Abstract
Herein, the synthesis and anticancer activity evaluation of a series of novel β-carbolines is reported. The reactivity of nitrosoalkenes towards indole was explored for the synthesis of novel tryptophan analogs where the carboxylic acid was replaced by a triazole moiety. This tryptamine was used in the synthesis of 3-(1,2,3-triazol-4-yl)-β-carbolines via Pictet–Spengler condensation followed by an oxidative step. A library of compounds, including the novel 3-(1,2,3-triazol-4-yl)-β-carbolines as well as methyl β-carboline-3-carboxylate and 3-tetrazolyl-β-carboline derivatives, was evaluated for their antiproliferative activity against colorectal cancer cell lines. The 3-(1H-tetrazol-5-yl)-β-carbolines stood out as the most active compounds, with values of half-maximal inhibitory concentration (IC50) ranging from 3.3 µM to 9.6 µM against colorectal adenocarcinoma HCT116 and HT29 cell lines. The results also revealed a mechanism of action independent of the p53 pathway. Further studies with the 3-tetrazolyl-β-carboline derivative, which showed high selectivity for cancer cells, revealed IC50 values below 8 μM against pancreatic adenocarcinoma PANC-1, melanoma A375, hepatocarcinoma HEPG2, and breast adenocarcinoma MCF-7 cell lines. Collectively, this work discloses the 3-tetrazolyl-β-carboline derivative as a promising anticancer agent worthy of being further explored in future works.
1. Introduction
Cancer is one of the leading causes of death worldwide. In 2020, the number of new cases of cancer was estimated to be around 19.3 million, with approximately 10 million deaths globally being attributed to cancer [1]. Moreover, colorectal cancer (CRC) is the third most common cancer type, and one of the deadliest (in 2020, CRC accounted for over 2 million new cancer cases and 1 million deaths worldwide). Therefore, the continuous effort towards the discovery of novel and effective anticancer agents against CRC is of great importance [1].
β-Carbolines are a class of indole-based natural and synthetic compounds bearing a 9H-pyrido[3,4-b]indole scaffold, with a wide range of biological activities, such as anti-HIV [2,3,4], antibacterial [3,4,5], antimalarial [6,7], and anticancer [8,9], among others [10,11,12]. It is noteworthy to mention their anticancer activity, which has been extensively studied in recent years by several groups, with promising results [5,8,9,13,14,15,16,17,18].
In recent years, several synthetic methodologies have been applied to the development of novel β-carboline derivatives. However, the most commonly explored synthetic route involves the Pictet–Spengler reaction between an aldehyde and a tryptamine derivative, followed by oxidation of the initially formed tetrahydro-β-carboline [7,19,20]. In our group, the reactivity of nitrosoalkenes towards heterocycles has been extensively studied, and it was thought that it could be explored for the synthesis of functionalized tryptophan analogs [21,22,23]. The nitrosoalkenes are generated in situ through the treatment of α-bromooximes with sodium carbonate, followed by the hetero-Diels–Alder reaction with indoles, subsequent 1,2-oxazine ring-opening, and concomitant rearomatization to afford 3-alkylated indoles incorporating an oxime moiety [24,25]. This open-chain oxime is then reduced to afford the desired tryptamine analog. In fact, the synthesis of a tryptophan analog, where the carboxylic acid was replaced by the bioisosteric tetrazole group, was recently reported by our group, and was used as a building block for the synthesis of novel 3-tetrazolyl-β-carbolines (Scheme 1a) [8]. Using a similar synthetic strategy, 6-substituted-β-carboline-3-carboxylates were also synthesized via the Pictet–Spengler approach, using tryptophan ethyl esters functionalized in the indole moiety (Scheme 1a) [8].
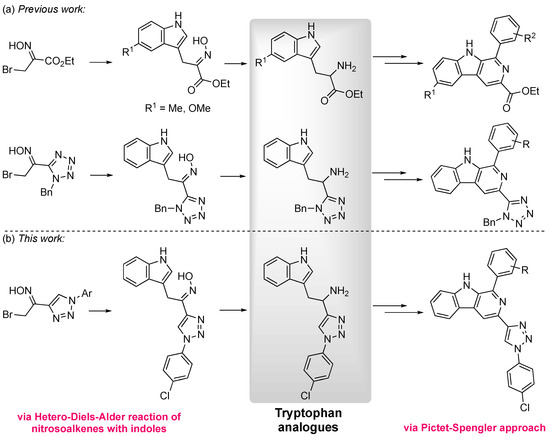
Scheme 1.
(a) Previous work on the synthesis of novel β-carbolines by our group [8]. (b) This work: synthesis of 3-triazolyl-β-carbolines.
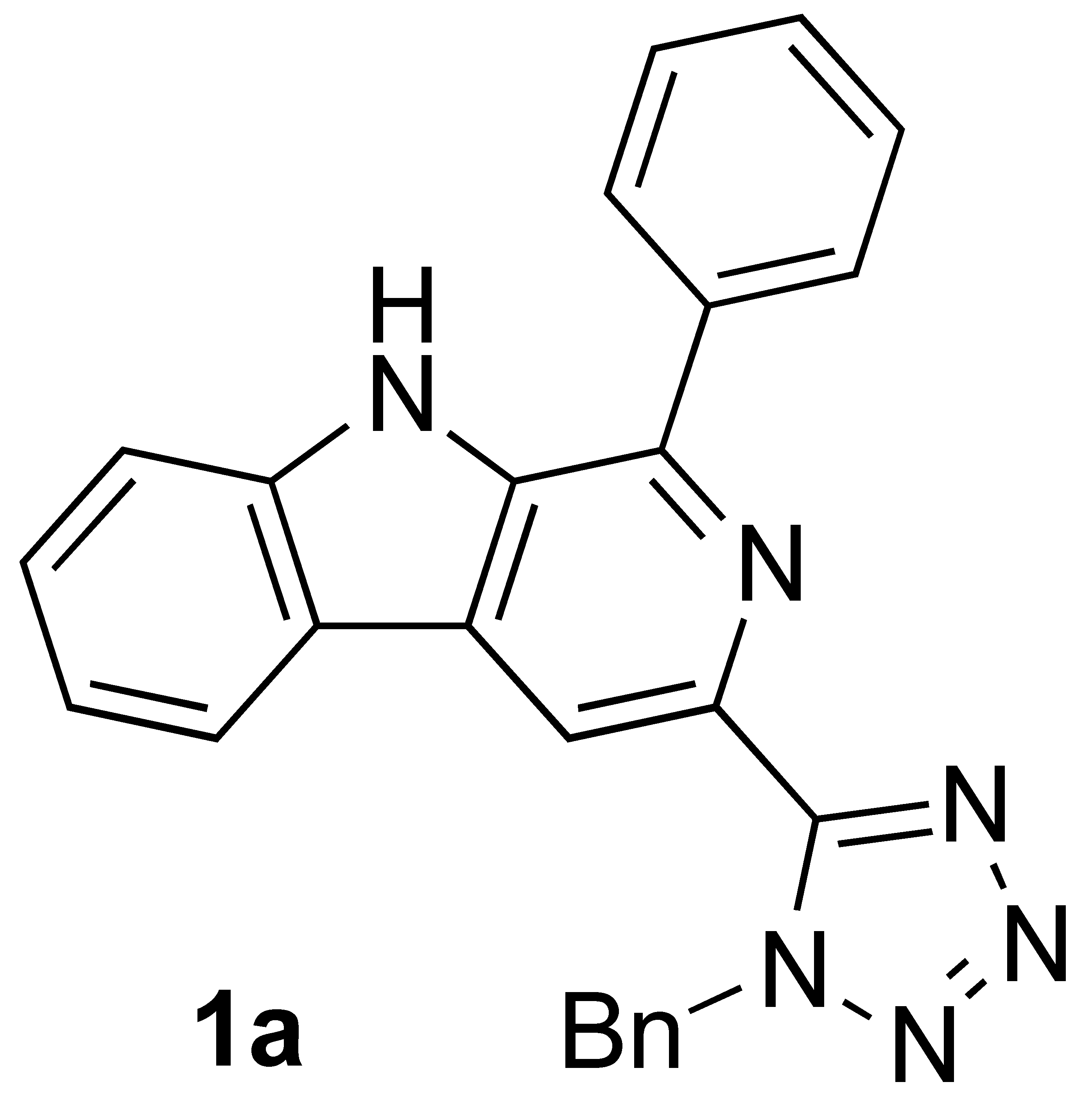
The activity of the synthesized 3-(1-benzyl-tetrazolyl)-β-carbolines 1a–c was evaluated against several human cancer cell lines, which evidenced their potential as anticancer agents (Figure 1) [8]. It is noteworthy to mention that the β-carboline derivative 1c, bearing a 4-fluorophenyl at C-1, was active against ovarian carcinoma (OVCAR-3), leukemia (K-562), renal adenocarcinoma (786-0), and breast adenocarcinoma (MCF-7), with half-maximal inhibitory concentration (IC50) values below 1 μM. The corresponding β-carboline 1d with the unprotected tetrazole ring showed significantly lower activity against most of the cancer cell lines studied, but was, nevertheless, selective and very active against ovarian carcinoma (OVCAR-3, IC50 = 0.27 μM). 3-Tetrazolyl-β-carboline 1b, bearing a 4-methoxyphenyl group at C-1, was also very active against several cancer cell lines, namely breast adenocarcinoma, lung carcinoma (NCI-H460), and ovarian carcinoma, with IC50 values between 1.32 and 1.62 µM. In contrast, compound 1a, containing a phenyl group at C-1, showed poor activity, except against breast cancer cell line (MCF-7), presenting an IC50 value of 4.40 µM.
