Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis
Abstract
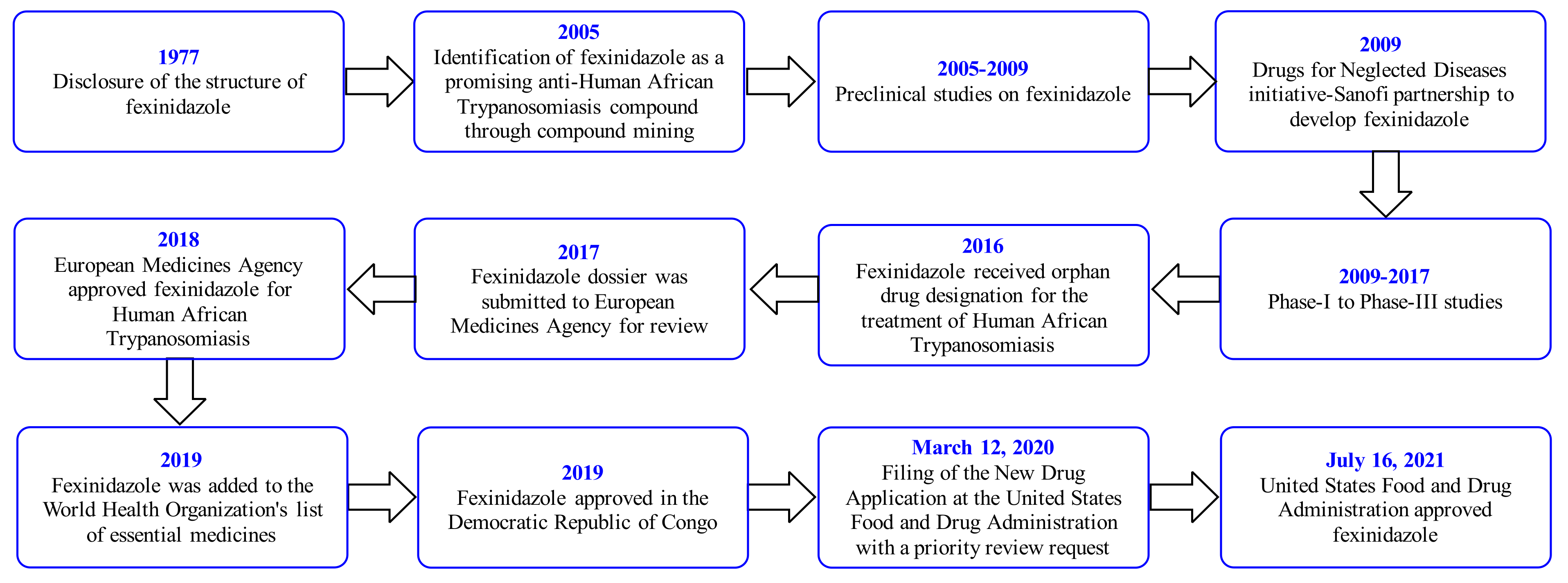
1. Introduction
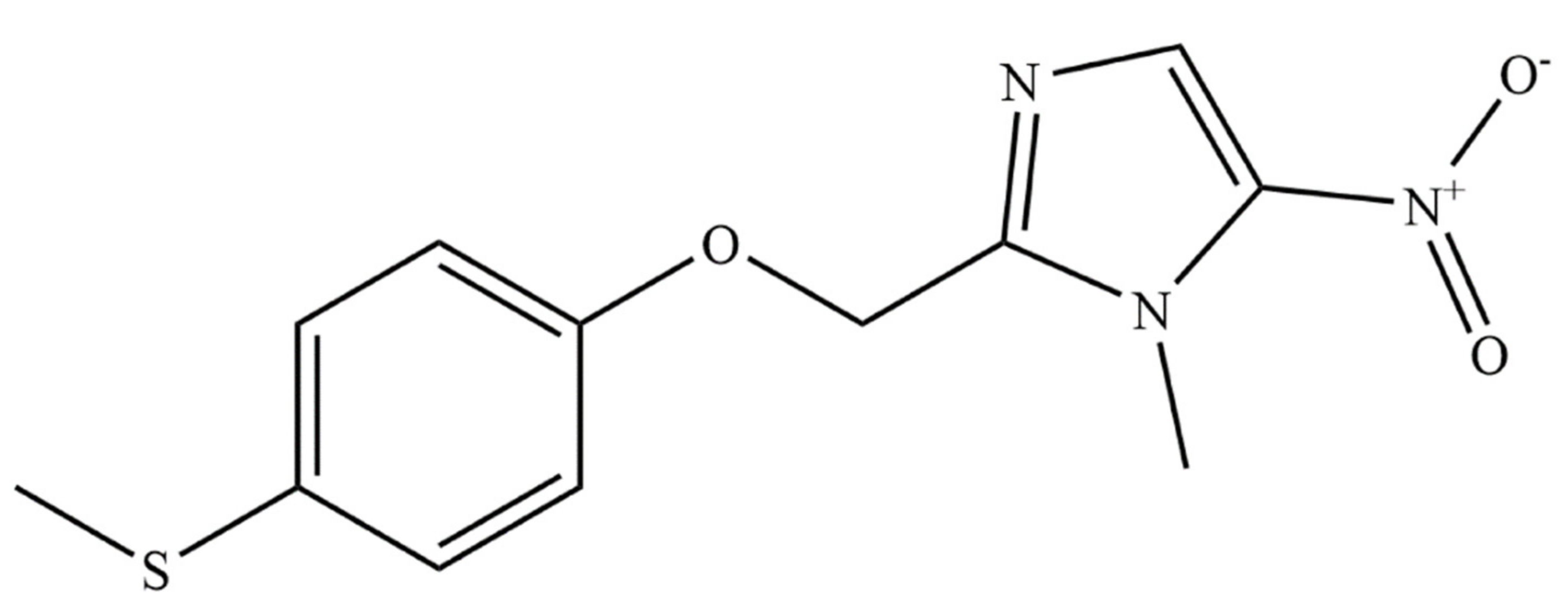
2. Fexinidazole (FEX)
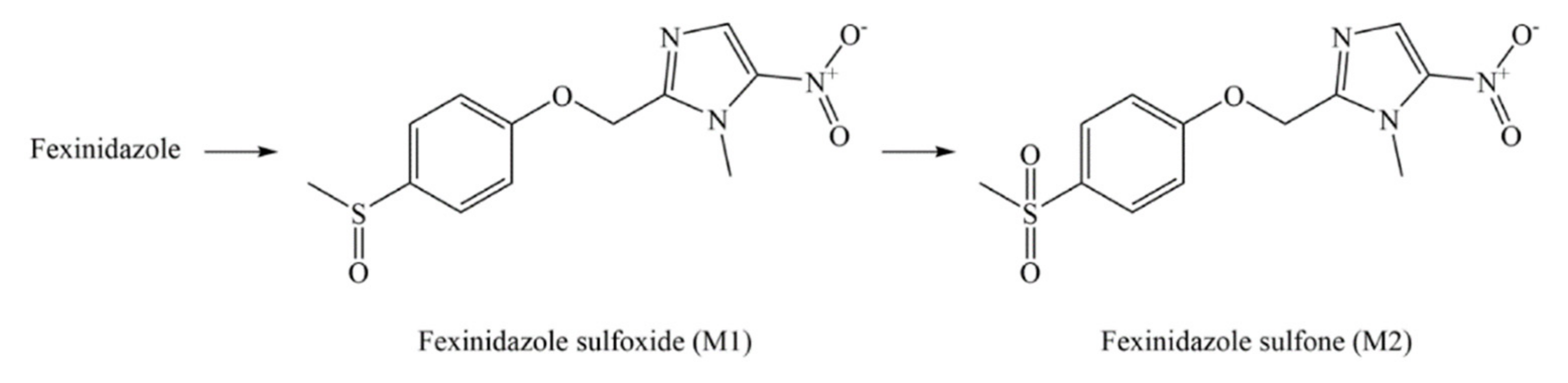
3. Pharmacology of FEX
4. Clinical Trials on FEX
| Condition | Phase (Number Enrolled) | Status (Study Start Date (SSD); Study Completion Date (SCD); Last Update Date (LUD)) | National Clinical Trial (NCT) Number/Other IDs (Sponsor/Collaborators; Funder Type; Location) |
|---|---|---|---|
| Chagas Disease and South American Trypanosomiasis | Phase 2 (140) | Unknown (SSD: July 2014; SCD: February 2016; LUD: 15 July 2015) | NCT02498782/DNDi-CH-FEXI-001 (Drugs for Neglected Diseases initiative; Other; Bolivia) |
| Trypanosomiasis (African) | Phase 1 (30) | Terminated (SSD: September 2011; SCD: February 2012; LUD: 31 March 2017) | NCT01483170/DNDiFEX003 (Drugs for Neglected Diseases initiative; Other; France) |
| Visceral Leishmaniasis | Phase 2 (14) | Terminated (SSD: November 2013; SCD: September 2015; LUD: 30 October 2015) | NCT01980199/FEXI VL001 (Drugs for Neglected Diseases initiative; Other; Sudan) |
| r-Human African Trypanosomiasis | Phase 2/3 (50) | Recruiting (SSD: 29 September 2019; SCD: March 2023; LUD: 30 August 2021) | NCT03974178/DNDi-FEX-07-HAT (Drugs for Neglected Diseases initiative; Other; Malawi and Uganda) |
