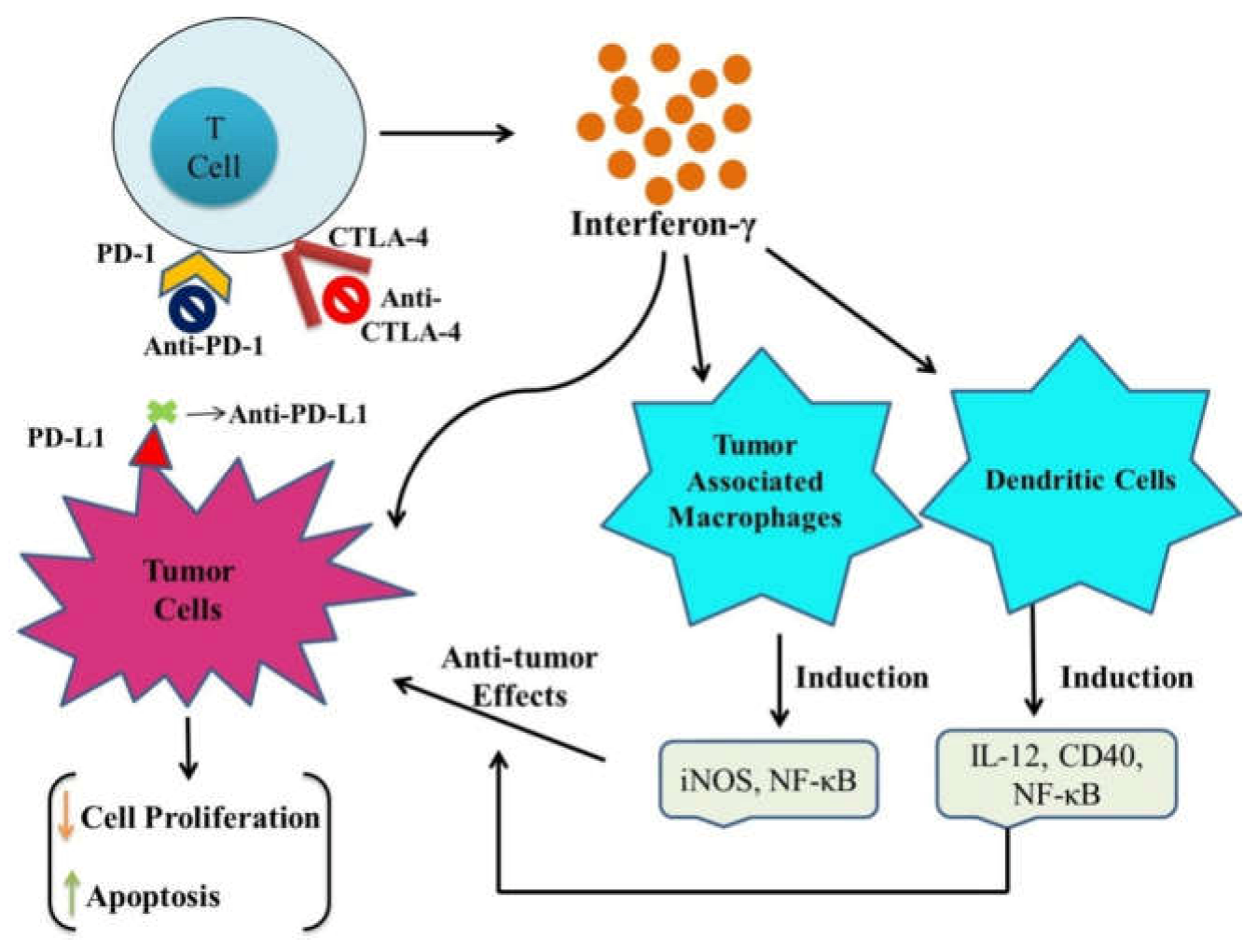
2. Emerging Immune Checkpoint Inhibitors
2.1. CTLA-4 (CD152) Inhibitors
2.2. PD-1 (CD279) Inhibitors
2.3. PD-L1 (CD274) Inhibitors
3. Immune Checkpoint Inhibitors in Combination with ICDs (Immunogenic Cell Death) Inducers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD-1/PD-L1 | programmed death 1/programmed death ligand 1 |
| CTLA-4 | cytotoxic T lymphocyte-associated antigen-4 |
| IDO | indoleamine 2.3-dioxygenase |
| HER2+ | human epidermal growth factor receptor 2 |
| SCLC | small cell lung cancer |
| TNF-α | tumor necrosis factor-α |
| CSCC | cutaneous squamous cell carcinoma |
| NCI | National Cancer Institute |
| FDA | Food and Drug Administration |
| CTLs | cytotoxic T lymphocytes |
| CTLA-4 | cytotoxic T lymphocyte-associated protein 4 |
| ROS | reactive oxygen species |
| IR | immune response |
| TCR | T cell receptor |
| TRIM | T cell interacting molecules |
References
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Wang, J.; Zhang, B. Anti-angiogenic agents in combination with immune checkpoint inhibitors: A promising strategy for cancer treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bhattacharya, P.; Prabhakar, B.S. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J. Autoimmun. 2018, 95, 77–99. [Google Scholar] [CrossRef]
- De Giglio, A.; Di Federico, A.; Nuvola, G.; Deiana, C.; Gelsomino, F. The Landscape of Immunotherapy in Advanced NSCLC: Driving Beyond PD-1/PD-L1 Inhibitors (CTLA-4, LAG3, IDO, OX40, TIGIT, Vaccines). Curr. Oncol. Rep. 2021, 27, 126. [Google Scholar] [CrossRef]
- Abdkarimi, S.; RaziSoofiyani, S.; Elham, G.; MashhadiAbdolahi, H.; Safarzadeh, E.; Baradaran, B. Targeting immune checkpoints: Building better therapeutic puzzle in pancreatic cancer combination therapy. Eur. J. Cancer Care 2020, 29, e13268. [Google Scholar] [CrossRef]
- Liu, D.; Gao, S.; Zhai, Y.; Yang, X.; Zhai, G. Research progress of tumor targeted drug delivery based on PD-1/PD-L1. Int. J. Pharm. 2022, 616, 121527. [Google Scholar] [CrossRef]
- Makkouk, A.; Chester, C.; Kohrt, H.E. Rationale for anti-CD137 cancer immunotherapy. Eur. J. Cancer 2016, 54, 112–119. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Strome, S.E. The role of the PD-L1: PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014, 50, 627–632. [Google Scholar] [CrossRef]
- Chikuma, S. CTLA-4, an essential immune-checkpoint for T-cell activation. Emerg. Concepts Target. Immune Checkp. Cancer Autoimmun. 2017, 410, 99–126. [Google Scholar]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, N.; Crown, J.; Collins, D.M. Immune checkpoint inhibitors: Key trials and an emerging role in breast cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Peeraphatdit, T.; Wang, J.; Odenwald, M.A.; Hu, S.; Hart, J.; Charlton, M.R. Hepatotoxicity from immune checkpoint inhibitors: A systematic review and management recommendation. Hepatology 2020, 72, 315–329. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Carlino, M.S.; Underhill, C.; Palmer, J.; Power, D.; Cebon, J.; Behren, A. Evaluation of TMB as a predictive biomarker in patients with solid cancers treated with anti-PD-1/CTLA-4 combination immunotherapy. Cancer Cell 2021, 39, 592–593. [Google Scholar] [CrossRef]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and cellular functions of CTLA-4. Regul. Cancer Immune Checkp. 2020, 1248, 7–32. [Google Scholar]
- Forde, P.M.; Scherpereel, A.; Tsao, A.S. Use of immune checkpoint inhibitors in mesothelioma. Curr. Treat. Options Oncol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [Green Version]
