Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
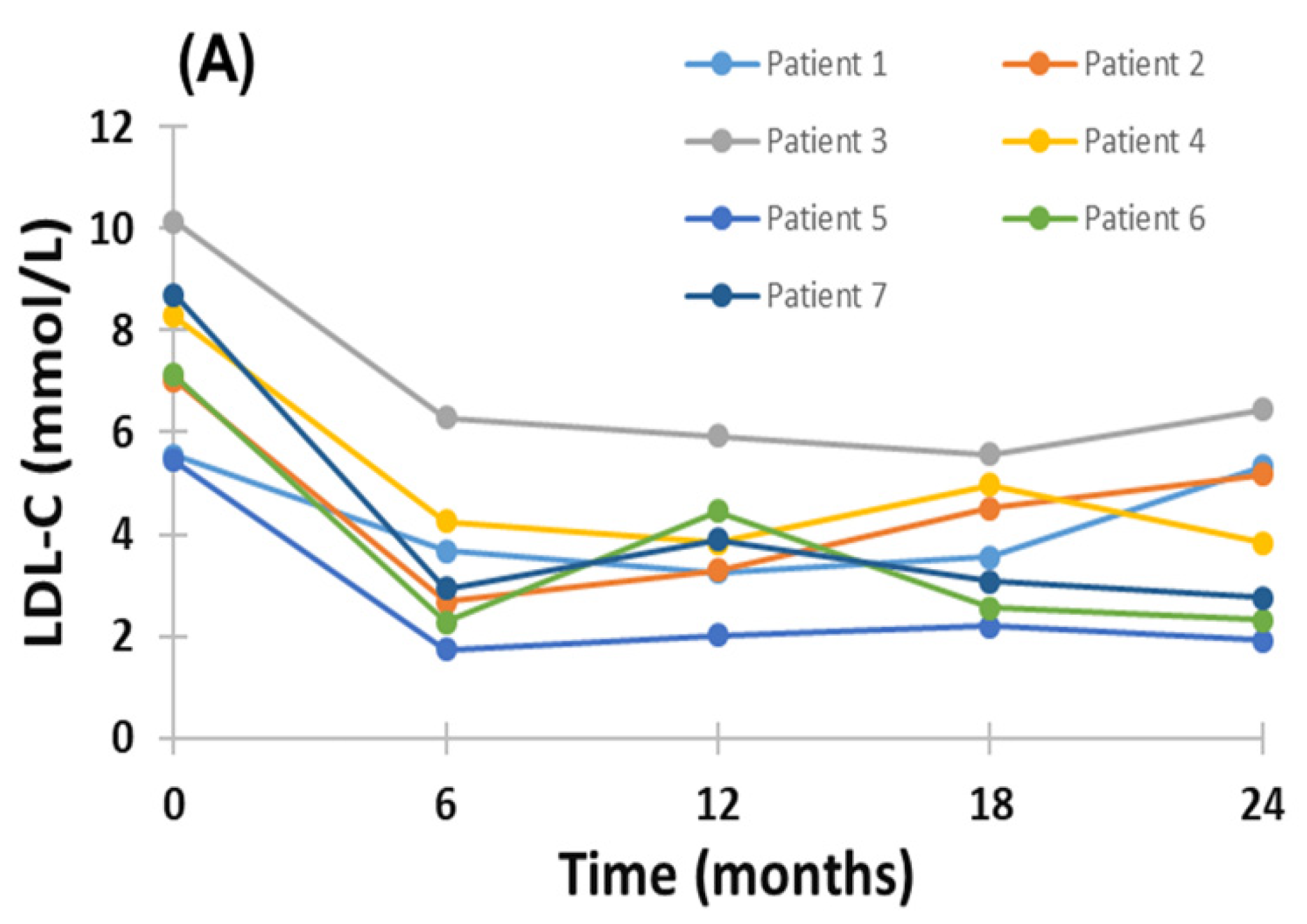
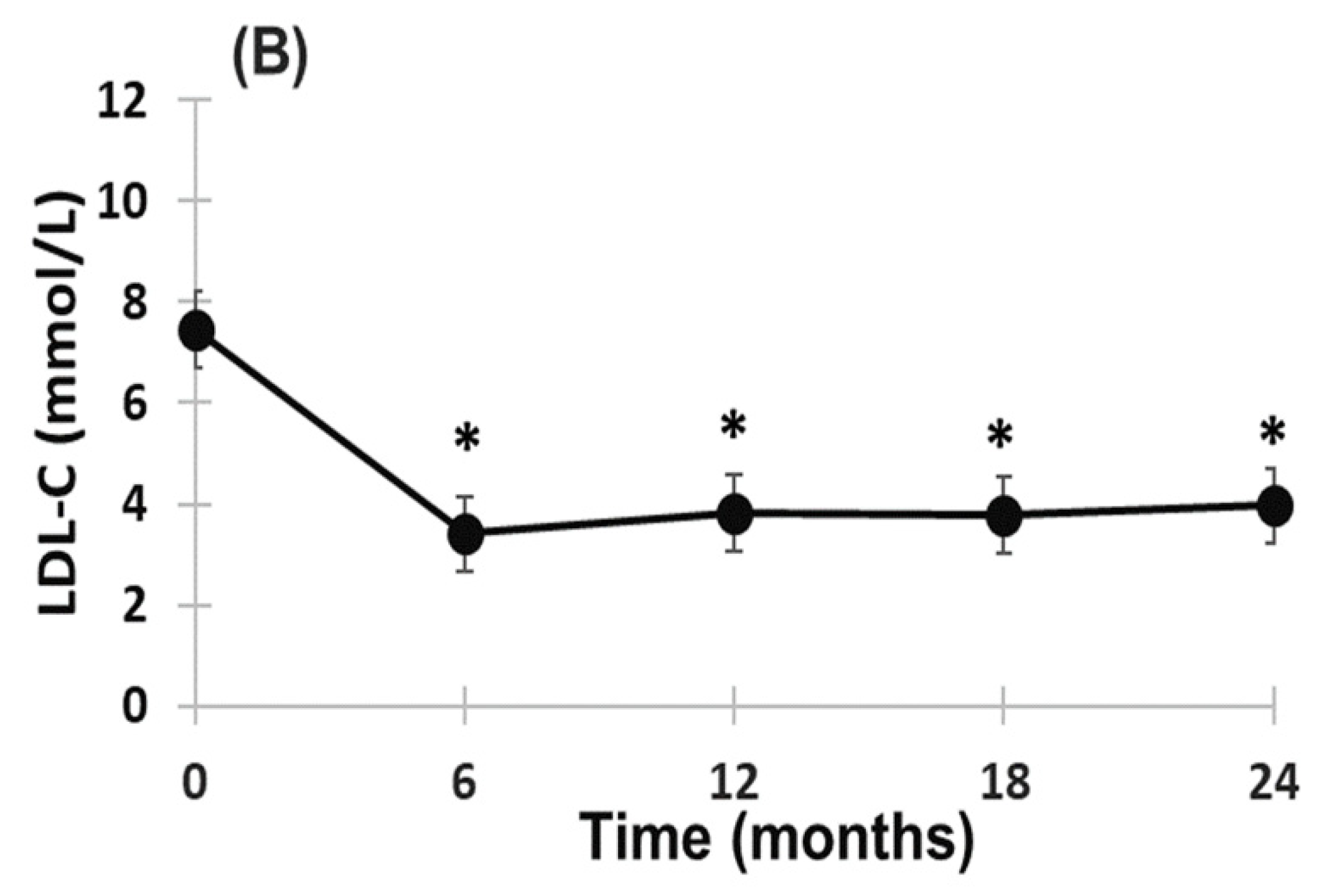
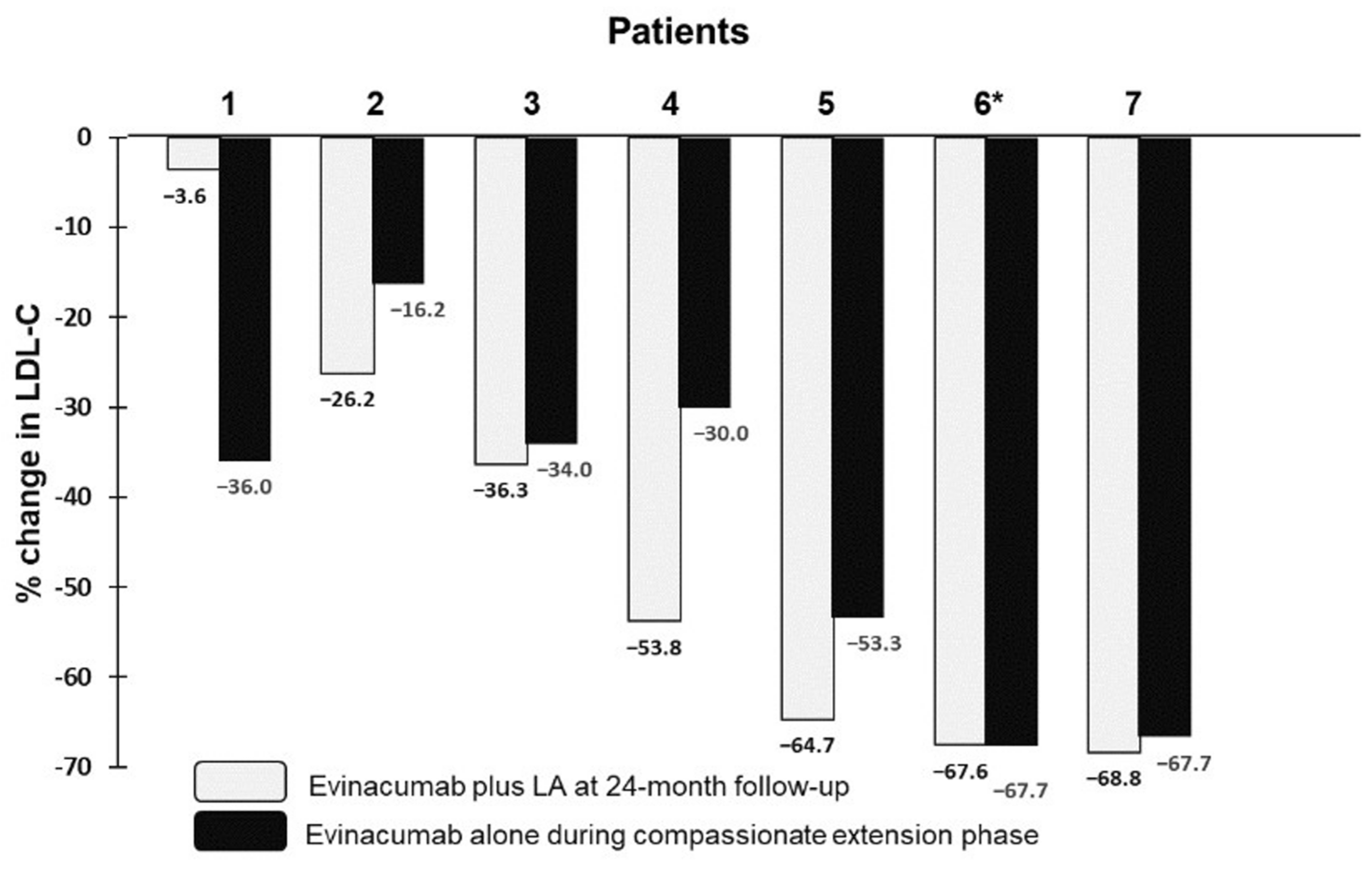
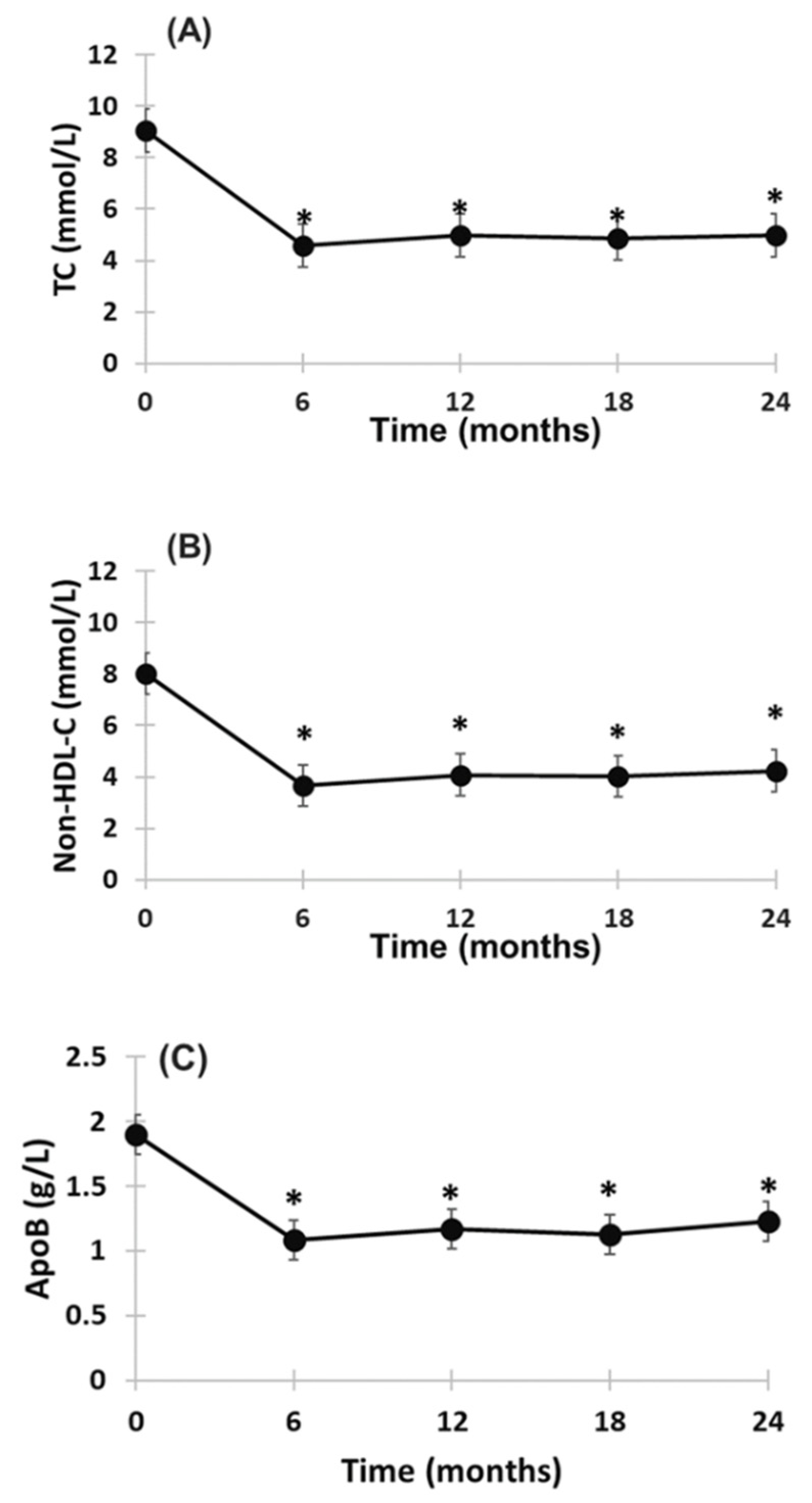
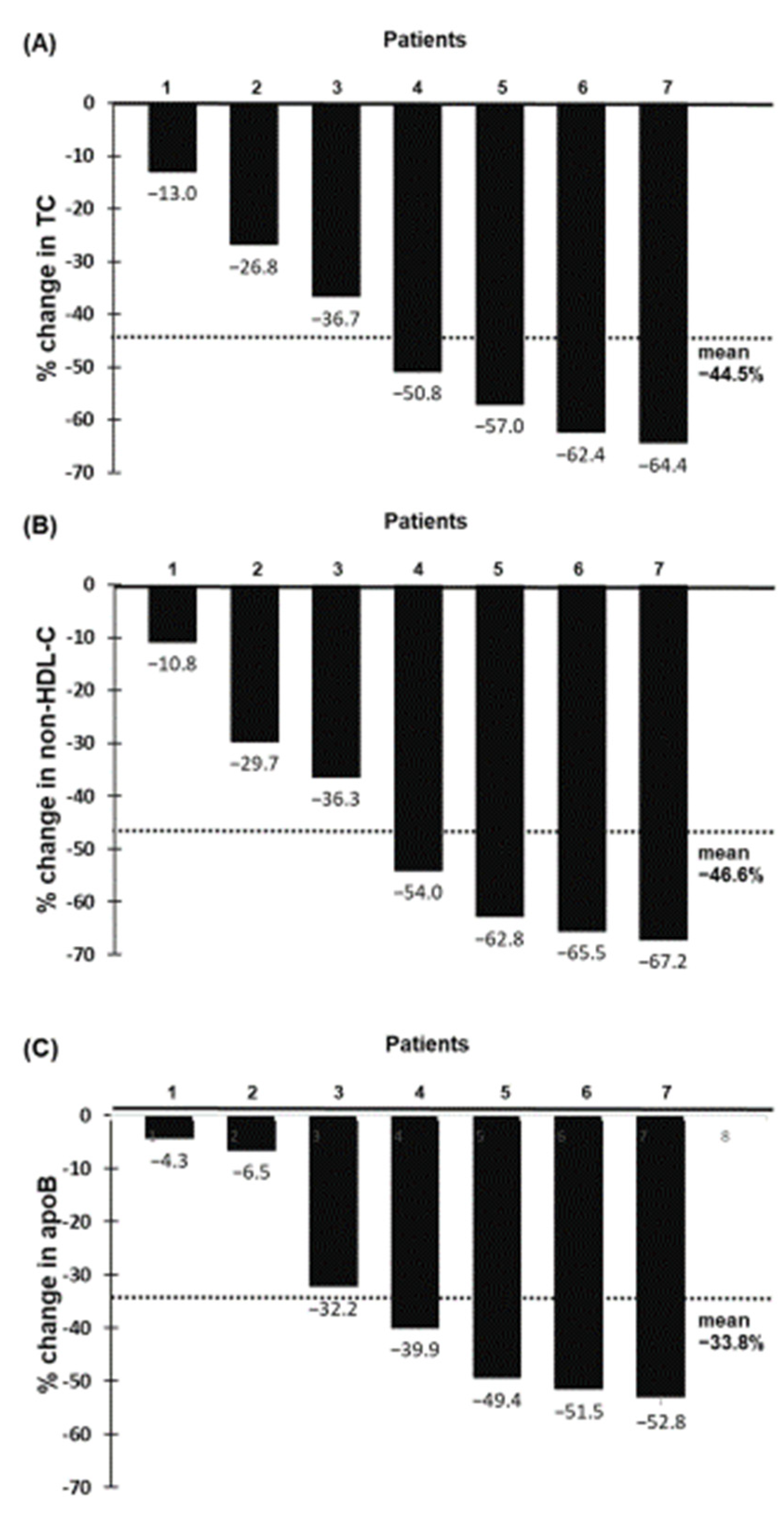
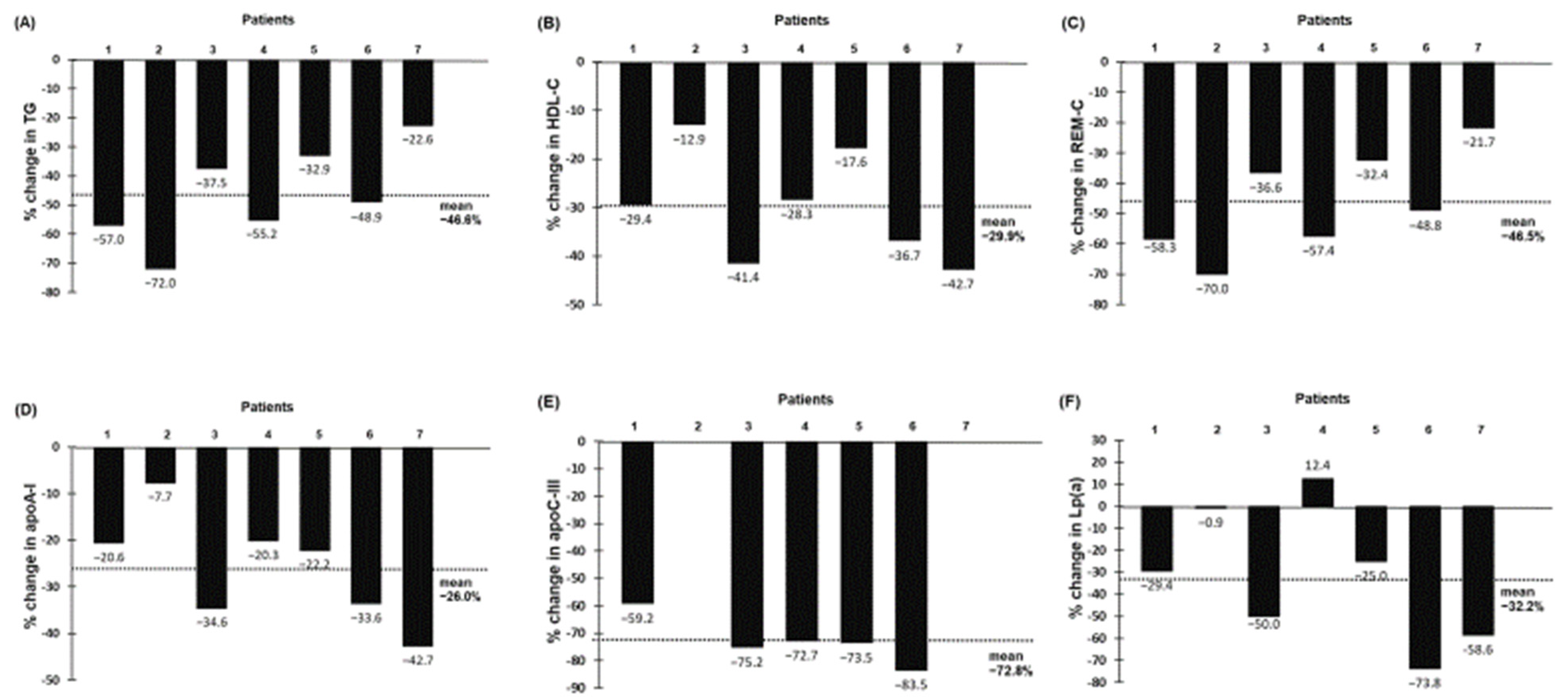
2.2. Effect of Evinacumab and Lipoprotein Apheresis
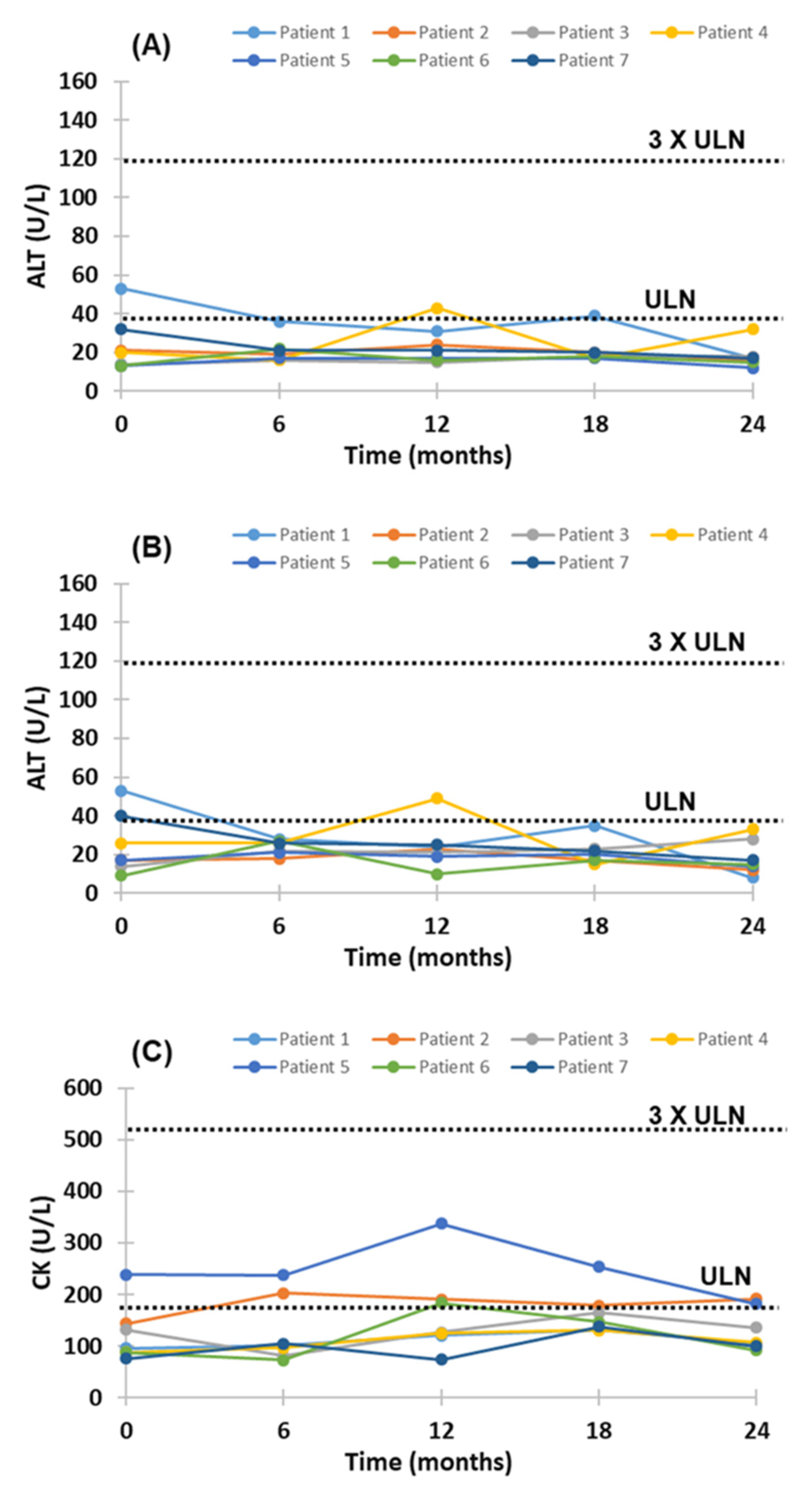
2.3. Adverse Effects of Evinacumab Therapy
3. Discussion
3.1. Previous Studies
3.2. Mechanisms
3.3. Strengths and Limitations
3.4. Clinical Implication
4. Materials and Methods
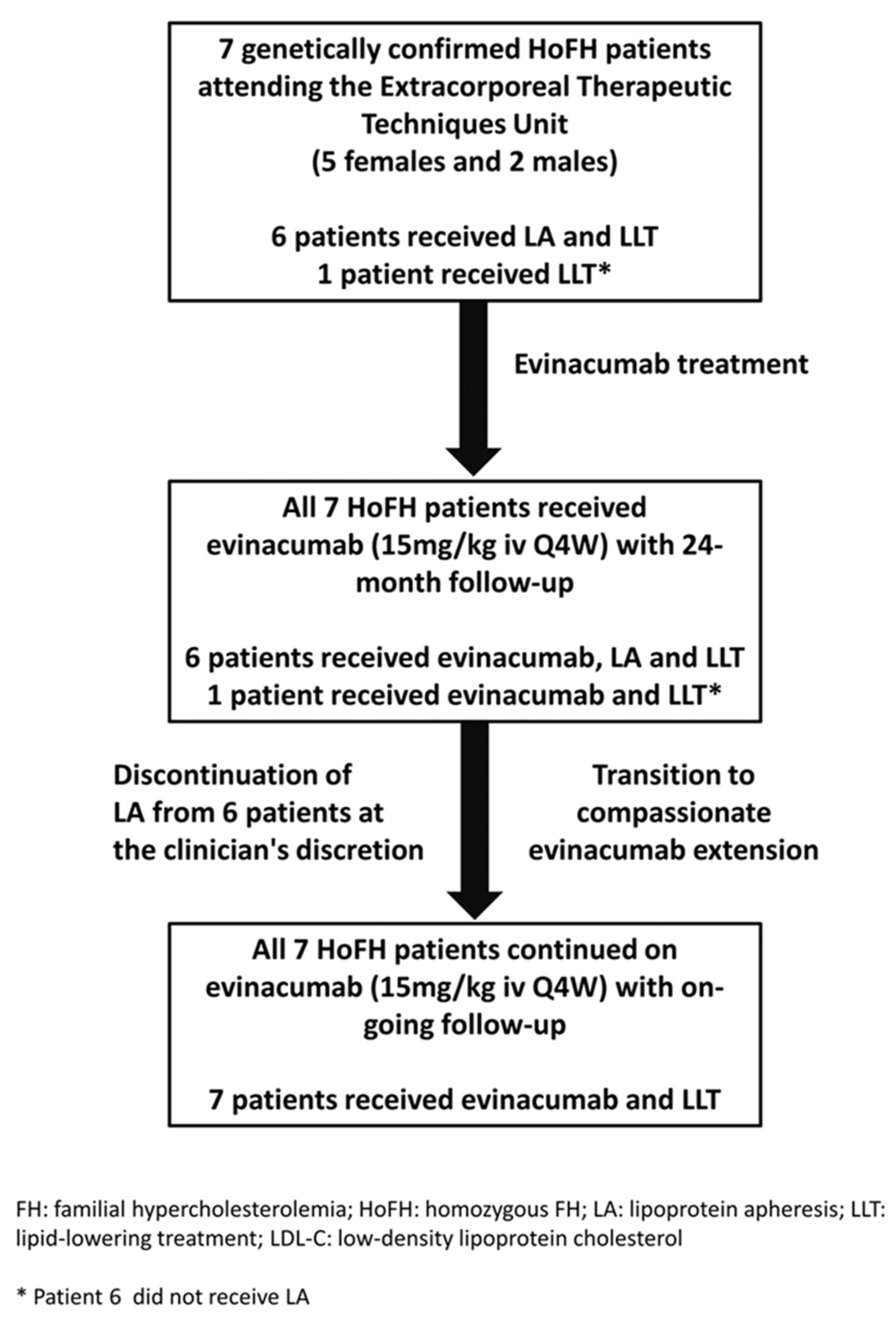
4.1. Patients and Study Design
4.2. Biochemical Analyses
4.3. Safety Monitoring
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuchel, M.; Bruckert, E.; Ginsberg, H.N.; Raal, F.J.; Santos, R.D.; Hegele, R.A.; Kuivenhoven, J.A.; Nordestgaard, B.G.; Descamps, O.S.; Steinhagen-Thiessen, E. Homozygous familial hypercholesterolaemia: New insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 2014, 35, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Moorjani, S.; Roy, M.; Torres, A.; Bétard, C.; Gagné, C.; Lambert, M.; Brun, D.; Davignon, J.; Lupien, P. Mutations of low-density-lipoprotein-receptor gene, variation in plasma cholesterol, and expression of coronary heart disease in homozygous familial hypercholesterolaemia. Lancet 1993, 341, 1303–1306. [Google Scholar] [CrossRef]