| Trypanosomiasis (African) | Phase 1 (30) | Completed (SSD: March 2015; SCD: June 2015; LUD: 8 October 2015) | NCT02571062/DNDiHATFEX008 (Drugs for Neglected Diseases initiative; Other; France) |
| Human African Trypanosomiasis | Phase 2/3 (394) | Completed (SSD: October 2012; SCD: 26 April 2017; LUD: 20 February 2018) | NCT01685827/DNDiFEX004 (Drugs for Neglected Diseases initiative; Other; Batangafo, Bagata, Congo, etc.) |
| Human African Trypanosomiasis | Phase 2/3 (125) | Completed (SSD: May 3, 2014; SCD: 27 June 2017; LUD: 24 June 2020) | NCT02184689/DNDiHATFEX006 (Drugs for Neglected Diseases initiative; Other; Congo) |
| Human African Trypanosomiasis | Phase 2/3 (230) | Completed (SSD: 30 April 2014; SCD: 25 April 2017; LUD: 24 June 2020) | NCT02169557/DNDiHATFEX005 (Drugs for Neglected Diseases initiative; Other; Congo) |
| Human African Trypanosomiasis | Phase 1 (108) | Completed (SSD: September 2009; SCD: October 2010; LUD: 6 April 2017) | NCT00982904/DNDiFEX001 (Drugs for Neglected Diseases initiative and Sanofi; Other/Industry; France) |
| Human African Trypanosomiasis and Trypanosomiasis (Gambian) | Phase 3 (174) | Completed (SSD: 17 November 2016; SCD: 1 February 2021; LUD: 11 October 2021) | NCT03025789/DNDi-FEX-09-HAT (Drugs for Neglected Diseases initiative and Sanofi; Other/Industry; Congo, Mbuji-Mayi, Bagata, etc.) |
