Chemical Profile and Skin-Beneficial Activities of the Petal Extracts of Paeonia tenuifolia L. from Serbia
Abstract
1. Introduction
2. Results
2.1. Chemical Profile
2.2. UV-Vis Chemical Analysis
2.3. Antioxidant Activity
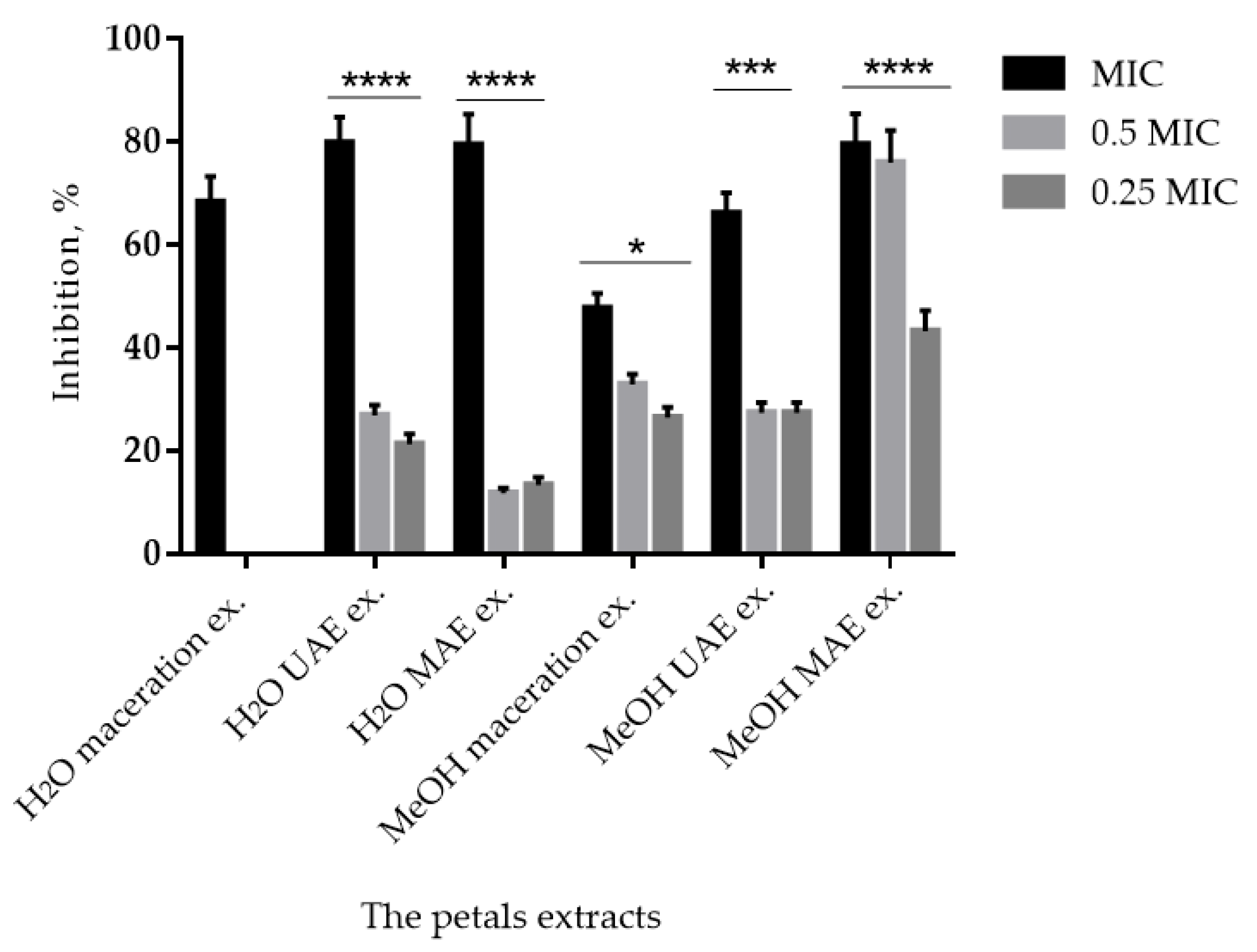
2.4. Antimicrobial and Antibiofilm Activities
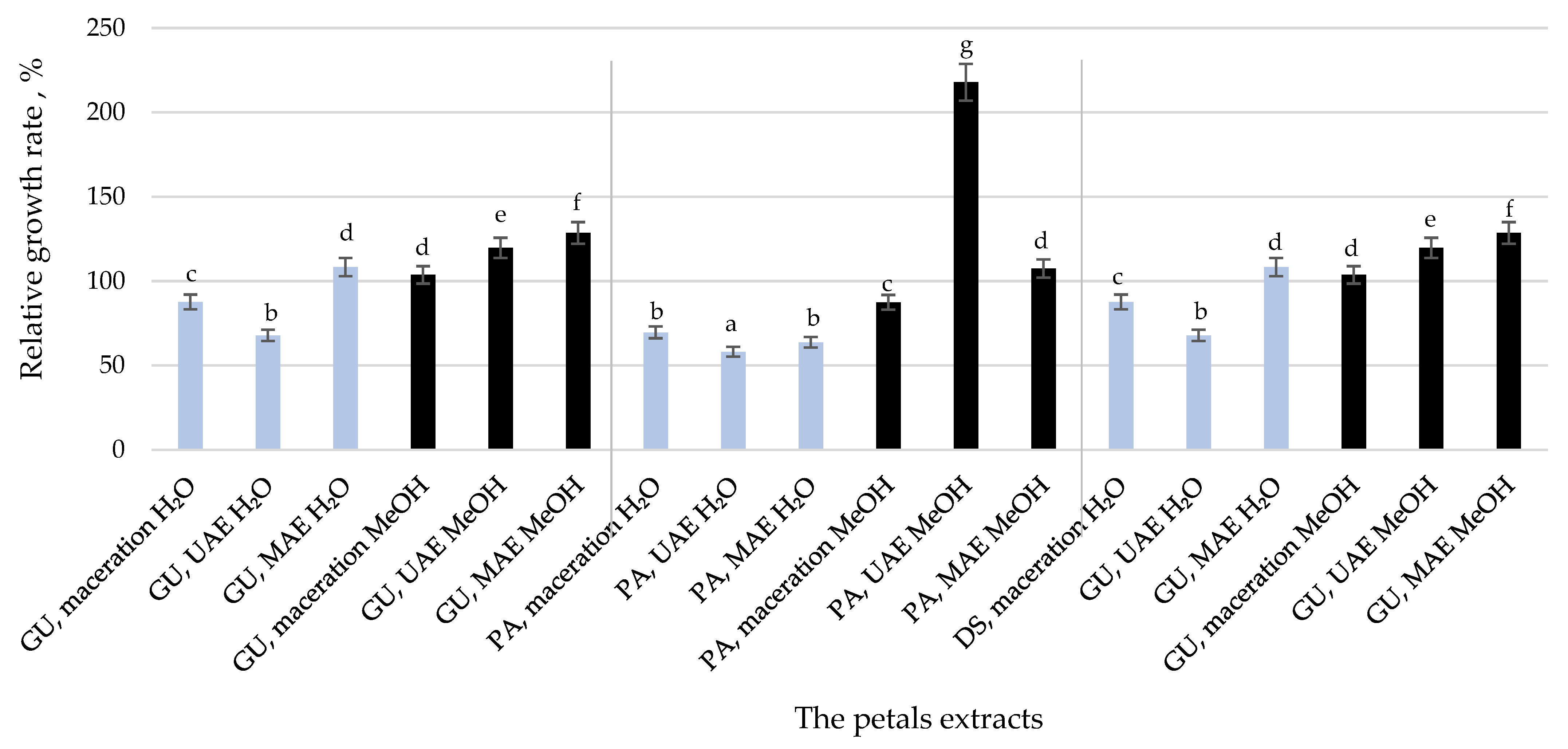
2.5. Cytotoxicity of Paeonia tenuifolia Petals Extracts
2.6. Wound Healing
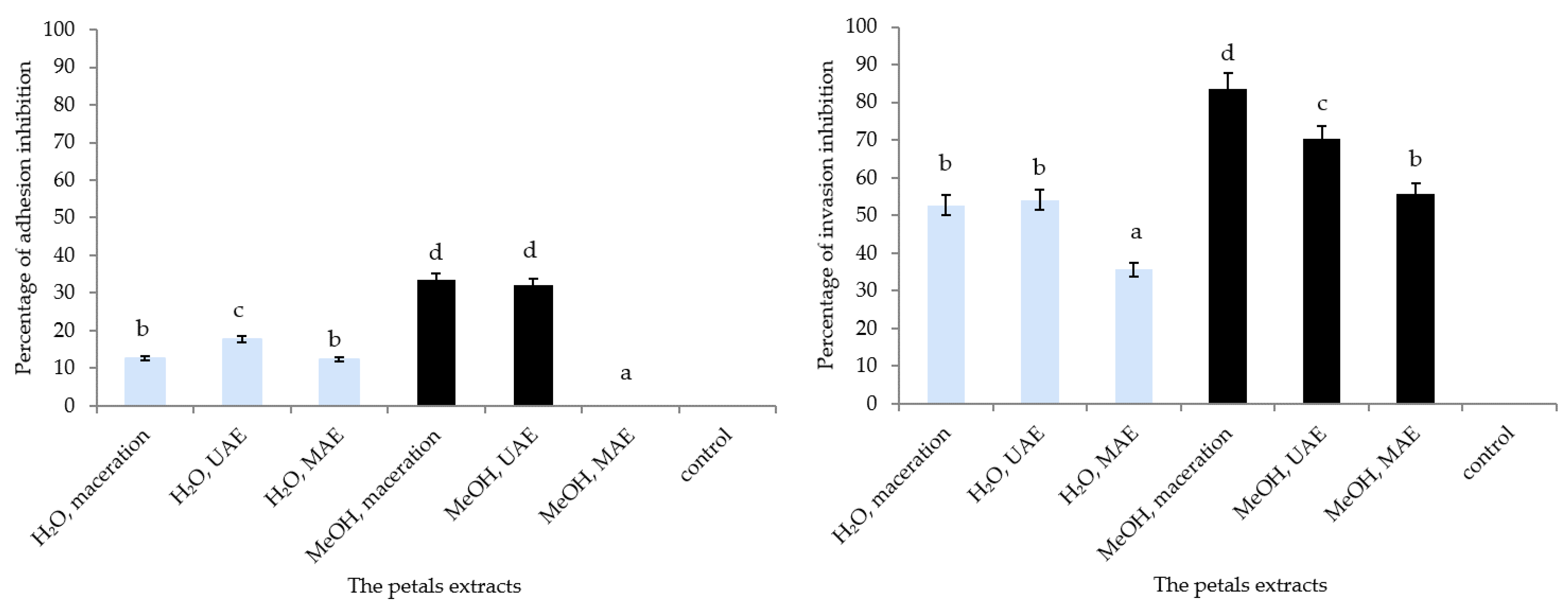
2.7. Adhesion and Invasion Capacities to HaCaT Cells by Staphylococcus lugdunensis
3. Discussion
3.1. Chemical Composition of the Petal Extracts Determined by UV-Vis Spectrometry
3.2. Antioxidant Activity of P. tenuifolia Extracts
3.3. Antimicrobial and Antibiofilm Activities of P. tenuifolia Extracts
3.4. Cytotoxicity and Wound Healing Capacity of the P. tenuifolia Petals Extracts
3.5. Adhesion and Invasion Capacities to HaCaT Cells by S. lugdunensis
4. Materials and Methods
4.1. Origin of Plant Material
4.2. Extraction of Plant Material
4.2.1. Extraction by Maceration Method
4.2.2. Ultrasound-Assisted Extraction (UAE)
4.2.3. Microwave-Assisted Extraction (MAE)
4.3. Chemical Analysis
4.3.1. Chemicals
4.3.2. UHPLC-LTQ-Orbitrap MS
4.3.3. UHPLC/MS Target Analysis of Active Compounds
4.3.4. Determination of the Content of Active Constituents in the Extracts
Total Polyphenol Content
Total Flavonoid Content
4.3.5. Antioxidant Assay
Ferric Reducing Antioxidant Power Assay
Cupric Ion Reducing Antioxidant Capacity Assay
ABTS Assay
DPPH Assay
4.3.6. Antimicrobial and Antibiofilm Activities of Methanolic and Aqueous Extracts