- I Kramer, A.; E Akioyamen, L.; Lee, S.; Bélanger, A.; Ruel, I.; Hales, L.; Genest, J.; Brunham, L.R. Major adverse cardiovascular events in homozygous familial hypercholesterolaemia: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Tromp, T.R.; Hartgers, M.L.; Hovingh, G.K.; Vallejo-Vaz, A.J.; Ray, K.K.; Soran, H.; Freiberger, T.; Bertolini, S.; Harada-Shiba, M.; Blom, D.J.; et al. Homozygous familial hypercholesterolaemia International Clinical Collaborators. Worldwide experience of homozygous familial hypercholesterolaemia: Retrospective cohort study. Lancet 2022, 399, 719–728. [Google Scholar] [CrossRef]
- Raal, F.J.; Pilcher, G.J.; Panz, V.R.; van Deventer, H.E.; Brice, B.C.; Blom, D.J.; Marais, A.D. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation 2011, 124, 2202–2207. [Google Scholar] [CrossRef]
- Blom, D.J.; Cuchel, M.; Ager, M.; Phillips, H. Target achievement and cardiovascular event rates with Lomitapide in homozygous Familial Hypercholesterolaemia. Orphanet. J. Rare Dis. 2018, 13, 96. [Google Scholar] [CrossRef]