- Chambers, C.A.; Sullivan, T.J.; Allison, J.P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 1997, 7, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.J.; Liu, Z.; Cheng, Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef]
- Simeone, E.; Ascierto, P.A. Immunomodulating antibodies in the treatment of metastatic melanoma: The experience with anti-CTLA-4, anti-CD137, and anti-PD-1. J. Immunotoxicol. 2012, 9, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinai, J.M.; Janakiram, M.; Chen, F.; Chen, W.; Kaplan, M.; Zang, X. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol. Sci. 2015, 36, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J. Immune checkpoint proteins: A new therapeutic paradigm for cancer—preclinical background: CTLA-4 and PD-1 blockade. Semin. Oncol. 2020, 37, 430–439. [Google Scholar] [CrossRef]
- Vidyarthi, A.; Agnihotri, T.; Khan, N.; Singh, S.; Tewari, M.K.; Radotra, B.D.; Chatterjee, D.; Agrewala, J.N. Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity. Cancer Immunol. Immunother. 2019, 68, 1995–2004. [Google Scholar] [CrossRef]
- Ivashko, I.N.; Kolesar, J.M. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. Am. J. Health-Syst. Pharm. 2016, 73, 193–201. [Google Scholar] [CrossRef]
- Foord, E.; Klynning, C.; Schoutrop, E.; Förster, J.M.; Krieg, J.; Mörtberg, A.; Müller, M.R.; Herzog, C.; Schiegg, D.; Villemagne, D.; et al. Profound Functional Suppression of Tumor-Infiltrating T-Cells in Ovarian Cancer Patients Can Be Reversed Using PD-1-Blocking Antibodies or DARPin® Proteins. J. Immunol. Res. 2020, 2020, 7375947. [Google Scholar] [CrossRef]
- Leonetti, A.; Wever, B.; Mazzaschi, G.; Assaraf, Y.G.; Rolfo, C.; Quaini, F.; Tiseo, M.; Giovannetti, E. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist. Updates 2019, 46, 100644. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Waidmann, O.; Trojan, J. Nivolumab for the treatment of hepatocellular carcinoma. Expert Rev. Anticancer. Ther. 2018, 18, 1169–1175. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.R.; Petersen, E.; Patel, R.; Migden, M.R. Cemiplimab-rwlc as first and only treatment for advanced cutaneous squamous cell carcinoma. Expert Rev. Clin. Pharmacol. 2019, 12, 947–951. [Google Scholar] [CrossRef]
- Hoy, S.M. Sintilimab: First global approval. Drugs 2019, 79, 341–346. [Google Scholar] [CrossRef]
- Song, Y.; Gao, Q.; Zhang, H.; Fan, L.; Zhou, J.; Zou, D.; Li, W.; Yang, H.; Liu, T.; Wang, Q.; et al. Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: Results of a phase 2, single-arm, multicenter study. Leukemia 2020, 34, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Guo, J.; Zhang, Q.; Pan, H.; Yuan, Y.; Bai, Y.; Liu, T.; Zhou, Q.; Zhao, J.; Shu, Y.; et al. Tislelizumab in Chinese patients with advanced solid tumors: An open-label, non-comparative, phase 1/2 study. J. Immunother. Cancer 2020, 8, e000437. [Google Scholar] [CrossRef]
- Keam, S.J. Toripalimab: First global approval. Drugs 2019, 79, 573–578. [Google Scholar] [CrossRef]
- Johnson, D.; Ma, B.B. Targeting the PD-1/PD-L1 interaction in nasopharyngeal carcinoma. Oral Oncol. 2021, 113, 105127. [Google Scholar] [CrossRef]
- Even, C.; Wang, H.-M.; Li, S.-H.; Ngan, R.K.-C.; Dechaphunkul, A.; Zhang, L.; Yen, C.-J.; Chan, P.C.; Chakrabandhu, S.; Ma, B.B.; et al. Phase II, Randomized Study of Spartalizumab (PDR001), an Anti–PD-1 Antibody, versus Chemotherapy in Patients with Recurrent/Metastatic Nasopharyngeal Cancer. Clin. Cancer Res. 2021, 27, 6413–6423. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Gowrishankar, K.; Gunatilake, D.; Gallagher, S.J.; Tiffen, J.; Rizos, H.; Hersey, P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS ONE 2015, 10, e0123410. [Google Scholar] [CrossRef] [Green Version]
