Antidepressant-like Effects of BDNF and NGF Individual Loop Dipeptide Mimetics Depend on the Signal Transmission Patterns Associated with Trk
Abstract
:1. Introduction
2. Results
2.1. GSB-106 Was the Only Mimetic That Possesses Antidepressant-like Activity at Acute Administration
2.2. At Subchronic Administration, Not Only GSB-106 but Also GSB-214, GK-2 and GK-6 Exhibit Antidepressant-like Activity
2.3. The PI3K Inhibitor Prevents the Antidepressant-like Effect of GSB-106
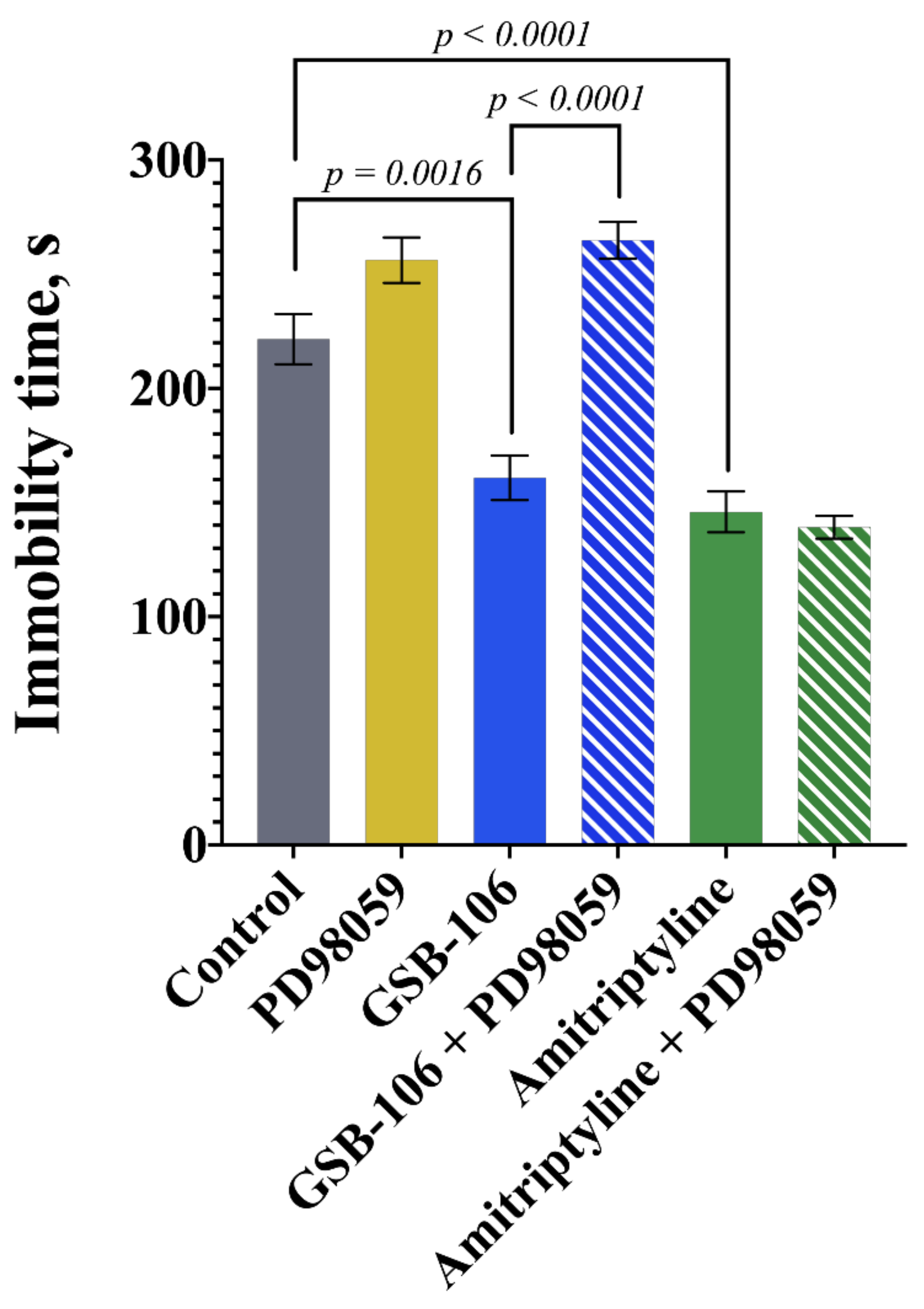
2.4. The MEK1/2 Inhibitor Prevents the Antidepressant-like Effect of GSB-106
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.2.1. Tested Compounds
4.2.2. Drug Reference Standard and Placebo
4.2.3. Signaling Pathway Blockers
4.3. The Forced Swim Test
4.3.1. Design of the Experiment with the Acute Administration of the Mimetics
4.3.2. Design of the Experiments with the Subchronic Administration of the Mimetics
4.4. Pharmacological Inhibitory Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Depression—WHO | World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 10 January 2022).