| Pharmacokinetic in Healthy Volunteers | Phase 1 (12) | Completed (SSD: February 2011; SCD: April 2011; LUD: 31 March 2017) | NCT01340157/DNDiFEX002 (Drugs for Neglected Diseases initiative and Sanofi; Other/Industry; France) |
| Chagas’ Disease (Chronic) | Phase 2 (45) | Completed (SSD: 13 November 2017; SCD: 28 August 2019; LUD: 23 September 2020) | NCT03587766/DNDi-FEX-12-CH (Drugs for Neglected Diseases initiative; Other; Spain) |
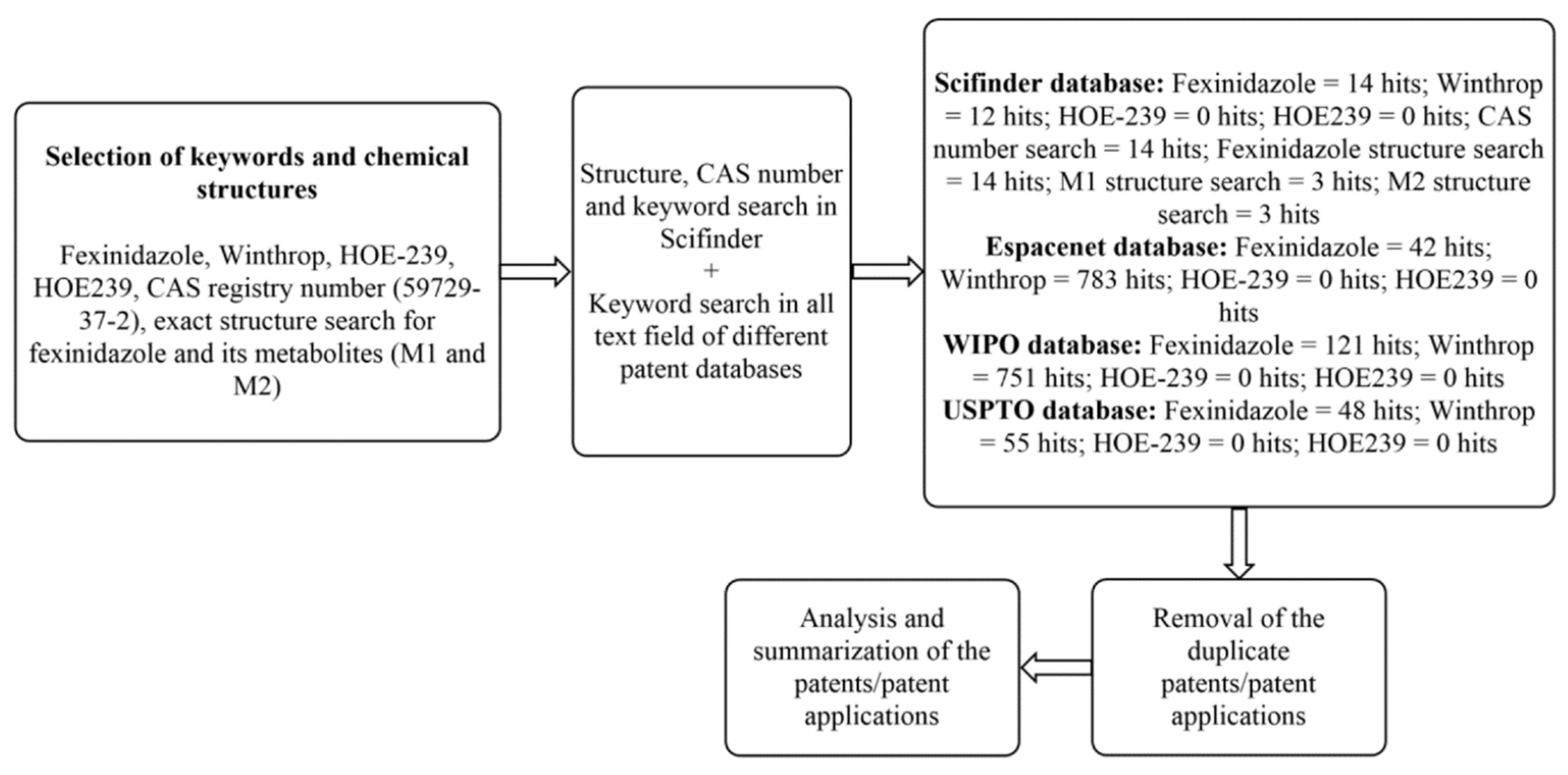
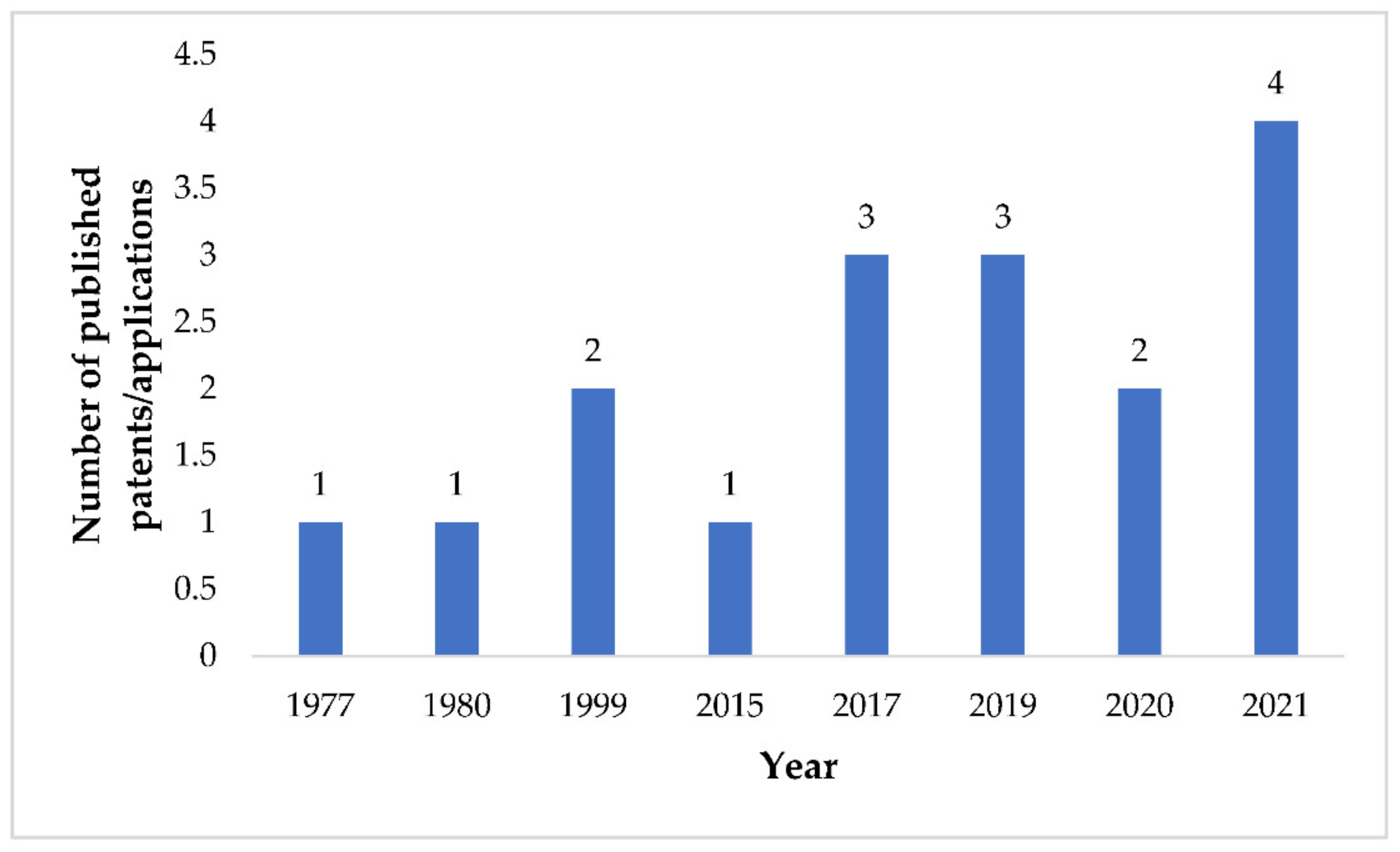
5. Patent Searching
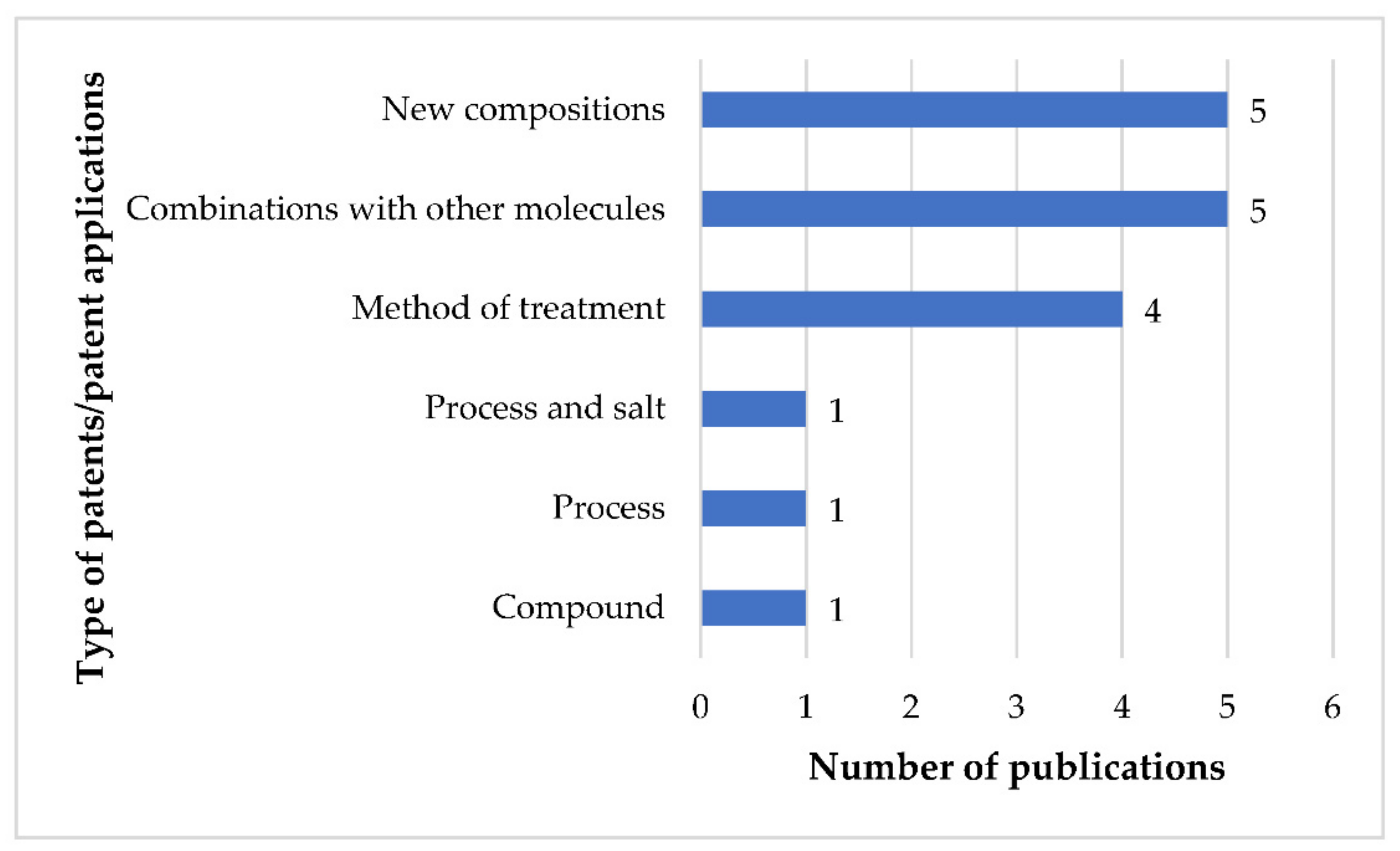
6. Patent Analysis
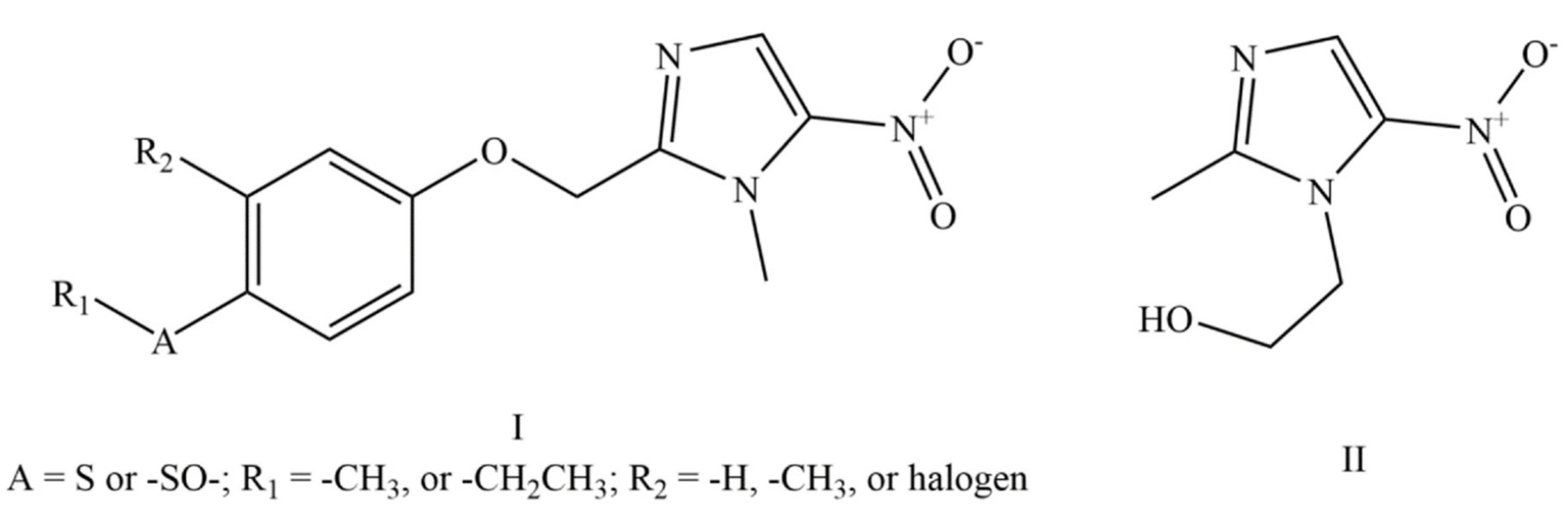
6.1. Compound Patent
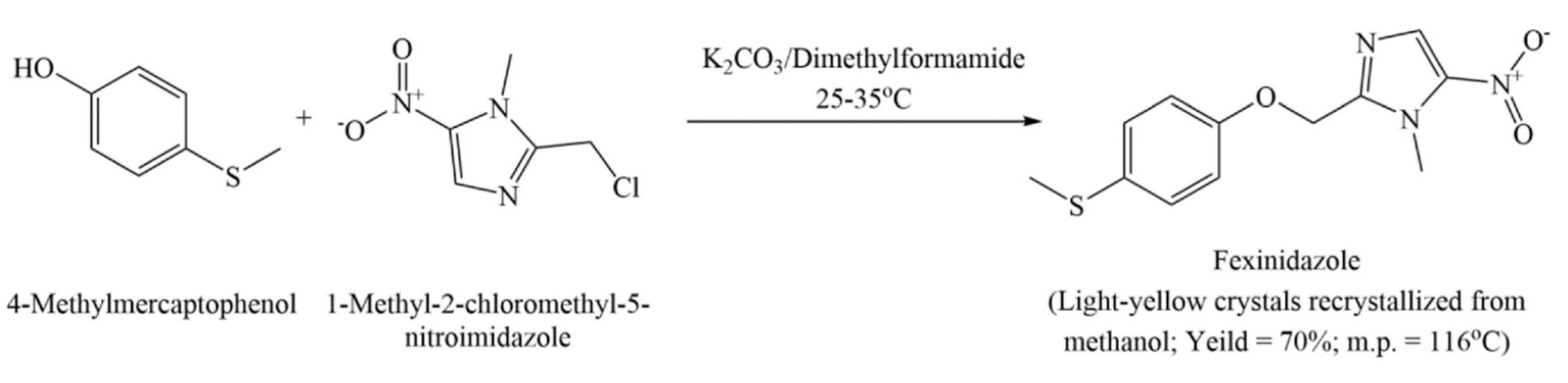
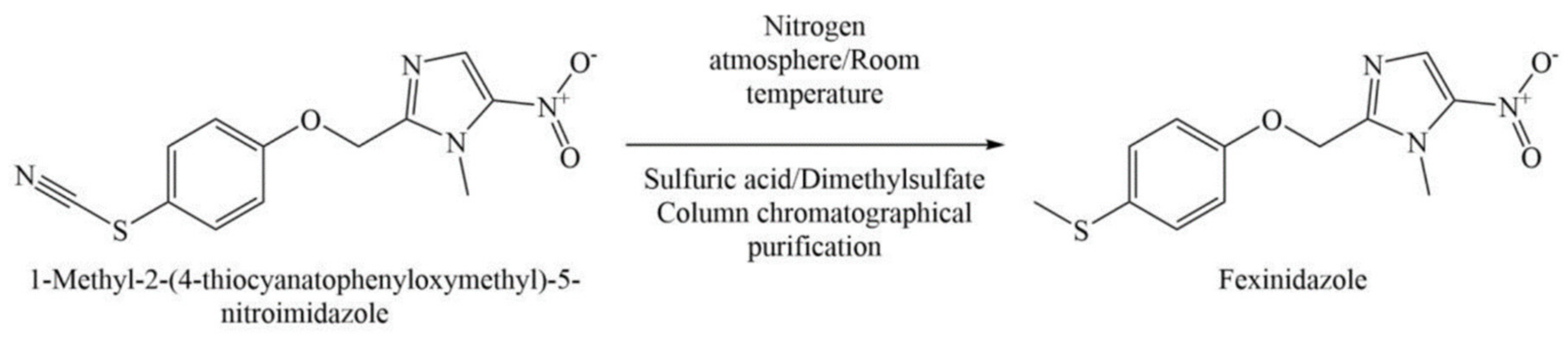
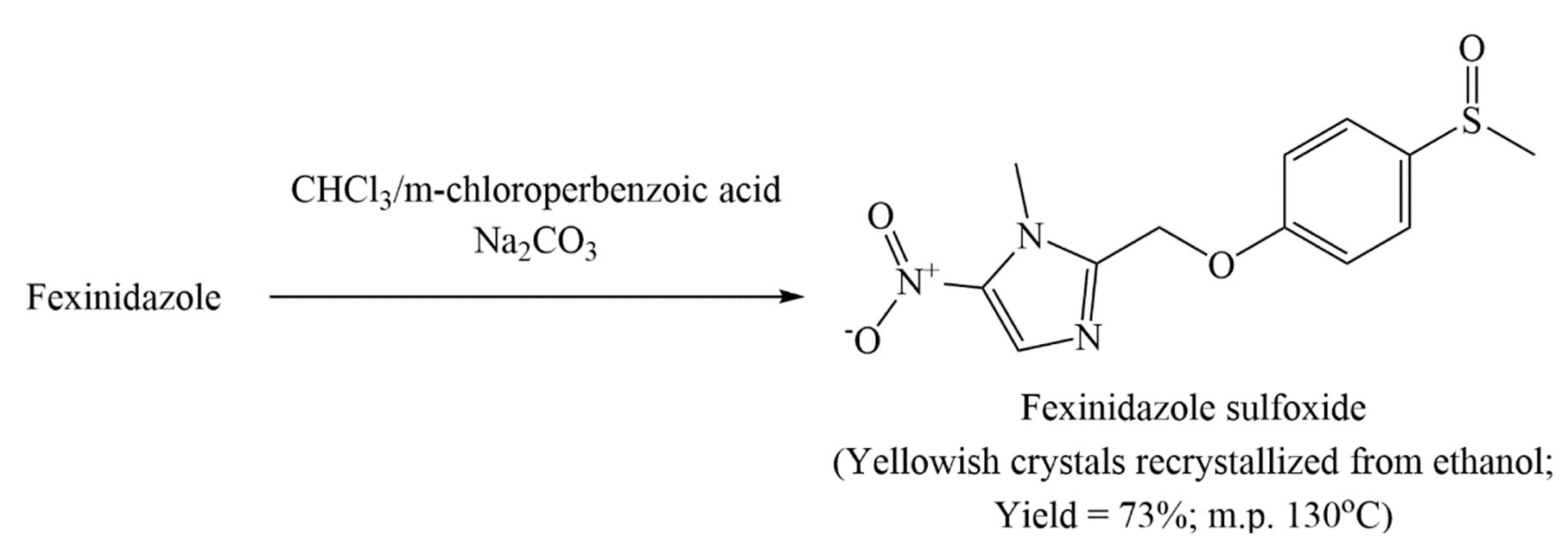
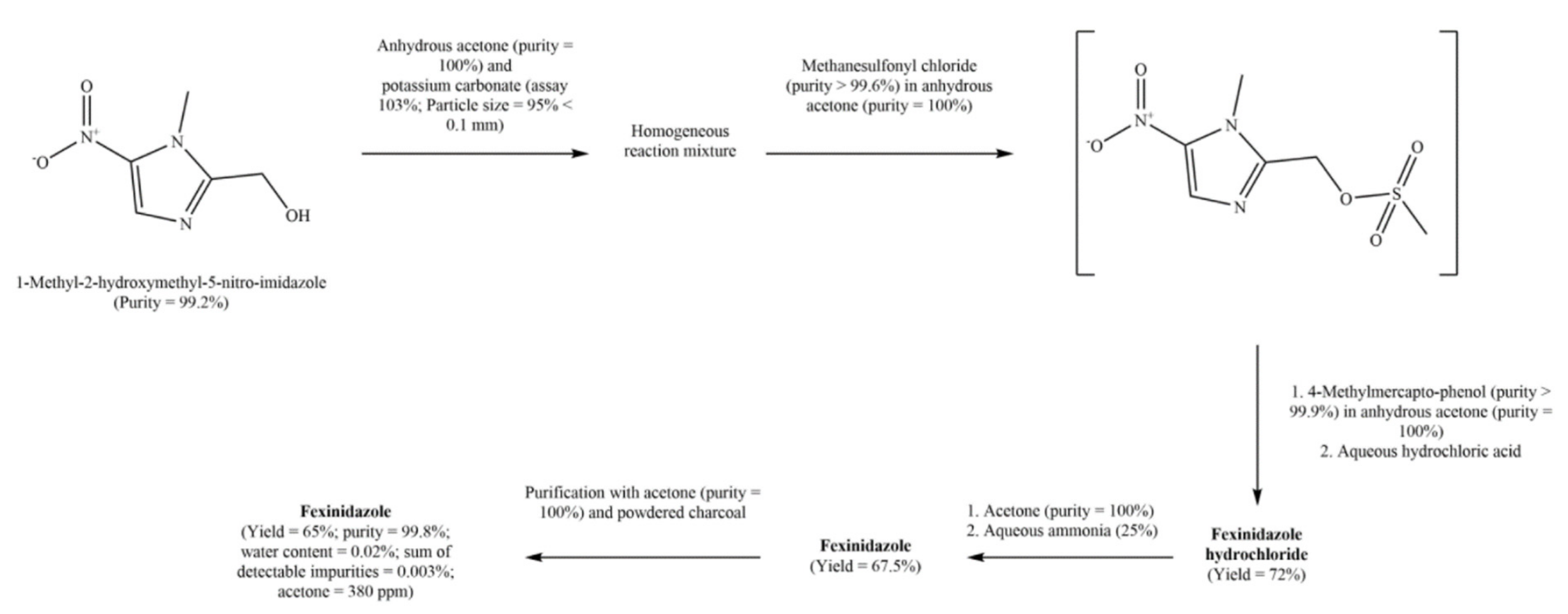
6.2. Process Patents
6.3. Patents/Applications Related to the Method of Treatment
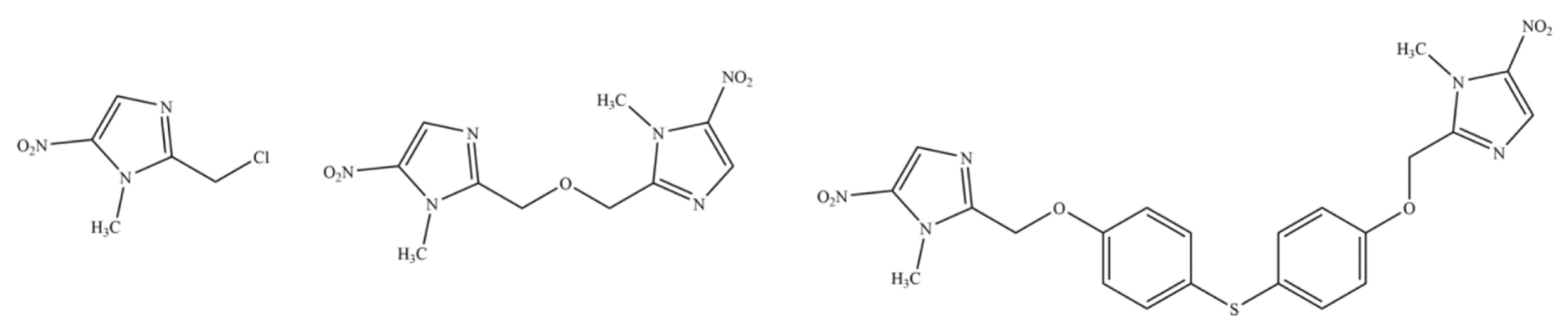
6.4. Patents/Applications Related to the Combination of FEX with Other Molecules
6.5. Patents/Applications Related to Novel Compositions of FEX
7. Conclusions