- Oliva, S.; Troia, R.; D’Agostino, M.; Boccadoro, M.; Gay, F. Promises and pitfalls in the use of PD-1/PD-L1 inhibitors in multiple myeloma. Front. Immunol. 2018, 9, 2749. [Google Scholar] [CrossRef]
- Shah, N.J.; Kelly, W.J.; Liu, S.V.; Choquette, K.; Spira, A. Product review on the Anti-PD-L1 antibody atezolizumab. Hum. Vaccines Immunother. 2018, 14, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.; Cordes, L.; Brownell, I. Avelumab: A new standard for treating metastatic Merkel cell carcinoma. Expert Rev. Anticancer. Ther. 2018, 18, 319–326. [Google Scholar] [CrossRef]
- Li, D.; Cheng, S.; Zou, S.; Zhu, D.; Zhu, T.; Wang, P.; Zhu, X. Immuno-PET imaging of 89Zr labeled anti-PD-L1 domain antibody. Mol. Pharm. 2018, 15, 1674–1681. [Google Scholar] [CrossRef]
- Inman, B.A.; Longo, T.A.; Ramalingam, S.; Harrison, M.R. Atezolizumab: A PD-L1–blocking antibody for bladder cancer. Clin. Cancer Res. 2017, 23, 1886–1890. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.; Bumbaca, D.; Pastuskovas, C.V.; Boswell, C.A.; West, D.; Cowan, K.J.; Chiu, H.; McBride, J.; Johnson, C.; Xin, Y.; et al. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. mAbs 2016, 8, 593–603. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, H.; Zhang, T.; Yang, X.; Zhong, J.; Wang, Y.; Chi, Y.; Wu, M.; An, T.; Li, J.; et al. Plasma cytokines interleukin-18 and CXC motif chemokine ligand 10 are indicative of the anti-programmed cell death protein-1 treatment response in lung cancer patients. Ann. Transl. Med. 2021, 9, 33. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; Van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Schmid, P.; Arén, O.; Arrieta, O.; Gottfried, M.; Jazieh, A.; Ramlau, R.; Timcheva, K.; Martin, C.; McIntosh, S. 192TiP: NEPTUNE: A global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy versus standard of care (SoC) platinum-based chemotherapy in the first-line treatment of patients (pts) with advanced or metastatic NSCLC. J. Thorac. Oncol. 2016, 11, S140–S141. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and characterization of MEDI4736, an antagonistic anti–PD-L1 monoclonal antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Grenga, I.; Donahue, R.N.; Lepone, L.M.; Richards, J.; Schlom, J. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin. Transl. Immunol. 2016, 5, e83. [Google Scholar] [CrossRef] [Green Version]
- Apolo, A.B.; Infante, J.R.; Balmanoukian, A.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Ravaud, A.; Mita, A.C.; et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: Results from a multicenter, phase ib study. J. Clin. Oncol. 2017, 35, 2117. [Google Scholar] [CrossRef]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- Taneja, S.S. Re: Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. J. Urol. 2012, 188, 2148–2149. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 2019, 23, 4854–4865. [Google Scholar] [CrossRef]
- Kuai, R.; Yuan, W.; Son, S.; Nam, J.; Xu, Y.; Fan, Y.; Schwendeman, A.; Moon, J.J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018, 4, eaao1736. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.P.; Wang, X.; Ahmed, A.; Jiang, W.; Ji, Y.; Meng, H.; Nel, A.E. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano 2018, 12, 11041–11061. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xia, R.; Huang, Y.; Zhao, W.; Li, J.; Zhang, X.; Wang, P.; Venkataramanan, R.; Fan, J.; Xie, W.; et al. An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat. Commun. 2016, 7, 13443. [Google Scholar] [CrossRef]
