Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1]heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4)
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Modelling
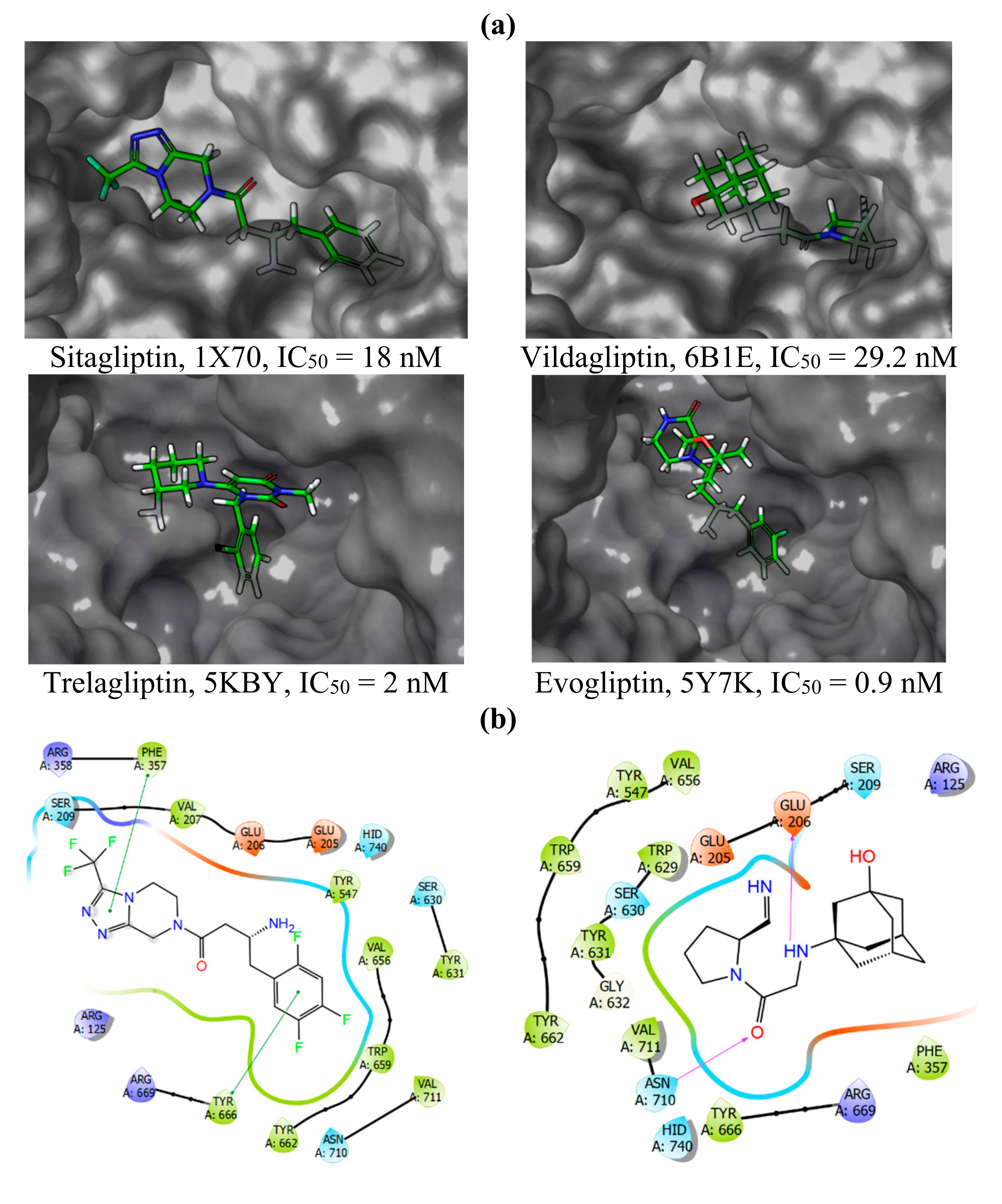