Antibacterial Activity
Antifungal Activity
Bacterial Biofilm Inhibitory Activity
4.3.7. Cytotoxicity of the Extracts
4.3.8. Scratch Wound Healing Assay
4.3.9. Effects of the on the Adhesion and Invasion Capacities of S. lugdunensis to HaCaT Cells
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A Comprehensive Review on Traditional Uses, Phytochemistry, Pharmacological Activities, Clinical Application, and Toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Pan, K.Y.; Turland, N.J. Paeoniaceae, 6th ed.; Wu, Z.Y., Raven, P.H., Eds.; Science Press and Missouri Botanic Garden Press: Beijing, China, 2001; Volume 6. [Google Scholar]
- Lazarević, P.; Stojanović, V. Wild Peonies (Paeonia L.): In Serbia: The Distribution, State of Populations, Threats and Protection. Zaštita Prir. 2012, 62, 19–44. [Google Scholar]
- Marković, T.; Prijić, Ž.; Xue, J.; Zhang, X.; Radanović, D.; Ren, X.; Filipović, V.; Lukić, M.; Gordanić, S. The Seed Traits Associated with Dormancy and Germination of Herbaceous Peonies, Focusing on Species Native in Serbia and China. Horticulturae 2022, 8, 585. [Google Scholar] [CrossRef]
- Stevanović, V. The Red Data Book of Flora of Serbia 1. Extinct and Critically Endangered Taxa, 1st ed.; Stevanovic Vladimir, Ed.; Ministry of Environmental Protection of Republic of Serbia: Belgrade, Serbia, 1999. [Google Scholar]
- Official Gazette of RS. The Law on Nature Protection. Available online: https://www.cms.int/cami/en/document/law-nature-protection-%E2%80%9Cofficial-gazette-rs%E2%80%9D-no-3609-8810-9110-1416-and-9518 (accessed on 10 November 2022).