- Feng, B.; Zhou, F.; Hou, B.; Wang, D.; Wang, T.; Fu, Y.; Ma, Y.; Yu, H.; Li, Y. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv. Mater. 2018, 30, 1803001. [Google Scholar] [CrossRef]
- Mei, K.C.; Liao, Y.P.; Jiang, J.; Chiang, M.; Khazaieli, M.; Liu, X.; Wang, X.; Liu, Q.; Chang, C.H.; Zhang, X.; et al. Liposomal delivery of mitoxantrone and a cholesterylindoximodprodrug provides effective chemo-immunotherapy in multiple solid tumors. ACS Nano 2020, 14, 13343–13366. [Google Scholar] [CrossRef]
- Sun, J.-J.; Chen, Y.-C.; Huang, Y.-X.; Zhao, W.-C.; Liu, Y.-H.; Venkataramanan, R.; Lu, B.-F.; Li, S. Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol. Sin. 2017, 38, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Sun, J.; Xu, J.; Moharil, P.; Chen, J.; Xu, J.; Zhu, J.; Li, J.; Huang, Y.; Xu, P.; et al. Dual functional immunostimulatory polymeric prodrug carrier with pendent indoximod for enhanced cancer immunochemotherapy. Acta Biomater. 2019, 90, 300–313. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Sun, Y.; Wan, C.; Zhang, Z.; Dai, X.; Lin, Z.; He, Q.; Yang, Z.; Huang, P.; et al. Co-delivery of bee venom melittin and a photosensitizer with an organic–inorganic hybrid nanocarrier for photodynamic therapy and immunotherapy. ACS Nano 2019, 13, 12638–12652. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic therapy mediated by nontoxic core–shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [Green Version]
- Ge, R.; Liu, C.; Zhang, X.; Wang, W.; Li, B.; Liu, J.; Liu, Y.; Sun, H.; Zhang, D.; Hou, Y.; et al. Photothermal-activatable Fe3O4 superparticlenanodrug carriers with PD-L1 immune checkpoint blockade for anti-metastatic cancer immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 20342–20355. [Google Scholar] [CrossRef]
- Sun, W.; Du, Y.; Liang, X.; Yu, C.; Fang, J.; Lu, W.; Guo, X.; Tian, J.; Jin, Y.; Zheng, J. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials 2019, 217, 119264. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, J.; Giżynski, K.; Song, M.G.; Grzybowski, B.A. Interference-like patterns of static magnetic fields imprinted into polymer/nanoparticle composites. Nat. Commun. 2017, 8, 1564. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Luo, Y.; Tian, X.; Ma, S.; Sun, Y.; You, C.; Gong, Y.; Xie, C. Impact of radiotherapy concurrent with anti-PD-1 therapy on the lung tissue of tumor-bearing mice. Radiat. Res. 2019, 191, 271–277. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Qin, X.; Liu, J. Photothermal-chemotherapy integrated nanoparticles with tumor microenvironment response enhanced the induction of immunogenic cell death for colorectal cancer efficient treatment. ACS Appl. Mater. Interfaces 2019, 11, 43393–43408. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Chan, C.; Han, W.; Guo, N.; Weichselbaum, R.R.; Lin, W. Immunostimulatorynanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat. Commun. 2019, 10, 1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Ma, Y.; Wang, S.; Li, Y. Tumor microenvironment-activatableprodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv. Mater. 2019, 31, 1805888. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choi, H.; Yu, B.; Kim, D.H. Synergistic local combination of radiation and anti-programmed death ligand 1 immunotherapy using radiation-responsive splintery metallic nanocarriers. ACS Nano 2020, 14, 13115–13126. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, W.; Kwak, K.; Choi, H.; Kim, D.H. Electric pulse responsive magnetic nanoclusters loaded with indoleamine 2,3-dioxygenase inhibitor for synergistic immuno-ablation cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 54415–54425. [Google Scholar] [CrossRef]