- Bajaj, A.; Cuchel, M. Advancements in the treatment of homozygous familial hypercholesterolemia. J. Atheroscler. Thromb. 2022, 29, 1125–1135. [Google Scholar] [CrossRef]
- Santos, R.D.; Gidding, S.S.; A Hegele, R.; A Cuchel, M.; Barter, P.J.; Watts, G.F.; Baum, S.J.; Catapano, A.L.; Chapman, M.J.; Defesche, J.C.; et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: A consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016, 4, 850–861. [Google Scholar] [CrossRef]
- Thompson, G.R. The scientific basis and future of lipoprotein apheresis. Ther. Apher. Dial. 2022, 26, 32–36. [Google Scholar] [CrossRef]
- Kersten, S. Angiopoietin-like 3 in lipoprotein metabolism. Nat. Rev. Endocrinol. 2017, 13, 731–739. [Google Scholar] [CrossRef]
- Burks, K.H.; Basu, D.; Goldberg, I.J.; Stitziel, N.O. Angiopoietin-like 3: An important protein in regulating lipoprotein levels. Best Pr. Res. Clin. Endocrinol. Metab. 2022, 101688. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Recent insights into low-density lipoprotein metabolism and therapy. Curr. Opin. Clin. Nutr. Metab. Care. 2021, 24, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Zimetti, F.; Adorni, M.P.; Sirtori, C.R.; Lupo, M.G.; Ferri, N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol. Res. 2020, 153, 104653. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.; Croteau, L.; Lauzière, A.; Gaudet, D. Lessons learned from the evinacumab trials in the treatment of homozygous familial hypercholesterolemia. Future Cardiol. 2022, 18, 507–518. [Google Scholar] [CrossRef]
- Adam, R.C.; Mintah, I.J.; Alexa-Braun, C.A.; Shihanian, L.M.; Lee, J.S.; Banerjee, P.; Hamon, S.C.; Kim, H.I.; Cohen, J.C.; Hobbs, H.H.; et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J. Lipid Res. 2020, 61, 1271–1286. [Google Scholar] [CrossRef]
- Reeskamp, L.F.; Millar, J.S.; Wu, L.; Jansen, H.; van Harskamp, D.; Schierbeek, H.; Gipe, D.A.; Rader, D.J.; Dallinga-Thie, G.M.; Hovingh, G.K.; et al. ANGPTL3 inhibition with evinacumab results in faster clearance of IDL and LDL apoB in patients with homozygous familial hypercholesterolemia. Arter. Thromb Vasc. Biol. 2021, 41, 1753–1759. [Google Scholar] [CrossRef]
- Gaudet, D.; Gipe, D.A.; Pordy, R.; Ahmad, Z.; Cuchel, M.; Shah, P.K.; Chyu, K.-Y.; Sasiela, W.J.; Chan, K.-C.; Brisson, D.; et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. New Engl. J. Med. 2017, 377, 296–297. [Google Scholar] [CrossRef]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.