Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance
Abstract
1. Introduction
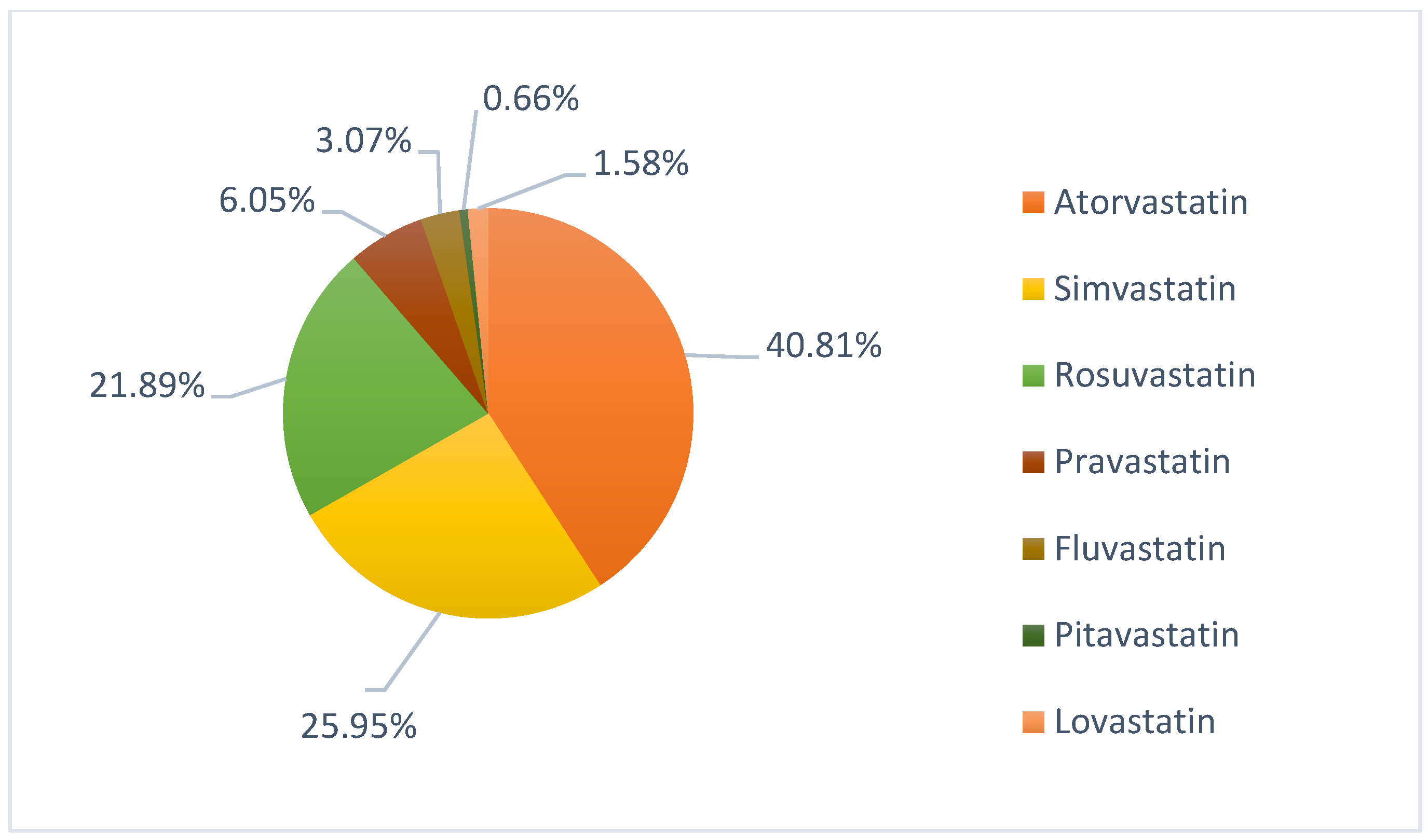
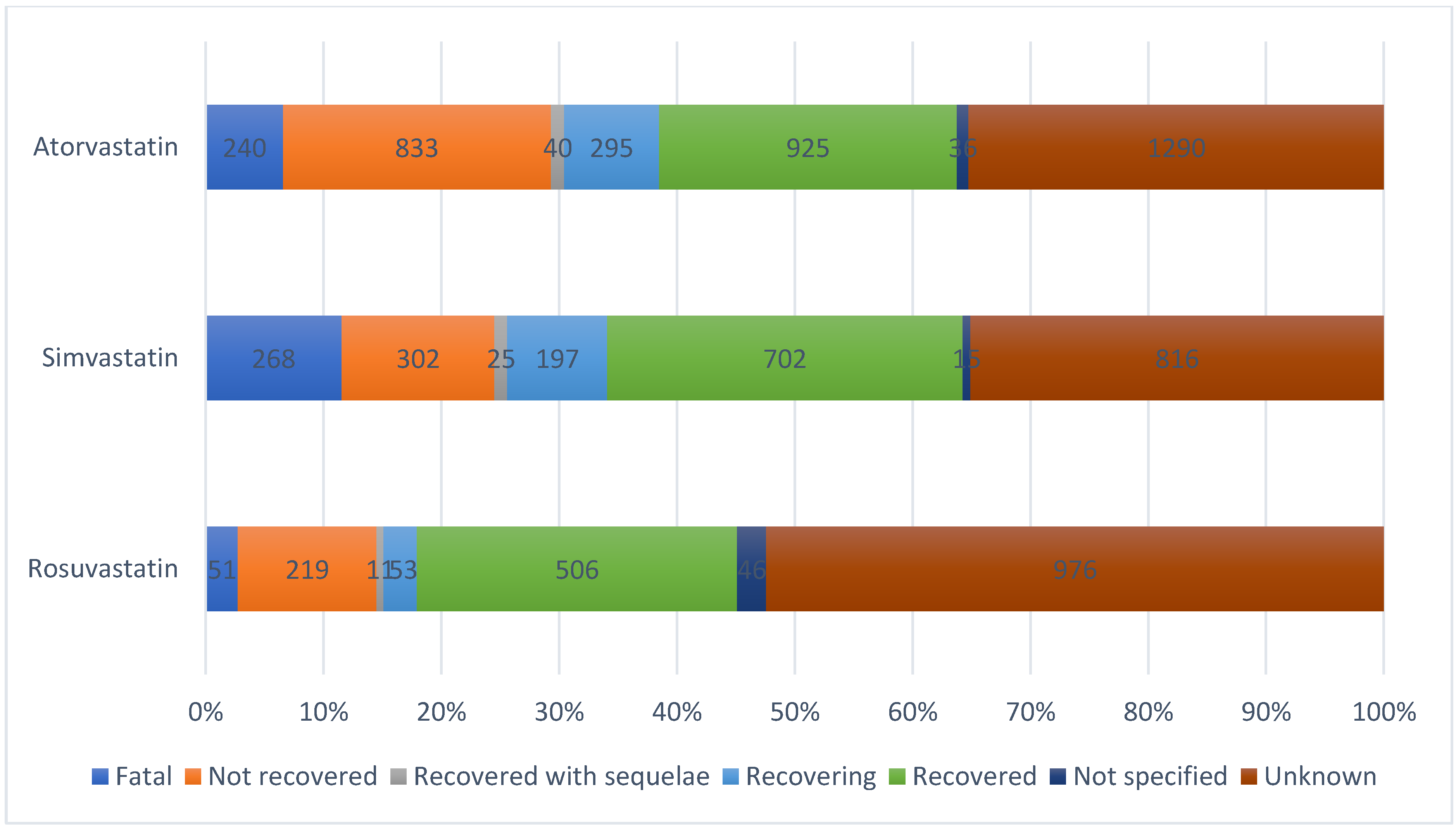
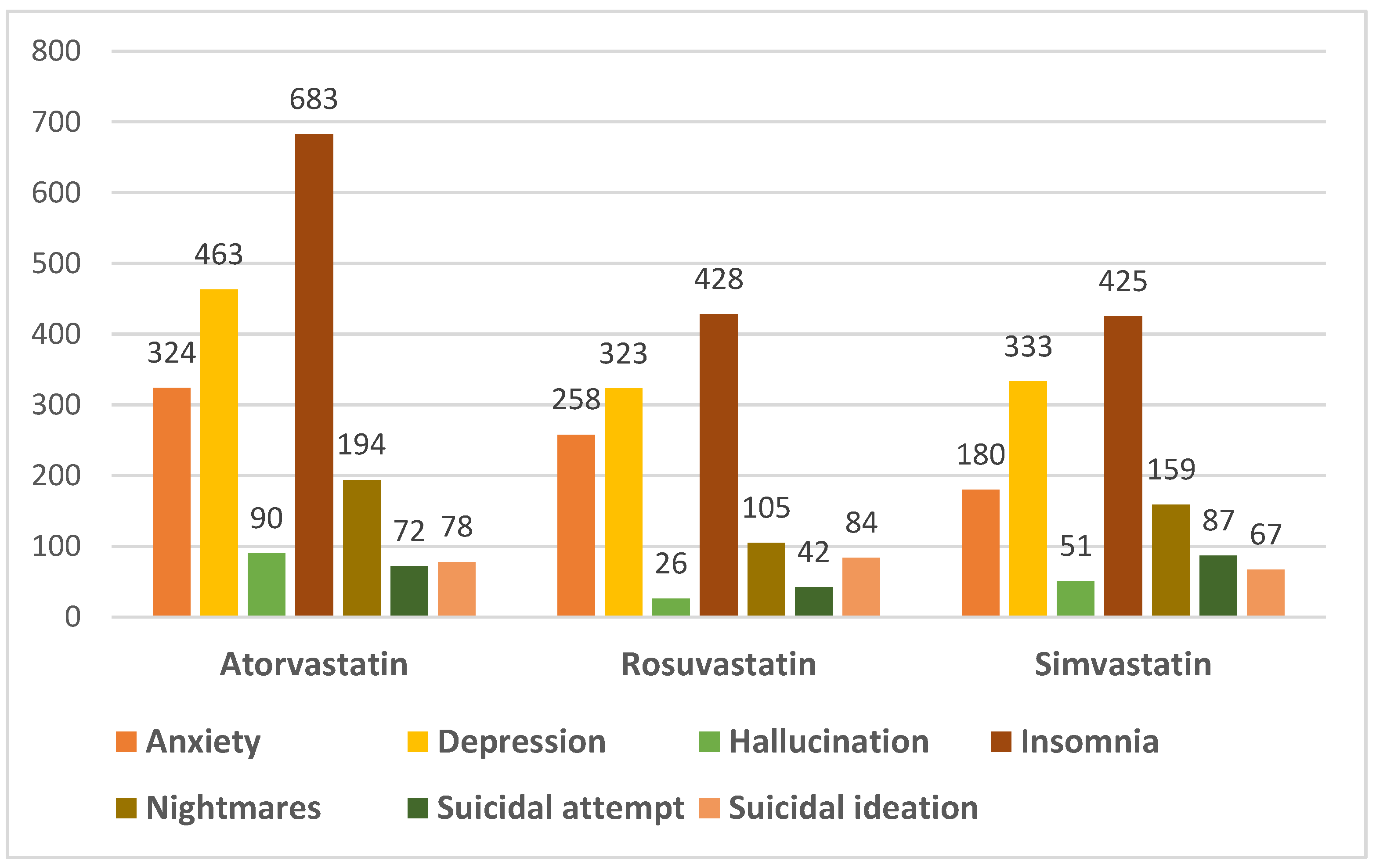
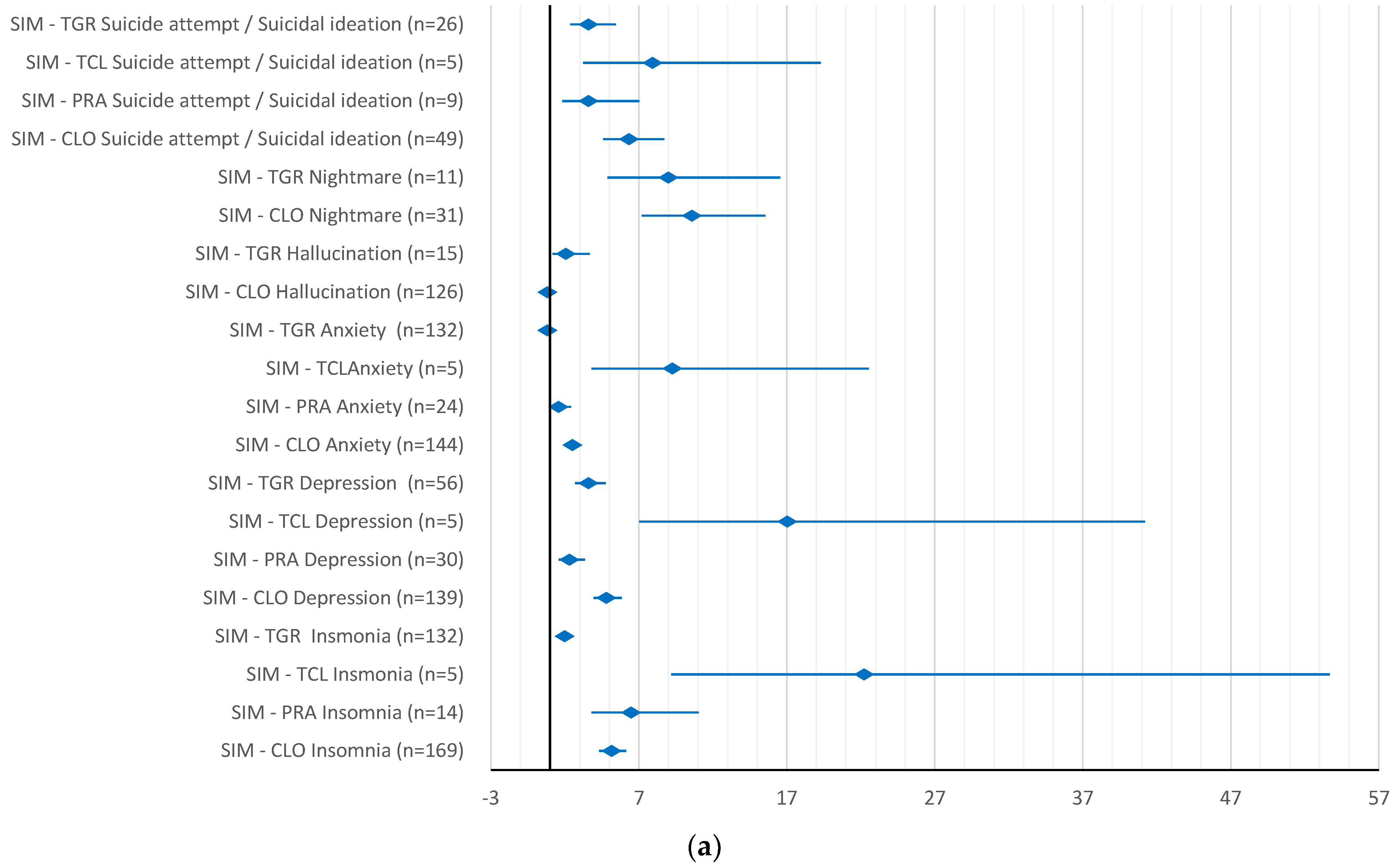
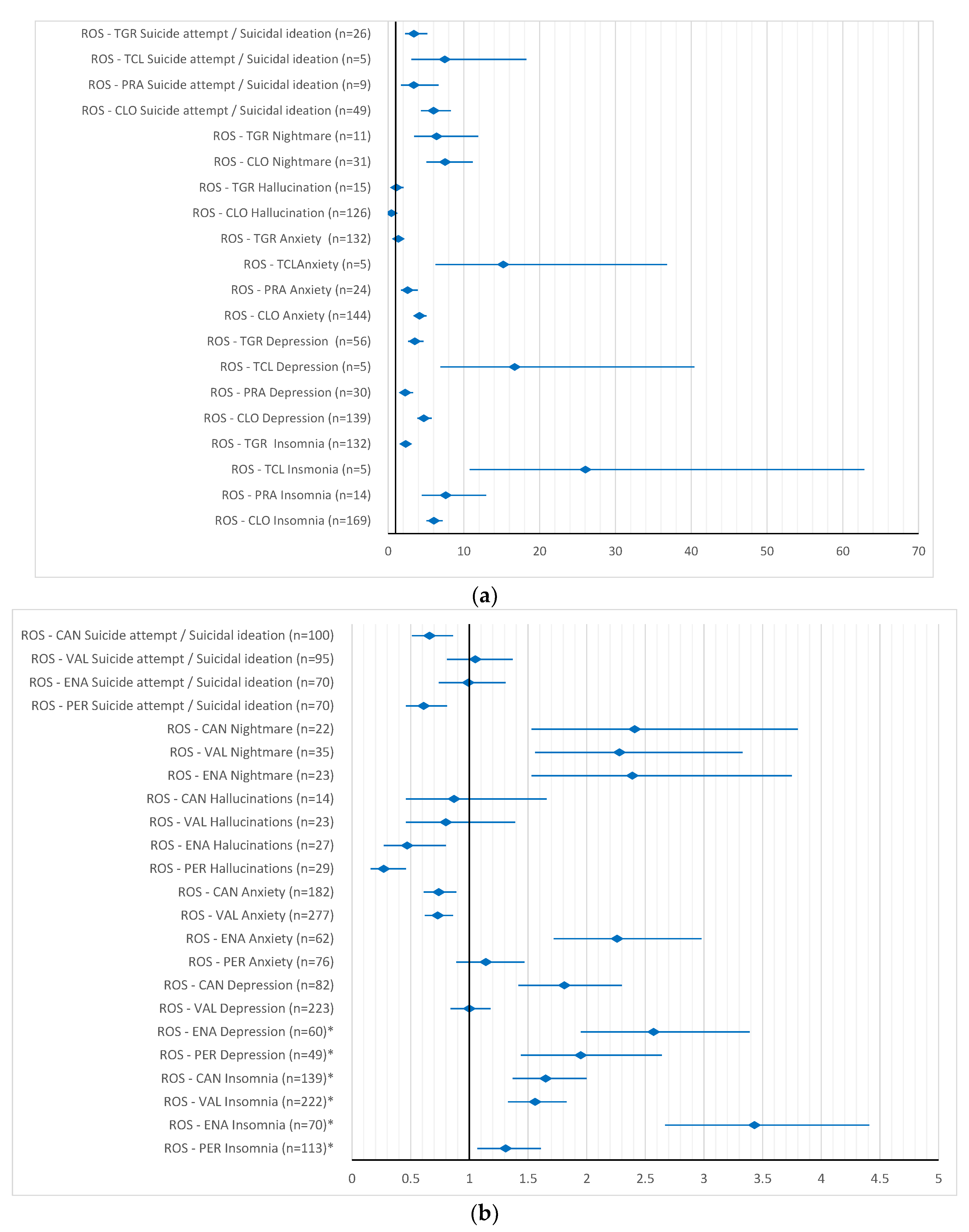
2. Results
3. Discussion
Limitations of This Study
4. Materials and Methods
4.1. Study Design
4.2. Material
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Lv, X.; Xie, N.; Fang, Z.; Ren, W.; Gong, Y.; Jin, Y.; Zhang, J. Time trends analysis of statin prescription prevalence, therapy initiation, dose intensity, and utilization from the hospital information system of Jinshan Hospital, Shanghai (2012–2018). BMC Cardiovasc. Disord. 