- Zhai, Q.; Chen, Y.; Xu, J.; Huang, Y.; Sun, J.; Liu, Y.; Zhang, X.; Li, S.; Tang, S. Lymphoma immunochemotherapy: Targeted delivery of doxorubicin via a dual functional nanocarrier. Mol. Pharm. 2017, 14, 3888–3895. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.; Melero, I.; Kopecka, J.; Sarmento-Ribeiro, A.B.; García-Aranda, M.; De Las Rivas, J. Cancer immunotherapy resistance based on immune checkpoints inhibitors: Targets, biomarkers, and remedies. Drug Resist. Updates 2020, 53, 100718. [Google Scholar] [CrossRef]
- Woods, D.M.; Sodré, A.L.; Villagra, A.; Sarnaik, A.; Sotomayor, E.M.; Weber, J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol. Res. 2015, 3, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Zhao, W.; Yan, C.; Watson, C.C.; Massengill, M.; Xie, M.; Massengill, C.; Noyes, D.R.; Martinez, G.V.; Afzal, R.; et al. HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin. Cancer Res. 2016, 22, 4119–4132. [Google Scholar] [CrossRef] [Green Version]
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol. Immunother. 2016, 65, 779–786. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Shao, D.; Chang, Z.; Wang, L.; Hu, H.; Zheng, X.; Li, X.; Chen, F.; Tu, Z.; et al. Janus nanobullets combine photodynamic therapy and magnetic hyperthermia to potentiate synergetic anti-metastatic immunotherapy. Adv. Sci. 2019, 6, 1901690. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Maxwell, R.; Xia, Y.; Cardarelli, P.; Oyasu, M.; Belcaid, Z.; Kim, E.; Hung, A.; Luksik, A.S.; Garzon-Muvdi, T.; et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J. Neuro-Oncol. 2019, 143, 241–249. [Google Scholar] [CrossRef]
| Cancer | FDA Approved Agents | Clinical Trial (s) |
|---|---|---|
| Merkel cell carcinoma (Skin cancer) | Avelumab (2017) | JAVELIN phase 2 |
| Pembrolizumab (2018) | KEYNOTE-017 phase 2 | |
| Melanoma (Skin Cancer) | Pembrolizumab (2014) | KEYNOTE-001 phase 1 |
| Nivolumab (2014) | CheckMate-037 | |
| Nivolumab + ipilimumab (2015) | CheckMate-069 | |
| Pembrolizumab (2015) | KEYNOTE-006 phase 3 | |
| Nivolumab + ipilimumab (2016) | CheckMate-067 phase 3 | |
| Nivolumab (2017) | CheckMate-238 phase 3 | |
| Primary mediastinal large B-cell lymphoma (Blood cancer) | Pembrolizumab (2018) | KEYNOTE-170 phase 2 |
| Classical Hodgkin lymphoma (Blood Cancer) | Nivolumab (2016) | CheckMate-039 phase 1 and Checkmate-205 phase 2 |
| Pembrolizumab (2017) | KEYNOTE-087 phase 2 | |
| Small cell lung cancer | Nivolumab (2018) | CheckMate-032 phase ½ |
| Non-small cell lung cancer | Nivolumab (2015) | CheckMate-017 phase 3 |
| Pembrolizumab (2015) | CheckMate-057 phase 3 | |
| Atezolizumab (2016) | POPLAR phase 2 and OAK phase 3 | |
| Pembrolizumab (2016) | KEYNOTE-024 phase 3 | |
| Pembrolizumab + Caroplatin + Pemetrexed (2017) | KEYNOTE-024 phase 3 | |
| Durvalumab (2018) | KEYNOTE-021 phase 2 | |
| Pembrolizumab + Pemetrexed + Platinum (2018) | PACIFIC phase 3 KEYNOTE-189 phase 3 | |
| Microsatellite instability-high and DNA mismatch repair deficiency unresectable solid tumors (Gastrointestinal Cancer) | Pembrolizumab (2017) | KEYNOTE-164 phase 2 |