-C.; Gipe, D.A.; et al. Evinacumab for homozygous familial hypercholesterolemia. New Engl. J. Med. 2020, 383, 711–720. [Google Scholar] [CrossRef]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.C.; Khilla, N.; et al. The longer-term efficacy of evinacumab in patients with homozygous familial hypercholesterolemia. Circulation 2020, 142, A14267. [Google Scholar] [CrossRef]
- Jeraj, N.; Huang, S.S.; Kennedy, B.A.; Hegele, R.A. Treatment of homozygous familial hypercholesterolemia with evinacumab. CJC Open 2021, 4, 347–349. [Google Scholar] [CrossRef]
- Reeskamp, L.F.; Nurmohamed, N.S.; Bom, M.J.; Planken, R.N.; Driessen, R.S.; van Diemen, P.A.; Luirink, I.K.; Groothoff, J.W.; Kuipers, I.M.; Knaapen, P.; et al. Marked plaque regression in homozygous familial hypercholesterolemia. Atherosclerosis 2021, 327, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Chan, K.-C.; Tarabocchia, M.; Benito-Vicente, A.; Alves, A.C.; Uribe, K.B.; Bourbon, M.; Skiba, P.J.; Pordy, R.; Gipe, D.A.; et al. Functional analysis of LDLR (low-density lipoprotein receptor) variants in patient lymphocytes to assess the effect of evinacumab in homozygous familial hypercholesterolemia patients with a spectrum of LDLR activity. Arter. Thromb. Vasc. Biol. 2019, 39, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Chemello, K.; Chan, D.C.; Lambert, G.; Watts, G.F. Recent advances in demystifying the