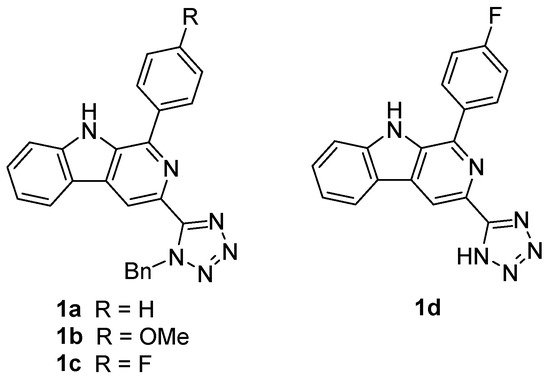
Figure 1.
3-Tetrazolyl-β-carbolines obtained from the tetrazolyl tryptophan analog via Pictet–Spengler, followed by oxidation approach.
Over the past decade, 1,2,3-triazoles derivatives have been extensively studied in medicinal chemistry due to their wide range of biological activities [26,27,28,29,30,31], being found in several well-known drugs (Figure 1a) [32,33,34]. In addition to their antifungal [35], antibacterial [36,37,38], anti-HIV [39], and antimalarial [40] activity, these five-membered heterocycles have been widely recognized for their anticancer activity [41,42,43,44]. The great biological potential of 1,2,3-triazoles is related to their high chemical stability, structural rigidity, ability to form hydrogen bonds in the biological environment, strong dipole moment, and the ability to mimic other groups such as amides, esters, and carboxylic acids [45,46]. Recently, reports on the synthesis and biological activity of β-carboline and 1,2,3-triazole hybrids have been disclosed. For instance, compounds with the general structure 2 showed antibacterial activity and high cytotoxicity against the Hela and HepG2 cell lines [47]. On the other hand, β-carbolines 3 [48] and the harmine derivative 4 [49] showed interesting anticancer activity against the MCF-7 cell line (Figure 2b).
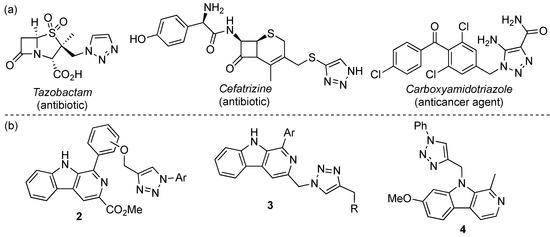
Figure 2.
Known drugs (a) and β-carbolines with anticancer activity (b) containing a 1,2,3-triazole motif.
Taking our previous promising results into account, we decided to carry out further studies on the anticancer activity of 3-tetrazolyl-β-carbolines, namely against several colon cancer cell lines. Moreover, the first synthesis of a novel tryptophan analog where the carboxylic acid was replaced by a 1,2,3-triazole moiety, as well the synthesis of a library of novel 3-triazolyl-β-carbolines, were carried out (Scheme 1b). The evaluation of the latter β-carbolines, as well as of a range of methyl β-carboline-3-carboxylates, as anticancer agents against human colon cancer cells is also reported.
2. Results and Discussion
2.1. Chemistry
In order to develop the proposed 3-triazolyl-β-carboline derivatives, firstly, the α-bromooxime 5 was prepared following previously reported procedures [21]. Treatment of oxime 5 with sodium carbonate led to the in situ formation of the nitrosoalkene 6, which reacted with indole (7) to give 3-alkylated indole 9 via hetero-Diels–Alder reaction, followed by a 1,2-oxazine ring-opening reaction. Oxime 9 reacted further with another molecule of nitrosoalkene 6 to afford functionalized indole 10, which was isolated in 57% yield (overall yield from 5). The reduction of the alkylated oxime 10 was achieved using an excess of metallic zinc/aqueous formic acid in tetrahydrofuran at room temperature for 24 h, affording the novel tryptamine 11 an 81% yield (Scheme 2).
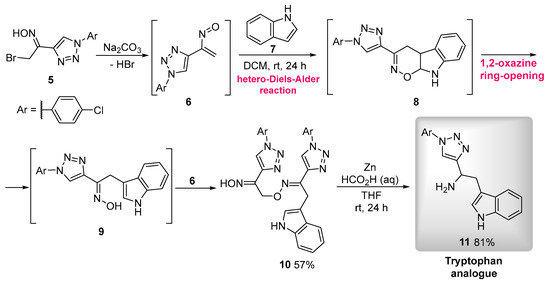
Scheme 2.
Synthesis of a tryptamine bearing a 1,2,3-triazole group.
Tryptophan analog 11 was used in the synthesis of 3-triazolyl-tetrahydro-β-carbolines 12 via the Pictet–Spengler approach. Trifluoroacetic acid-catalyzed condensation of tryptamine 11 with aromatic aldehydes (benzaldehyde, p-fluorobenzaldehyde, p-methoxybenzaldehyde, p-dimethylaminobenzaldehyde, m-nitrobenzaldehyde, o-chlorobenzaldehyde), carried out in dry dichloromethane for 24-60 h at room temperature, afforded the corresponding 3-triazolyl-tetrahydro-β-carbolines 12 in good yields (36–77%), isolated as mixtures of cis/trans isomers. The isomeric ratios were determined through NMR analysis of the isolated mixtures and the stereochemistry established by comparison with data previously reported for other β-carboline derivatives [8]. These 3-triazolyl-tetrahydro-β-carbolines underwent oxidation upon treatment with sulfur in DMF at reflux for 24 h, giving the target β-carbolines 13a–f in moderate to good yields (37-52%) (Scheme 3).
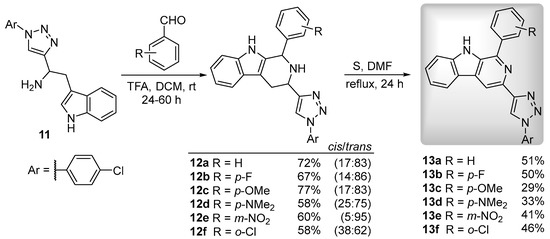
Scheme 3.
Synthesis of 3-triazolyl-β-carbolines 13a–f.