- Panin, A.V. K Voprosu o Vnutrividovoy Strukture Pib Ona Tonkolistnogo (Paeonia tenuifolia L.) v Saratovskoy Oblasti [On the Intraspecific Structure of the Paeonia tenuifolia L. in the Saratov Region]. Bull. Bot. Gard. Saratov State Univ. 2008, 44–45. [Google Scholar]
- Lara-Cortés, E.; Osorio-Díaz, P.; Jiménez-Aparicio, A.; Bautista-Bañios, S. Nutritional Content, Functional Properties and Conservation of Edible Flowers. Review. Arch Lat. Nutr. 2013, 63, 197–208. [Google Scholar]
- Grzeszczuk, M.; Stefaniak, A.; Pachlowska, A. Biological Value of Various Edible Flower Species. Acta Sci. Pol. Hortorum Cultus 2016, 15, 109–119. [Google Scholar]
- Chen, N.-H.; Wei, S. Factors Influencing Consumers’ Attitudes towards the Consumption of Edible Flowers. Food Qual. Prefer. 2017, 56, 93–100. [Google Scholar] [CrossRef]
- Smiljković, M.; Dias, M.I.; Stojković, D.; Barros, L.; Bukvički, D.; Ferreira, I.C.F.R.; Soković, M. Characterization of Phenolic Compounds in Tincture of Edible Nepeta Nuda: Development of Antimicrobial Mouthwash. Food Funct. 2018, 9, 5417–5425. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, X.; Hua, Y.; Tao, J.; Zhou, C. Effects of Different Harvest Times on Nutritional Component of Herbaceous Peony Flower Petals. J. Chem. 2020, 2020, 4942805. [Google Scholar] [CrossRef]
- Tamura, M. Paeoniaceae. In Flowering Plants·Eudicots; Springer: Berlin/Heidelberg, Germany, 2007; pp. 265–269. [Google Scholar]
- He, C.; Peng, Y.; Zhang, Y.; Xu, L.; Gu, J.; Xiao, P. Phytochemical and Biological Studies of Paeoniaceae. Chem. Biodivers. 2010, 7, 805–838. [Google Scholar] [CrossRef]
- Wu, S.; Wu, D.; Chen, Y. Chemical Constituents and Bioactivities of Plants from the Genus paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Spiller, H.A.; Sawyer, T.S. Toxicology of Oral Antidiabetic Medications. Am. J. Health-Syst. Pharm. 2006, 63, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Aejazuddin, D.Q.M. Herbal Medicine: A Comprehensive Review. Int. J. Pharm. Res. 2016, 8, 1–5. [Google Scholar]
- Mohammadhosseini, M.; Frezza, C.; Venditti, A.; Mahdavi, B. An Overview of the Genus Aloysia Paláu (Verbenaceae): Essential Oil Composition, Ethnobotany and Biological Activities. Nat. Prod. Res. 2022, 36, 5091–5107. [Google Scholar] [CrossRef] [PubMed]
- Mirkov, I.; Stojković, D.; Aleksandrov, A.P.; Ivanov, M.; Kostić, M.; Glamočlija, J.; Soković, M. Plant Extracts and Isolated Compounds Reduce Parameters of Oxidative Stress Induced by Heavy Metals: An up-to-Date Review on Animal Studies. Curr. Pharm. Des. 2020, 26, 1799–1815. [Google Scholar] [CrossRef]
- Kostić, M.; Ivanov, M.; Stojković, D.; Ćirić, A.; Soković, M. Antibacterial and Antibiofilm Activity of Selected Polyphenolic Compounds: An in Vitro Study on Staphylococcus aureus. Lek. Sirovine 2020, 40, 57–61. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Chaudhari, P.M.; Kawade, P.v; Funne, S.M. Cosmeceuticals—A Review. Int. J. Pharm. Technol. 2011, 3, 774–798. [Google Scholar]
- Frank, K.L.; del Pozo, J.L.; Patel, R. From Clinical Microbiology to Infection Pathogenesis: How Daring to Be Different Works for Staphylococcus lugdunensis. Clin. Microbiol. Rev. 2008, 21, 111–133. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Dong, Y.; Sun, G.; Jiang, A.; Li, Y. Cleavage Rules of Mass Spectrometry Fragments and Rapid Identification of Chemical Components of Radix Paeoniae Alba Using UHPLC-Q-TOF-MS. Phytochem. Anal. 2021, 32, 836–849. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Meng, M.; Wang, P.; Feng, Y.; Jia, J.; Qin, X. Exploration of Chemical Composition and Absorption Characteristics of Chaigui Granules Based on UHPLC-Q-Orbitrap-MS/MS. J. Pharm. Biomed. Anal. 2020, 187, 113293. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.-Z.; Yu, R.; Tang, J.-M.; Zhou, Y.; Zheng, T.-T.; Ni, J.; Sun, D.-Y.; Liu, P.; Niu, L.-X.; Zhang, Y.-L. Comparative Investigation on Metabolites and Biological Activities of Paeonia ostii Stamens from Different Geographical Regions of China. Ind. Crops Prod. 2021, 172, 114038. [Google Scholar] [CrossRef]
- Xiong, P.; Qin, S.; Li, K.; Liu, M.; Zhu, L.; Peng, J.; Shi, S.; Tang, S.; Tian, A.; Cai, W. Identification of the Tannins in Traditional Chinese Medicine Paeoniae Radix Alba by UHPLC-Q-Exactive Orbitrap MS. Arab. J. Chem. 2021, 14, 103398. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Yang, Y.; Lv, M.; Li, S.; Teixeira da Silva, J.A.; Wang, L.; Yu, X. Analysis of Chemical Components in the Roots of Eight Intersubgeneric Hybrids of Paeonia. Chem. Biodivers. 2021, 18, e2000848. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Zhang, Y.; Jin, Q.; Zhang, S.; Wu, G.; Chen, L.; Zhang, H.; Wang, X. Identification and Characterisation of Bioactive Compounds from the Seed Kernels and Hulls of Paeonia lactiflora Pall by UPLC-QTOF-MS. Food Res. Int. 2021, 139, 109916. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Ma, Y.; Wang, R.; Zhao, D. To Explore the Radix Paeoniae Rubra-Flos Carthami Herb Pair’s Potential Mechanism in the Treatment of Ischemic Stroke by Network Pharmacology and Molecular Docking Technology. Medicine 2021, 100, e27752. [Google Scholar] [CrossRef]