metabolism of lipoprotein(a). Atherosclerosis 2022, 349, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Stefanutti, C.; Pang, J.; Di Giacomo, S.; Wu, X.; Wang, X.; Morozzi, C.; Watts, G.F.; Lin, J. A cross-national investigation of cardiovascular survival in homozygous familial hypercholesterolemia: The Sino-Roman Study. J. Clin. Lipidol. 2019, 13, 608–617. [Google Scholar] [CrossRef]
- Stefanutti, C.; Julius, U.; Watts, G.F.; Harada-Shiba, M.; Cossu, M.; Schettler, V.J.; De Silvestro, G.; Soran, H.; Van Lennep, J.R.; Pisciotta, L.; et al. Toward an international consensus-Integrating lipoprotein apheresis and new lipid-lowering drugs. J. Clin. Lipidol. 2017, 11, 858–871. [Google Scholar] [CrossRef]
- US Food & Drug. FDA Approves Add-on Therapy for Patients with Genetic Form of Severely High Cholesterol. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-patients-genetic-form-severely-high-cholesterol (accessed on 15 August 2022).
- European Medicines Agency. Evkeeza. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evkeeza (accessed on 15 August 2022).
- Kroon, A.A.; van’t Hof, M.A.; Demacker, P.N.; Stalenhoef, A.F. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis 2000, 152, 519–526. [Google Scholar] [CrossRef]