2.2. Anticancer Activity
The synthesized 3-triazolyl-β-carbolines 13a–f and 3-tetrazolyl-β-carbolines 1a–c [8] were evaluated for their anticancer activity in several colon cancer cells. In order to better evaluate and establish structure–activity relationships, methyl β-carboline-3-carboxylates 14a–e were also synthesized from L-tryptophan methyl ester following a previously reported procedure, and their evaluation as colorectal anticancer agents was carried out (Figure 3) [50].
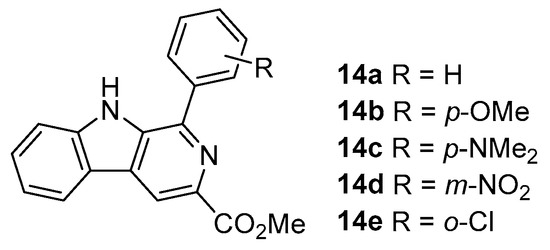
Figure 3.
Structures of the studied methyl β-carboline-3-carboxylates 14a–e.
Impairment of the tumor suppressor p53 protein pathway is a critical event in cancer. Therefore, re-establishing p53 activity has become one of the most appealing therapeutic anticancer strategies [51,52,53]. Moreover, some β-carboline derivatives are known for their capability to activate the p53 activity [54,55]. In this context, we set out to determine whether the anticancer activity of the synthesized β-carbolines could involve selective activation of the p53 pathway. For that, the antiproliferative activity of β-carbolines 1a–c, 13a–f, and 14a–e was evaluated against the human colorectal carcinoma HCT116 cell line expressing wild type (wt) p53, as well as the respective p53 knockout HCT116 derivative. The comparison of the activity of the compounds was made by analyzing the corresponding IC50 values calculated from the dose–response curves (Table 1).

Table 1.
Antiproliferative activity of β-carbolines 13a–f, 1a–c, and 14a–e against colorectal HCT116 carcinoma cells with and without (HCT116 p53−/−) wt p53.
In this initial screening, it was possible to observe that, despite our initial assumptions, the 3-triazolyl-β-carboline derivatives 13a–f were not active against the studied cancer cell lines, with IC50 values ranging from 32 µM to values higher than 50 µM. Furthermore, among the methyl β-carboline-3-carboxylates, β-carboline 14c was the one that presented the most promising results, with IC50 values of 15 µM and 18 µM against p53+/+ and p53−/− HCT116 cancer cells. The remaining methyl β-carboline-3-carboxylates were not active (IC50 > 50 µM).
The 3-(1H-tetrazol-5-yl)-β-carbolines 1a-c proved to be the most active compounds, with values of IC50 ranging from 3.3 µM to 4.6 µM against both cancer cell lines studied. It should be highlighted that the replacement of the methyl ester group of β-carbolines 14a and 14b by the bioisosteric benzyl-tetrazole group, leading to 3-(1H-tetrazol-5-yl)-β-carbolines 1a and 1b, respectively, resulted in a huge increase in anticancer activity. However, the results allowed us to conclude that none of the tested 3-(1H-tetrazol-5-yl)-β-carbolines selectively activate the p53 pathway.
The study was extended to evaluation of the anticancer activity of the 3-triazolyl- and 3-tetrazolyl-β-carbolines (13a–f and 1a–c) against other human colorectal adenocarcinoma cell lines (SW837 and HT29), as well as the cytotoxicity against a normal human colon cell line (CCD-18Co), to determine the selectivity of these derivatives to cancer cells. The results of this study are summarized in Table 2.

Table 2.
Antiproliferative activity of β-carbolines 13a-f and 1a-c against colorectal adenocarcinoma and normal colon cell lines.
From this study, we could confirm that the introduction of a triazole moiety directly attached to the β-carboline core has a negative impact on anticancer activity. In fact, it was observed that the 3-triazolyl-β-carboline derivatives 13a–f were not active against the SW837 and HT29 cancer cell lines, nor did they show cytotoxicity against normal colon cell lines.
Interestingly, a different activity profile was observed with the 3-tetrazolyl-β-carboline derivatives 1a–c. These β-carbolines proved to be active against the HT29 cells, showing IC50 values ranging from 5.9 to 9.6 µM. However, moderate activity was observed against the SW837 cells (IC50 from 29 µM to 44 µM).
The study of the cytotoxicity against a normal colon cell line (CCD-18Co) revealed that 3-tetrazolyl-β-carboline 1a has the highest selectivity for cancer cells, showing an IC50 value of 26 µM in these cells. In fact, this selectivity of compound 1a to cancer cells was further evidenced in the normal human foreskin fibroblasts (HFF-1) cell line, in which compound 1a presented an IC50 value of 29 μM (Table 3). Moreover, the results showed that the antiproliferative activity of compound 1a was associated with apoptotic cell death (Figure 4).

Table 3.
Antiproliferative activity of β-carboline 1a against pancreatic, melanoma, hepatocarcinoma, and breast cancer cells.
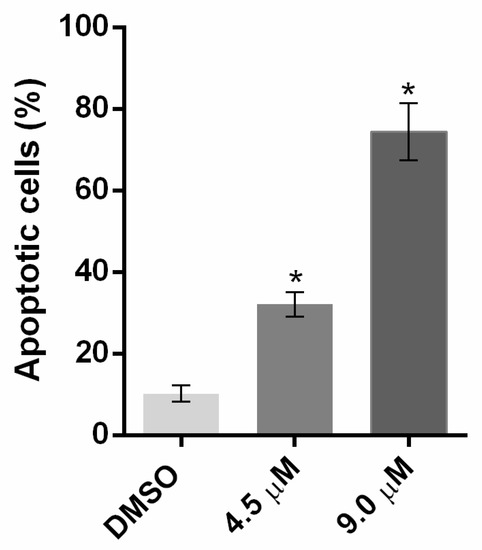
Figure 4.
Effect of compound 1a on apoptosis in HCT116 after 48 h of treatment; the percentage of Annexin-positive cells was analyzed by flow cytometry using PI and Annexin-V staining; data are mean ± SEM of 2 independent experiments. Values are significantly different from DMSO (* p < 0.05, unpaired Student’s t-test).
The exact mechanism of action for these molecules was not studied; however, the selectivity of these compounds against cancer cells may be explained by the ability of β-carboline derivatives to bind to the DNA by intercalation. In fact, the DNA intercalation of β-carboline derivatives is associated with structural damage to the DNA of cancer cells, as well as with an inhibition of the DNA repair mechanism, inducing apoptosis [18,56,57].
Taking this into account, the anticancer activity of compound 1a was further analyzed by testing its antiproliferative activity against distinct cell lines from distinct tumor tissues. As observed in Table 3, compound 1a showed IC50 values below 8 μM against pancreatic carcinoma PANC-1, melanoma A375, hepatocarcinoma HEPG2, and breast adenocarcinoma MCF-7 cell lines. These results indicate that compound 1a may be highly effective against distinct cancer types.
3. Experimental Section
3.1. Chemistry
3.1.1. General Information
NMR spectra were recorded on a Bruker Avance III instrument, operating at 400 MHz (1H) or 100 MHz (13C). Chemical shifts are expressed in ppm relative to tetramethylsilane (TMS), and coupling constants (J) are in Hz. Infrared spectra (IR) were recorded using a Fourier Transform spectrometer coupled with a diamond Attenuated Total Reflectance (ATR) sampling accessory. High-resolution mass spectra (HRMS) were obtained on a TOF VG Autospect M spectrometer with electrospray ionization (ESI). Melting points were recorded in open glass capillaries. Thin Layer Chromatography (TLC) was performed using precoated silica gel plates. Flash chromatography was performed using silica gel 60 as a stationary phase. 3-Tetrazolyl-β-carbolines 1 [8], 3-triazolyl-α-bromooxime 10 [8], and β-carboline-3-carboxylates 14 [50] were prepared as described in the literature.