- Oidovsambuu, S.; Kim, C.Y.; Kang, K.; Dulamjav, B.; Jigjidsuren, T.; Nho, C.W. Protective Effect of Paeonia Anomala Extracts and Constituents against Tert-Butylhydroperoxide-Induced Oxidative Stress in HepG2 Cells. Planta Med. 2013, 29, 116–122. [Google Scholar] [CrossRef]
- Sut, S.; Zengin, G.; Dall’Acqua, S.; Gazdová, M.; Šmejkal, K.; Bulut, G.; Dogan, A.; Haznedaroglu, M.Z.; Aumeeruddy, M.Z.; Maggi, F. Paeonia Arietina and Paeonia kesrounansis Bioactive Constituents: NMR, LC-DAD-MS Fingerprinting and in Vitro Assays. J. Pharm. Biomed. Anal. 2019, 165, 1–11. [Google Scholar] [CrossRef]
- Michalea, R.; Stathopoulou, K.; Polychronopoulos, P.; Benaki, D.; Mikros, E.; Aligiannis, N. Efficient Identification of Acetylcholinesterase and Hyaluronidase Inhibitors from Paeonia parnassica Extracts through a Heterocovariance Approach. J. Ethnopharmacol. 2020, 257, 111547. [Google Scholar] [CrossRef]
- Li, C.; Du, H.; Wang, L.; Shu, Q.; Zheng, Y.; Xu, Y.; Zhang, J.; Zhang, J.; Yang, R.; Ge, Y. Flavonoid Composition and Antioxidant Activity of Tree Peony (Paeonia Section Moutan) Yellow Flowers. J. Agric. Food Chem. 2009, 57, 8496–8503. [Google Scholar] [CrossRef]
- Wang, L.-S.; Hashimoto, F.; Shiraishi, A.; Aoki, N.; Li, J.-J.; Sakata, Y. Chemical Taxonomy of the Xibei Tree Peony from China by Floral Pigmentation. J. Plant Res. 2004, 117, 47–55. [Google Scholar] [PubMed]
- Hua, M.; Ma, H.; Tan, R.; Yuan, X.; Chen, J.; Yang, W.; Wang, Y.; Kong, J.; Hu, Y.; Yang, Y. Determination of Anthocyanins and Flavonols in Paeonia delavayi by High-Performance Liquid Chromatography with Diode Array and Mass Spectrometric Detection. Anal. Lett. 2018, 51, 2331–2339. [Google Scholar] [CrossRef]
- Ulubelen, A.; Çetin, E.T.; Isildatici, S.; Ozturk, S. Phytochemical Investigation of Paeonia decora. Lloydia 1968, 31, 249. [Google Scholar]
- Wang, X.; Tang, Y.H.; Luan, Y.T.; Zhang, H.C.; Zhao, D.Q.; Tao, J. Flavonoids Composition and Transcriptome Analysis in Herbaceous Peony (Paeonia lactiflora) of Double-Colored Flowers. Russ. J. Plant Physiol. 2022, 69, 66. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Zhao, D.-Q.; Han, C.-X.; Tao, J. Biochemical and Molecular Responses of Herbaceous Peony to High Temperature Stress. Can. J. Plant Sci. 2016, 96, 474–484. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Ji, K.-X.; Zeng, Q.-Y.; Bhuiya, M.-W.; Su, S.; Shu, Q.-Y.; Ren, H.-X.; Liu, Z.-A.; Wang, L.-S. Methylation Mediated by an Anthocyanin, O-Methyltransferase, Is Involved in Purple Flower Coloration in Paeonia. J. Exp. Bot. 2015, 66, 6563–6577. [Google Scholar] [CrossRef]
- Wang, X.-L.; Jiao, W.; Liao, X.; Peng, S.-L.; Ding, L.-S. Monoterpene Glycosides from the Roots of Paeonia lactiflora. Chin. Chem. Lett. 2006, 17, 916–918. [Google Scholar]
- Demir, A.; Turumtay, H.; Emirik, M.; Sandalli, C.; Kanbolat, Ş.; Özgen, U.; Turumtay, E.A. Paeoniflorigenone Purified from Paeonia daurica Roots Potently Inhibits Viral and Bacterial DNA Polymerases: Investigation by Experimental Validation and Docking Simulation. Med. Chem. Res. 2019, 28, 2232–2245. [Google Scholar] [CrossRef]
- Stosic, D.; Gorunovic, M. Peoniflorine in the underground organs of the peony, Paeonia-tenuifolia L. (Paeoniaceae). Pharmazie 1989, 44, 510. [Google Scholar]
- Pan, Y.; Gao, Z.; Huang, X.-Y.; Chen, J.-J.; Geng, C.-A. Chemical and Biological Comparison of Different Parts of Paeonia Suffruticosa (Mudan) Based on LCMS-IT-TOF and Multi-Evaluation in Vitro. Ind. Crops Prod. 2020, 144, 112028. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Z.; He, S.; He, D.; Liu, Y.; Fu, Z. Research on the Relationship between Phenolic Acids and Rooting of Tree Peony (Paeonia suffruticosa) Plantlets in Vitro. Sci. Hortic. 2017, 224, 53–60. [Google Scholar] [CrossRef]
