Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease
Abstract
1. Introduction
2. Renal Proximal Tubular Cells
2.1. Physiological Characteristics of Proximal Tubule Cells
2.2. Relationship with DKD Progression
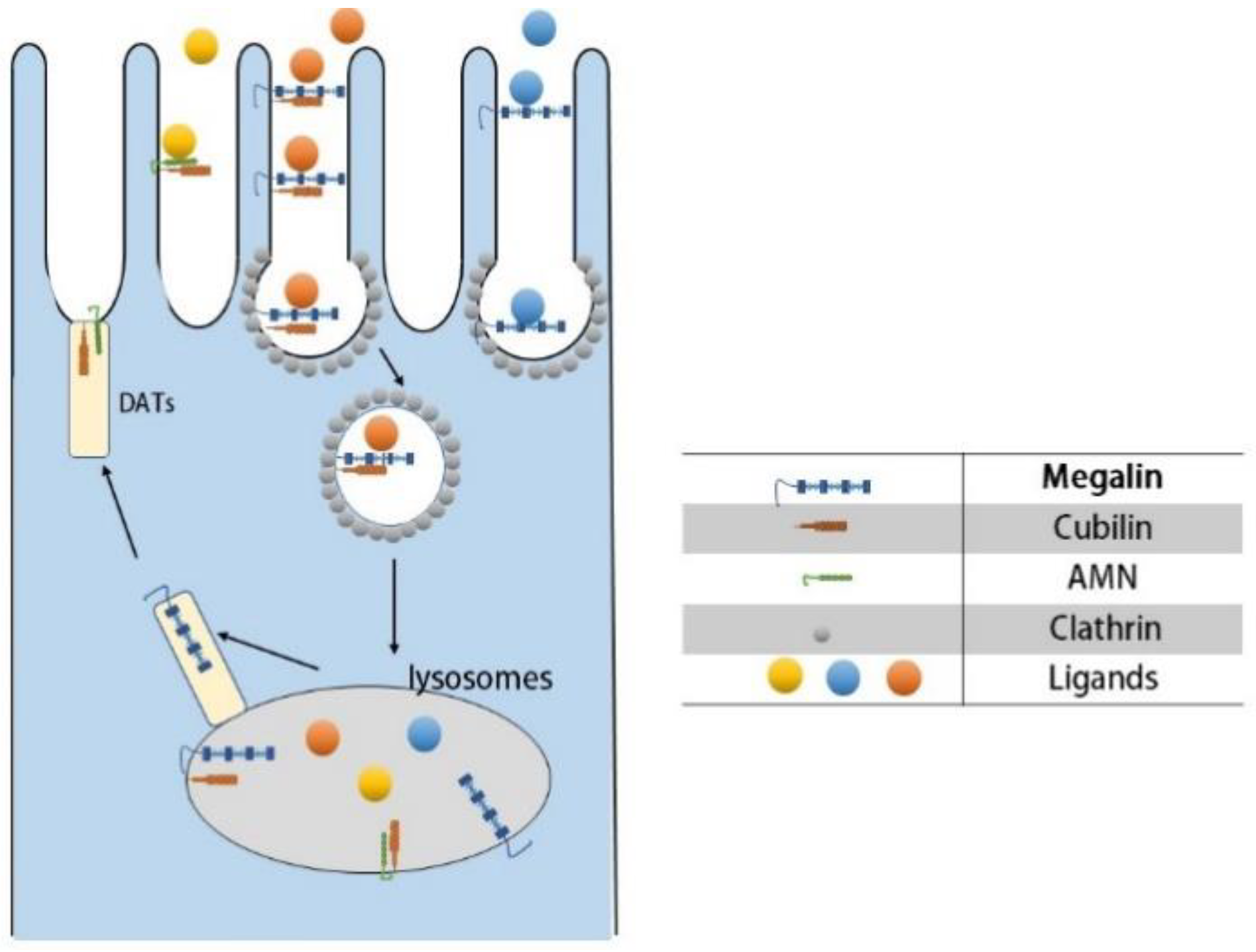
2.3. Mechanism of Proximal Tubule Uptake of Drugs
- (A)
- Ligand–receptor binding: Cubilin, due to its lack of transmembrane and cytoplasmic structural domains, must form a dual receptor complex with megelin or amnionless (AMN) for endocytosis to occur. Different ligands bind to the corresponding receptors with different affinities.