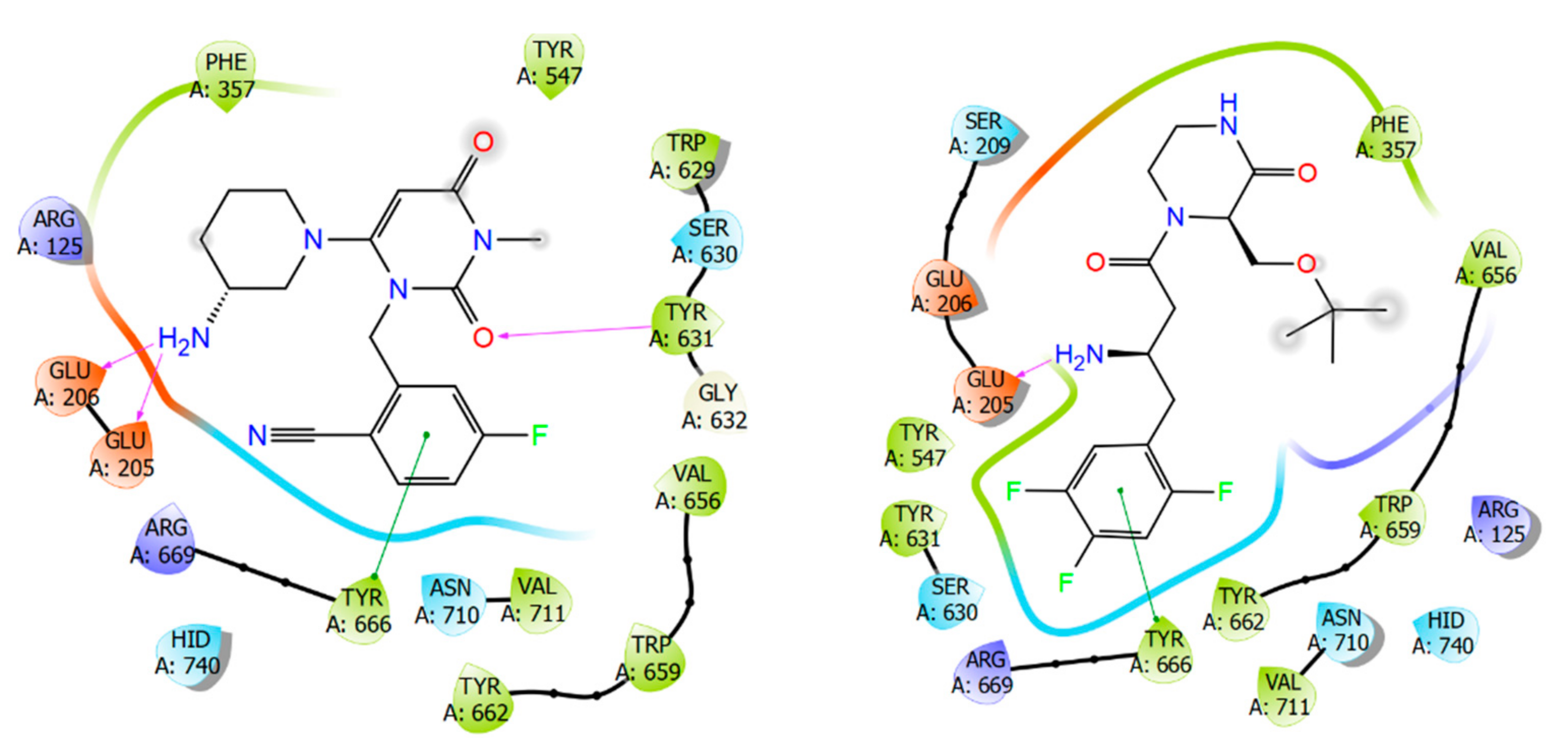
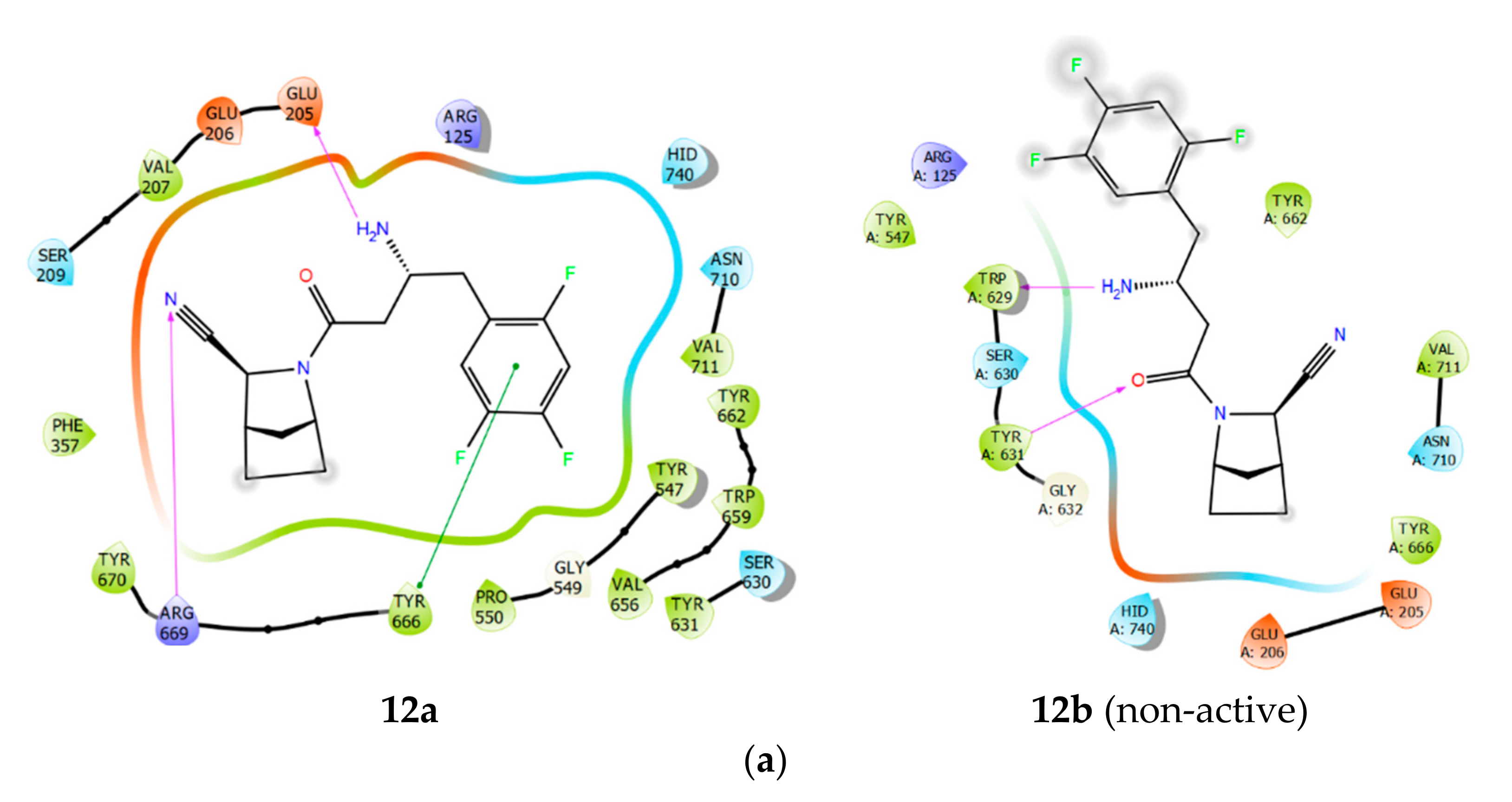
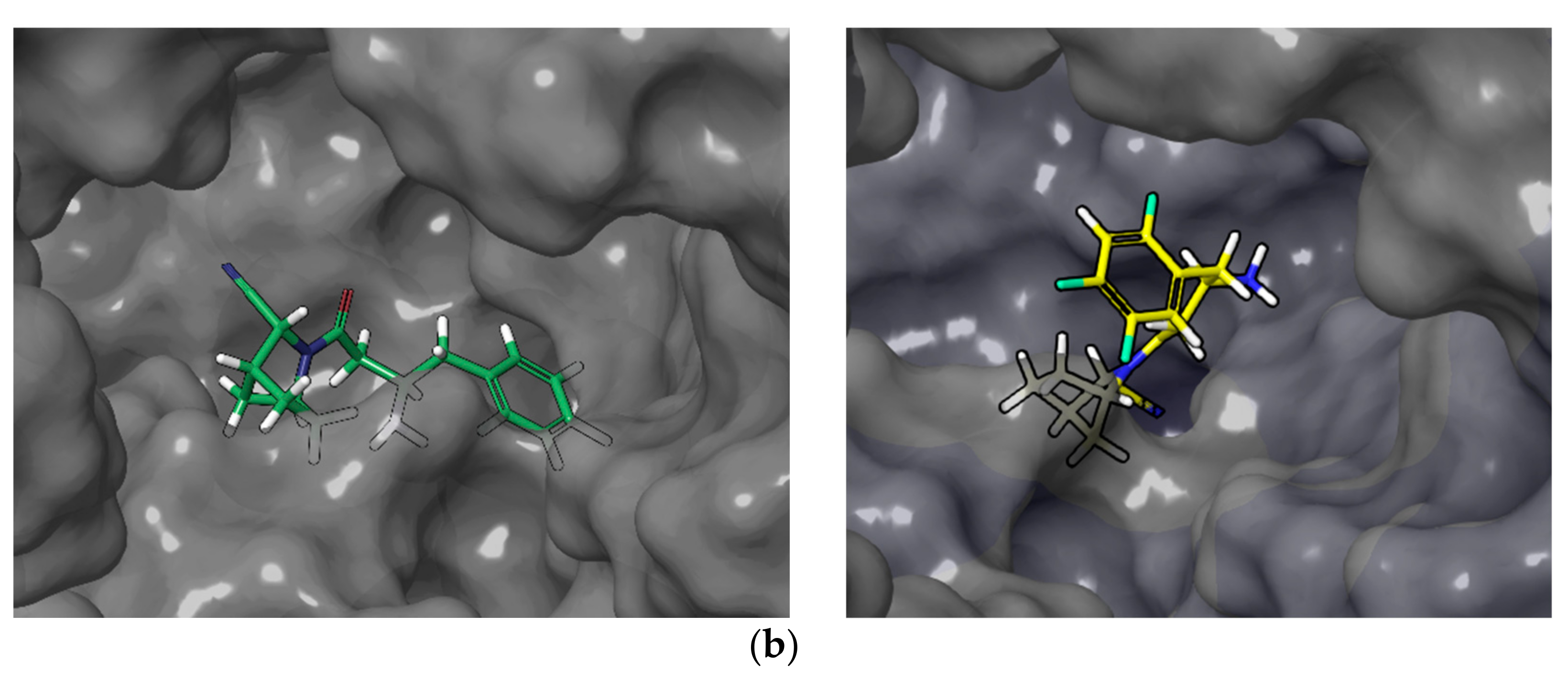
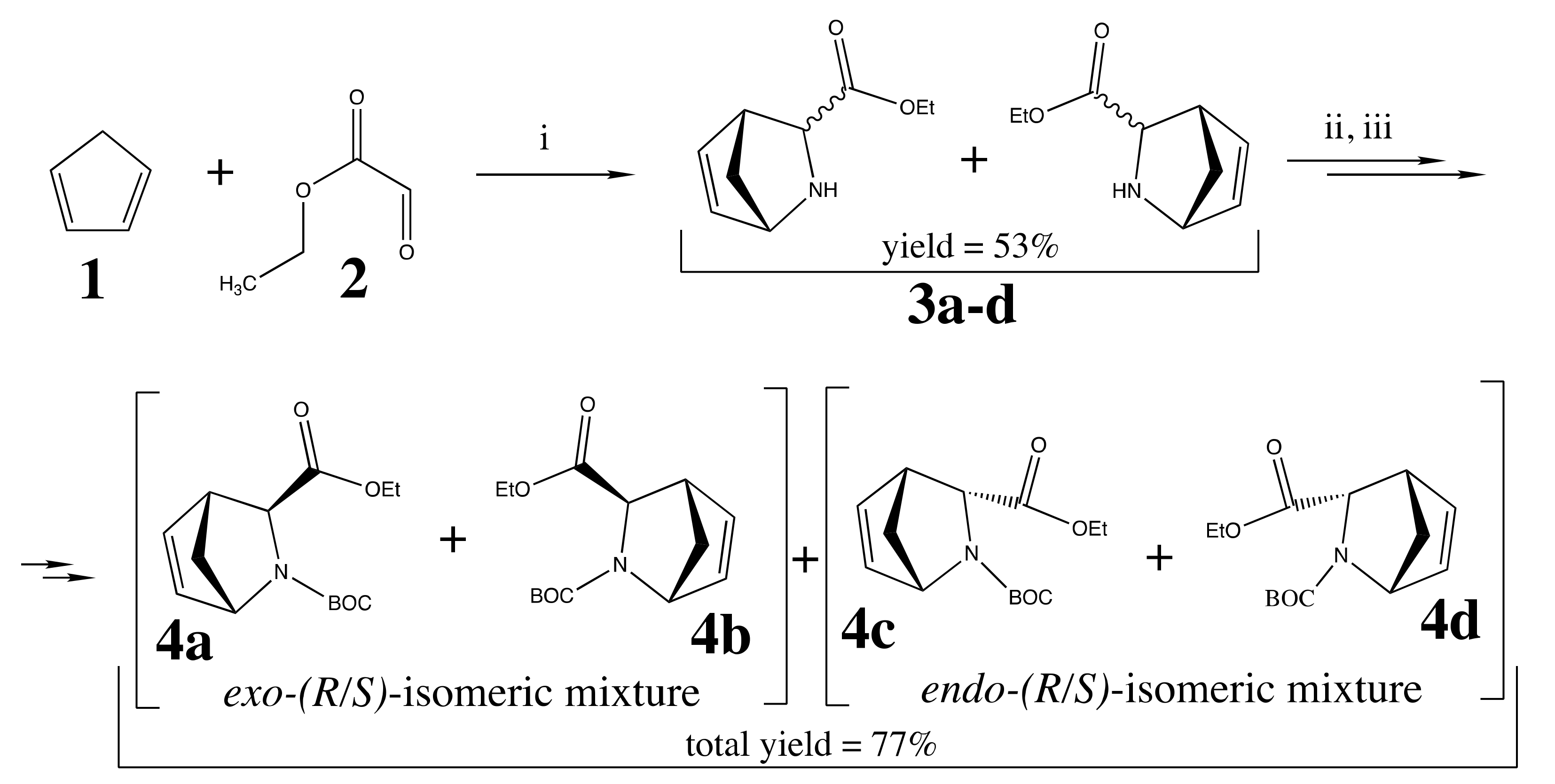