2020, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Blais, J.E.; Wei, Y.; Yap, K.K.; Alwafi, H.; Ma, T.-T.; Brauer, R.; Lau, W.C.; Man, K.K.; Siu, C.W.; Tan, K.C.; et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis 2021, 328, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Paulose-Ram, R.; Burt, V.L.; Kit, B.K. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief 2014, 177, 1–8. [Google Scholar]
- Schultz, B.G.; Patten, D.K.; Berlau, D.J. The role of statins in both cognitive impairment and protection against dementia: A tale of two mechanisms. Transl. Neurodegener. 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Whitmer, R. Statin use and risk of Alzheimer disease: A new view on an old relationship. Neurology 2018, 90, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.A.; Golomb, B.A. Statin-associated adverse cognitive effects: Survey results from 171 patients. Pharmacotherapy 2009, 3329, 800–811. [Google Scholar] [CrossRef]
- Cham, S.; Koslik, H.J.; Golomb, B.A. Mood, Personality, and Behavior Changes during Treatment with Statins: A Case Series. Drug Saf.-Case Rep. 2016, 3, 1. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clin. 2018, 150, 398–402. [Google Scholar] [CrossRef]
- Jukema, J.W.; Cannon, C.P.; De Craen, A.J.M.; Westendorp, R.G.J.; Trompet, S. The controversies of statin therapy: Weighing the evidence. J. Am. Coll. Cardiol. 2012, 60, 875–881. [Google Scholar] [CrossRef]
- Agostini, J.V.; Tinetti, M.E.; Han, L.; McAvay, G.; Foody, J.M.; Concato, J. Effects of Statin Use on Muscle Strength, Cognition, and Depressive Symptoms in Older Adults. J. Am. Geriatr. Soc. 2007, 55, 420–425. [Google Scholar] [CrossRef]
- Alberton, M.; Wu, P.; Druyts, E.; Briel, M.; Mills, E.J. Adverse events associated with individual statin treatments for cardiovascular disease: An indirect comparison meta-analysis. QJM Int. J. Med. 2012, 105, 145–157. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Safety Communication: Important Safety Label Changes to Cholesterol-Lowering Statin Drugs. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-important-safety-label-changes-cholesterol-lowering-statin-drugs (accessed on 16 April 2022).