- Thompson, G.R.; Barbir, M.; Davies, D.; Dobral, P.; Gesinde, M.; Livingston, M.; Mandry, P.; Marais, A.; Matthews, S.; Neuwirth, C.; et al. Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis 2010, 208, 317–321. [Google Scholar] [CrossRef]
| Patient | Age | Gender | FH Diagnosis | Mutation | Co-Morbidities | Lipid-Lowering |
|---|---|---|---|---|---|---|
| (years) | Treatment | |||||
| 1 | 63 | Female | Compound | c.1474 G > A, Asp471Asn | Premature CAD | Simvastatin 40 mg/day |
| HeFH | c.2094 C > G, Cys677Trp | Hypertension | Ezetimibe 10 mg/day | |||
| Alirocumab 150 mg/sc/15 days | ||||||
| 2 | 36 | Male | True HoFH | c.1056 C > G, | Ezetimibe 10 mg/day | |
| p.C352W(C331W) receptor | Lomitapide 20 mg/day | |||||
| defective | Evolocumab 140 mg/sc/15 days | |||||
| 3 | 14 | Male | True HoFH | c.1478-1479 del 2bp (CT) | Premature CAD | Rosuvastatin 40 mg/day |
| receptor negative | Ezetimibe 10 mg/day | |||||
| 4 | 52 | Female | True HoFH | c.2054 C > T, Pro664Leu | Premature CAD | Simvastatin 40 mg/day |
| receptor defective | Hypertension | Ezetimibe 10 mg/day | ||||
| Evolocumab 140 mg/sc/15 days | ||||||
| 5 | 53 | Female | True HoFH | c.2054 C > T, | Aortic valve disease | Rosuvastatin 20 mg/day |
| p.P685L (P664L) | Hypertension | Ezetimibe 10 mg/day | ||||