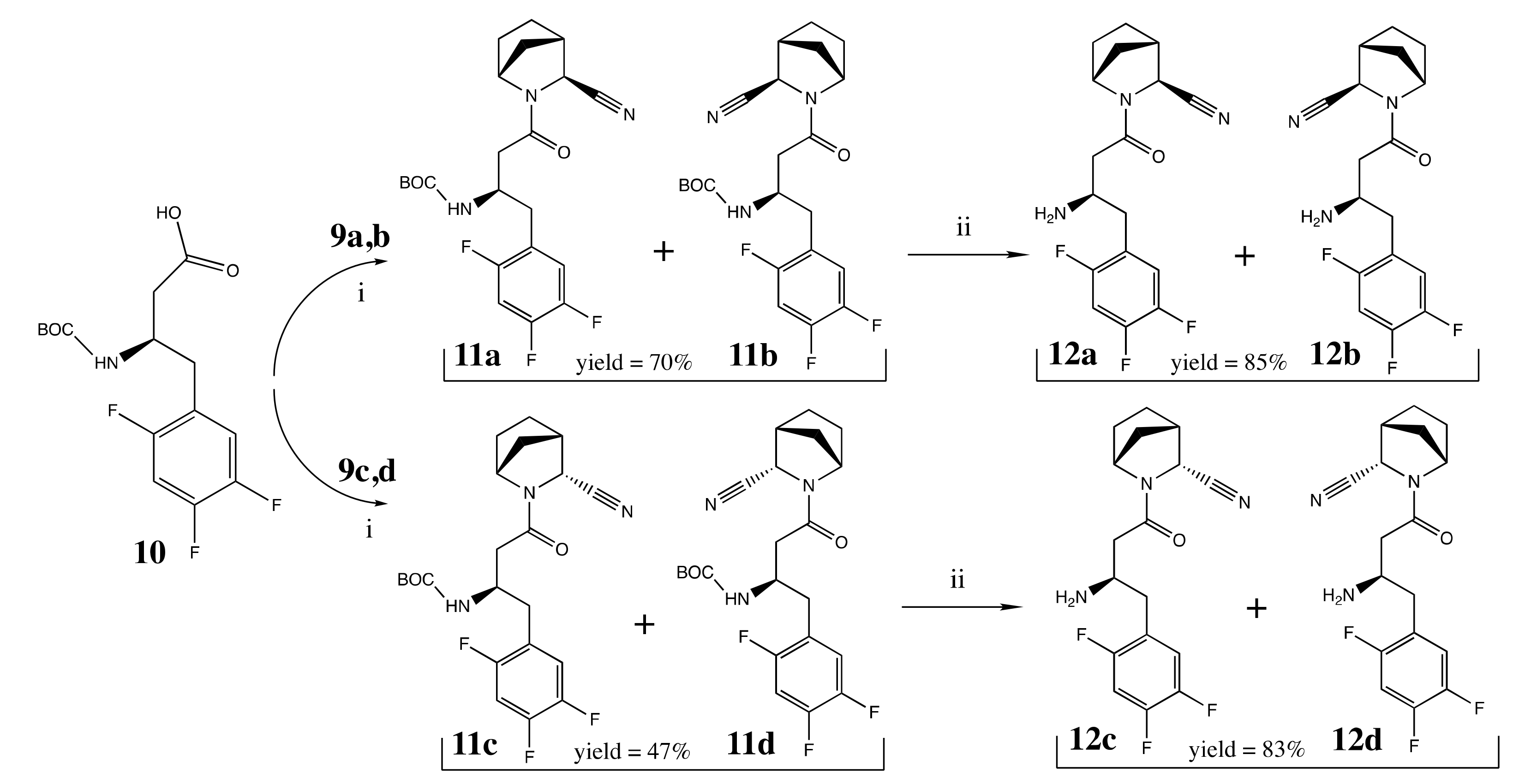
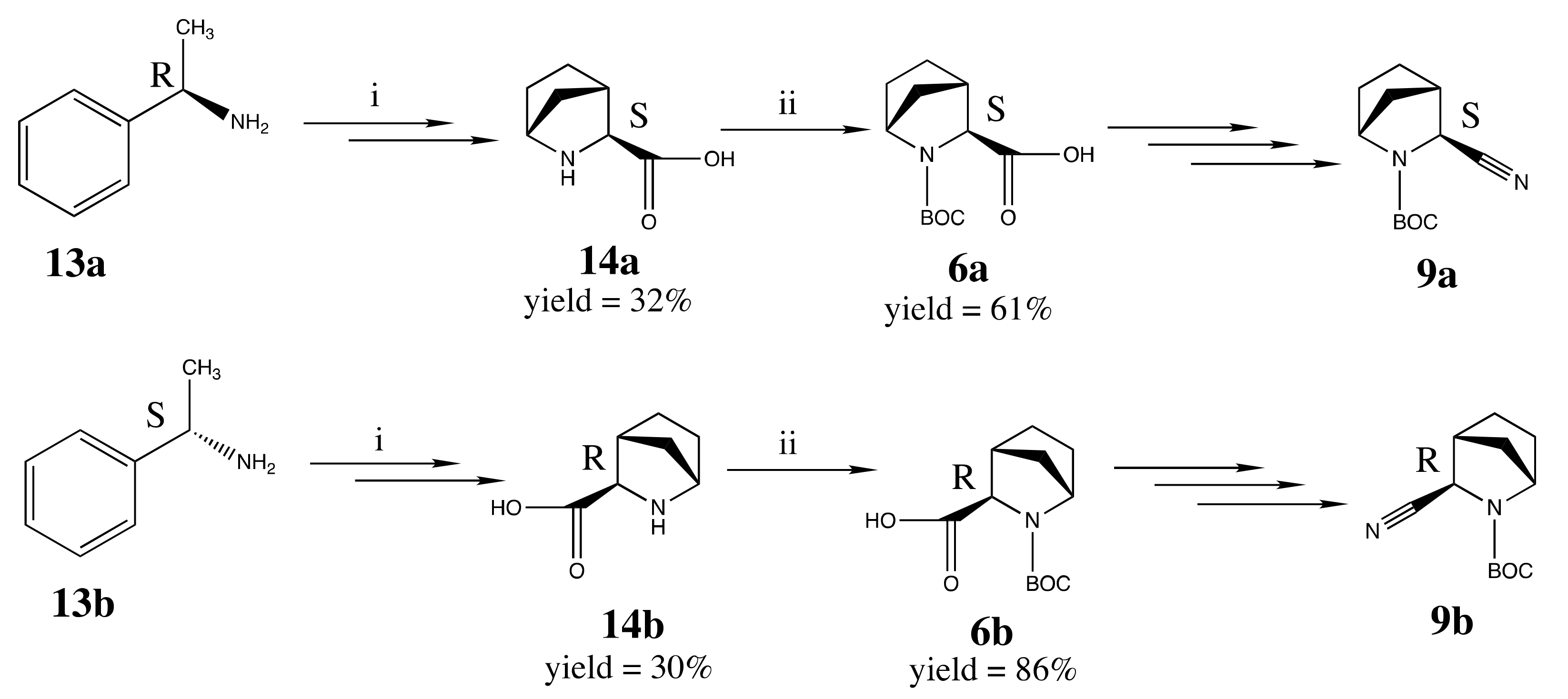
2.2. Chemical Synthesis
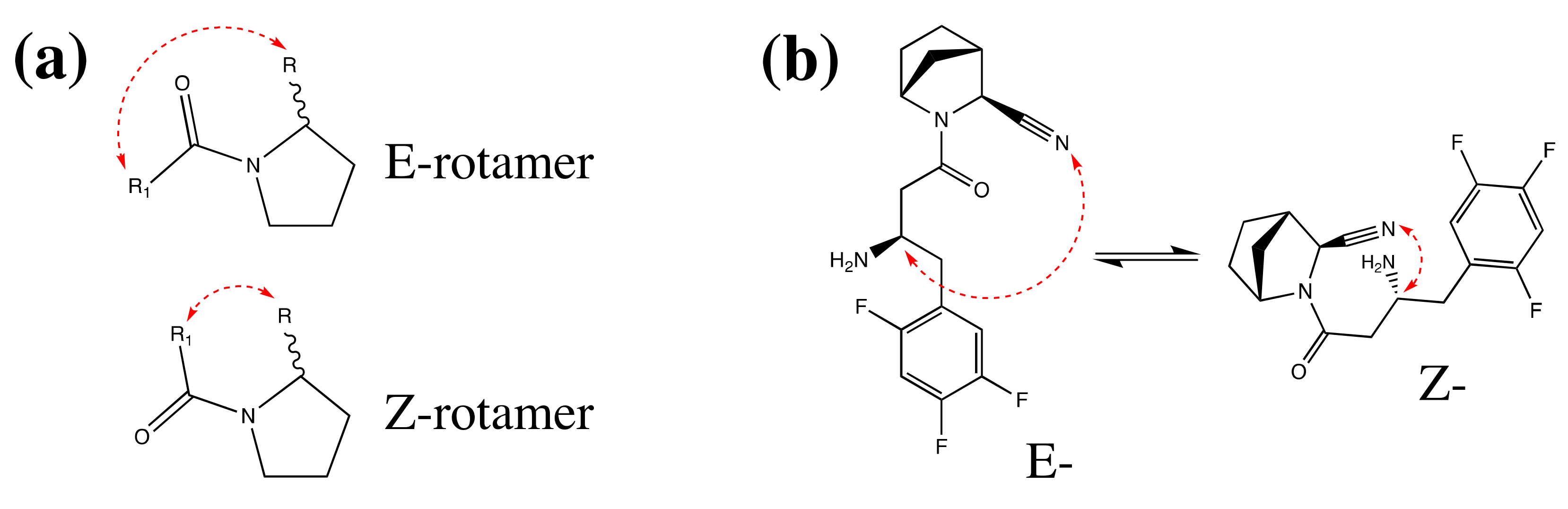
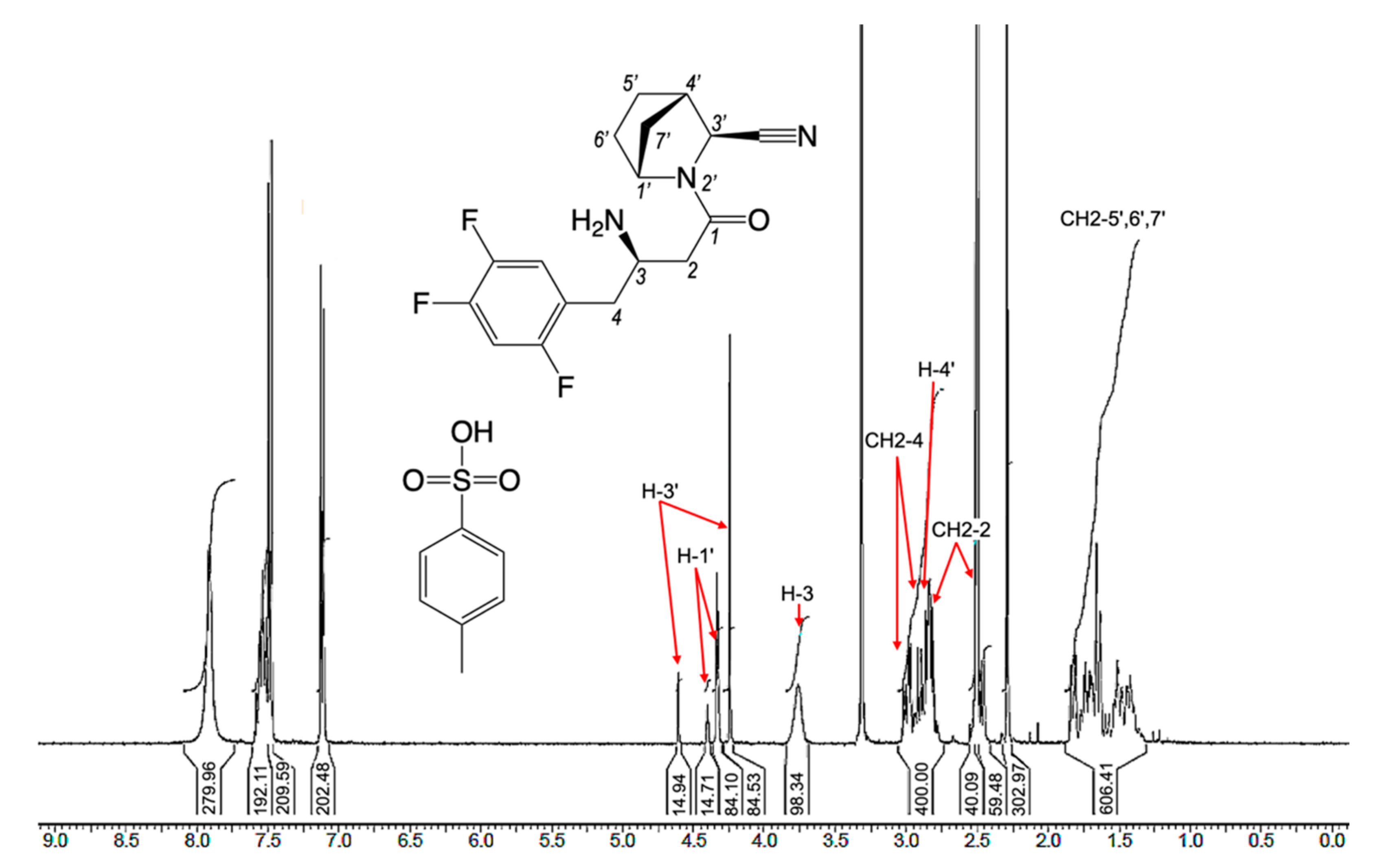
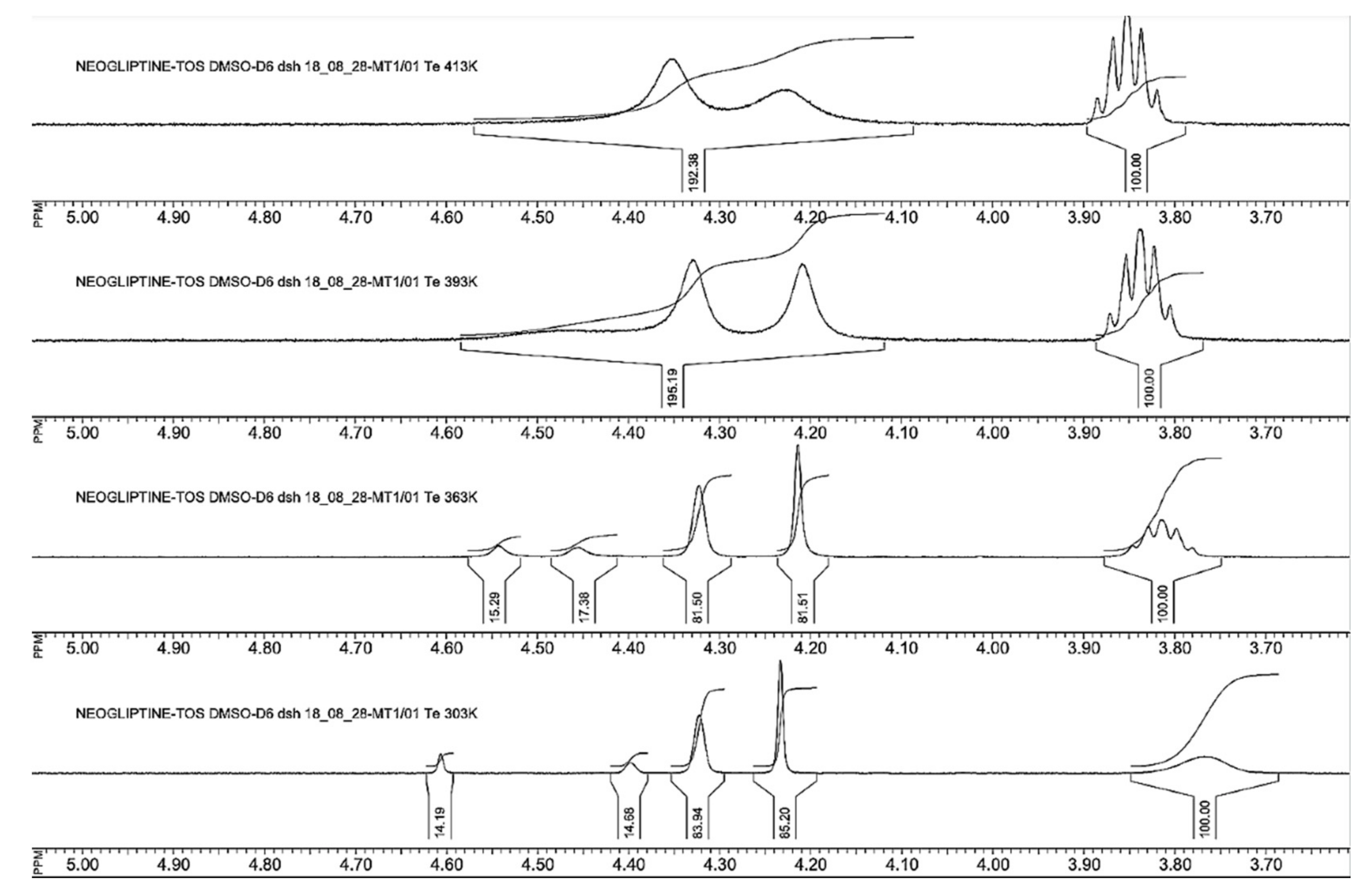
2.3. Structure and Purity Confirmation
2.4. Inhibitory Activity Evaluation
3. Materials and Methods
3.1. Molecular Modelling
3.1.1. Molecular Modelling Hardware
- All calculations were processed at Sechenov First Moscow State Medical University, using the following hardware:
- Fujitsu RX4770M3 CPU-server. Intel(R) Xeon(R) CPU E7-8867 v4 @ 2.40GHz 18C/36T x4, 1024Gb RAM.