8. Expert Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagcchi, S. WHO manual on neglected tropical diseases. Lancet Infect. Dis. 2021, 21, E1498. [Google Scholar] [CrossRef]
- Kennedy, P.G.E. Update on human African trypanosomiasis (sleeping sickness). J. Neurol. 2019, 266, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- Costi, M.P.; Costantino, L.; Ferrari, S.; Linciano, P.; Da Silva, A.C. Triaminopyrimidine Derivatives with Antiparasitic Activity. WO2020188437A1, 24 September 2020. [Google Scholar]
- Kennedy, P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013, 12, 186–194. [Google Scholar] [CrossRef]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Kennedy, P.G. Human African trypanosomiasis of the CNS: Current issues and challenges. J. Clin. Investig. 2004, 113, 496–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bisser, S.; Lumbala, C.; Nguertoum, E.; Kande, V.; Flevaud, L.; Vatunga, G.; Boelaert, M.; Büscher, P.; Josenando, T.; Bessell, P.R.; et al. Sensitivity and specificity of a prototype rapid diagnostic test for the detection of Trypanosoma brucei gambiense infection: A multi-centric prospective study. PLoS Negl. Trop. Dis. 2016, 10, e0004608. [Google Scholar] [CrossRef] [PubMed]
- Lumbala, C.; Biéler, S.; Kayembe, S.; Makabuza, J.; Ongarello, S.; Ndung’u, J.M. Prospective evaluation of a rapid diagnostic test for Trypanosoma brucei gambiense infection developed using recombinant antigens. PLoS Negl. Trop. Dis. 2018, 12, e0006386. [Google Scholar] [CrossRef]