- Frodl, T. Recent advances in predicting responses to antidepressant treatment. F1000Research 2017, 6, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lader, M. Limitations of current medical treatments for depression: Disturbed circadian rhythms as a possible therapeutic target. Eur. Neuropsychopharmacol. 2007, 17, 743–755. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 6871089. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 35, 2195–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyakova, M.; Stuke, K.; Schuemberg, K.; Mueller, K.; Schoenknecht, P.; Schroeter, M.L. BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. J. Affect. Disord. 2015, 174, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.N.; Ren, X.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Dwivedi, Y. Brain derived neurotrophic factor and tyrosine kinase B receptor signalling in postmortem brain of teenage suicide victims. Int. J. Neuropsychopharmacol. 2008, 11, 1047–1061. [Google Scholar] [CrossRef] [Green Version]
- Karege, F.; Vaudan, G.; Schwald, M.; Perroud, N.; La Harpe, R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Mol. Brain Res. 2005, 136, 29–37. [Google Scholar] [CrossRef]
- Mondal, A.C.; Fatima, M. Direct and indirect evidences of BDNF and NGF as key modulators in depression: Role of antidepressants treatment. Int. J. Neurosci. 2019, 129, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin. Med. Insights Pathol. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Shirayama, Y.; Chen, A.C.-H.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [CrossRef] [Green Version]
- Overstreet, D.H.; Fredericks, K.; Knapp, D.; Breese, G.; McMichael, J. Nerve growth factor (NGF) has novel antidepressant-like properties in rats. Pharmacol. Biochem. Behav. 2010, 94, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siuciak, J.A.; Boylan, C.; Fritsche, M.; Altar, C.A.; Lindsay, R.M. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996, 710, 11–20. [Google Scholar] [CrossRef]
- Szapacs, M.E.; Mathews, T.A.; Tessarollo, L.; Ernest Lyons, W.; Mamounas, L.A.; Andrews, A.M. Exploring the relationship between serotonin and brain-derived neurotrophic factor: Analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J. Neurosci. Methods 2004, 140, 81–92. [Google Scholar] [CrossRef]
- Li, Y.; Luikart, B.W.; Birnbaum, S.; Chen, J.; Kwon, C.H.; Kernie, S.G.; Bassel-Duby, R.; Parada, L.F. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 2008, 59, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Monteggia, L.M.; Barrot, M.; Powell, C.M.; Berton, O.; Galanis, V.; Gemelli, T.; Meuth, S.; Nagy, A.; Greene, R.W.; Nestler, E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA 2004, 101, 10827–10832. [Google Scholar] [CrossRef] [Green Version]
- Sairanen, M.; Lucas, G.; Ernfors, P.; Castrén, M.; Castrén, E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005, 25, 1089–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, F.M.; Massa, S.M. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug. Discov. 2013, 12, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J.; Lu, D.; Chadwick, W.; Siddiqui, S.; Azmi, A.; Etienne, H.; Jushaj, A.; van Gastel, J.; Martin, B.; Maudsley, S. Development of Precision Small-Molecule Proneurotrophic Therapies for Neurodegenerative Diseases. Vitam. Horm. 2017, 104, 263–311. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Hashimoto, K. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 2015, 232, 4325–4335. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Povarnina, P.Y.; Antipova, T.A.; Firsova, Y.N.; Konstantinopolsky, M.A.; Seredenin, S.B. Dimeric dipeptide mimetics of the nerve growth factor Loop 4 and Loop 1 activate TRKA with different patterns of intracellular signal transduction. J. Biomed. Sci. 2015, 22, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudasheva, T.A.; Tarasiuk, A.V.; Pomogaibo, S.V.; Logvinov, I.O.; Povarnina, P.I.; Antipova, T.A.; Seredenin, S.B. Design and synthesis of dipeptide mimetics of brain-derived neurotrophic factor. Bioorg. Khim. 2012, 38, 280–290. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Paoletti, F.; de Chiara, C.; Kelly, G.; Covaceuszach, S.; Malerba, F.; Yan, R.; Lamba, D.; Cattaneo, A.; Pastore, A. Conformational Rigidity within Plasticity Promotes Differential Target Recognition of Nerve Growth Factor. Front. Mol. Biosci. 2016, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- McDonald, N.Q.; Lapatto, R.; Murray-Rust, J.; Gunning, J.; Wlodawer, A.; Blundell, T.L. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature 1991, 354, 411–414. [Google Scholar] [CrossRef]
- Robinson, R.C.; Radziejewski, C.; Spraggon, G.; Greenwald, J.; Kostura, M.R.; Burtnick, L.D.; Stuart, D.I.; Choe, S.; Jones, E.Y. The structures of the neurotrophin 4 homodimer and the brain-derived neurotrophic factor/neurotrophin 4 heterodimer reveal a common Trk-binding site. Protein Sci. 1999, 8, 2589–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattarawarapan, M.; Burgess, K. Molecular basis of neurotrophin-receptor interactions. J. Med. Chem. 2003, 46, 5277–5291. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, N.M.; Tarasyuk, A.V.; Shumskii, A.N.; Povarnina, P.Y.; Kruglov, S.V.; Antipova, T.A.; Gudasheva, T.A.; Seredenin, S.B. Synthesis and biological properties of a new dipeptide mimetic of brain-derived neurotrophic factor loop 2. Pharm. Chem. J. 2018, 52, 763–770. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Povarnina, P.; Logvinov, I.O.; Antipova, T.A.; Seredenin, S.B. Mimetics of brain-derived neurotrophic factor loops 1 and 4 are active in a model of ischemic stroke in rats. Drug Des. Dev. Ther. 2016, 10, 3545–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudasheva, T.A.; Logvinov, I.O.; Nikolaev, S.V.; Antipova, T.A.; Povarnina, P.Y.; Seredenin, S.B. Dipeptide Mimetics of Different NGF and BDNF Loops Activate PLC-γ1. Dokl. Biochem. Biophys. 2020, 494, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Gudasheva, T.A.; Tallerova, A.V.; Mezhlumyan, A.G.; Antipova, T.A.; Logvinov, I.O.; Firsova, Y.N.; Povarnina, P.Y.; Seredenin, S.B. Low-molecular weight bdnf mimetic, dimeric dipeptide GSB-106, reverses depressive symptoms in mouse chronic social defeat stress. Biomolecules 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Vakhitova, Y.V.; Kalinina, T.S.; Zainullina, L.F.; Lusta, A.Y.; Volkova, A.V.; Kudryashov, N.V.; Gudasheva, T.A.; Shimshirt, A.A.; Kadnikov, I.A.; Voronin, M.V.; et al. Analysis of Antidepressant-like Effects and Action Mechanisms of GSB-106, a Small Molecule, Affecting the TrkB Signaling. Int. J. Mol. Sci. 2021, 22, 13381. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Detke, M.J.; Johnson, J.; Lucki, I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp. Clin. Psychopharmacol. 1997, 5, 107–112. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.H.; Hung, T.H.; Chen, C.C.; Ke, C.H.; Lee, C.Y.; Wang, P.Y.; Chen, S.F. Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLoS ONE 2014, 9, e113397. [Google Scholar] [CrossRef]