- Fujitsu RX2540M4 GPU-server. Intel(R) Xeon(R) Gold 6138 CPU @ 2.00GHz 20C/80T x2, 1024Gb RAM, 2xTesla P100 16Gb.
- Fujitsu Celsius R940 GPU-server. Intel(R) Xeon(R) CPU E5-2690 v4 @ 2.60GHz 14C/28T x2, 256Gb RAM, 2xGTX 1080Ti 11Gb.
3.1.2. Used Software
- Maestro. Schrödinger Release 2020-3: Maestro, Schrödinger, LLC, New York, NY, USA, 2020.
- LigPrep. Schrödinger Release 2020-3: LigPrep, Schrödinger, LLC, New York, NY, USA, 2020.
- Prime. Schrödinger Release 2020-3: Prime, Schrödinger, LLC, New York, NY, USA, 2020.
- Glide module. Schrödinger Release 2020-3: Glide, Schrödinger, LLC, New York, NY, USA, 2020.
3.2. Chemical Synthesis
3.3. LC/MS, NMR, and Elemental Analysis
3.4. Inhibitory Activity Evaluation
3.5. Experimental Section
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2019, 16, 829–842. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Almén, M.S.; Schiöth, H.B. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 579–590. [Google Scholar] [CrossRef]
- Kaur, P.; Mittal, A.; Nayak, S.K.; Vyas, M.; Mishra, V.; Khatik, G.L. Current strategies and drug targets in the management of type 2 diabetes mellitus. Curr. Drug Targets 2018, 19, 1738–1766. [Google Scholar] [CrossRef]
- Green, B.D.; Flatt, P.R.; Bailey, C.J. Dipeptidyl peptidase IV (DPP IV) inhibitors: A newly emerging drug class for the treatment of type 2 diabetes. Diab. Vasc. Dis. Res. 2006, 3, 159–165. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Liu, C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Obes. Metab. 2014, 16, 30–37. [Google Scholar] [CrossRef]
- Ahrén, B. DPP-4 inhibition and the path to clinical proof. Front. Endocrinol. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Shimizu, S.; Hosooka, T.; Matsuda, T.; Asahara, S.; Koyanagi-Kimura, M.; Kanno, A.; Bartolome, A.; Etoh, H.; Fuchita, M.; Teruyama, K.; et al. DPP4 inhibitor vildagliptin preserves β-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J. Mol. Endocrinol. 2012, 49, 125–135. [Google Scholar] [CrossRef]
- De, S.; Banerjee, S.; Kumar SK, A.; Paira, P. Critical role of dipeptidyl peptidase IV: A therapeutic target for diabetes and cancer. Mini Rev. Med. Chem. 2018, 19, 88–97. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 1–20. [Google Scholar] [CrossRef]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef]
- Upadhyay, J.; Gajjar, A. Analysis of crystal structures of dipeptidyl peptidase 4 (Dpp 4) Co- crystallized with diverse inhibitors. Int. J. Pharm. Sci. Res. 2018, 9, 4460–4471. [Google Scholar] [CrossRef]
- Thornberry, N.; Weber, A. Discovery of JANUVIA (Sitagliptin), a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Curr. Top. Med. Chem. 2007, 7, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Augeri, D.J.; Robl, J.A.; Betebenner, D.A.; Magnin, D.R.; Khanna, A.; Robertson, J.G.; Wang, A.; Simpkins, L.M.; Taunk, P.; Huang, Q.; et al. Discovery and preclinical profile of saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005, 48, 5025–5037. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Z.; Wallace, M.B.; Stafford, J.A.; Kaldor, S.W.; Kassel, D.B.; Navre, M.; Shi, L.; Skene, R.J.; Asakawa, T.; et al. Discovery of alogliptin: A potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J. Med. Chem. 2008, 51, 4357. [Google Scholar] [CrossRef]
- Eckhardt, M.; Langkopf, E.; Mark, M.; Tadayyon, M.; Thomas, L.; Nar, H.; Pfrengle, W.; Guth, B.; Lotz, R.; Sieger, P.; et al. 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin- 2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2007, 50, 6450–6453. [Google Scholar] [CrossRef]
- Packer, M. Do DPP-4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity? Clues from laboratory models and clinical trials. Circ. Res. 2018, 122, 928–932. [Google Scholar] [CrossRef]
- Hussain, H.; Abbas, G.; Green, I.R.; Ali, I. Dipeptidyl peptidase IV inhibitors as a potential target for diabetes: Patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 535–553. [Google Scholar] [CrossRef]
- Mishra, A.; Cross, M.; Hofmann, A.; Coster, M.J.; Karim, A.; Sattar, A. Identification of a novel scaffold for inhibition of dipeptidyl peptidase-4. J. Comput. Biol. 2019, 26, 1470–1486. [Google Scholar] [CrossRef]
- Schwehm, C.; Li, J.; Song, H.; Hu, X.; Kellam, B.; Stocks, M.J. Synthesis of new DPP-4 inhibitors based on a novel tricyclic scaffold. ACS Med. Chem. Lett. 2015, 6, 324–328. [Google Scholar] [CrossRef]
- Berger, J.P.; SinhaRoy, R.; Pocai, A.; Kelly, T.M.; Scapin, G.; Gao, Y.D.; Pryor, K.A.D.; Wu, J.K.; Eiermann, G.J.; Xu, S.S. A comparative study of the binding properties, dipeptidyl peptidase-4 (DPP-4) inhibitory activity and glucose-lowering efficacy of the DPP-4 inhibitors alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin in mice. Endocrinol. Diabetes Metab. 2018, 1, e00002. [Google Scholar] [CrossRef]
- Musoev, A.; Numonov, S.; You, Z.; Gao, H. Discovery of novel DPP-IV inhibitors as potential candidates for the treatment of type 2 diabetes mellitus predicted by 3D QSAR pharmacophore models, molecular docking and de novo evolution. Molecules 2019, 24, 2870. [Google Scholar] [CrossRef] [PubMed]