- (B)
- Vesicle transport: After receptor–ligand binding, lattice proteins wrap the vesicles and transport them to the corresponding organelles (e.g., lysosomes) for further processing.
- (C)
- Receptor recycling: After vesicle release of ligand, the receptor is recycled to the cell membrane by the apical tubules (DATs).
3. Targeted Drug Delivery Strategy
3.1. Protein-Based and Peptide-Based Carriers
3.2. Polymeric Carriers
3.3. Small-Molecule Prodrugs
3.4. Nanoparticles
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Zhang, W.; Chen, X.; Hsu, C.-Y. Trends in end-stage kidney disease in Shanghai, China. Kidney Int. 2019, 95, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Tang, S.C.-W.; Kashihara, N.; Kim, Y.-S.; Togtokh, A.; Yang, C.-W.; Zhao, M.-H. Disease burden and challenges of chronic kidney disease in North and East Asia. Kidney Int. 2018, 94, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; Deng, X.; Xiang, D.; Zhu, Y.; Xu, P.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front. Endocrinol. 2021, 12, 672350. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.; Stefánsson, B.; Reyner, D.; Rossing, P.; Sjöström, C.D.; Wheeler, D.C.; Langkilde, A.M.; Heerspink, H.J.L. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 429–441. [Google Scholar]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar]
- Mann, J.F.E.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef]
- Cherney, D.; Perkins, B.A.; Lytvyn, Y.; Heerspink, H.; Rodríguez-Ortiz, M.E.; Mischak, H. The effect of sodium/glucose cotransporter 2 (SGLT2) inhibition on the urinary proteome. PLoS ONE 2017, 12, e0186910. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Van Raalte, D.H.; Heerspink, H.L.; Cherney, D.Z.I. The New Biology of Diabetic Kidney Disease-Mechanisms and Therapeutic Implications. Endocr. Rev. 2020, 41, 202–231. [Google Scholar]
- Ravindran, S.; Munusamy, S. Renoprotective mechanisms of sodium-glucose co-transporter 2 (SGLT2) inhibitors against the progression of diabetic kidney disease. J. Cell. Physiol. 2022, 237, 1182–1205. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Liu, J.; Zhou, L. Luteolin Attenuates Diabetic Nephropathy through Suppressing Inflammatory Response and Oxidative Stress by Inhibiting STAT3 Pathway. Exp. Clin. Endocrinol. Diabetes 2021, 129, 729–739. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Liu, Z.; He, L. Adiponectin promotes repair of renal tubular epithelial cells by regulating mitochondrial biogenesis and function. Metabolism 2022, 128, 154959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Xiao, L.; Liu, G.; Sun, L.; He, L. STC-1 ameliorates renal injury in diabetic nephropathy by inhibiting the expression of BNIP3 through the AMPK/SIRT3 pathway. Lab. Invest. 2019, 99, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Peng, X.-F.; Liu, G.-Y.; Liu, J.-S.; Sun, L.; Liu, H.; Xiao, L.; He, L.-Y. Protein arginine methyltranferase-1 induces ER stress and epithelial-mesenchymal transition in renal tubular epithelial cells and contributes to diabetic nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Qiu, X.; He, L.; Liu, L. MicroRNA-122-5p ameliorates tubular injury in diabetic nephropathy via FIH-1/HIF-1α pathway. Ren. Fail. 2022, 44, 293–303. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug. Deliv. Rev. 2020, 158, 4–16. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Qiu, X.; Dong, S.; He, J.; Liu, J.; Xu, W.; Huang, S.; Hu, X.; Xiang, D.-X. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact. Mater. 2023, 20, 548–560. [Google Scholar] [CrossRef]