- Hafiz, S.; Kyriakopoulos, C. Pentamidine. 19 June 2021. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557586/ (accessed on 20 December 2021). [PubMed]
- Wiedemar, N.; Hauser, D.A.; Mäser, P. 100 years of Suramin. Antimicrob. Agents Chemother. 2020, 64, e01168-19. [Google Scholar]
- Thakare, R.; Dasgupta, A.; Chopra, S. Update on nifurtimox for treatment of Chagas disease. Drugs Today 2021, 57, 251–263. [Google Scholar] [CrossRef]
- Jobanputra, K.S.; Rajpal, A.V.; Nagpur, N.G. Eflornithine. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 365–366. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Horn, D. Melarsoprol resistance in African trypanosomiasis. Trends Parasitol. 2018, 34, 481–492. [Google Scholar] [CrossRef]
- Hidalgo, J.; Ortiz, J.F.; Fabara, S.P.; Eissa-Garcés, A.; Reddy, D.; Collins, K.D.; Tirupathi, R. Efficacy and toxicity of fexinidazole and nifurtimox plus eflornithine in the treatment of African trypanosomiasis: A systematic review. Cureus 2021, 13, 16881. [Google Scholar] [CrossRef]
- Developing Products for Rare Diseases & Conditions. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=513915 (accessed on 2 November 2021).