3.1.2. 1-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-2-(1H-indol-3-yl)ethanamine (11)
Oxime 10 (1.25 g, 2.13 mmol) was dissolved in the smallest amount of THF, 70% aqueous formic acid (40 mL) was added, and the solution was cooled to 0°C. Then, zinc powder (4.18 g, 63.9 mmol) was added portion-wise over 30 min. The reaction mixture was stirred for 24 h at room temperature. After this time, the mixture was filtered on a Celite pad and the celite was washed with ethyl acetate (3 × 15 mL). The filtrate was neutralized with a concentrated ammonia solution to pH 8, and then extracted with ethyl acetate (3 × 40 mL). The combined organic phases were washed with water, then dried over anhydrous Na2SO4, and the solvent evaporated off. The product was purified by flash chromatography [dichloromethane/methanol (90:10)], giving amine 11 as a brown oil (0.58 g, 81%). IR (ATR) 738, 828, 989, 1041, 1093, 1230, 1499, 1726, 2918, 3056, and 3141 cm−1. 1H NMR (CDCl3) δ: 3.10 (dd, J = 14.4 and 8.8 Hz, 1H), 3.38 (dd, J = 14.4 and 5.22 Hz, 1H), 3.42 (s, 1H), 4.53 (dd, J = 8.4 and 5.2 Hz, 1H), 6.98 (br s, 1H), 7.01–7.05 (m, 1H), 7.10–7.14 (m, 1H), 7.30 (br d, J = 8.0 Hz, 1H), 7.35-7.39 (m, 2H), 7.48-7.52 (m, 3H), 7.74 (s, 1H), 8.93 (s, 1H). 13C NMR (CDCl3) δ: 34.3, 48.9, 111.2, 112.2, 118.8, 118.9, 119.6, 121.6, 122.2, 123.1, 127.6, 129.9, 134.3, 135.7, 136.3, 153.3. HRMS (ESI-TOF) m/z for C18H17ClN5 [M+H+] calculated 338.1167, and found 338.1162.
1H NMR spectrum and 13C NMR spectrum of amine 11 are shown in Figure S1 and S2.
3.1.3. General Procedure for the Synthesis of Tetrahydro-β-carbolines 12
Trifluoroacetic acid (2 equiv.) was added to a solution of amine 11 (1 equiv.) and the appropriate aldehyde (1 equiv.) to dry dichloromethane (8 mL/mmol). The reaction mixture was stirred at room temperature, monitored by TLC, until all of the amine 11 was consumed. Upon completion, the reaction mixture was concentrated under reduced pressure, and the residue was dissolved in ethyl acetate (7 mL) and neutralized with an aqueous solution of Na2CO3 10%. The resulting solution was extracted with ethyl acetate (3 × 25 mL), dried over anhydrous Na2SO4, and the solvent evaporated off. The products were purified by recrystallization in MeOH and obtained as a mixture of cis/trans isomers, unless otherwise stated.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-tetrahydro-β-carboline (12a)
This was obtained from the reaction of amine 11 (72 mg, 0.21 mmol) with benzaldehyde (22 μL, 0.21 mmol), as described in the general procedure (reaction time: 28 h), as a mixture of cis/trans isomers [65 mg, 71%, (17:83)]. Major component (trans-12a): IR (ATR): 748, 828, 1049, 1222, 1499 and 3398 cm−1. 1H NMR (Acetone-d6) δ: 3.10 (ddd, J = 14.8, 10.8 and 2.4 Hz, 1H), 3.30 (ddd, J = 15.0, 4.0 and 2.0 Hz, 1H), 4.54 (dd, J = 10.8 and 3.6 Hz, 1H), 5.46 (s, 1H), 7.00–7.05 (m, 2H), 7.26–7.28 (m, 1H), 7.31–7.35 (m, 3H), 7.44–7.47 (m, 2H), 7.50–7.52 (m, 1H), 7.61–7.64 (m, 2H), 7.96–8.00 (m, 2H), 8.60 (s, 1H), 9.53 (br s, 1H). HRMS (ESI-TOF) m/z for C25H21ClN5 [M+H+] calculated 426.1480, and found 426.1473.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(p-fluorophenyl)-tetrahydro-β-carboline (12b)
This was obtained from the reaction of amine 11 (73 mg, 0.22 mmol) with p-fluorobenzaldehyde (24 μL, 0.22 mmol), as described in the general procedure (reaction time: 40 h), as a mixture of cis/trans isomers [61 mg, 63%, (14:86)]. Major component (trans-12b): IR (ATR): 749, 832, 1137, 1204, 1500, 1655 and 3456 cm−1. 1H NMR (Acetone-d6) δ: 3.06–3.14 (m, 1H), 3.29 (ddd, J = 14.8, 4.0 and 1.6 Hz, 1H), 4.54 (dd, J = 10.8 and 4.0 Hz, 1H), 5.49 (s, 1H), 7.00–7.13 (m, 4H), 7.25–7.28 (m, 1H), 7.47–7.52 (m, 3H), 7.61–7.65 (m, 2H), 7.96–8.00 (m, 2H), 8.60 (s, 1H), 9.55 (br s, 1H). HRMS (ESI-TOF) m/z for C25H20ClFN5 [M+H+] calculated 444.1386, and found 444.1389.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(p-methoxyphenyl)-tetrahydro-β-carboline (12c)
This was obtained from the reaction of amine 11 (44 mg, 0.13 mmol) with p-methoxybenzaldehyde (15 μL, 0.13 mmol), as described in the general procedure (reaction time: 40 h). The product was purified by flash chromatography [ethyl acetate/hexane (1:1)] and obtained as a mixture of cis/trans isomers [45 mg, 77%, (12:88)]. Major component (trans-12c): IR (ATR): 746, 830, 1136, 1183, 1203, 1502, 1672 and 2839 cm−1. 1H NMR (Acetone-d6) δ: 3.09 (ddd, J = 14.8, 10.8 and 2.4 Hz, 1H), 3.29 (ddd, J = 14.8, 3.6 and 1.6 Hz, 1H), 3.79 (s, 3H), 4.53 (dd, J = 11.2 and 3.6 Hz, 1H), 5.41 (s, 1H), 6.88–6.92 (m, 2H), 6.99–7.05 (m, 2H), 7.27–7.29 (m, 1H), 7.33–7.37 (m, 2H), 7.49–7.52 (m, 1H), 7.60–7.64 (m, 2H), 7.95–7.99 (m, 2H), 8.58 (s, 1H), 9.47 (s, 1H). HRMS (EI-TOF) m/z for C26H23ClN5O [M+H+] calculated 456.1586, and found 456.1580.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(p-dimethylaminophenyl)-tetrahydro-β-carboline (12d)
This was obtained from the reaction of amine 11 (89 mg, 0.26 mmol) with p-(dimethylamino)benzaldehyde (39 mg, 0.26 mmol), as described in the general procedure (reaction time: 58 h). The product was purified by flash chromatography [ethyl acetate/hexane (1:1)] and obtained as a mixture of cis/trans isomers [43 mg, 36%, (30:70)]. Major component (trans-12d): IR (ATR): 739, 829, 1035, 1094, 1438, 1500, 1654 cm−1. 1H NMR (Acetone-d6) δ: 2.92 (s, 6H), 3.05–3.09 (m, 1H), 3.27–3.32 (m, 1H), 4.50–4.53 (m, 1H), 5.32 (s, 1H), 6.67–6.73 (m, 2H), 7.02–7.07 (m, 2H), 7.22–7.25 (m, 2H), 7.29–7.32 (m, 1H), 7.50–7.53 (m, 1H), 7.59–7.63 (m, 2H), 7.93–7.97 (m, 2H), 8.55 (s, 1H), 9.46 (s, 1H). HRMS (ESI-TOF) m/z for C27H26ClN6 [M+H+] calculated 469.1902, and found 469.1898.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(m-nitrophenyl)-tetrahydro-β-carboline (12e)