- Houck, J.D.; Dawson, T.K.; Kennedy, A.J.; Kharel, Y.; Naimon, N.D.; Field, S.D.; Lynch, K.R.; Macdonald, T.L. Structural requirements and docking analysis of amidine-based sphingosine kinase 1 inhibitors containing oxadiazoles. ACS Med. Chem. Lett. 2016, 7, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.D.; Bhadada, S.V.; Ghate, M.D. Design, synthesis and anti-diabetic activity of triazolotriazine derivatives as dipeptidyl peptidase-4 (DPP-4) inhibitors. Bioorg. Chem. 2017, 72, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Shahar Yar, M. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.B.; Qian, X.; Biftu, T.; Singh, S.; Gao, Y.D.; Scapin, G.; Patel, S.; Leiting, B.; Patel, R.; Wu, J.; et al. Discovery of new binding elements in DPP-4 inhibition and their applications in novel DPP-4 inhibitor design. Bioorganic Med. Chem. Lett. 2008, 18, 3706–3710. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.J.; Jiang, B.; Guo, S.J.; Li, X.Q.; Chen, X.C.; Luo, J.; Li, C.; Wang, Y.; Shi, D.Y. Design, synthesis and biological evaluation of novel pyrimidinedione derivatives as DPP-4 inhibitors. Bioorganic Med. Chem. Lett. 2018, 28, 2131–2135. [Google Scholar] [CrossRef]
- Su, H.; Boulton, D.W.; Barros, A., Jr.; Wang, L.; Cao, K.; Bonacorsi, S.J., Jr.; Iyer, R.A.; Humphreys, W.G.; Christopher, L.J. Characterization of the in vitro and in vivo metabolism and disposition and cytochrome P450 inhibition/induction profile of saxagliptin in human. Drug Metab. Dispos. 2012, 40, 1345–1356. [Google Scholar] [CrossRef]
- Link, J.O.; Taylor, J.G.; Xu, L.; Mitchell, M.; Guo, H.; Liu, H.; Kato, D.; Kirschberg, T.; Sun, J.; Squires, N.; et al. Discovery of ledipasvir (GS-5885): A potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J. Med. Chem. 2014, 57, 2033–2046. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Huang, N. A survey of the role of nitrile groups in protein-ligand interactions. Future Med. Chem. 2018, 10, 2713–2727. [Google Scholar] [CrossRef]
- Zeng, D.K.; Xiao, Q.; Li, F.Q.; Tang, Y.Z.; Jia, C.L.; Tang, X.W. Cardiovascular risk of sitagliptin in treating patients with type 2 diabetes mellitus. Biosci. Rep. 2019, 39, 2713–2728. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Maneesha Khalse, A.B. A review on cardiovascular outcome studies of dipeptidyl peptidase-4 inhibitors. J. Clin. Endocrinol. Metab. 2018, 22, 689–695. [Google Scholar] [CrossRef]
- Ishida, Y.; Murayama, H.; Shinfuku, Y.; Taniguchi, T.; Sasajima, T.; Oyama, N. Cardiovascular safety and effectiveness of vildagliptin in patients with type 2 diabetes mellitus: A 3-year, large-scale post-marketing surveillance in Japan. Expert Opin. Drug Saf. 2020, 19, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Hursthouse, M.B.; Malik, K.A.; Hibbs, D.E.; Roberts, S.M.; Seago, A.J.; Šik, V.; Storer, R. Reactions of ethyl 2-acetyl-2-azabicyclo[2.2.1]hept-5-ene-3-carboxylate and 4-acetylamino-2-oxabicyclo[3.3.0]oct-7-en-3-one with some electrophiles. J. Chem. Soc. Perkin Trans. 1 1995, 19, 2419–2425. [Google Scholar] [CrossRef]
- Balog, J.A.; Fairfax, D.J.; Martin, G.S.; Salvati, M.E.; Xiao, H.Y. Tricycloundecane Compounds Useful as Modulators of Nuclear Hormone Receptor Function. U.S. Patent 7,550,458, 23 June 2009. [Google Scholar]
- Genentech, Inc. Substituted Heterocyclic Sulfonamide Compounds Useful as TRPAl Modulators. WO Patent 2015/052264, 16 April 2015. [Google Scholar]
- Agouron Pharmaceuticals, Inc. Compunds, Compositions, and Methods for Stimulating Neuronal Growth and Elongation. WO Patent 01/40185, 7 June 2001. [Google Scholar]
- Chu, S.S.; Alegria, L.A.; Bleckman, T.M.; Chong, W.K.M.; Duvadie, R.K.; Li, L.; Reich, S.H.; Romines, W.H.; Wallace, M.B.; Yang, Y. Thiazole Benzamide Derivatives and Pharmaceutical Compositions for Inhibiting Cell Proliferation, and Methods for Their Use. WO Patent 03/004467, 16 January 2003. [Google Scholar]
- Vincent, M.; Remond, G.; Portevin, B.; Herve, Y.; Lepagnol, J.; Biton, C. New Heterocyclic Tripeptide Compounds. U.S. Patent 5,098,888, 24 March 1992. [Google Scholar]
- Blondet, D.; Morin, C. Total synthesis of (±)-8-aza-9a,9b-dicarbaprostaglandin H1. J. Chem. Soc. Perkin Trans. 1984, 1, 1085–1090. [Google Scholar] [CrossRef]
- Stella, L.; Abraham, H.; Feneau-Dupont, J.; Tinant, B.; Declercq, J. Asymmetric aza-diels-alder reaction using the chiral 1-phenyl ethyl imine of methyl glyoxylate. Tetrahedron Lett. 1990, 31, 2603–2606. [Google Scholar] [CrossRef]
- McKinnell, R.M.; Long, D.D. Hepatitis C Virus Inhibitors. WO Patent 2013/163270, 31 October 2013. [Google Scholar]
- Alonso, D.; Bertilsson, S.; Johnsson, S.; Nordin, S.; Södergren, M.; Andersson, P. New expedient route to both enantiomers of nonproteinogenic α-amino acid derivatives from the unsaturated 2-aza-bicyclo moiety. J. Org. Chem. 1999, 64, 2276–2280. [Google Scholar] [CrossRef]
- Abbott Laboratories. Furopyridine, Thienopyridine, Pyrrolopyridine and Related Pyrimidine, Pyridazine and Triazines Compounds Useful in Controlling Chemical Synaptic Transmission. WO Patent 97/05139, 13 February 1997. [Google Scholar]
- Novartis, A.G.; IRM LLC. Ether Derivatives of Bicyclic Heteroaryls. WO Patent 2011/029915, 17 March 2011. [Google Scholar]
- Tararov, V.I.; Kadyrov, R.; Kadyrova, Z.; Dubrovina, N.; Börner, A. An improved synthesis of enantiopure 2-azabicyclo[2.2.1]heptane-3-carboxylic acid. Tetrahedron Asymmetry 2002, 13, 25–28. [Google Scholar] [CrossRef]