| Nivolumab (2017) | CheckMate-142 phase 2 | |
| Nivolumab + ipilimumab (2018) | CheckMate-142 phase 2 | |
| Hepatocellular carcinoma | Nivolumab (2017) | CheckMate-040 phase ½ |
| Pembrolizumab (2018) | KEYNOTE-224 phase 2 | |
| Gastric cancer | Pembrolizumab (2017) | KEYNOTE-059 phase 2 |
| Renal cell cancer | Nivolumab (2015) | CheckMate-025 phase 3 |
| Nivolumab + ipilimumab (2018) | CheckMate-025 phase 3 | |
| Urothelial cancer (Renal Cancer) | Atezolizumab (2016) | IMVigor 210 phase 2 |
| Nivolumab (2017) | CheckMate-275 phase 2 | |
| Atezolizumab (2017) | IMVigor 210 phase 2 | |
| Durvalumab (2017) | Study 1108 phase 2 | |
| Avelumab (2017) | JAVELIN solid tumor phase 1 | |
| Pembrolizumab (2017) | KEYNOTE-052 phase 2 KEYNOTE-045 phase 3 | |
| Cervical cancer | Pembrolizumab (2018) | KEYNOTE-158 phase 2 |
| Head and Neck squamous cell carcinoma | Pembrolizumab (2016) | KEYNOTE-012 phase 1b |
| Nivolumab (2016) | CheckMate-141 phase 3 |
| Emerging PD-1 Inhibitors | Features | Cancer Type | Reference |
|---|---|---|---|
| Cemiplimab | Fully human hinge stabilized IgG4 anti-PD-1 antibody | CSCC patients (both metastatic or locally advanced) | [32] |
| Sintilimab | PD-1 targeted human IgG4 mAb (monoclonal antibody) | Gastric carcinoma (NCT03745170) | [33] |
| Lymphoma (NCT04052659) | |||
| Oesophageal carcinoma (NCT03946969) | |||
| NSCLC (NCT03830411) | |||
| Nasopharyngeal cancer (NCT03700476) | |||
| Tislelizumab | PD-1 targeted humanized IgG4 mAb | Hodgkin’s lymphoma (both relapsed and refractory) | [34] |
| Nasopharyngeal carcinoma (NCT03924986) | [35] | ||
| UC (NCT03967977) | |||
| Gastroesophageal or gastric junction cancer (NCT03777657) | |||
| Lymphoma (NCT03493451) | |||
| Oesophageal carcinoma (NCT03957590) | |||
| NSCLC (NCT03358875) | |||
| Toripalimab | Humanized IgG4 anti PD-1 mAb | Metastatic melanoma patients who did not respond to systemic therapies | [36] |
| Liver cancer (NCT03949231) | [37] | ||
| Oesophageal cancer (NCT03829969) | |||
| Neck and head cancer (NCT03952065), | |||
| Melanoma (NCT03941795), | |||
| NSCLC (NCT03924050), | |||
| Neuroendocrine carcinoma of the bladder (NCT03992911) | |||
| Nasopharyngeal carcinoma (NCT03581786) | |||
| Spartaliumab | IgG4 PD-1 targeted mAb | Phase III COMBI-I trial (NCT02967692) in BRAFV600 mutant metastatic or unresectable melanoma | [38] |
| triple-negative breast cancer treatment (TNBC; NCT03499899) | |||
| RCC (NCT04028245) | |||
| NSCLC (NCT03647488) | |||
| Nasopharyngeal carcinoma and colorectal cancer (NCT03891953) |
| Inhibitor | Role in Cancers | References |
|---|---|---|
| Atezolizumab (MPDL3280) Fully humanized IgG1 monoclonal antibody having a modified Fc domain that prohibits the depletion of PD-L1 expressing T cells | It blocks PD-L1 interaction with both B7.1 and PD-1. | [45] |
| Atezolizumab treatment increased immunity against tumors via reducing immunosuppressive signals present in the tumor microenvironment. | [46] | |
| Highly effective against several hematologic malignancies and solid tumors. | [47] | |
| Several preclinical studies have reported increased CD8+ T, IL-18, CXCL11, IFN cells, and reduced IL-6 cytokines. | [48] | |
| In a phase 1 study, three dosing schedules of this drug were tested against various recurrent melanomas, renal cell carcinoma, non-small cell lung carcinoma, gastric cancer, and neck and head squamous cell cancer. In addition, phase II trials have reported a 10% overall response rate in patients with enhanced PD-L1 expression levels. | [49] | |