This was obtained from the reaction of amine 11 (79 mg, 0.24 mmol) with m-nitrobenzaldehyde (36 mg, 0.24 mmol), as described in the general procedure (reaction time: 28 h), as a mixture of cis/trans isomers [67 mg, 61%, (5:95)]. Major component (trans-12e): IR (ATR): 747, 829, 1029, 1094, 1229, 1500 and 3307 cm−1. 1H NMR (Acetone-d6) δ: 3.16 (ddd, J = 14.8, 10.8 and 2.4 Hz, 1H), 3.30 (ddd, J = 15.2, 4.0 and 2.0 Hz, 1H), 4.60 (dd, J = 10.8 and 4.0 Hz, 1H), 5.69 (s, 1H), 7.01–7.08 (m, 2H), 7.23–7.25 (m, 1H), 7.52–7.54 (m, 1H), 7.62–7.67 (m, 3H), 7.93–7.99 (m, 3H), 8.19 (ddd, J = 8.4, 2.4 and 1.2 Hz, 1H), 8.35–8.36 (m, 1H), 8.65 (s, 1H), 9.69 (br s, 1H). 13C NMR (Acetone-d6) δ: 52.4, 59.4, 109.7, 112.0, 118.8, 119.8, 120.4, 122.2, 122.5, 123.6, 124.5, 128.1, 130.6, 130.7, 134.3, 135.4, 136.3, 137.1, 137.9, 145.9, 149.3, 152.5. HRMS (ESI-TOF) m/z for C25H20ClN6O2 [M+H+] calculated 471.1331, and found 471.1323.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(o-chlorophenyl)-tetrahydro-β-carboline (12f)
This was obtained from the reaction of amine 11 (133 mg, 0.39 mmol) with o-chlorobenzaldehyde (44 μL, 0.39 mmol), as described in the general procedure (reaction time: 40 h). The product was purified by flash chromatography [ethyl acetate/hexane (1:2)] and obtained as a mixture of cis/trans isomers [70 mg, 39%, (50:50)]. Component trans-12f: IR (ATR): 739, 830, 989, 1036, 1095, 1438, 1500, 2858 and 3281 cm−1. 1H NMR (Acetone-d6) δ 3.09–3.17 (m, 1H), 3.29–3.36 (m, 1H), 4.57 (dd, J = 10.6 and 4.0 Hz, 1H), 5.99 (s, 1H), 6.91 (dd, J = 8.0 and 2.0 Hz, 1H), 7.02–7.20 (m, 2H), 7.22–7.31 (m, 2H), 7.34–7.47 (m, 2H), 7.52–7.59 (m, 3H), 7.90–7.94 (m, 2H), 8.55 (s, 1H), 9.66 (s, 1H). HRMS (ESI-TOF) m/z for C25H20Cl2N5 [M+H+] calculated 460.1090, and found 460.1082.
1H NMR spectrum and 13C NMR spectrum of tetrahydro-β-carbolines 12a-f are shown in Figures S3–S14.
3.1.4. General Procedure for the Synthesis of β-Carbolines 13
Sulfur powder (3 equiv.) was added to a solution of the appropriate tetrahydro-β-carboline 12 (1 equiv.) in dimethylformamide (20 mL/mmol). The reaction mixture was stirred under reflux for 24 h. After this time, the solvent was evaporated off. The product was precipitated by addition of diethyl ether, then filtered and dried under vacuum to obtain the products in a pure form, unless otherwise stated.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-β-carboline (13a)
This was obtained from tetrahydro-β-carboline 12a (81 mg, 0.19 mmol) as a dark yellow solid (28 mg, 51%). mp 207.5–207.9 °C (from diethyl ether). IR (ATR): 738, 826, 1027, 1098, 1496, 1630 and 1670 cm−1. 1H NMR (DMSO-d6) δ: 7.29–7.33 (m, 1H), 7.56–7.61 (m, 2H), 7.65–7.72 (m, 5H), 8.12–8.18 (m, 4H), 8.42 (d, J = 8.0 Hz, 1H), 8.88 (s, 1H), 9.31 (s, 1H), 11.69 (s, 1H). 13C NMR (DMSO-d6) δ: 110.3, 112.6, 119.9, 120.3, 121.0, 121.8, 122.0, 128.6, 128.7, 128.8, 128.9, 129.8, 130.3, 132.6, 132.9, 135.6, 137.7, 138.7, 141.7, 141.8, 149.5. HRMS (ESI-TOF) m/z for C25H17ClN5 [M+H+] calculated 422.1167, and found 422.1164.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(p-fluorophenyl)-β-carboline (13b)
This was obtained from tetrahydro-β-carboline 12b (72 mg, 0.16 mmol) as a dark yellow solid (36 mg, 50%). mp 237.3–238.1°C (from diethyl ether). IR (ATR): 738, 829, 1037, 1094, 1199, 1401, 1498, 1630, 1655 and 3062 cm−1. 1H NMR (DMSO-d6) δ: 7.31 (t, J = 8.0 Hz, 1H), 7.46–7.52 (m, 2H), 7.58–7.62 (m, 1H), 7.67–7.72 (m, 3H), 8.12–8.16 (m, 2H), 8.21–8.24 (m, 2H), 8.43 (d, J = 8.0 Hz, 1H), 8.87 (s, 1H), 9.32 (s, 1H), 11.69 (s, 1H). 13C NMR (DMSO-d6) δ: 110.3, 112.6, 115.6 (d, J = 21.3 Hz), 120.0, 120.3, 121.0, 121.8, 122.0, 128.7, 129.8, 130.5, 130.9 (d, J = 8.4 Hz), 132.6, 132.9, 134.1 (d, J = 2.7 Hz), 135.5, 138.6, 140.7, 141.8, 149.3, 162.6 (d, J = 244.4 Hz). HRMS (ESI-TOF) m/z for C25H16ClFN5 [M+H+] calculated 440.1073, and found 440.1077.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(p-methoxyphenyl)-β-carboline (13c)
This was obtained from tetrahydro-β-carboline 12c (71 mg, 0.16 mmol). The product was purified by flash chromatography [ethyl acetate/hexane (1:1)] and obtained as a brown solid (26 mg, 37%). mp 219.7-221.3°C (from diethyl ether). IR (ATR): 738, 826, 1023, 1093, 1170, 1498, 1607, 1628 and 3036 cm−1. 1H NMR (Acetone-d6) δ: 3.91 (s, 3H), 7.12–7.15 (m, 2H), 7.33 (ddd, J = 8.0, 6.8 and 0.8 Hz, 1H), 7.57 (ddd, J = 8.0, 6.8 and 1.2 Hz, 1H), 7.64–7.69 (m, 3H), 8.07–8.10 (m, 2H), 8.11–8.14 (m, 2H), 8.39 (d, J = 8.0 Hz, 1H), 8.84 (s, 1H), 9.04 (s, 1H), 10.78 (s, 1H). 13C NMR (Acetone-d6) δ: 55.8, 110.3, 113.2, 115.0, 120.4, 120.9, 122.6, 122.7, 122.8, 129.2, 130.7, 130.8, 131.4, 132.0, 133.8, 134.3, 137.1, 140.8, 142.6, 143.2, 151.4, 161.3. HRMS (ESI-TOF) m/z for C26H19OClN5 [M+H+] calculated 452.1273, and found 452.1269.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(p-dimethylaminophenyl)-β-carboline (13d)
This was obtained from tetrahydro-β-carboline 12d (40 mg, 0.09 mmol) as an orange solid (20 mg, 48%). mp 244.2-245.6°C (from diethyl ether). IR (ATR): 747, 828, 1021, 1465, 1603 and 2800 cm−1. 1H NMR (DMSO-d6) δ: 2.85 and 3.08 (s, 6H), 6.90 and 7.02 (d, J = 8.0 Hz, 1H), 7.32-7.36 (m, 1H), 7.62-7.73 (m, 4H), 7.94–8.08 (m, 4H), 8.42 (d, J = 6.8 Hz, 1H), 8.87 and 8.88 (s, 1H), 9.39 and 9.42 (s, 1H), 11.94 and 12.00 (s, 1H). 13C NMR (DMSO-d6) δ: 34.4, 110.4, 112.3, 112.9, 120.4, 120.8, 121.2, 121.8, 122.3, 129.4, 129.9, 130.2, 130.5, 131.0, 132.0, 133.2, 135.3, 141.3, 142.6, 151.1. HRMS (ESI-TOF) m/z for C27H22ClN6 [M+H+] calculated 465.1589, and found 465.1584.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(m-nitrophenyl)-β-carboline (13e)
This was obtained from tetrahydro-β-carboline 12e (33 mg, 0.07 mmol). The product was purified by flash chromatography [ethyl acetate/hexane (1:1)] and obtained as a yellow solid (17 mg, 52%). mp 237.3-239.1°C (from diethyl ether). IR (ATR): 737, 827, 1026, 1093, 1244, 1346, 1400, 1499 and 1526 cm−1. 1H NMR (DMSO-d6) δ: 7.33 (ddd, J = 7.6, 6.4 and 0.8 Hz, 1H), 7.61 (ddd, J = 7.6, 6.8 and 0.8 Hz, 1H), 7.66-7.72 (m, 3H), 7.94 (t, J = 8.0 Hz, 1H), 8.10–8.14 (m, 2H), 8.41 (ddd, J = 8.4, 2.4 and 0.8 Hz, 1H), 8.44 (d, J = 7.6 Hz, 1H), 8.57 (dt, J = 8.0 and 1.2 Hz, 1H), 8.85 (t, J = 1.6 Hz, 1H), 8.93 (s, 1H), 9.31 (s, 1H), 11.83 (s, 1H).13C NMR (DMSO-d6) δ: 111.1, 112.4, 120.1, 120.3, 121.0, 121.8, 122.1, 123.2, 123.4, 128.8, 129.8, 130.3, 130.7, 132.9, 132.9, 135.1, 135.5, 1329.2, 139.4, 139.4, 141.7, 148.2, 149.3. HRMS (ESI-TOF) m/z for C25H16O2ClN6 [M+H+] calculated 467.1018, and found 467.1017.