- Crestor. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/referral/crestor-5-mg-article-29-referral-annex-i-ii-iii_en.pdf (accessed on 11 March 2022).
- Sortis. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/referral/sortis-emea/h/29-pad/1255-article-29-paediatrics-referral-annex-i-ii-iii_en.pdf (accessed on 11 March 2022).
- Zocord. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/referral/zocord-article-30-referral-annex-i-ii-iii_en.pdf (accessed on 11 March 2022).
- Lescol. Summary of Product characteristics. Available online: https://www.ema.europa.eu/en/documents/referral/lescol-article-30-referrals-annex-i-iii_en.pdf (accessed on 11 March 2022).
- Parker, B.A.; Polk, D.M.; Rabdiya, V.; Meda, S.A.; Anderson, K.; Hawkins, K.A.; Pearlson, G.D.; Thompson, P.D. Changes in Memory Function and Neuronal Activation Associated with Atorvastatin Therapy. Pharmacotherapy 2010, 30, 625. [Google Scholar] [CrossRef]
- Zamrini, E.; McGwin, G.; Roseman, J.M. Association between Statin Use and Alzheimer’s Disease. Neuroepidemiology 2004, 23, 94–98. [Google Scholar] [CrossRef]
- McFarland, A.; Anoopkumar-Dukie, S.; Arora, D.; Grant, G.; McDermott, C.; Perkins, A.; Davey, A. Molecular Mechanisms Underlying the Effects of Statins in the Central Nervous System. Int. J. Mol. Sci. 2014, 15, 20607–20637. [Google Scholar] [CrossRef] [PubMed]
- Thelen, K.M.; Rentsch, K.M.; Gutteck, U.; Heverin, M.; Olin, M.; Andersson, U.; von Eckardstein, A.; Björkhem, I.; Lütjohann, D. Brain Cholesterol Synthesis in Mice Is Affected by High Dose of Simvastatin but Not of Pravastatin. J. Pharmacol. Exp. Ther. 2006, 316, 1146–1152. [Google Scholar] [CrossRef]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- Cibičková, L. Statins and their influence on brain cholesterol. J. Clin. Lipidol. 2011, 5, 373–379. [Google Scholar] [CrossRef]
- Quinn, K.L.; Macdonald, E.M.; Mamdani, M.M.; Diong, C.; Juurlink, D.N. Lipophilic Statins and the Risk of Intracranial Hemorrhage Following Ischemic Stroke: A Population-Based Study. Drug Saf. 2017, 40, 887–893. [Google Scholar] [CrossRef]
- McGuinness, B.; Craig, D.; Bullock, R.; Passmore, P. Statins for the prevention of dementia. Cochrane Database Syst. Rev. 2016, 2016, CD003160. [Google Scholar] [CrossRef]
- Wilmot, K.A.; Khan, A.; Krishnan, S.; Eapen, D.J.; Sperling, L. Statins in the elderly: A patient-focused approach. Clin. Cardiol. 2015, 38, 56–61. [Google Scholar] [CrossRef]
- Jeong, S.M.; Shin, D.W.; Yoo, T.G.; Cho, M.H.; Jang, W.; Lee, J.; Kim, S.Y. Association between statin use and Alzheimer’s disease with dose response relationship. Sci. Rep. 2021, 11, 15280. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Kang, H.-J.; Jhon, M.; Kim, J.-W.; Lee, J.-Y.; Walker, A.J.; Agustini, B.; Kim, J.-M.; Berk, M. Statins and Inflammation: New Therapeutic Opportunities in Psychiatry. Front. Psychiatry 2019, 10, 103. [Google Scholar] [CrossRef]
- Garcia-Doval, I.; Segovia, E.; Hunter, H.; Frew, J.; Naldi, L. The value of case reports in pharmacovigilance. Br. J. Dermatol. 2020, 183, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Meaney, S. Brain Cholesterol: Long Secret Life Behind a Barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, H. Low serum cholesterol and suicide. Lancet 1992, 339, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Schinnar, R.; Karlawish, J.; Hennessy, S.; Teal, V.; Bilker, W.B. Statin Therapy and Risk of Acute Memory Impairment. JAMA Intern. Med. 2015, 175, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, J.; Meerlo, P.; Koolhaas, J.M.; Lancel, M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. 2012, 13, 327–334. [Google Scholar] [CrossRef]
- Li, R.; Wang, T.J.; Lyu, P.Y.; Liu, Y.; Chen, W.H.; Fan, M.Y.; Xu, J. Effects of Plasma Lipids and Statins on Cognitive Function. Chin. Med. J. 2018, 131, 471–476. [Google Scholar] [CrossRef]
- Davison, K.M.; Kaplan, B.J. Lipophilic Statin Use and Suicidal Ideation in a Sample of Adults With Mood Disorders. Crisis 2014, 35, 278–282. [Google Scholar] [CrossRef]
- di Mauro, G.; Zinzi, A.; Scavone, C.; Mascolo, A.; Gaio, M.; Sportiello, L.; Ferrajolo, C.; Rafaniello, C.; Rossi, F.; Capuano, A. PCSK9 Inhibitors and Neurocognitive Adverse Drug Reactions: Analysis of Individual Case Safety Reports from the Eudravigilance Database. Drug Saf. 2021, 44, 337–349. [Google Scholar] [CrossRef]