- Andero, R.; Heldt, S.A.; Ye, K.; Liu, X.; Armario, A.; Ressler, K.J. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am. J. Psychiatry 2011, 168, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Díaz Barriga, G.; Giralt, A.; Anglada-Huguet, M.; Gaja-Capdevila, N.; Orlandi, J.G.; Soriano, J.; Canals, J.M.; Alberch, J. 7,8-dihydroxyflavone ameliorates cognitive and motor deficits in a Huntington’s disease mouse model through specific activation of the PLCγ1 pathway. Hum. Mol. Genet. 2017, 26, 3144–3160. [Google Scholar] [CrossRef]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; MacDonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar] [CrossRef]
- Rantamäki, T.; Hendolin, P.; Kankaanpää, A.; Mijatovic, J.; Piepponen, P.; Domenici, E.; Chao, M.V.; Männistö, P.T.; Castrén, E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology 2007, 32, 2152–2162. [Google Scholar] [CrossRef] [Green Version]
- Rantamäki, T.; Vesa, L.; Antila, H.; Di Lieto, A.; Tammela, P.; Schmitt, A.; Lesch, K.P.; Rios, M.; Castrén, E. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS ONE 2011, 6, e20567. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeffer, C.A.; Klann, E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010, 33, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, J.M.; Duman, R.S. Activation of mammalian target of rapamycin and synaptogenesis: Role in the actions of rapid-acting antidepressants. Biol. Psychiatry 2013, 73, 1189–1198. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; Haji, N.; De Gregorio, D.D.; Matta-Camacho, E.; Eslamizade, M.J.; Popic, J.; Sharma, V.; Cao, R.; Rummel, C.; Tanti, A.; et al. Translational control of depression-like behavior via phosphorylation of eukaryotic translation initiation factor 4E. Nat. Commun. 2018, 9, 2459. [Google Scholar] [CrossRef]
- Genheden, M.; Kenney, J.W.; Johnston, H.E.; Manousopoulou, A.; Garbis, S.D.; Proud, C.G. BDNF stimulation of protein synthesis in cortical neurons requires the MAP kinase-interacting kinase MNK1. J. Neurosci. 2015, 35, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Li, L.Y.; Zou, X.L.; Song, X.B.; Hu, Y.L.; Feng, Z.T.; Wang, T.T. Immunohistochemical distribution of NGF, BDNF, NT-3, and NT-4 in adult rhesus monkey brains. J. Histochem. Cytochem. 2007, 55, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruel-Jungerman, E.; Veyrac, A.; Dufour, F.; Horwood, J.; Laroche, S.; Davis, S. Inhibition of PI3K-Akt Signaling Blocks Exercise-Mediated Enhancement of Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus. PLoS ONE 2009, 4, e7901. [Google Scholar] [CrossRef] [Green Version]
- Koh, S.H.; Lo, E.H. The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. J. Clin. Neurol. 2015, 11, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Zainullina, L.F.; Vakhitova, Y.V.; Lusta, A.Y.; Gudasheva, T.A.; Seredenin, S.B. Dimeric mimetic of BDNF loop 4 promotes survival of serum-deprived cell through TrkB-dependent apoptosis suppression. Sci. Rep. 2021, 11, 7781. [Google Scholar] [CrossRef] [PubMed]