3-(1-(p-Chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(o-chlorophenyl)-β-carboline (13f)
This was obtained from tetrahydro-β-carboline 12f (45 mg, 0.10 mmol) as a dark yellow solid (18 mg, 40%). mp 219.7-221.3°C (from diethyl ether). IR (ATR): 738, 823, 1037, 1090, 1498, 1625 and 3070 cm−1. 1H NMR (DMSO-d6) δ: 7.27-7.33 (m, 1H), 7.57-7.73 (m, 8H), 8.11–8.14 (m, 2H), 8.43 (d, J = 8.0 Hz, 1H), 8.94 (s, 1H), 9.23 (s, 1H), 11.47 (s, 1H). 13C NMR (DMSO-d6) δ: 110.8, 112.2, 119.7, 120.0, 120.9, 121.7, 122.1, 127.4, 128.6, 129.2, 129.7, 129.8, 130.4, 132.0, 132.6, 132.8, 133.6, 135.5, 136.9, 138.6, 141.3, 141.5, 149.5. HRMS (ESI-TOF) m/z for C25H16Cl2N5 [M+H+] calculated 456.0777, and found 456.0776.
1H NMR and 13C NMR spectrum ofβ-carbolines 13a-f are shown in Figures S15-S26.
3.2. Anticancer Activity
3.2.1. Human Cell Lines
Human colorectal adenocarcinoma HCT116 cell lines expressing p53 and its p53-null isogenic derivative (HCT116 p53−/−) were provided by B. Vogelstein (The Johns Hopkins Kimmel Cancer Center, Baltimore, MD, USA). Human colorectal adenocarcinoma SW837, HT29, pancreatic carcinoma PANC-1, breast adenocarcinoma MCF-7, hepatocarcinoma HEPG2, melanoma A375, normal human foreskin fibroblasts HFF-1, and normal human colon CCD-18Co cell lines were purchased from ATCC (Rockville, MD, USA). The cancer cell lines were routinely cultured with RPMI-1640 medium from Biowest (HCT116, HCT116 p53−/−, SW837, HT29, A375, MCF-7, HEPG-2, and HFF-1) or DMEM with 5% glucose (HFF-1) and supplemented with 10% fetal bovine serum from Gibco (Alfagene, Lisboa, Portugal). All cells were maintained in a humidified incubator at 37 °C and 5% CO2.
3.2.2. Sulforhodamine B (SRB) Assay
Human cell lines were seeded in 96-well plates at a density of 5.0 × 103 (HCT116 p53 −/−, HCT116 wt, HT29, SW837, CCD-18Co, HFF-1, and MCF-7 cell lines) or 4.5 × 103 (A375, HEPG2 and PANC-1 cell lines) cells per well, for 24 h. Cells were treated with the appropriate compound, with serial dilutions ranging from 0.2 to 50 µM, for 48 h. Following the incubation, the effects on cell proliferation were evaluated through the SRB assay as stated by Ramos et al. [58]. The IC50 values were determined using the software GraphPad Prism version 9.0.
3.2.3. Annexin-V Assay
The analysis of apoptotic cell death was performed essentially as described by Ramos et al. [58]. Briefly, 1.5 × 105 HCT116 cells/well were seeded in 6-well plates, allowed to adhere overnight, and then treated with 4.5 and 9.0 μM of compound 1a. After 48 h of treatment, cells were stained using the Annexin V-FITC Apoptosis Detection Kit I from BD Biosciences (Enzifarma, Porto, Portugal), according to the manufacturer’s instructions. The AccuriTM C6 flow cytometer and the BD Accuri C6 software (BD Biosciences) were used.
4. Conclusions
The synthesis of β-carboline derivatives and their activity against several human colorectal adenocarcinoma cell lines has been disclosed. A synthetic route to novel 3-(1,2,3-triazol-4-yl)-β-carboline derivatives was established via the Pictet–Spengler approach using a tryptophan analog, where the carboxylic acid was replaced by a triazole moiety. In order to better evaluate and establish structure–activity relationships, methyl β-carboline-3-carboxylates and 3-tetrazolyl-β-carbolines were also synthesized from the corresponding tryptamine analogs.
The antiproliferative activity of the synthesized β-carbolines against colorectal cancer cells revealed that the 3-(1H-tetrazol-5-yl)-β-carbolines, particularly compound 1a, were the most active molecules, and that they act through a p53-independent apoptotic pathway. Moreover, compound 1a demonstrated a high selectivity to cancer cells, which may be attributed to the DNA intercalating ability of β-carbolines, with subsequent inhibition of DNA repair mechanisms, in cancer cell lines.
The disclosed results also indicate that the presence of a triazole moiety at β-carboline’s C-3 carbon severely hinders the overall biological activity of these heterocycles, while a tetrazole moiety at the same position greatly increases the cytotoxicity against human colon cancer cell lines when compared with the methyl ester β-carboline derivatives.
Further studies on the 3-tetrazolyl-β-carboline derivative, with the highest selectivity for cancer cells, unveiled an interesting anticancer profile by targeting cancer cells from distinct tissues.
This study, therefore, unveils a promising anticancer agent worthy of being explored in future works.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15121510/s1, Figure S1: 1H NMR spectrum of amine 11, Figure S2: 13C NMR spectrum of amine 11. Figures S3–S14: 1H NMR and 13C NMR spectrum of tetrahydro-β-carbolines 12a-f. Figures S15–S26: 1H NMR and 13C NMR spectrum of β-carbolines 13a-f.