| Evolocumab 140 mg/sc/15 days | ||||||
| 6 | 50 | Female | True HoFH | c.2054 C > T, | Hypertension | Rosuvastatin 40 mg/day |
| p.P685L (P664L) | Ezetimibe 10 mg/day | |||||
| 7 | 34 | Female | Compound | Deletion of promotor and | Rosuvastatin 20 mg/day | |
| HeFH | Ex 1-2, c.1775 G > A, | Ezetimibe 10 mg/day | ||||
| Gly571Glu | Evolocumab 420 mg/sc/30days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanutti, C.; Chan, D.C.; Di Giacomo, S.; Morozzi, C.; Watts, G.F. Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience. Pharmaceuticals 2022, 15, 1389. https://doi.org/10.3390/ph15111389
Stefanutti C, Chan DC, Di Giacomo S, Morozzi C, Watts GF. Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience. Pharmaceuticals. 2022; 15(11):1389. https://doi.org/10.3390/ph15111389
Chicago/Turabian StyleStefanutti, Claudia, Dick C. Chan, Serafina Di Giacomo, Claudia Morozzi, and Gerald F. Watts. 2022. "Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience" Pharmaceuticals 15, no. 11: 1389. https://doi.org/10.3390/ph15111389
APA StyleStefanutti, C., Chan, D. C., Di Giacomo, S., Morozzi, C., & Watts, G. F. (2022). Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience. Pharmaceuticals, 15(11), 1389. https://doi.org/10.3390/ph15111389