- Tatley, M.; Savage, R. Psychiatric Adverse Reactions with Statins, Fibrates and Ezetimibe. Drug Saf. 2007, 30, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sahebzamani, M.F.; Munro, C.L.; Marroquin, O.C.; Diamond, D.M.; Keller, E.; Kip, K.E. Examination of the FDA Warning for Statins and Cognitive Dysfunction. J. Pharmacovigil. 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Dave, C.V.; Winterstein, A.G.; Park, H.; Cook, R.L.; Hartzema, A.G. Comparative risk of lipophilic and hydrophilic statins on incident depression: A retrospective cohort study. J. Affect. Disord. 2018, 238, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, K.; Burzyńska, M.; Rydlewska-Liszkowska, I.; Sienkiewicz, J.; Gaszyńska, E. The Importance of Direct Patient Reporting of Adverse Drug Reactions in the Safety Monitoring Process. Int. J. Environ. Res. Public Health 2021, 19, 413. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Fujimoto, M.; Yamazaki, K.; Takamoto, M.; Hosomi, K. Association of Statin Use with Sleep Disturbances: Data Mining of a Spontaneous Reporting Database and a Prescription Database. Drug Saf. 2014, 37, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Tuccori, M.; Lapi, F.; Testi, A.; Coli, D.; Moretti, U.; Vannacci, A.; Motola, D.; Salvo, F.; Rivolta, A.L.; Blandizzi, C.; et al. Statin-Associated Psychiatric Adverse Events. Drug Saf. 2008, 31, 1115–1123. [Google Scholar] [CrossRef]
- Molero, Y.; Cipriani, A.; Larsson, H.; Lichtenstein, P.; D’Onofrio, B.M.; Fazel, S. Associations between statin use and suicidality, depression, anxiety, and seizures: A Swedish total-population cohort study. Lancet Psychiatry 2020, 7, 982–990. [Google Scholar] [CrossRef]
- Li, H.-H.; Lin, C.-L.; Huang, C.-N. Neuroprotective effects of statins against amyloid β-induced neurotoxicity. Neural Regen. Res. 2018, 13, 198–206. [Google Scholar] [CrossRef]
- Kosowski, M.; Smolarczyk-Kosowska, J.; Hachuła, M.; Maligłówka, M.; Basiak, M.; Machnik, G.; Pudlo, R.; Okopień, B. The Effects of Statins on Neurotransmission and Their Neuroprotective Role in Neurological and Psychiatric Disorders. Molecules 2021, 26, 2838. [Google Scholar] [CrossRef]
- Parsaik, A.K.; Singh, B.M.; Hassan, M.; Singh, K.; Mascarenhas, S.S.; Williams, M.D.; Lapid, M.I.; Richardson, J.W.; West, C.P.; Rummans, T.A. Statins use and risk of depression: A systematic review and meta-analysis. J. Affect. Disord. 2014, 160, 62–67. [Google Scholar] [CrossRef]
- O’Neil, A.; Sanna, L.; Redlich, C.; Sanderson, K.; Jacka, F.; Williams, L.J.; Pasco, J.A.; Berk, M. The impact of statins on psychological wellbeing: A systematic review and meta-analysis. BMC Med. 2012, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Yatham, M.S.; Yatham, K.S.; Ravindran, A.V.; Sullivan, F. Do statins have an effect on depressive symptoms? A systematic review and meta-analysis. J. Affect. Disord. 2019, 257, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rached, F.; Santos, R.D. The Role of Statins in Current Guidelines. Curr. Atheroscler. Rep. 2020, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, N.; Lee, D.H.; Kim, H.-S. Analysis of National Pharmacovigilance Data Associated with Statin Use in Korea. Basic Clin. Pharmacol. Toxicol. 2017, 121, 409–413. [Google Scholar] [CrossRef]
- Cífková, R.; Krajčoviechová, A. Dyslipidemia and Cardiovascular Disease in Women. Curr. Cardiol. Rep. 2015, 17, 52. [Google Scholar] [CrossRef]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just about Sex Hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef]
- Korhonen, M.J.; Pentti, J.; Hartikainen, J.; Kivimäki, M.; Vahtera, J. Somatic symptoms of anxiety and nonadherence to statin therapy. Int. J. Cardiol. 2016, 214, 493–499. [Google Scholar] [CrossRef]
- Golomb, B.A.; Dimsdale, J.E.; Koslik, H.J.; Evans, M.A.; Lu, X.; Rossi, S.; Mills, P.J.; White, H.L.; Criqui, M.H. Statin Effects on Aggression: Results from the UCSD Statin Study, a Randomized Control Trial. PLoS ONE 2015, 10, e0124451. [Google Scholar] [CrossRef][Green Version]
- Schooling, C.M.; Au Yeung, S.L.; Freeman, G.; Cowling, B.J. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013, 11, 57. [Google Scholar] [CrossRef]
- Olson, M.B.; Kelsey, S.F.; Matthews, K.A.; Bairey Merz, C.N.; Eteiba, W.; McGorray, S.P.; Cornell, C.E.; Vido, D.A.; Muldoon, M.F. Lipid-Lowering Medication Use and Aggression Scores in Women: A Report from the NHLBI-Sponsored WISE Study. J. Women’s Health 2008, 17, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Botts, S.; Ryan, M. Depression. In Drug-Induced Diseases: Prevention, Detection, and Management; Tisdale, J.E., Miller, D.A., Eds.; American Society of Health-System Pharmacists: Bethesda, Maryland, 2018; pp. 375–398. ISBN 9781585285303. [Google Scholar]
- EudraVigilance-European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/en/index.html (accessed on 3 July 2021).