Author Contributions
Conceptualization, S.M.M.L., L.S. and T.M.V.D.P.e.M.; methodology, J.L.P.R. and J.B.L.; formal analysis, S.M.M.L., L.S. and T.M.V.D.P.e.M.; investigation, J.L.P.R. and J.B.L.; writing—original draft preparation, J.L.P.R.; writing—review and editing, S.M.M.L., L.S. and T.M.V.D.P.e.M.; supervision, S.M.M.L., L.S. and T.M.V.D.P.e.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Fundação para a Ciência e Tecnologia” (FCT) within the scope of the projects UIDB/00313/2020, UIDP/00313/2020 (National Funds), UID/QUI/50006/2020 (LAQV/REQUIMTE), and IMS complementary funds. Project PTDC/QUI-QOR/0103/2021 was financed by FCT, I.P./MCTES, by national funds (PIDDAC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge to the Coimbra Chemistry Centre-Institute of Molecular Sciences (CQC-IMS), supported by the Portuguese Agency for Scientific Research, “Fundação para a Ciência e a Tecnologia” (FCT). João L. P. Ribeiro thanks FCT for the PhD fellowship PD/BD/143160/2019 (MedChemTrain Programme). We also acknowledge the Nuclear Magnetic Resonance Laboratory of the Coimbra Chemistry Centre, University of Coimbra, for obtaining the NMR spectroscopic data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ashok, P.; Chander, S.; Balzarini, J.; Pannecouque, C.; Murugesan, S. Design, synthesis of new β-carboline derivatives and their selective anti-HIV-2 activity. Bioorg. Med. Chem. Lett. 2015, 25, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Faheem; Kumar, K.B.; Sekhar, G.C.K.V.; Kunjiappan, S.; Jamalis, J.; Balaña-Fouce, R.; Sankaranarayanan, M. Recent Update on the Anti-infective Potential of β-carboline Analogs. Mini-Rev. Med. Chem. 2021, 21, 398–425. [Google Scholar] [CrossRef]
- Wang, J.; Gong, F.; Liang, T.; Xie, Z.; Yang, Y.; Cao, C.; Gao, J.; Lu, T.; Chen, X. A review of synthetic bioactive tetrahydro-β-carbolines: A medicinal chemistry perspective. Eur. J. Med. Chem. 2021, 225, 113815. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana Reddy, P.O.; Hridhay, M.; Nikhil, K.; Khan, S.; Jha, P.N.; Shah, K.; Kumar, D. Synthesis and investigations into the anticancer and antibacterial activity studies of β-carboline chalcones and their bromide salts. Bioorg. Med. Chem. Lett. 2018, 28, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Gorki, V.; Walter, N.S.; Singh, R.; Chauhan, M.; Dhingra, N.; Salunke, D.B.; Kaur, S. β-Carboline Derivatives Tackling Malaria: Biological Evaluation and Docking Analysis. ACS Omega 2020, 5, 17993–18006. [Google Scholar] [CrossRef]
- Szabó, T.; Volk, B.; Milen, M. Recent Advances in the Synthesis of β-Carboline Alkaloids. Molecules 2021, 26, 663. [Google Scholar] [CrossRef]
- Panice, M.R.; Lopes, S.M.M.; Figueiredo, M.C.; Goes Ruiz, A.L.T.; Foglio, M.A.; Nazari Formagio, A.S.; Sarragiotto, M.H.; Pinho e Melo, T.M.V.D. New 3-tetrazolyl-β-carbolines and β-carboline-3-carboxylates with anti-cancer activity. Eur. J. Med. Chem. 2019, 179, 123–132. [Google Scholar] [CrossRef]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The importance of indole and azaindole scaffold in the development of antitumor agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Culjat, M.; Huizenga, M.N.; Forcelli, P.A. Age-dependent anticonvulsant actions of perampanel and brivaracetam in the methyl-6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate (DMCM) model of seizures in developing rats. Pharmacol. Rep. 2021, 73, 296–302. [Google Scholar] [CrossRef]
- Farzin, D.; Mansouri, N. Antidepressant-like effect of harmane and other β-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 2006, 16, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Tewari, U.; Sharma, D.; Srivastava, S.; Kumar, B.K.; Faheem; Murugesan, S. Anti-Tubercular Insights of Carbolines—A Decade Critique. ChemistrySelect 2021, 6, 2428–2445. [Google Scholar] [CrossRef]
- Pavić, K.; Beus, M.; Poje, G.; Uzelac, L.; Kralj, M.; Rajić, Z. Synthesis and Biological Evaluation of Harmirins, Novel Harmine–Coumarin Hybrids as Potential Anticancer Agents. Molecules 2021, 26, 6490. [Google Scholar] [CrossRef] [PubMed]
- Ayipo, Y.O.; Osunniran, W.A.; Mordi, M.N. Metal complexes of β-carboline: Advances in anticancer therapeutics. Coord. Chem. Rev. 2021, 432, 213746. [Google Scholar] [CrossRef]
- Luo, B.; Song, X. A comprehensive overview of β-carbolines and its derivatives as anticancer agents. Eur. J. Med. Chem. 2021, 224, 113688. [Google Scholar] [CrossRef]
- Pontes, O.; Oliveira-Pinto, S.; Baltazar, F.; Costa, M. Renal cell carcinoma therapy: Current and new drug candidates. Drug Discov. Today 2022, 27, 304–314. [Google Scholar] [CrossRef]
- Soni, J.P.; Yeole, Y.; Shankaraiah, N. β-Carboline-based molecular hybrids as anticancer agents: A brief sketch. RSC Med. Chem. 2021, 12, 730–750. [Google Scholar] [CrossRef]
- Aaghaz, S.; Sharma, K.; Jain, R.; Kamal, A. β-Carbolines as potential anticancer agents. Eur. J. Med. Chem. 2021, 216, 113321. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, K.; Kumar, V. Recent insights into synthetic β-carbolines with anti-cancer activities. Eur. J. Med. Chem. 2017, 142, 48–73. [Google Scholar] [CrossRef]
- Maity, P.; Adhikari, D.; Jana, A.K. An overview on synthetic entries to tetrahydro-β-carbolines. Tetrahedron 2019, 75, 965–1028. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Novais, J.S.; Costa, D.C.S.; Castro, H.C.; Figueiredo, A.M.S.; Ferreira, V.F.; Pinho e Melo, T.M.V.D.; da Silva, F.d.C. Hetero-Diels-Alder reactions of novel 3-triazolyl-nitrosoalkenes as an approach to functionalized 1,2,3-triazoles with antibacterial profile. Eur. J. Med. Chem. 2018, 143, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.M.; Lemos, A.; Melo, T.M.V.D.P.e. A hetero-Diels–Alder approach to functionalized 1H-tetrazoles: Synthesis of tetrazolyl-1,2-oxazines, -oximes and 5-(1-aminoalkyl)-1H-tetrazoles. Tetrahedron Lett. 2010, 51, 6756–6759. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Palacios, F.; Lemos, A.; Pinho e Melo, T.M.V.D. Diels–Alder reactions of 3-(1H-tetrazol-5-yl)-nitrosoalkenes: Synthesis of functionalized 5-(substituted)-1H-tetrazoles. Tetrahedron 2011, 67, 8902–8909. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Nunes, S.C.C.; Carato, C.C.; Pais, A.A.C.C.; Pinho e Melo, T.M.V.D. Reactivity of 1-arylnitrosoethylenes towards indole derivatives. Monatsh. Chem. 2016, 147, 1565–1573. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Cardoso, A.L.; Lemos, A.; Pinho e Melo, T.M.V.D. Recent Advances in the Chemistry of Conjugated Nitrosoalkenes and Azoalkenes. Chem. Rev. 2018, 118, 11324–11352. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, B.; Mehra, V.; Kumar, V. Recent accomplishments on the synthetic/biological facets of pharmacologically active 1H-1,2,3-triazoles. Eur. J. Med. Chem. 2021, 212, 113069. [Google Scholar] [CrossRef]
- Kumar, S.; Khokra, S.L.; Yadav, A. Triazole analogues as potential pharmacological agents: A brief review. Future J. Pharm. Sci. 2021, 7, 106. [Google Scholar] [CrossRef]