- Eming, S.A.; Dissemond, J. Wound Healing. In Braun-Falco´s Dermatology; Plewig, G., French, L., Ruzicka, T., Kaufmann, R., Hertl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Siddhuraju, P.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Chandola, V.; Chandra, S.; Nautiyal, A.R.; Concenço, G. Antioxidant Potential and Impact of Different Extraction Solvents on the Free, Esterified and Insoluble-Bound Phenolics, Flavonoid and Tannin Content of Trillium govanianum Wall Ex D. Don, a Rare Himalayan Herb. Vegetos 2022, 35, 953–960. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-Assisted Extraction of Polyphenols (Flavanone glycosides) from Orange (Citrus Sinensis L.) Peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus Sinensis Peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. Degradation during Application of Ultrasound in Food Processing: A Review. Food Control. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Pingret, D.; Durand, G.; Fabiano-Tixier, A.-S.; Rockenbauer, A.; Ginies, C.; Chemat, F. Degradation of Edible Oil during Food Processing by Ultrasound: Electron Paramagnetic Resonance, Physicochemical, and Sensory Appreciation. J. Agric. Food Chem. 2012, 60, 7761–7768. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total Phenolic, Flavonoid and Antioxidant Activity of 23 Edible Flowers Subjected to in Vitro Digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Weixing, L.; Shunbo, Y.; Hui, C.; Yanmin, H.; Jun, T.; Chunhua, Z. Nutritional Evaluation of Herbaceous Peony (Paeonia lactiflora Pall.) Petals. Emir. J. Food Agric. 2017, 29, 518–531. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, M.; Zhao, D.; Jun, T.A.O. Flavonoid Content and Expression Analysis of Flavonoid Biosynthetic Genes in Herbaceous Peony (Paeonia lactiflora Pall.) with Double Colors. J. Integr. Agric. 2016, 15, 2023–2031. [Google Scholar] [CrossRef]
- Oancea, S.; Perju, M.; Olosutean, H. Influence of Enzyme-Aided Extraction and Ultrasonication on the Phenolics Content and Antioxidant Activity of Paeonia officinalis L. Petals. J. Serb. Chem. Soc. 2020, 85, 845–856. [Google Scholar] [CrossRef]
- Batinic, P. In Vitro Evaluation of Antioxidative Activities of the Extracts of Petals of Paeonia Lactiflora and Calendula officinalis Incorporated in the New Forms of Biobased Carriers. Food Feed Res. 2022, 49, 23–35. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Wang, Z.; Wang, J. The Antioxidant Activity, α-Glucosidase and Acetylcholinesterase Inhibition Activity, and Chemical Composition of Paeonia delavayi Petal. Food Qual. Saf. 2022, 6, fyac020. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Liu, X.; Yang, W.; Li, M.; Li, J.; Li, F. Aqueous Extracts of Tree Peony Petals: Renin and Angiotensin I-Converting Enzyme Inhibitory Activities in Different Colours and Flowering Stages. RSC Adv. 2022, 12, 7735–7741. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, B.; Zhang, Z.; Yao, S. Focused Microwave-Assisted Solvent Extraction and HPLC Determination of Effective Constituents in Eucommia Ulmodies Oliv. (E. Ulmodies). Talanta 2004, 63, 659–665. [Google Scholar] [CrossRef]
- Guo, Z.; Jin, Q.; Fan, G.; Duan, Y.; Qin, C.; Wen, M. Microwave-Assisted Extraction of Effective Constituents from a Chinese Herbal Medicine Radix Puerariae. Anal. Chim. Acta. 2001, 436, 41–47. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzalez, R.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility of Phenolic Compounds and Antioxidant Capacity of Chia (Salvia Hispanica L.) Seeds. Plant Foods Hum. Nutr. 2018, 73, 47–53. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on Antioxidant Activity of Certain Plants in Thailand: Mechanism of Antioxidant Action of Guava Leaf Extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Aruoma, O.I. Methodological Considerations for Characterizing Potential Antioxidant Actions of Bioactive Components in Plant Foods. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 523, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative Evaluation of 11 Essential Oils of Different Origin as Functional Antioxidants, Antiradicals and Antimicrobials in Foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of Antioxidant Activity by Using Different in Vitro Methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC–DAD Analysis of Phenolic Compounds in Pomace Extracts from Five Grape Cultivars: Evaluation of Their Antioxidant, Antiradical and Antifungal Activities in Orange and Apple Juices. Food Chem. 2011, 126, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Li, S.; Li, S.-K.; Gan, R.-Y.; Song, F.-L.; Kuang, L.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of Infusions from 223 Medicinal Plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Dian-Nashiela, F.; Noriham, A.; Nooraain, H.; Azizah, A.H. Antioxidant Activity of Herbal Tea Prepared from Cosmos Caudatus Leaves at Different Maturity Stages. Int. Food Res. J. 2015, 22, 1189–1194. [Google Scholar]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Li, X.; Wang, L. Effect of Extraction Method on Structure and Antioxidant Activity of Hohenbuehelia serotina Polysaccharides. Int. J. Biol. Macromol. 2016, 83, 270–276. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Lobo, M.G.; González, M. The Effect of Extraction Temperature, Time and Number of Steps on the Antioxidant Capacity of Methanolic Banana Peel Extracts. Sep. Purif. Technol. 2010, 71, 347–355. [Google Scholar] [CrossRef]