- European Medicines Agency Guideline on Good Pharmacovigilance Practices (GVP). Available online: http://www.jfda.jo/Download/JPC/TheGoodPharmacovigilancePracticev2.pdf (accessed on 21 September 2021).
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.-M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Data Source. EudraVigilance-European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/en/data_source.html (accessed on 10 July 2021).
- Screening for Adverse Reactions in EudraVigilance. European Medicine Agency. Available online: https://www.ema.europa.eu/en/documents/other/screening-adverse-reactions-eudravigilance_en.pdf (accessed on 14 November 2022).
- Grundmark, B.; Holmberg, L.; Garmo, H.; Zethelius, B. Reducing the noise in signal detection of adverse drug reactions by standardizing the background: A pilot study on analyses of proportional reporting ratios-by-therapeutic area. Eur. J. Clin. Pharmacol. 2014, 70, 627–635. [Google Scholar] [CrossRef] [PubMed]


| Name | Active Substance | Undesirable Effects | Frequencies * | Reference |
|---|---|---|---|---|
| CRESTOR® (5 mg, 10 mg, 20 mg, 40 mg) | Rosuvastatin | Depression | Not known | [13] |
| SORTIS® (10 mg, 20 mg, 40 mg, 80 mg) | Atorvastatin | Nightmares | Uncommon | [14] |
| Insomnia | Not known | |||
| Depression | Not known | |||
| ZOCORD® (10 mg, 20 mg, 40 mg) | Simvastatin | Insomnia | Very rare | [15] |
| Depression | Not known | |||
| Sleep disturbance | Not known | |||
| Nightmares | Not known | |||
| LESCOL® (20 mg, 40 mg, 80 mg) | Fluvastatin | Insomnia | Common | [16] |
| Memory loss | Not known | |||
| Sleep disturbance | Not known | |||
| Nightmares | Not known | |||
| Depression | Not known |
| Atorvastatin n = 3659 N, (%) | Simvastatin n = 2326 N, (%) | Rosuvastatin n = 1962 N, (%) | ||
|---|---|---|---|---|
| Age category | 18–64 years | 1756 (47.99) | 1155 (49.66) | 943 (48.06) |
| 65–85 years | 1284 (35.09) | 810 (34.82) | 693 (35.32) | |
| Gender | Male | 1522 (41.60) | 1111 (47.76) | 805 (41.03) |
| Female | 2039 (55.73) | 1129 (48.54) | 1122 (57.19) | |
| Reporter group | Health professionals | 2172 (59.36) | 1732 (74.46) | 1126 (57.39) |
| Non-health professionals | 1422 (38.86) | 573 (24.63) | 835 (42.56) | |
| Seriousness | Serious ADRs | 2911 (79.56) | 1718 (73.86) | 1581 (80.58) |
| Non-serious ADRs | 740 (20.22) | 596 (25.62) | 380 (19.37) | |
| Countries | EEA | 2175 (59.44) | 1294 (55.63) | 602 (30.68) |
| Non-EEA | 1454 (39.74) | 1032 (44.37) | 1360 (69.32) | |
| Statins | Number of ICSRs That Do Not Mention Another Suspected Drug/Interacting Drug (%) | Number of ICSRs That Do Not Mention Another Concomitant Drug (%) |
|---|---|---|
| Atorvastatin | 1983 (54.20) | 1765 (48.24) |
| Simvastatin | 557 (23.95) | 809 (34.78) |
| Rosuvastatin | 845 (43.07) | 764 (38.94) |
| Atorvastatin | ||||||
| Type of side effects | Anxiety n = 324 N, (%) | Depression n = 463 N, (%) | Hallucination n = 90 N, (%) | Insomnia n = 683 N, (%) | Suicidal attempt/ideation n = 150 N,(%) | Nightmares n = 194 N, (%) |
| Age Group N (%) | ||||||
| Not specified | 30 (9.26) | 62 (13.39) | 15 (16.67) | 88 (12.88) | 21 (14.00) | 24 (12.37) |
| <18 years | 0 | 0 | 0 | 0 | 7 (4.67) | 0 |
| 18–64 years | 196 (60.49) | 248 (53.56) | 22 (24.44) | 309 (45.24) | 87 (58.00) | 77 (39.69) |
| 65–85 years | 94 (29.01) | 139 (30.02) | 40 (44.44) | 264 (38.65) | 32 (21.33) | 87 (44.85) |
| >85 years | 2 (0.62) | 14 (3.02) | 13 (14.44) | 22 (3.22) | 2 (1.33) | 6 (3.09) |
| Sex N (%) | ||||||
| M | 102 (31.48) | 210 (45.36) | 37 (41.11) | 286 (41.87) | 68 (45.33) | 93 (47.94) |
| F | 218 (67.28) | 243 (52.48) | 48 (53.33) | 376 (55.05) | 77 (51.33) | 99 (51.03) |
| Not specified | 4 (1.23) | 10 (2.16) | 5 (5.56) | 21 (3.07) | 5 (3.33) | 2 (1.03) |
| Outcome N (%) | ||||||