- Povarnina, P.Y. Neuroprotective Activity of Dipeptide BDNF Mimetics, which Differently Activate TRKB-Related Signaling Pathways under Conditions of Experimental Ischemic Stroke. Eksp. Klin. Farmakol. 2020, 83, 8–12. (In Russian) [Google Scholar] [CrossRef]
- Tirasa, P. The nerve growth factor administrated as eye drops activates mature and precursor cells in subventricular zone of adult rats. Arch. Ital. Biol. 2011, 149, 205–213. [Google Scholar] [CrossRef]
- Seredenin, S.B.; Gudasheva, T.A. The development of a pharmacologically active low-molecular mimetic of the nerve growth factor. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2015, 115, 63–70. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, R.E.; Kovács, K.J.; Nunez, M.G.; Larson, A.A. Depressive behavior in the forced swim test can be induced by TRPV1 receptor activity and is dependent on NMDA receptors. Pharmacol. Res. 2014, 79, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.X.; Cai, G.Q.; Cai, Y.Q.; Sheng, Z.J.; Jiang, J.; Mei, Z.; Wang, Z.G.; Guo, L.; Fei, J. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology 2007, 32, 1531–1539. [Google Scholar] [CrossRef]
- Li, S.; Yi, Z.; Deng, M.; Scott, M.J.; Yang, C.; Li, W.; Lei, Z.; Santerre, N.M.; Loughran, P.; Billiar, T.R. TSLP protects against liver I/R injury via activation of the PI3K/Akt pathway. JCI Insight 2019, 4, e129013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, T.L.; Wang, C.; Gu, J.L.; Ma, X.L.; Kumar, S.; Lee, J.C.; Feuerstein, G.Z.; Thomas, H.; Maleeff, B.; Ohlstein, E.H. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ. Res. 2000, 86, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. “Behavioural despair” in rats and mice: Strain differences and the effects of imipramine. Eur. J. Pharmacol. 1978, 51, 291–294. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Herrera-Mundo, N.; Sykes, C.E.; Francescutti, D.M.; Kuhn, D.M. Mice genetically depleted of brain serotonin do not display a depression-like behavioral phenotype. ACS Chem. Neurosci. 2014, 5, 908–919. [Google Scholar] [CrossRef] [Green Version]
- Clemons, A.P.; Holstein, D.M.; Galli, A.; Saunders, C. Cerulein-induced acute pancreatitis in the rat is significantly ameliorated by treatment with MEK1/2 inhibitors U0126 and PD98059. Pancreas 2002, 25, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Lobato, K.R.; Binfaré, R.W.; Freitas, A.E.; Costa, A.P.; Martín-de-Saavedra, M.D.; Leal, R.B.; Lopez, M.G.; Rodrigues, A.L. Involvement of PI3K, GSK-3β and PPARγ in the antidepressant-like effect of folic acid in the forced swimming test in mice. J. Psychopharmacol. 2012, 26, 714–723. [Google Scholar] [CrossRef]
- Manosso, L.M.; Moretti, M.; Ribeiro, C.M.; Gonçalves, F.M.; Leal, R.B.; Rodrigues, A.L. Antidepressant-like effect of zinc is dependent on signaling pathways implicated in BDNF modulation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 59, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, J.M.; Hake, P.W.; Denenberg, A.; Nowell, M.; Piraino, G.; Zingarelli, B. Phosphorylation of the extracellular signal-regulated kinase (ERK)-1/2 is associated with the downregulation of peroxisome proliferator-activated receptor (PPAR)-γ during polymicrobial sepsis. Mol. Med. 2010, 16, 491–497. [Google Scholar] [CrossRef]