- Prescribing Information of Fexinidazole. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214429s000lbl.pdf (accessed on 2 November 2021).
- Fairlamb, A.H. Fexinidazole for the treatment of human African trypanosomiasis. Drugs Today 2019, 55, 705–712. [Google Scholar] [CrossRef]
- Neau, P.; Hänel, H.; Lameyre, V.; Strub-Wourgaft, N.; Kuykens, L. Innovative partnerships for the elimination of human African Trypanosomiasis and the development of fexinidazole. Trop. Med. Infect. Dis. 2020, 5, 17. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef]
- Quality Review of Fexinidazole. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214429Orig1s000ChemR.pdf (accessed on 2 November 2021).
- Erhardt, W.; Wolfgang, R. 1-Methyl-2-(phenyl-oxymethyl)-5-nitro-imidazoles and Process for Their Manufacture. U.S. Patent US4042705A, 16 August 1977. [Google Scholar]
- Torreele, E.; Bourdin Trunz, B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazue, G.; Bray, M.A.; Pecoul, B. Fexinidazole—A new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e923. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Patterson, S.; Stojanovski, L.; Simeons, F.R.; Norval, S.; Kime, R.; Read, K.D.; Fairlamb, A.H. The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci. Transl. Med. 2012, 4, 119re1. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Tarral, A.; Blesson, S.; Mordt, O.V.; Torreele, E.; Sassella, D.; Bray, M.A.; Hovsepian, L.; Evene, E.; Gualano, V.; Felices, M.; et al. Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: First-in-human studies. Clin. Pharmacokinet. 2014, 53, 565–580. [Google Scholar] [CrossRef]
- Kaiser, M.; Bray, M.A.; Cal, M.; Bourdin Trunz, B.; Torreele, E.; Brun, R. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob. Agents Chemother. 2011, 55, 5602–5608. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials on Fexinidazole. Available online: htttps://clinicaltrial.gov (accessed on 6 November 2021).
- Espacenet Database. Available online: https://worldwide.espacenet.com/patent/search (accessed on 6 November 2021).
- USPTO Full Text-Image Database. Available online: https://patft.uspto.gov/netahtml/PTO/search-bool.html (accessed on 6 November 2021).
- Patenetscope Database. Available online: https://patentscope.wipo.int/search/en/search.jsf (accessed on 6 November 2021).
- Wagner, A.B. SciFinder Scholar 2006: An empirical analysis of research topic query processing. J. Chem. Inf. Model 2006, 46, 767–774. [Google Scholar] [CrossRef]
- Erhardt, W. Process for the Manufacture of 1-alkyl-2-(phenoxymethyl)-5-nitroimidazoles. CA1079738A, 17 June 1980. [Google Scholar]
- Parkanyi, Z.; Alattyani, E.; Bugir, Z.; Harsanyi, M. Method for Preparing Phenyloxymethyl-Nitro-Imidazole Derivatives and Use of Same. U.S. Patent US9758488B2, 12 September 2017. [Google Scholar]
- Natarajan, P.; Chaudhary, R.; Venugopalan, P. Silver(I)-Promoted ipso-nitration of carboxylic acids by nitronium tetrafluoroborate. J. Org. Chem. 2015, 80, 10498–10504. [Google Scholar] [CrossRef] [PubMed]
- Samant, B.S.; Sukhthankar, M.G. Compounds containing 2-substituted imidazole ring for treatment against human African trypanosomiasis. Bioorg. Med. Chem. Lett. 2011, 21, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Pollmeier, M.; Blair, J.L. Method for Treating and Curing Leishmaniosis Using Fexinidazole. U.S. Patent US9585871B2, 7 March 2017. [Google Scholar]
- Blake, D.; Naughton, D.; Stratford, I.; Adams, G. Treatment of Inflammatory Conditions. WO9912547A1, 18 March 1999. [Google Scholar]
- Blake, D.; Naughton, D.; Stratford, I.; Adams, G. Treatment of Inflammatory Conditions. WO9912548A1, 18 March 1999. [Google Scholar]
- Xiao, Z.; Yu, D.X.; Pouton, C.W.; He, Z. Nitroimidazole Formulations. U.S. Patent US2021220335A1, 22 July 2021. [Google Scholar]
- Fairlamb, A.; Patterson, S.; Wylie, S.; Read, K. Treatment of Parasitic Disease. WO2017072523A1, 4 May 2017. [Google Scholar]
- Page, S.; Stevens, A.; McCluskey, A.; Keenan, M.; Abraham, R. Methods for Treating Protozoan Infections. U.S. Patent US10392363B2, 27 August 2019. [Google Scholar]
- Page, S.; Stevens, A.; McCluskey, A.; Keenan, M.; Abraham, R. Methods for Treating Protozoan Infections. U.S. Patent US10562880B2, 18 February 2020. [Google Scholar]
- Page, S.; Stevens, A.; McCluskey, A.; Keenan, M.; Abraham, R. Methods for Treating Protozoan Infections. U.S. Patent US10752606B2, 25 August 2020. [Google Scholar]
- Jiricek, J.; Ng, S.P.; Rao, S.P.S. Cyanotriazole Compounds and Uses Thereof. WO2019244049A1, 26 December 2019. [Google Scholar]
- Lellouche, J.P.; Michaeli, S.; Israel, L.L.; Harel, Y.; Dolitzky, A.; Ostrovsky, S. Core-Shell Particles Comprising Metal Oxide and Lanthanide Element. WO2019043701A1, 7 March 2019. [Google Scholar]
- Carricarte, V.; Roldan, E. Nanostructured Nanoparticles Comprising One or More Active Ingredients for the Treatment of Diseases Caused by Trypanosomes and for the Treatment of Tumours of Neural Origin, Compositions Comprising Same, a Preparation Method and Therapeutic Use Thereof. U.S. Patent US2021322329A1, 21 October 2021. [Google Scholar]
- Scott, E.A.; Yi, S.; Karabin, N.; Li, X. Compositions and Methods of Using Propylene Sulfide-Based Polymers for Treatment of Chagas Disease. U.S. Patent US2021052498A1, 25 February 2021. [Google Scholar]
- Patterson, J. Engineered Platelets for Targeted Delivery of a Therapeutic Agent. WO2021123775A3, 10 September 2021. [Google Scholar]
- Brennan, A.B.; Long, C.J.; Bagan, J.W.; Schumacher, J.F.; Spiecker, M.M. Surface Topographies for Non-Toxic Bioadhesion Control. U.S. Patent US9016221B2, 25 April 2015. [Google Scholar]
- De Koning, H.P. The drugs of sleeping sickness: Their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Bottieau, E.; Clerinx, J. Human African trypanosomiasis: Progress and stagnation. Infect. Dis. Clin. N. Am. 2019, 33, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Plurad, D.S.; Geesman, G.; Mahmoud, A.; Sheets, N.; Chawla-Kondal, B.; Ayutyanont, N.; Ghostine, S. The effect of trauma center verification level on traumatic brain injury outcome after implementation of the orange book. Am. J. Surg. 2021, 221, 637–641. [Google Scholar] [CrossRef]
- Imran, M.; Kumar Arora, M.; Asdaq, S.M.; Khan, S.A.; Alaqel, S.I.; Alshammari, M.K.; Alshehri, M.M.; Alshrari, A.S.; Mateq Ali, A.; Al-Shammeri, A.M.; et al. Discovery, development, and patent trends on molnupiravir: A prospective oral treatment for COVID-19. Molecules 2021, 26, 5795. [Google Scholar] [CrossRef]
- Imran, M.; Asdaq, S.M.; Khan, S.A.; Unnikrishnan Meenakshi, D.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Mohzari, Y.; Alrashed, A.; AlMotairi, M.; et al. Innovations and patent trends in the development of USFDA approved protein kinase inhibitors in the last two decades. Pharmaceuticals 2021, 14, 710. [Google Scholar] [CrossRef]
- Imran, M.; Alshrari, A.S.; Asdaq, S.M.B.; Abida. Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review. J. Infect. Public Health 2021, 14, 1075–1086. [Google Scholar] [CrossRef]
- Rodgers, J.; Jones, A.; Gibaud, S.; Bradley, B.; McCabe, C.; Barrett, M.P.; Gettinby, G.; Kennedy, P.G. Melarsoprol cyclodextrin inclusion complexes as promising oral candidates for the treatment of human African trypanosomiasis. PLoS Negl. Trop. Dis. 2011, 5, 1308. [Google Scholar] [CrossRef]
- Medicines for the People. Available online: https://dndi.org/wp-content/uploads/2021/08/DNDi-AnnualReport-2020.pdf (accessed on 12 January 2021).