| Atezolizumab obtained FDA approval for metastatic or local advanced urothelial cancer against cisplatin therapy in May 2016. | [50] | |
| Commonly reported adverse effects include fatigue, pyrexia, reduced appetite, diarrhea, nausea, arthralgia, rash, pruritus, and headache. | ||
| Obtained FDA approval in October 2016 for NSCLC patients undergoing platinum-based chemotherapy | [51] | |
| In December 2018, atezolizumab obtained FDA approval for NSq NSCLC (non-squamous and non-small cell lung carcinoma) patients along with chemotherapy and bevacizumab treatments. | [52] | |
| In March 2019, it obtained further FDA approval for small cell lung cancer patients and chemotherapy. Further FDA has also granted its approval for metastatic or local advanced PD-L1 + ve TNBC patients along with nab-paclitaxel treatment. | [53] | |
| Durvalumab (MEDI4736) Fully humanized IgG1 monoclonal antibody that blocks the interaction of CD80 molecules with PD-1. | Potent inhibitor with subnanomolar activities against PD-L1. | [54] |
| In vivo studies having co implanted T cells have shown significant inhibition of human tumor growth in a xenograft model. | [55] | |
| In May 2017, FDA approved urothelial cancer patients (metastatic or locally advanced) following platinum-based chemotherapies. | [56] | |
| In February 2018, durvalumab received FDA approval for unresectable NSCLC (stage III) patients undergoing platinum-based chemotherapies. | [57] | |
| Avelumab (MSB0010718C) Fully humanized IgG1 mAb blocking the interaction of PD-L1 with B7.1 and PD 1 (Inhibitory T cell receptor) | Avelumab treatments result in cytokine production or adaptive or cell-mediated antitumor IR (immune response). | [58] |
| The wild-type Fc region helps the NK cells induce tumor-directed ADCC (antibody-dependent cell-mediated cytotoxicity). | [59] | |
| In March 2017, the FDA approved its use for patients with metastatic Merkel cell carcinoma (MCC). | [60] | |
| In May 2017, the FDA approved its usage for patients with metastatic urothelial cancer following platinum-based chemotherapy. | [61] | |
| In May 2019, the FDA approved its use for patients with advanced RCC (renal cell carcinoma) axitinib treatment. | [62] | |
| BMS-936559 (MDX-1105) Fully humanized IgG4 mAb that inhibits binding of PD-L1with CD80 and PD-1 | Commonly reported side effects include infusion reaction, arthralgia, fatigue, rash, headache, and pruritus. | [63] |
| Studies showed an overall response rate of 17% in NSCLC, RCC, and melanoma patients. | ||
| CK-301 Fully humanized IgG4 mAb of IgG1 that blocks the interaction of PD-L1 with B7.1 and PD-1 | Comprises the functional Fc domain capable of ADCC induction and CDC (complement-dependent cytotoxicity) mediated Killing of PD-L1+ lymphoma cells. | [64] |
| Cancer | ICD Inducers | ICIs | Anticancer Efficacy | Reference |
|---|---|---|---|---|
| Breast cancer | Doxil | IND (an IDO-1 inhibitor) and anti-PD-1 antibody | It induced superior synergistic anticancer response in comparison to DOX-only liposome along with reduced tumor volume | [67] |
| Paclitaxel | NLG919 (an IDO-1 inhibitor) | Well-controlled tumor growth with a prolonged median survival time of mice | [68] | |
| OXA | NLG919 (an IDO-1 inhibitor) | High efficiency of combined drugs over tumor growth regression compared to free medicines and prevented metastasis in tumor-bearing mice. | [69] | |
| MIT | ND (an IDO-1 inhibitor) | Significant decrease in tumor size and increased survival rate in the treated animal. | [70] | |