| Neurotrophins Mimetics | Dipeptide Code | Basis Hairpin Loop | Activation of Trk Receptor and Post-Receptor Signaling Pathways |
|---|---|---|---|
| NGF mimetics | GK-6 | 1st | TrkA, PI3K/AKT, MAPK/ERK, PLCγ [23,31] |
| GK-2 | 4th | TrkA, PI3K/AKT, PLCγ [23,31] | |
| BDNF mimetics | GSB-214 | 1st | TrkB, PI3K/AKT, PLCγ [30,31] |
| GTS-201 | 2nd | TrkB, MAPK/ERK, PLCγ [29,31] | |
| GSB-106 | 4th | TrkB, PI3K/AKT, MAPK/ERK, PLCγ [30,31] |
| Group | Dose, mg/kg, i.p. | Immobility Time, s 1 | Immobility Time, % of Control |
|---|---|---|---|
| Control | 0 | 220.8 ± 13.8 | 100 |
| GSB-106 | 0.1 | 169.4 ± 8.2 * | 76.7 |
| GSB-106 | 1.0 | 177.0 ± 11.2 * | 80.2 |
| Amitriptyline | 10.0 | 159.2 ± 9.8 * | 72.1 |
| Control | 0 | 226.1 ± 6.7 | 100 |
| GSB-214 | 0.1 | 223.8 ± 8.5 | 98.9 |
| GSB-214 | 1.0 | 230.9 ± 7.5 | 102.1 |
| Control | 0 | 228.5 ± 9.7 | 100 |
| GTS-201 | 0.1 | 241.3 ± 11.3 | 105.6 |
| GTS-201 | 1.0 | 209.8 ± 10.0 | 91.8 |
| GTS-201 | 5.0 | 228.1 ± 9.3 | 99.8 |
| Control | 0 | 214.4 ± 15.3 | 100 |
| GK-2 | 0.5 | 234.8 ± 9.5 | 105.1 |
| GK-2 | 1.0 | 225.3 ± 4.7 | 111.3 |
| Control | 0 | 204.1 ± 15.9 | 100 |
| GK-6 | 1.0 | 216.8 ± 13.1 | 106.2 |
| GK-6 | 2.0 | 176.9 ± 18.0 | 86.7 |
| GK-6 | 5.0 | 202.6 ± 9.5 | 99.3 |
| Amitriptyline | 10.0 | 133.2 ± 10.4 * | 65.3 |
| Group | Dose, mg/kg, i.p. | Immobility Time, s 1 | Immobility Time, % of Control |
|---|---|---|---|
| Control | 0 | 205.0 ± 9.1 | 100 |
| GSB-106 | 1,0 | 172.7 ± 11.4 # | 84.2 |
| Control | 0 | 242.6 ± 6.6 | 100 |
| Amitriptyline | 10,0 | 205.2 ± 8.4 * | 84.6 |
| GSB-214 | 1,0 | 198.4 ± 8.1 * | 81.8 |
| GSB-201 | 1,0 | 218.0 ± 7.7 | 89.9 |
| GK-2 | 0,5 | 225.5 ± 10.5 | 93.0 |
| GK-2 | 1,0 | 210.5 ± 4.4 * | 86.8 |
| GK-2 | 5,0 | 239.3 ± 6.8 | 98.6 |
| GK-6 | 2,0 | 207.0 ± 11.0 * | 85.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezhlumyan, A.G.; Tallerova, A.V.; Povarnina, P.Y.; Tarasiuk, A.V.; Sazonova, N.M.; Gudasheva, T.A.; Seredenin, S.B. Antidepressant-like Effects of BDNF and NGF Individual Loop Dipeptide Mimetics Depend on the Signal Transmission Patterns Associated with Trk. Pharmaceuticals 2022, 15, 284. https://doi.org/10.3390/ph15030284
Mezhlumyan AG, Tallerova AV, Povarnina PY, Tarasiuk AV, Sazonova NM, Gudasheva TA, Seredenin SB. Antidepressant-like Effects of BDNF and NGF Individual Loop Dipeptide Mimetics Depend on the Signal Transmission Patterns Associated with Trk. Pharmaceuticals. 2022; 15(3):284. https://doi.org/10.3390/ph15030284
Chicago/Turabian StyleMezhlumyan, Armen G., Anna V. Tallerova, Polina Y. Povarnina, Aleksey V. Tarasiuk, Nellya M. Sazonova, Tatiana A. Gudasheva, and Sergey B. Seredenin. 2022. "Antidepressant-like Effects of BDNF and NGF Individual Loop Dipeptide Mimetics Depend on the Signal Transmission Patterns Associated with Trk" Pharmaceuticals 15, no. 3: 284. https://doi.org/10.3390/ph15030284
APA StyleMezhlumyan, A. G., Tallerova, A. V., Povarnina, P. Y., Tarasiuk, A. V., Sazonova, N. M., Gudasheva, T. A., & Seredenin, S. B. (2022). Antidepressant-like Effects of BDNF and NGF Individual Loop Dipeptide Mimetics Depend on the Signal Transmission Patterns Associated with Trk. Pharmaceuticals, 15(3), 284. https://doi.org/10.3390/ph15030284