- Dixit, D.; Verma, P.K.; Marwaha, R.K. A review on ‘triazoles’: Their chemistry, synthesis and pharmacological potentials. J. Iran Chem. Soc. 2021, 18, 2535–2565. [Google Scholar] [CrossRef]
- Forezi, L.S.M.; Lima, C.G.S.; Amaral, A.A.P.; Ferreira, P.G.; Souza, M.C.B.V.; Cunha, A.C.; Silva, F.C.d.; Ferreira, V.F. Bioactive 1,2,3-Triazoles: An Account on their Synthesis, Structural Diversity and Biological Applications. Chem. Rec. 2021, 21, 1–27. [Google Scholar] [CrossRef]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef]
- Saroha, B.; Kumar, G.; Kumar, R.; Kumari, M.; Kumar, S. A minireview of 1,2,3-triazole hybrids with O-heterocycles as leads in medicinal chemistry. Chem. Biol. Drug Des. 2022, 100, 843–869. [Google Scholar] [CrossRef] [PubMed]
- Selig, D.J.; DeLuca, J.P.; Chung, K.K.; Pruskowski, K.A.; Livezey, J.R.; Nadeau, R.J.; Por, E.D.; Akers, K.S. Pharmacokinetics of piperacillin and tazobactam in critically Ill patients treated with continuous kidney replacement therapy: A mini-review and population pharmacokinetic analysis. J. Clin. Pharm. Ther. 2022, 47, 1091–1102. [Google Scholar] [CrossRef]
- Coenen, S.; Ferech, M.; Dvorakova, K.; Hendrickx, E.; Suetens, C.; Goossens, H.; ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient cephalosporin use in Europe. J. Antimicrob. Chemother. 2006, 58, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Wang, J.; Cheng, Y.; Shi, J.; Cui, L.; Zhang, H.; Huang, Y.; Liu, W.; Chen, L.; Zhu, J.; et al. A phase III, randomized, double-blind, controlled trial of carboxyamidotriazole plus chemotherapy for the treatment of advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2020, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.; Tratrat, C.; Kochkar, H.; Nair, B.A. Recent Advances in the Development of 1,2,3-Triazole-containing Derivatives as Potential Antifungal Agents and Inhibitors of Lanoster ol 14α-Demethylase. Curr. Top. Med. Chem. 2021, 21, 462–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef]
- Upadhyay, C.H. Coumarin-1,2,3-triazole Hybrid Molecules: An Emerging Scaffold for Combating Drug Resistance. Curr. Top. Med. Chem. 2021, 21, 737–752. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J. The Antibacterial Activity of 1,2,3-triazole- and 1,2,4-Triazole-containing Hybrids against Staphylococcus aureus: An Updated Review (2020- Present). Curr. Top. Med. Chem. 2022, 22, 41–63. [Google Scholar] [CrossRef]
- Feng, L.-S.; Zheng, M.-J.; Zhao, F.; Liu, D. 1,2,3-Triazole hybrids with anti-HIV-1 activity. Arch. Pharm. 2021, 354, e2000163. [Google Scholar] [CrossRef]
- Chu, X.-M.; Wang, C.; Wang, W.-L.; Liang, L.-L.; Liu, W.; Gong, K.-K.; Sun, K.-L. Triazole derivatives and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2019, 166, 206–223. [Google Scholar] [CrossRef]
- Sachdeva, H.; Saquib, M.; Tanwar, K. Design and Development of Triazole Derivatives as Prospective Anticancer Agents: A Review. Anti-Cancer Agents Med. Chem. 2022, 22, 3269–3279. [Google Scholar] [CrossRef]
- Kala, N.; Rahate, P.K. Triazole as Potent Anti-cancer Agent—A Pharmacophoric Scaffold. Curr. Cancer Ther. Rev. 2022, 18, 95–117. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Bumbrah, S.G.; Chawla, A.P.; Gurnule, B.W.; Shejul, K.S. Recent Advances in Synthesis and Anticancer Potential of Triazole-Containing Scaffolds. Anti-Cancer Agents Med. Chem. 2022, 22, 2852–2875. [Google Scholar] [CrossRef]
- Qin, B.; Bai, Q.; Yan, D.; Yin, F.; Zhu, Z.; Xia, C.; Yang, Y.; Zhao, Y. Discovery of novel mRNA demethylase FTO inhibitors against esophageal cancer. J. Enzym. Inhib. Med. Chem. 2022, 37, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.S.; Abell, A. 1,2,3-Triazoles in Peptidomimetic Chemistry. Eur. J. Org. Chem. 2011, 2399–2411. [Google Scholar] [CrossRef]
- Salehi, P.; Babanezhad-Harikandei, K.; Bararjanian, M.; Al-Harrasi, A.; Esmaeili, M.-A.; Aliahmadi, A. Synthesis of novel 1,2,3-triazole tethered 1,3-disubstituted β-carboline derivatives and their cytotoxic and antibacterial activities. Med. Chem. Res. 2016, 25, 1895–1907. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Jadala, C.; Nekkanti, S.; Senwar, K.R.; Nagesh, N.; Shrivastava, S.; Naidu, V.G.M.; Sathish, M.; Kamal, A. Design and synthesis of C3-tethered 1,2,3-triazolo-β-carboline derivatives: Anticancer activity, DNA-binding ability, viscosity and molecular modeling studies. Bioorg. Chem. 2016, 64, 42–50. [Google Scholar] [CrossRef]
- Filali, I.; Belkacem, M.A.; Ben Nejma, A.; Souchard, J.P.; Ben Jannet, H.; Bouajila, J. Synthesis, cytotoxic, anti-lipoxygenase and anti-acetylcholinesterase capacities of novel derivatives from harmine. J. Enzym. Inhib. Med. Chem. 2016, 31, 23–33. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Tonin, L.T.D.; Foglio, M.A.; Madjarof, C.; de Carvalho, J.E.; da Costa, W.F.; Cardoso, F.P.; Sarragiotto, M.H. Synthesis and antitumoral activity of novel 3-(2-substituted-1,3,4-oxadiazol-5-yl) and 3-(5-substituted-1,2,4-triazol-3-yl) β-carboline derivatives. Biorg. Med. Chem. 2008, 16, 9660–9667. [Google Scholar] [CrossRef]
- Fischer, M. Census and evaluation of p53 target genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Vousden, K.H. Mutant p53 in Cancer: New Functions and Therapeutic Opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Dai, F.; Chen, Y.; Song, Y.; Huang, L.; Zhai, D.; Dong, Y.; Lai, L.; Zhang, T.; Li, D.; Pang, X.; et al. A Natural Small Molecule Harmine Inhibits Angiogenesis and Suppresses Tumour Growth through Activation of p53 in Endothelial Cells. PLoS ONE 2012, 7, e52162. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Xu, C.; Luo, L.; Cao, J.; Feng, J.; Xue, Y.; Zhu, Q.; Ju, C.; Li, F.; Zhang, Y.; et al. Novel β-Carboline/Hydroxamic Acid Hybrids Targeting Both Histone Deacetylase and DNA Display High Anticancer Activity via Regulation of the p53 Signaling Pathway. J. Med. Chem. 2015, 58, 9214–9227. [Google Scholar] [CrossRef] [PubMed]
- Mota, N.S.R.S.; Kviecinski, M.R.; Felipe, K.B.; Grinevicius, V.M.A.S.; Siminski, T.; Almeida, G.M.; Zeferino, R.C.; Pich, C.T.; Filho, D.W.; Pedrosa, R.C. β-carboline alkaloid harmine induces DNA damage and triggers apoptosis by a mitochondrial pathway: Study in silico, in vitro and in vivo. Int. J. Funct. Nutr. 2020, 1, 1. [Google Scholar] [CrossRef]
- Miao, J.; Meng, C.; Wu, H.; Shan, W.; Wang, H.; Ling, C.; Zhang, J.; Yang, T. Novel Hybrid CHC from β-carboline and N-Hydroxyacrylamide Overcomes Drug-Resistant Hepatocellular Carcinoma by Promoting Apoptosis, DNA Damage, and Cell Cycle Arrest. Front. Pharmacol. 2021, 11, 626065. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Soares, M.I.L.; Silva, J.; Raimundo, L.; Calheiros, J.; Gomes, C.; Reis, F.; Monteiro, F.A.; Nunes, C.; Reis, S.; et al. A selective p53 activator and anticancer agent to improve colorectal cancer therapy. Cell Rep. 2021, 35, 108982. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).