- Luque-Garcıa, J.L.; de Castro, M.D.L. Ultrasound: A Powerful Tool for Leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of Extraction of Natural Products Using Ultrasonic Irradiations—A Review of Current Status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the Extraction Process of Polyphenols from Thymus serpyllum L. Herb Using Maceration, Heat-and Ultrasound-Assisted Techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Vilkhu, K.; Manasseh, R.; Mawson, R.; Ashokkumar, M. Ultrasonic Recovery and Modification of Food Ingredients. In Ultrasound Technologies for Food and Bioprocessing; Springer: Berlin/Heidelberg, Germany, 2011; pp. 345–368. [Google Scholar]
- Mohapatra, P.; Ray, A.; Jena, S.; Nayak, S.; Mohanty, S. Influence of Extraction Methods and Solvent System on the Chemical Composition and Antioxidant Activity of Centella asiatica L. Leaves. Biocatal. Agric. Biotechnol. 2021, 33, 101971. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Senkardes, I.; Dogan, A.; Seebaluck-Sandoram, R.; Rengasamy, K.R.R.; Sinan, K.I. Chemical Composition and Bio-Functional Perspectives of Erica arborea L. Extracts Obtained by Different Extraction Techniques: Innovative Insights. Ind. Crops Prod. 2019, 142, 111843. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total Phenolic Content and Antioxidant Capacity of Extracts Obtained from Six Important Fruit Residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics Composition and Antioxidant Activity of Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A Comprehensive Review of CUPRAC Methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of Antioxidant Activity of Medicinal Plants Containing Polyphenol Compounds. Comparison of Two Extraction Systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of Mango Byproducts: Study of the Effect of Extraction Solvent and Temperature on Their Antioxidant Properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.-L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Antimicrobial Gallic Acid from Caesalpinia mimosoides Lamk. Food Chem. 2007, 100, 1044–1048. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Guan, Y.; Wang, Y.; Yun, Y. In Vitro Activity of Gallic Acid against Candida Albicans Biofilms. Zhongguo Zhong Yao Za Zhi 2009, 34, 1137–1140. [Google Scholar]
- Isefuku, S.; Joyner, C.J.; Simpson, A.; Hamish, R.W. Gentamicin May Have an Adverse Effect on Osteogenesis. J. Orthop. Trauma 2003, 17, 212–216. [Google Scholar] [CrossRef]
- Chaves, B.J.; Tadi, P. Gentamicin; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Hsu, H.; Sheth, C.C.; Veses, V. Herbal Extracts with Antifungal Activity against Candida albicans: A Systematic Review. Mini Rev. Med. Chem. 2021, 21, 90–117. [Google Scholar] [CrossRef]
- Choi, S.H.; Gu, M.B. Phenolic Toxicity—Detection and Classification through the Use of a Recombinant Bioluminescent Escherichia coli. Environ. Toxicol. Chem. Int. J. 2001, 20, 248–255. [Google Scholar] [CrossRef]
- Picerno, P.; Mencherini, T.; Sansone, F.; del Gaudio, P.; Granata, I.; Porta, A.; Aquino, R.P. Screening of a Polar Extract of Paeonia rockii: Composition and Antioxidant and Antifungal Activities. J. Ethnopharmacol. 2011, 138, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New Insights into Oxidative Stress and Inflammation during Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Palíková, I.; Heinrich, J.; Bednář, P.; Marhol, P.; Křen, V.; Cvak, L.; Valentová, K.; Ružička, F.; Holá, V.; Kolář, M. Constituents and Antimicrobial Properties of Blue Honeysuckle: A Novel Source for Phenolic Antioxidants. J. Agric. Food Chem. 2008, 56, 11883–11889. [Google Scholar] [CrossRef] [PubMed]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Zagórska-Dziok, M. Antioxidant Activity and Cytotoxicity of Jerusalem Artichoke Tubers and Leaves Extract on HaCaT and BJ Fibroblast Cells. Lipids Health Dis. 2018, 17, 280. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin Wound Healing and Phytomedicine: A Review. Ski. Pharm. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef]
- Negahdari, S.; Galehdari, H.; Kesmati, M.; Rezaie, A.; Shariati, G. Wound Healing Activity of Extracts and Formulations of Aloe Vera, Henna, Adiantum Capillus-Veneris, and Myrrh on Mouse Dermal Fibroblast Cells. Int. J. Prev. Med. 2017, 8, 18. [Google Scholar]