- Kleinmann, N.; Matin, S.F.; Pierorazio, P.M.; Gore, J.L.; Shabsigh, A.; Hu, B.; Chamie, K.; Godoy, G.; Hubosky, S.; Rivera, M.; et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): An open-label, single-arm, phase 3 trial. Lancet Oncol. 2020, 21, 776–785. [Google Scholar] [CrossRef]
- Haas, M.; Moolenaar, F.; Meijer, D.K.F.; de Zeeuw, D. Specific drug delivery to the kidney. Cardiovasc. Drugs Ther. 2002, 16, 489–496. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Renal function in diabetic disease models: The tubular system in the pathophysiology of the diabetic kidney. Annu. Rev. Physiol. 2012, 74, 351–375. [Google Scholar] [CrossRef]
- Qian, Y.; Feldman, E.; Pennathur, S.; Kretzler, M.; Brosius, F.C. From fibrosis to sclerosis: Mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 2008, 57, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Ponchiardi, C.; Mauer, M.; Najafian, B. Temporal profile of diabetic nephropathy pathologic changes. Curr. Diab. Rep. 2013, 13, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-P.; Hu, Y.; Lin, J.-C.; Fu, H.-L.; Lim, L.Y.; Yuan, Z.-X. Targeting strategies for drug delivery to the kidney: From renal glomeruli to tubules. Med. Res. Rev. 2019, 39, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Lan, R.; Liu, Y.; Chen, W.; Wu, M.; Saikumar, P.; Weinberg, J.M.; Venkatachalam, M.A. Proximal tubule LPA1 and LPA2 receptors use divergent signaling pathways to additively increase profibrotic cytokine secretion. Am. J. Physiol. Renal. Physiol. 2021, 320, F359–F374. [Google Scholar] [CrossRef] [PubMed]
- Stormark, T.A.; Strømmen, K.; Iversen, B.M.; Matre, K. Three-dimensional ultrasonography can detect the modulation of kidney volume in two-kidney, one-clip hypertensive rats. Ultrasound. Med. Biol. 2007, 33, 1882–1888. [Google Scholar] [CrossRef]
- Zou, Z.; Chung, B.; Nguyen, T.; Mentone, S.; Thomson, B.; Biemesderfer, D. Linking receptor-mediated endocytosis and cell signaling: Evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J. Biol. Chem. 2004, 279, 34302–34310. [Google Scholar] [CrossRef]
- Christensen, E.I.; Gburek, J. Protein reabsorption in renal proximal tubule-function and dysfunction in kidney pathophysiology. Pediatr. Nephrol. 2004, 19, 714–721. [Google Scholar] [CrossRef]
- Dai, H.; Liu, Q.; Liu, B. Research Progress on Mechanism of Podocyte Depletion in Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 2615286. [Google Scholar] [CrossRef]
- Finch, N.C.; Fawaz, S.S.; Neal, C.R.; Butler, M.J.; Lee, V.K.; Salmon, A.J.; Lay, A.C.; Stevens, M.; Dayalan, L.; Band, H.; et al. Reduced Glomerular Filtration in Diabetes Is Attributable to Loss of Density and Increased Resistance of Glomerular Endothelial Cell Fenestrations. J. Am. Soc. Nephrol. 2022, 33, 1120–1136. [Google Scholar] [CrossRef]
- Kim, D.; Li, H.Y.; Lee, J.H.; Oh, Y.S.; Jun, H.-S. Lysophosphatidic acid increases mesangial cell proliferation in models of diabetic nephropathy via Rac1/MAPK/KLF5 signaling. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Gurung, P.; Wang, T.; Li, L.; Zhang, R.; Li, H.; Guo, R.; Han, Q.; Zhang, J.; et al. The relationship between the thickness of glomerular basement membrane and renal outcomes in patients with diabetic nephropathy. Acta Diabetol. 