- Gao, J.M.; Qian, Z.Y.; Hide, G.; Lai, D.H.; Lun, Z.R.; Wu, Z.D. Human African trypanosomiasis: The current situation in endemic regions and the risks for non-endemic regions from imported cases. Parasitology 2020, 147, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.B.; Chen, H.X.; Wang, M.W. Innovation in neglected tropical disease drug discovery and development. Infect. Dis. Poverty 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Mäser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.; Barrett, M.P. New drugs for human African trypanosomiasis: A twenty first century success story. Trop. Med. Infect. Dis. 2020, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Ozioko, K.U.; Okoye, C.I.; Obiezue, R.N.; Idika, I.K.; Awudu, R.A.; Ezewudo, B.I.; Ezea, C.O. Accelerating towards human African trypanosomiasis elimination: Issues and opportunities. J. Vector Borne Dis. 2020, 57, 105–113. [Google Scholar] [CrossRef] [PubMed]
| Drug (Dosage Form and Administration) | Comments |
|---|---|
| Treatment of Stage 1 of Human African Trypanosomiasis | |
| Pentamidine (Solution for inhalation/Injection) | A skilled and trained professional is needed for drug administration. It is administered as a single daily intramuscular/intravenous injection for seven days. It can cause severe hypotension after intramuscular/intravenous administration, hypoglycemia, acute pancreatitis, and cardiac arrhythmias, and is effective against stage-1 of g-human African trypanosomiasis only because it does not cross the blood–brain barrier efficiently [9]. |
| Suramin (Intravenous, injection) | A skilled and trained professional is needed for drug administration. It is mainly used for stage-1 of r-human African trypanosomiasis, and rarely used for stage-1 g-human African trypanosomiasis. It can cause renal toxicity and anaphylactic reactions [10]. |
| Treatment of Stage 2 of Human African Trypanosomiasis | |
| Nifurtimox (Tablet, Oral) | The combination of nifurtimox with eflornithine is the first-line treatment for stage-2 of human African trypanosomiasis. It has potential for genotoxicity, carcinogenicity, fetal toxicity, worsening of neurological and psychiatric conditions, hypersensitivity, decreased appetite and weight loss, and porphyria [11]. |
| Eflornithine (Intravenous, injection) | A skilled and trained professional is needed for drug administration and requires long therapy. It can cause fever, pruritus, hypertension, cough, anorexia, nausea, vomiting, diarrhea, abdominal pain, headaches, and is the second-line treatment for stage-2 of g-human African trypanosomiasis [12]. |
| Melarosoprol (Intravenous, injection) | A skilled and trained professional is needed for drug administration and is effective for stage-2 g-human African trypanosomiasis. Its administration is painful and toxic. The adverse events may be life-threatening including encephalopathic syndrome [13]. |
| Nifurtimox-eflornithine combination therapy (Oral Nifurtimox + Intravenous Eflornithine) | A skilled and trained professional is needed for drug administration. It needs systematic hospitalization and is mainly used for stage-2 of g-human African trypanosomiasis [14]. |
| Proprietary Name (Application Number; Applicant) | Dosage Form; Route; Strength | Marketing Status (Exclusivities) | Recommended Dosage | |
|---|---|---|---|---|
| Greater than or Equal to 35 kg | Greater than or Equal to 20 and Less than 35 kg | |||
| Fexinidazole (N214429; Sanofi Aventis) | Immediate release tablet; Oral; 600 mg | Prescription (New Chemical Entity (NCE) and Orphan Drug Exclusivity (ODE-359) expiring on 16 July 2026, and 16 July 2028, respectively) | Loading dose (1800 mg, 3 tablets) for four days followed by the maintenance dose (1200 mg, 2 tablets) for 6 days | Loading dose (1200 mg, 2 tablets) for four days followed by the maintenance dose (600 mg, 1 tablet) for 6 days |
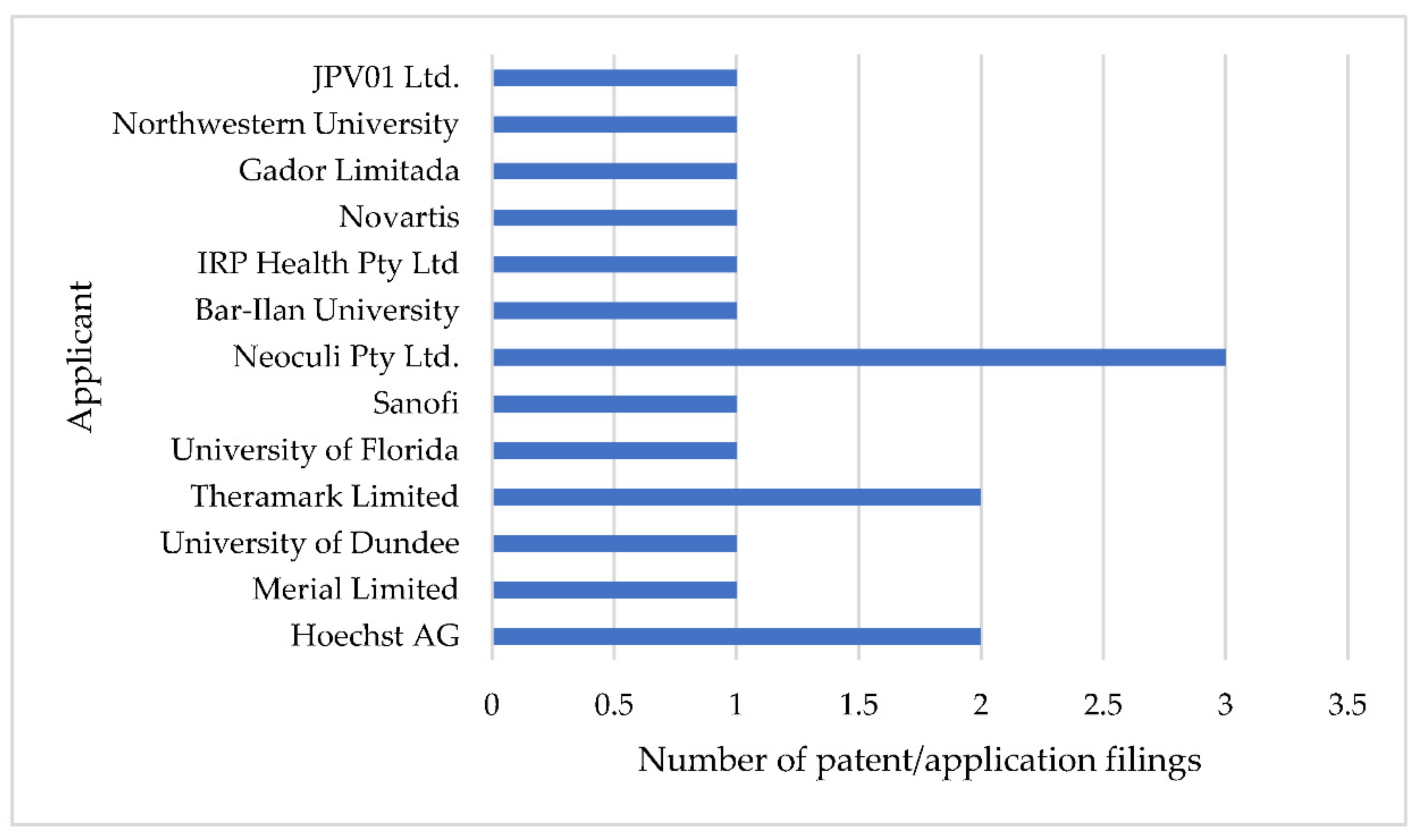
| Patent/Patent Application Number (Assignee; Publication Date; Priority Country; Estimated Expiry Date) | Legal Status on 6 November 2021 (International Patent Classification) | Family Members on 6 November 2021 |
|---|---|---|
| US4042705A (Hoechst AG; August 16, 1977; Germany; Expired) | Expired patent (A61K31/415, A61P31/04, C07D233/94, (IPC1-7): A61K31/415, C07D233/94) | AT359059B, AU500640B2, CH605813A5, CH605819A5, DK142412C, EG12284A, ES449589A1, FI61185C, FR2317925B1, GB1541280A, GR66090B, HU173463B, IE43692B1, IL50023A, IT1064924B, JPS5231074A, LU75359A1, MX3539E, MY8100120A, NL7607495A, NO145136B, NO145136C, NZ181421A, PT65350B, SE414927B |
| CA1079738A (Hoechst AG; 17 June 1980; Germany; Expired) | Expired patent (C07D233/94, (IPC1-7): C07D233/94) | AT361468B, ATA128477A, CH624942A5, DK144524C, EG13828A, ES456117A2, FI770599A, IT1115608B, LU76834A1, NL7701838A, NO770648L, SE7702126L |