- Chithra, P.; Sajithlal, G.B.; Chandrakasan, G. Influence of Aloe Vera on Collagen Turnover in Healing of Dermal Wounds in Rats. Indian J. Exp. Biol. 1998, 36, 896–901. [Google Scholar]
- Santoro, M.M.; Gaudino, G. Cellular and Molecular Facets of Keratinocyte Reepithelization during Wound Healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Şenkardes, I.; Dogan, A.; Sinan, K.I.; Uysal, S.; Aumeeruddy-Elalfi, Z. Modern and Traditional Extraction Techniques Affect Chemical Composition and Bioactivity of Tanacetum parthenium (L.) Sch. Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; Montoro, P.; Piacente, S.; Pizza, C.; Dönmez, A.; Çalış, İ. Identification by HPLC-PAD-MS and Quantification by HPLC-PAD of Phenylethanoid Glycosides of Five Phlomis Species. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aghakhani Kaaji, F.; Kharazian, N. Flavonoid Diversity and Morphological Variations among Seven Phlomis Species in Zagros, Iran. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 415–431. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Park, J.-W.; Lee, Y.-J.; Yoon, S. Total Flavonoids and Phenolics in Fermented Soy Products and Their Effects on Antioxidant Activities Determined by Different Assays. J. Korean Soc. Food Cult. 2007, 22, 353–358. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Milošević, M.D.; Marinković, A.D.; Petrović, P.; Klaus, A.; Nikolić, M.G.; Prlainović, N.Ž.; Cvijetić, I.N. Synthesis, Characterization and SAR Studies of Bis (Imino) Pyridines as Antioxidants, Acetylcholinesterase Inhibitors and Antimicrobial Agents. Bioorg. Chem. 2020, 102, 104073. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojkovic, D.; Glamoclija, J.; Grdadolnik, S.G.; Sanglard, D.; Sokovic, M. Revealing the Astragalin Mode of Anticandidal Action. EXCLI J. 2020, 19, 1436. [Google Scholar]
- Stojković, D.; Dias, M.I.; Drakulić, D.; Barros, L.; Stevanović, M.; CFR Ferreira, I.; Soković, M.D. Methanolic Extract of the Herb Ononis spinosa L. Is an Antifungal Agent with No Cytotoxicity to Primary Human Cells. Pharmaceuticals 2020, 13, 78. [Google Scholar] [CrossRef]
- Stojković, D.S.; Kovačević-Grujičić, N.; Reis, F.S.; Davidović, S.; Barros, L.; Popović, J.; Petrović, I.; Pavić, A.; Glamočlija, J.; Ćirić, A. Chemical Composition of the Mushroom Meripilus giganteus Karst. and Bioactive Properties of Its Methanolic Extract. LWT-Food Sci. Technol. 2017, 79, 454–462. [Google Scholar] [CrossRef]
- Ahmed, G.F.; Elkhatib, W.F.; Noreddin, A.M. Inhibition of Pseudomonas Aeruginosa PAO1 Adhesion to and Invasion of A549 Lung Epithelial Cells by Natural Extracts. J. Infect. Public Health 2014, 7, 436–444. [Google Scholar] [CrossRef] [PubMed]

| Origin of Plant Material | Extraction Medium | Extraction Method | TPC [mg GAE/g] | TFC [mg CE/g] |
|---|---|---|---|---|
| Gulenovci | H2O | Maceration | 31.34 ± 0.23 e | 24.62 ± 0.11 b |
| UAE | 22.82 ± 0.21 c | 15.75 ± 0.20 f | ||
| MAE | 28.22 ± 0.27 a | 23.09 ± 0.21 a | ||
| MeOH | Maceration | 31.85 ± 0.31 e | 24.50 ± 0.19 c | |
| UAE | 32.14 ± 0.22 d | 20.23 ± 0.25 e | ||
| MAE | 35.24 ± 0.23 b | 21.44 ± 0.11 d | ||
| Pančevo | H2O | Maceration | 24.04 ± 0.32 c | 16.54 ± 0.23 f |
| UAE | 18.91 ± 0.47 e | 12.31 ± 0.19 e | ||
| MAE | 22.51 ± 0.25 d | 16.78 ± 0.25 d | ||
| MeOH | Maceration | 18.43 ± 0.24 e | 12.69 ± 0.18 f | |
| UAE | 22.08 ± 0.26 d | 15.78 ± 0.27 f | ||
| MAE | 28.15 ± 0.33 b | 19.73 ± 0.23 c | ||
| Deliblato sand | H2O | Maceration | 30.15 ± 0.33 d | 24.61 ± 0.32 d |
| UAE | 32.94 ± 0.26 e | 25.44 ± 0.37 c | ||
| MAE | 33.26 ± 0.15 e | 28.48 ± 0.18 d | ||
| MeOH | Maceration | 23.54 ± 0.20 d | 16.01 ± 0.29 e,f | |
| UAE | 32.83 ± 0.19 e | 23.72 ± 0.44 e | ||
| MAE | 26.04 ± 0.26 c | 20.84 ± 0.41 f |
| Origin of Plant Material | Extraction Medium, Extraction Method | Antioxidant Assays | |||
|---|---|---|---|---|---|
| DPPH IC50 [mg/mL] | ABTS IC50 [mg/mL] | CUPRAC [mol TE/g] | FRAP [μmol Fe2+/g] | ||
| Gulenovci | H2O, maceration | 0.088 ± 0.001 c | 0.090 ± 0.001 b | 0.386 ± 0.002 a | 834.24 ± 6.4 e |
| H2O, UAE | 0.051 ± 0.001 a | 0.074 ± 0.002 a | 0.378 ± 0.001 b | 843.39 ± 5.6 e | |
| H2O, MAE | 0.063 ± 0.001 b | 0.092 ± 0.001 b | 0.327 ± 0.001 f | 796.56 ± 10.5 c | |
| MeOH, maceration | 0.123 ± 0.001 d | 0.099 ± 0.000 c | 0.345 ± 0.000 e | 830.22 ± 11.6 e | |
| MeOH, UAE | 0.124 ± 0.001 d | 0.099 ± 0.001 c | 0.349 ± 0.001 d | 776.43 ± 9.9 d | |
| MeOH, MAE | 0.124 ± 0.002 d | 0.098 ± 0.001 c | 0.358 ± 0.002 b | 840.46 ± 7.0 e | |
| Pančevo | H2O, maceration | 0.074 ± 0.002 b | 0.089 ± 0.001 b | 0.341 ± 0.001 f | 715.33 ± 5.8 e |
| H2O, UAE | 0.058 ± 0.001 a | 0.070 ± 0.002 a | 0.371 ± 0.001 b | 832.4 ± 13.9 b | |
| H2O, MAE | 0.061 ± 0.001 c | 0.088 ± 0.002 b | 0.385 ± 0.002 a | 751.19 ± 5.4 d | |