| Not recovered | 79 (24.38) | 80 (17.28) | 9 (10.00) | 147 (21.52) | 1 (0.67) | 41 (21.13) |
| Recovered | 47 (14.51) | 115 (24.84) | 32 (35.56) | 196 (28.70) | 70 (46.67) | 79 (40.72) |
| Recovered with sequelae | 11 (3.40) | 6 (1.30) | 2 (2.22) | 8 (1.17) | 0 | 2 (1.03) |
| Recovering | 19 (5.86) | 48 (10.37) | 5 (5.56) | 70 (10.25) | 11 (7.33) | 13 (6.70) |
| Fatal | 0 | 2 (0.43) | 0 | 0 | 2 (1.33) | 0 |
| Simvastatin | ||||||
| Type of side effect | Anxiety n = 180 N, (%) | Depression n = 333 N, (%) | Hallucination n = 51 N, (%) | Insomnia n = 425 N, (%) | Suicidal attempt/ideation n = 154 N, (%) | Nightmares n = 159 N, (%) |
| Age Group N (%) | ||||||
| Not specified | 23 (12.78) | 50 (15.02) | 7 (13.73) | 60 (14.12) | 27 (17.53) | 16 (10.06) |
| <18 years | 0 | 0 | 1 (1.96) | 0 | 3 (1.95) | 0 |
| 18–64 | 94 (52.22) | 183 (54.95) | 20 (39.22) | 215 (50.59) | 91 (59.09) | 64 (40.25) |
| 65–85 | 58 (32.22) | 94 (28.23) | 21 (41.18) | 148 (34.82) | 30 (19.48) | 72 (45.28) |
| >85 | 5 (2.78) | 6 (1.80) | 2 (3.92) | 2 (0.47) | 3 (1.95) | 0 |
| Sex N (%) | ||||||
| M | 58 (32.22) | 162 (48.65) | 24 (47.06) | 178 (41.88) | 72 (46.75) | 82 (51.57) |
| F | 110 (61.11) | 154 (46.25) | 25 (49.02) | 237 (55.76) | 74 (48.05) | 75 (47.17) |
| Not specified | 12 (6.67) | 5 (1.50) | 2 (3.92) | 10 (2.35) | 8 (5.19) | 2 (1.26) |
| Outcome N (%) | ||||||
| Not recovered | 23 (12.78) | 56 (16.82) | 4 (7.84) | 73 (17.18) | 6 (3.90) | 0 |
| Recovered | 40 (22.22) | 103 (30.93) | 22 (43.14) | 132 (31.06) | 48 (31.17) | 2 (1.26) |
| Recovered with sequelae | 4 (2.22) | 4 (1.20) | 0 | 6 (1.41) | 3 (1.95) | 4 (2.52) |
| Recovering | 20 (11.11) | 42 (12.61) | 1 (1.96) | 44 (10.35) | 14 (9.09) | 13 (8.18) |
| Fatal | 0 | 0 | 0 | 0 | 4 (2.60) | 0 |
| Rosuvastatin | ||||||
| Type of side effect | Anxiety n = 258 N, (%) | Depression n = 323 N, (%) | Hallucination n = 26 N, (%) | Insomnia n = 428 N, (%) | Suicidal attempt/ideation n = 126 N, (%) | Nightmares n = 105 N, (%) |
| Age Group N (%) | ||||||
| Not specified | 11 (4.26) | 30 (9.29) | 5 (19.23) | 46 (10.75) | 31 (24.60) | 11 (10.48) |
| <18 years | 0 | 0 | 0 | 0 | 5 (3.97) | 0 |
| 18–64 | 134(51.94) | 187 (57.89) | 6 (23.08) | 197(46.03) | 65 (51.59) | 39 (37.14) |
| 65–85 | 102(39.53) | 89 (27.55) | 12 (46.15) | 167(39.012 | 25 (19.84) | 52 (49.52) |
| >85 | 11 (4.26) | 16 (4.95) | 3 (11.54) | 18 (4.21) | 0 | 3 (2.86) |
| Sex N (%) | ||||||
| M | 97 (37.60) | 105 (32.51) | 6 (23.08) | 163(38.08) | 57 (45.24) | 50 (47.62) |
| F | 159(61.63) | 210 (65.02) | 18 (69.23) | 255(59.58) | 66 (52.38) | 54 (51.43) |
| Not specified | 2 (0.78) | 8 (2.48) | 2 (7.69) | 10 (2.34) | 3 (2.38) | 1 (0.95) |
| Outcome N (%) | ||||||
| Not recovered | 35 (13.57) | 44 (13.62) | 5 (19.23) | 92 (21.50) | 6 (4.76) | 19 (18.10) |
| Recovered | 51 (19.77) | 68 (21.05) | 7 (26.92) | 109 (25.47) | 42 (33.33) | 48 (45.71) |
| Recovered with sequelae | 0 | 2 (0.62) | 0 | 3 (0.70) | 0 | 1 (0.95) |
| Recovering | 18 (6.98) | 31 (9.60) | 1 (3.85) | 35 (8.18) | 5 (3.97) | 10 (9.52) |
| Fatal | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. https://doi.org/10.3390/ph15121536
Pop G, Farcaș A, Butucă A, Morgovan C, Arseniu AM, Pumnea M, Teodoru M, Gligor FG. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals. 2022; 15(12):1536. https://doi.org/10.3390/ph15121536
Chicago/Turabian StylePop, Gabriela, Andreea Farcaș, Anca Butucă, Claudiu Morgovan, Anca Maria Arseniu, Manuela Pumnea, Minodora Teodoru, and Felicia Gabriela Gligor. 2022. "Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance" Pharmaceuticals 15, no. 12: 1536. https://doi.org/10.3390/ph15121536
APA StylePop, G., Farcaș, A., Butucă, A., Morgovan, C., Arseniu, A. M., Pumnea, M., Teodoru, M., & Gligor, F. G. (2022). Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals, 15(12), 1536. https://doi.org/10.3390/ph15121536