- Södergren, M.; Bertilsson, S.; Andersson, P. Allylic alcohols via catalytic asymmetric epoxide rearrangement. J. Am. Chem. Soc. 2000, 122, 6610–6618. [Google Scholar] [CrossRef]
- Nabeno, M.; Akahoshi, F.; Kishida, H.; Miyaguchi, I.; Tanaka, Y.; Ishii, S.; Kadowaki, T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem. Biophys. Res. Commun. 2013, 434, 191–196. [Google Scholar] [CrossRef]
- Singh, S.K.; Manne, N.; Pal, M. Synthesis of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile: A key intermediate for dipeptidyl peptidase IV inhibitors. Beilstein J. Org. Chem. 2008, 4, 20. [Google Scholar] [CrossRef]
- Nordhoff, S.; Bulat, S.; Cerezo-Gálvez, S.; Hill, O.; Hoffmann-Enger, B.; López-Canet, M.; Rosenbaum, C.; Rummey, C.; Thiemann, M.; Matassa, V.G.; et al. The design of potent and selective inhibitors of DPP-4: Optimization of ADME properties by amide replacements. Bioorganic Med. Chem. Lett. 2009, 19, 6340–6345. [Google Scholar] [CrossRef] [PubMed]
- Kalhotra, P.; Chittepu, V.C.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Structure–activity relationship and molecular docking of natural product library reveal chrysin as a novel dipeptidyl peptidase-4 (DPP-4) inhibitor: An integrated in silico and in vitro study. Molecules 2018, 23, 1368. [Google Scholar] [CrossRef] [PubMed]
- Landersdorfer, C.B.; He, Y.L.; Jusko, W.J. Mechanism-based population pharmacokinetic modelling in diabetes: Vildagliptin as a tight binding inhibitor and substrate of dipeptidyl peptidase IV. Br. J. Clin. Pharmacol. 2012, 73, 391–401. [Google Scholar] [CrossRef]
- Wang, A.; Dorso, C.; Kopcho, L.; Locke, G.; Langish, R.; Harstad, E.; Shipkova, P.; Marcinkeviciene, J.; Hamann, L.; Kirby, M.S. Potency, selectivity and prolonged binding of saxagliptin to DPP4: Maintenance of DPP4 inhibition by saxagliptin in vitro and ex vivo when compared to a rapidly-dissociating DPP4 inhibitor. BMC Pharmacol. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Von Websky, K.; Reichetzeder, C.; Hocher, B. Linagliptin as add-on therapy to insulin for patients with type 2 diabetes. Vasc. Health Risk Manag. 2013, 9, 681–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komala, M.G.; Gross, S.; Zaky, A.; Pollock, C.; Panchapakesan, U. Linagliptin limits high glucose induced conversion of latent to active TGFβ through interaction with CIM6PR and limits renal tubulointerstitial fibronectin. PLoS ONE 2015, 10, e0141143. [Google Scholar] [CrossRef]



| Compound | FDA | Structure |
|---|---|---|
| Sitagliptin | + | 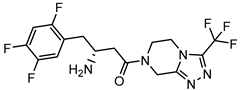 |
| Saxagliptin | + | 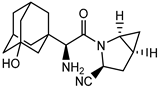 |
| Alogliptin | + | 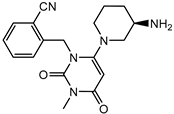 |
| Linagliptin | + | 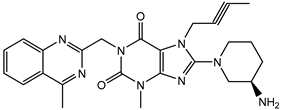 |
| Vildagliptin | − | 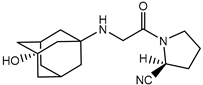 |
| Teneligliptin | − | 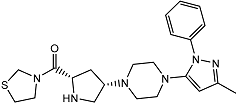 |
| Trelagliptin | − | 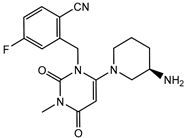 |
| Evogliptin | − | 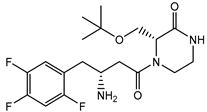 |
| Omarigliptin | − | 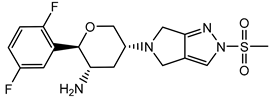 |
| Structure | GlideScore | Emodel | ∆Gbind (kcal/mol) | QPlogHERG |
|---|---|---|---|---|
| Sitagliptin | −5.80 | −63.96 | −17.05 | −4.351 |
| Trelagliptin | −5.68 | −54.94 | −16.35 | −5.078 |
| Evogliptin | −5.84 | −53.11 | −17.02 | −3.032 |
| Vildagliptin | −5.99 | −56.58 | −16.60 | −2.987 |
| 12a | −5.74 | −59,69 | −17.39 | −2.884 |
| 12b | −4.01 | −40.56 | −5.14 | −3.191 |
| 12c | −3.69 | −37.24 | −9.09 | −3.010 |
| 12d | −3.70 | −45.46 | −3.43 | −3.393 |
| Structure | DPP-4 IC50, nM | DPP-8 IC50, nM | DPP-9 IC50, nM |
|---|---|---|---|
| 12a (S-exo) | 16.8 ± 2.2 | >1000 | >1000 |
| 12b (R-exo) | >1000 | - | - |
| 12c (S-endo): 12d (R-endo), 1:1 mixture | >1000 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslov, I.O.; Zinevich, T.V.; Kirichenko, O.G.; Trukhan, M.V.; Shorshnev, S.V.; Tuaeva, N.O.; Gureev, M.A.; Dahlén, A.D.; Porozov, Y.B.; Schiöth, H.B.; et al. Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1]heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4). Pharmaceuticals 2022, 15, 273. https://doi.org/10.3390/ph15030273
Maslov IO, Zinevich TV, Kirichenko OG, Trukhan MV, Shorshnev SV, Tuaeva NO, Gureev MA, Dahlén AD, Porozov YB, Schiöth HB, et al. Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1]heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4). Pharmaceuticals. 2022; 15(3):273. https://doi.org/10.3390/ph15030273
Chicago/Turabian StyleMaslov, Ivan O., Tatiana V. Zinevich, Olga G. Kirichenko, Mikhail V. Trukhan, Sergey V. Shorshnev, Natalya O. Tuaeva, Maxim A. Gureev, Amelia D. Dahlén, Yuri B. Porozov, Helgi B. Schiöth, and et al. 2022. "Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1]heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4)" Pharmaceuticals 15, no. 3: 273. https://doi.org/10.3390/ph15030273
APA StyleMaslov, I. O., Zinevich, T. V., Kirichenko, O. G., Trukhan, M. V., Shorshnev, S. V., Tuaeva, N. O., Gureev, M. A., Dahlén, A. D., Porozov, Y. B., Schiöth, H. B., & Trukhan, V. M. (2022). Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1]heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4). Pharmaceuticals, 15(3), 273. https://doi.org/10.3390/ph15030273