| MeOH, maceration | 0.126 ± 0.001 d | 0.097 ± 0.001 c | 0.346 ± 0.001 e | 724.84 ± 10.3 e | |
| MeOH, UAE | 0.126 ± 0.001 d | 0.099 ± 0.000 c | 0.357 ± 0.001 d | 777.16 ± 9.8 c | |
| MeOH, MAE | 0.125 ± 0.002 d | 0.099 ± 0.000 c | 0.367 ± 0.001 c | 748.99 ± 8.2 d | |
| Deliblato sands | H2O, maceration | 0.103 ± 0.003 c | 0.098 ± 0.001 c | 0.319 ± 0.001 e | 759.24 ± 2.3 e |
| H2O, UAE | 0.094 ± 0.003 b | 0.092 ± 0.001 b | 0.344 ± 0.001 c | 815.58 ± 1.9 b | |
| H2O, MAE | 0.067 ± 0.001 a | 0.094 ± 0.001 a,b | 0.391 ± 0.000 b | 791.43 ± 7.8 c | |
| MeOH, maceration | 0.125 ± 0.001 d | 0.098 ± 0.001 c | 0.353 ± 0.001 f | 769.11 ± 10.8 e | |
| MeOH, UAE | 0.125 ± 0.001 d | 0.097 ± 0.001 c | 0.337 ± 0.001 d | 559.5 ± 11.3 d | |
| MeOH, MAE | 0.125 ± 0.002 d | 0.097 ± 0.001 c | 0.358 ± 0.001 f | 833.88 ± 8.6 a | |
| Origin of Plant Material | Extraction Medium, Extraction Method | Bacteria | |||||
|---|---|---|---|---|---|---|---|
| S. lugdunensis | S. aureus | P. vulgaris | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| Gulenovci | H2O, maceration | 0.125 | 0.25 | 0.5 | 1 | 0.5 | 1 |
| H2O, UAE | 0.5 | 1 | 2 | 4 | 2 | 4 | |
| H2O, MAE | 0.5 | 1 | 2 | 4 | 0.125 | 0.25 | |
| MeOH, maceration | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | |
| MeOH, UAE | 0.25 | 0.5 | 0.5 | 1 | 0.25 | 0.5 | |
| MeOH, MAE | 0.5 | 1 | 1 | 2 | 0.5 | 1 | |
| Pančevo | H2O, maceration | 0.5 | 1 | 4 | 8 | 2 | 4 |
| H2O, UAE | 1 | 2 | 0.25 | 0.5 | 2 | 4 | |
| H2O, MAE | 0.5 | 1 | 2 | 4 | 1 | 2 | |
| MeOH, maceration | 0.5 | 1 | 1 | 2 | 1 | 2 | |
| MeOH, UAE | 0.5 | 1 | 0.5 | 1 | 0.5 | 1 | |
| MeOH, MAE | 0.5 | 1 | 1 | 2 | 0.5 | 1 | |
| Deliblato sands | H2O, maceration | 0.5 | 1 | 2 | 4 | 2 | 4 |
| H2O, UAE | 0.5 | 1 | 1 | 2 | 1 | 2 | |
| H2O, MAE | 0.5 | 1 | 2 | 4 | 2 | 4 | |
| MeOH, maceration | 1 | 2 | 2 | 4 | 1 | 2 | |
| MeOH, UAE | 1 | 2 | 0.5 | 1 | 0.5 | 1 | |
| MeOH, MAE | 0.5 | 1 | 0.5 | 1 | 0.5 | 1 | |
| Control | Gentamicin | 0.008 | 0.016 | 1.33 | 2.66 | 0.066 | 0.133 |
| Origin of Plant Material | Extraction Medium, Extraction Method | Candida Species | |||||
|---|---|---|---|---|---|---|---|
| C. kefyr | C. krusei | C. albicans | |||||
| MIC | MFC | MIC | MFC | MIC | MFC | ||
| Gulenovci | H2O, maceration | 1 | 2 | 0.5 | 1 | 1 | 2 |
| H2O, UAE | 0.5 | 1 | 2 | 4 | 1 | 2 | |
| H2O, MAE | 0.5 | 1 | 1 | 2 | 1 | 2 | |
| MeOH, maceration | 1 | 2 | 0.5 | 1 | 1 | 2 | |
| MeOH, UAE | 0.5 | 1 | 1 | 2 | 1 | 2 | |
| MeOH, MAE | 0.5 | 1 | 1 | 2 | 0.5 | 1 | |
| Pančevo | H2O, maceration | 0.5 | 1 | 2 | 4 | 1 | 2 |
| H2O, UAE | 1 | 2 | 2 | 4 | 1 | 2 | |
| H2O, MAE | 0.5 | 1 | 1 | 2 | 1 | 2 | |
| MeOH, maceration | 0.5 | 1 | 1 | 2 | 0.5 | 1 | |
| MeOH, UAE | 0.5 | 1 | 1 | 2 | 1 | 2 | |
| MeOH, MAE | 1 | 2 | 1 | 2 | 0.5 | 1 | |
| Deliblato sands | H2O, maceration | 0.5 | 1 | 2 | 4 | 1 | 2 |
| H2O, UAE | 1 | 2 | 1 | 2 | 1 | 2 | |
| H2O, MAE | 0.5 | 1 | 2 | 4 | 1 | 2 | |
| MeOH, maceration | 1 | 2 | 0.5 | 1 | 1 | 2 | |
| MeOH, UAE | 1 | 2 | 1 | 2 | 1 | 2 | |
| MeOH, MAE | 0.5 | 1 | 1 | 2 | 0.5 | 1 | |
| Control | Ketoconazole | 0.05 | 0.1 | 0.05 | 0.1 | 0.05 | 0.1 |
| Origin of Plant Material | Extraction Medium | Extraction Method | Wound Healing (%) |
|---|---|---|---|
| Gulenovci | H2O | MAE | 26.14 ± 0.04 |
| MeOH | Maceratiom | 5.38 ± 1.2 | |
| UAE | 8.04 ± 0.11 | ||
| MAE | 0.08 ± 0.7 | ||
| Pančevo | MeOH | UAE | NA |
| MAE | 5.56 ± 0.09 | ||
| Deliblato sands | MeOH | Maceration | 19.19 ± 1.3 |
| MAE | NA | ||
| Control | 0.08 ± 0.12 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čutović, N.; Marković, T.; Kostić, M.; Gašić, U.; Prijić, Ž.; Ren, X.; Lukić, M.; Bugarski, B. Chemical Profile and Skin-Beneficial Activities of the Petal Extracts of Paeonia tenuifolia L. from Serbia. Pharmaceuticals 2022, 15, 1537. https://doi.org/10.3390/ph15121537
Čutović N, Marković T, Kostić M, Gašić U, Prijić Ž, Ren X, Lukić M, Bugarski B. Chemical Profile and Skin-Beneficial Activities of the Petal Extracts of Paeonia tenuifolia L. from Serbia. Pharmaceuticals. 2022; 15(12):1537. https://doi.org/10.3390/ph15121537
Chicago/Turabian StyleČutović, Natalija, Tatjana Marković, Marina Kostić, Uroš Gašić, Željana Prijić, Xiuxia Ren, Milan Lukić, and Branko Bugarski. 2022. "Chemical Profile and Skin-Beneficial Activities of the Petal Extracts of Paeonia tenuifolia L. from Serbia" Pharmaceuticals 15, no. 12: 1537. https://doi.org/10.3390/ph15121537
APA StyleČutović, N., Marković, T., Kostić, M., Gašić, U., Prijić, Ž., Ren, X., Lukić, M., & Bugarski, B. (2022). Chemical Profile and Skin-Beneficial Activities of the Petal Extracts of Paeonia tenuifolia L. from Serbia. Pharmaceuticals, 15(12), 1537. https://doi.org/10.3390/ph15121537