| Doxil | NLG919 (an IDO-1 inhibitor) | Increased tumor growth inhibitory potential and prolonging survival rate in treated mice. | [71] | |
| Doxil | IND (an IDO-1 inhibitor) | Improved immune response and tumor regression in tumor-bearing mice | [72] | |
| Ce6 (Photosensitizer) | Anti-PD-1 antibody | Increased ROS production via PDT and elevated tumor ICD; evoked immune response | [73] | |
| Photosensitizer (pyrolipid, a lipid the conjugate of pyropheophorbidea) | Anti-PD-L1 antibody | Stimulated systemic immune response and distant tumors were inhibited | [74] | |
| ICG | Anti-PD-L1 antibody | Prevented liver and lung metastasis via activation of antitumor immune system | [75] | |
| Camptothecin + polypyrrole | Anti-PD-L1 antibody | Combined treatment-induced potent tumor immunogenic cell death and enhanced antitumor immune response. Prevented tumor recurrences and metastasis | [76] | |
| DOX + Ce6 | Anti-PD-L1 antibody | Significant synergistic therapeutic effect was observed that eventually triggered the antitumor immune response and inhibited metastasis | [77] | |
| Ce6 + Magnetic hyperthermia | Anti-CTLA4 Antibody | Combinatorial treatment exhibited strong anticancer activity and elicited ICD along with eradication of metastatic tumors | [78] | |
| Colon Cancer | DOX | Anti-PD-1 Antibody | Treatment resulted in complete regression of persisted tumors in animals and inhibited tumor recurrence in survivors | [67] |
| Ce6 | Anti-CTLA4 Antibody | This resulted in ICD induction and inhibition of distant tumors | [79] | |
| DOX + photothermal reagent | Anti-PD-L1 antibody | Treatment resulted in tumor cell death and induced effective ICD. In addition, it prevented tumor growth with stimulated immune response | [80] | |
| OXA + photosensitizer | Anti-PD-L1 antibody | Treatment resulted in tumor cell death and provoked ICD resulting in tumor regression via a strong immune response. | [81] | |
| OXA + DHA | Anti-PD-L1 antibody | Treatment retarded tumor growth initially for one month, and no tumor recurrence was reported for about 120 days | [82] | |
| OXA + PPa | Anti-CD47 Antibody | The treatment potentially inhibited tumor (both primary and abscopal) growth and inhibited tumor recurrence and metastasis | [83] | |
| Prostate Cancer | Radiotherapy | Anti-PD-L1 antibody | Enhanced tumor ICD and tumor growth suppression, resulting in synergistic anticancer immune response | [84] |
| IRE | IDO-1 inhibitor | Induced tumor ICD and overturned tumor immunosuppression, leading to the elimination of both secondary and primary tumors. | [85] | |
| B-cell Lymphoma | DOX | IDO-1 inhibitor | Significant improvement in antitumor response in comparison to Doxil | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, P.; Khan, F.; Qari, H.A.; Upadhyay, T.K.; Alkhateeb, A.F.; Oves, M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals 2022, 15, 335. https://doi.org/10.3390/ph15030335
Pandey P, Khan F, Qari HA, Upadhyay TK, Alkhateeb AF, Oves M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals. 2022; 15(3):335. https://doi.org/10.3390/ph15030335
Chicago/Turabian StylePandey, Pratibha, Fahad Khan, Huda A. Qari, Tarun Kumar Upadhyay, Abdulhameed F. Alkhateeb, and Mohammad Oves. 2022. "Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4" Pharmaceuticals 15, no. 3: 335. https://doi.org/10.3390/ph15030335
APA StylePandey, P., Khan, F., Qari, H. A., Upadhyay, T. K., Alkhateeb, A. F., & Oves, M. (2022). Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals, 15(3), 335. https://doi.org/10.3390/ph15030335