| US9758488B2 (Sanofi; 12 September 2017; Europe; 24 December 2032) | Patented case (C07D233/94) | AP3759A, BR112015011446A2, CA2892334C, CN104797562B, CR20150286A, CY1118983T1, ECSP15025960A, EP2922822B1, ES2618800T3, HRP20170390T1, HUE032160T2, IL238934A, MX365587B, PL2922822T3, SG11201504047TA, SI2922822T1, WO2014079497A1, ZA201503063B |
| US9585871B2 (Merial Limited; 7 March 2017; USA; 31 January 2034) | Patented case (A61K31/4164) | AU2014212217B2, CY1120381T1, DK2950795T3, EP2950795B1, ES2681420T3, HK1211466A1, HRP20181181T1, HUE039467T2, IL240246A, LT2950795T, MX367952B, PL2950795T3, PT2950795T, RS57469B1, SI2950795T1, WO2014121064A1 |
| WO9912547A1 (Theramark Limited; 18 March 1999; United Kingdom; 8 September 2018) | Lapsed (A61K31/404, A61K31/407, A61K31/4155, A61K31/4174, A61K31/535, (IPC1-7): A61K31/40, A61K31/41, A61K31/415, A61K31/535) | AU9082698A, AU9082798A |
| WO9912548A1 (Theramark Limited; 18 March 1999; United Kingdom; 8 September 2018) | ||
| US2021220335A1 (IRP Health Pty Ltd.; 22 July 2021; Australia; 29 May 2039) | Under examination (A23K20/121, A23K20/137, A23K20/195, A23K50/30, A23L33/00, A23L33/10, A61K31/4164, A61K31/4375, A61K9/00, A61P31/04) | AU2019277198A1, CN112543635A, EP3810137A1, WO2019227149A1 |
| WO2017072523A1 (University of Dundee; 4 May 2017; United Kingdom; 28 October 2036) | Lapsed (A61K31/454, A61P33/02) | None |
| US10392363B2 (Neoculi Pty Ltd.; 27 August 2019; Australia; 28 August 2035) | Patented case (A61K31/155, A61K31/381, A61K31/404, A61K31/496, A61K31/505, A61K45/06, A61K9/00, C07C281/18, C07C335/40, C07D209/08, C07D239/50, C07D295/135, C07D333/58, A61K31/17, A61K31/341, A61K31/4045, A61K31/4192, A61K31/44, A61K31/498, C07C211/29, C07C251/24, C07C47/565, C07D209/14, C07D239/48, C07D241/20, C07D249/14, C07D251/54, C07D307/56, C07D311/58, C07D401/14) | AU2015311598A1, AU2020204441B2, BR112017004153A2, CA2959440A1, CN107106524B, EP3188722B1, JP2017528518A, RU2719593C2, RU2020113652A, US10829468B2, US2021009552A1, WO2016033635A1 |
| US10562880B2 (Neoculi Pty Ltd.; 18 February 2020; Australia; 28 August 2035) | ||
| US10752606B2 (Neoculi Pty Ltd.; 25 August 2020; Australia; 28 August 2035) | ||
| WO2019244049A1 (Novartis; 26 December 2019; USA; 18 June 2039) | National phase entry in many countries (A61K31/4196, A61K31/4439, A61P33/02, C07D401/14, C07D403/06) | AU2019291490A1, BR112020025538A2, CA3100954A1, CL2020003252A1, CN112313217A, CR20200619A, CU20200102A7, EA202190064A1, ECSP20080991A, EP3810598A1, JOP20200327A1, JP2021528397A, KR20210022646A, MA52977A, PE20210780A1, PH12020552186A1, SG11202012628XA |
| WO2019043701A1 (Bar-Ilan University; 7 March 2019; USA; 28 August 2038) | Entered into Europe (A61K31/155, A61K31/7105, A61K33/26, A61K38/00, A61K9/14, B82Y5/00, C01G49/02, C12N15/113) | EP3675875A1 |
| US2021322329A1 (Gador Limitada; 21 October 2021; Chile; 21 August 2038) | Under examination (A61K31/541, A61K9/51, A61P33/02) | CA3113862A1, EP3842029A1, WO2020037438A1 |
| US2021052498A1 (Northwestern University; 25 February 2021; USA; 29 July 2040) | Under examination (A61K31/4168, A61K47/34, A61K9/107, A61K9/127, A61P33/02) | None |
| WO2021123775A2 (JPV01 Ltd.; 24 June 2021; United Kingdom; 16 December 2040) | No national phase entry (C07K14/705, C07K16/28) | None |
| US9016221B2 (University of Florida Research Foundation; 28 April 2015; USA; 24 November 2025) | Patented case (A41D31/00, A61F2/02, A61L2/02, A61L27/00, B08B17/02, B08B17/06, B63B59/04, B64D15/00, A61F2/00, A61F2/12, A61F2/24, B63B1/36 | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Khan, S.A.; Alshammari, M.K.; Alqahtani, A.M.; Alanazi, T.A.; Kamal, M.; Jawaid, T.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis. Pharmaceuticals 2022, 15, 128. https://doi.org/10.3390/ph15020128
Imran M, Khan SA, Alshammari MK, Alqahtani AM, Alanazi TA, Kamal M, Jawaid T, Ghoneim MM, Alshehri S, Shakeel F. Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis. Pharmaceuticals. 2022; 15(2):128. https://doi.org/10.3390/ph15020128
Chicago/Turabian StyleImran, Mohd, Shah Alam Khan, Mohammed Kanan Alshammari, Ashwaq Muiedh Alqahtani, Turkiah Abdullah Alanazi, Mehnaz Kamal, Talha Jawaid, Mohammed M. Ghoneim, Sultan Alshehri, and Faiyaz Shakeel. 2022. "Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis" Pharmaceuticals 15, no. 2: 128. https://doi.org/10.3390/ph15020128
APA StyleImran, M., Khan, S. A., Alshammari, M. K., Alqahtani, A. M., Alanazi, T. A., Kamal, M., Jawaid, T., Ghoneim, M. M., Alshehri, S., & Shakeel, F. (2022). Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis. Pharmaceuticals, 15(2), 128. https://doi.org/10.3390/ph15020128