2018, 55, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Krolewski, A.S. Progressive renal decline: The new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 2015, 38, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, R.; Kohara, Y.; Matsubayashi, K.; Kitazawa, R.; Kitazawa, S. New Insights into the Pathogenesis of Diabetic Nephropathy: Proximal Renal Tubules Are Primary Target of Oxidative Stress in Diabetic Kidney. Acta Histochem. Cytochem. 2020, 53, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E. Proximal Tubulopathy: Prime Mover and Key Therapeutic Target in Diabetic Kidney Disease. Diabetes 2017, 66, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Birn, H.; Storm, T.; Weyer, K.; Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 2012, 27, 223–236. [Google Scholar] [CrossRef]
- Kaissling, B.; Hegyi, I.; Loffing, J.; Le Hir, M. Morphology of interstitial cells in the healthy kidney. Anat. Embryol. 1996, 193, 303–318. [Google Scholar] [CrossRef]
- Li, A.S.; Ingham, J.F.; Lennon, R. Genetic Disorders of the Glomerular Filtration Barrier. Clin. J. Am. Soc. Nephrol. 2020, 15, 1818–1828. [Google Scholar] [CrossRef]
- Akilesh, S.; Huber, T.B.; Wu, H.; Wang, G.; Hartleben, B.; Kopp, J.B.; Miner, J.H.; Roopenian, D.C.; Unanue, E.R.; Shaw, A.S. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 967–972. [Google Scholar] [CrossRef]
- Sverrisson, K.; Axelsson, J.; Rippe, A.; Asgeirsson, D.; Rippe, B. Dynamic, size-selective effects of protamine sulfate and hyaluronidase on the rat glomerular filtration barrier In Vivo. Am. J. Physiol. Renal. Physiol. 2014, 307, F1136–F1143. [Google Scholar] [CrossRef]
- Naylor, R.W.; Morais MR, P.T.; Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 2021, 17, 112–127. [Google Scholar]
- Sever, S. Role of actin cytoskeleton in podocytes. Pediatr. Nephrol. 2021, 36, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Pavenstädt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Iyer, G.; Koh, A.L.; Cheng, Z.; Ebenstein, Y.; Aharoni, A.; Keren, S.; Bentolila, L.A.; Li, J.; Rao, J.; et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small 2009, 5, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Satchell, S.C.; Braet, F. Glomerular endothelial cell fenestrations: An integral component of the glomerular filtration barrier. Am. J. Physiol. Renal. Physiol. 2009, 296, F947–F956. [Google Scholar] [CrossRef]
- Rabelink, T.J.; Wijewickrama, D.C.; De Koning, E.J. Peritubular endothelium: The Achilles heel of the kidney? Kidney Int. 2007, 72, 926–930. [Google Scholar] [CrossRef]
- Shaw, I.; Rider, S.; Mullins, J.; Hughes, J.; Péault, B. Pericytes in the renal vasculature: Roles in health and disease. Nat. Rev. Nephrol. 2018, 14, 521–534. [Google Scholar] [CrossRef]
- Stan, R.V.; Kubitza, M.; Palade, G.E. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc. Natl. Acad. Sci. USA 1999, 96, 13203–13207. [Google Scholar] [CrossRef]
- Alcorn, D.; Maric, C.; Mccausland, J. Development of the renal interstitium. Pediatr. Nephrol. 1999, 13, 347–354. [Google Scholar] [CrossRef]
- Bearer, E.L.; Orci, L. Endothelial fenestral diaphragms: A quick-freeze, deep-etch study. J. Cell Biol. 1985, 100, 418–428. [Google Scholar] [CrossRef]
- Ivanyuk, A.; Livio, F.; Biollaz, J.; Buclin, T. Renal Drug Transporters and Drug Interactions. Clin. Pharmacokinet. 2017, 56, 825–892. [Google Scholar]
- Nigam, S.K. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Yaneff, A.; Sahores, A.; Gómez, N.; Carozzo, A.; Shayo, C.; Davio, C. MRP4/ABCC4 As a New Therapeutic Target: Meta-Analysis to Determine cAMP Binding Sites as a Tool for Drug Design. Curr. Med. Chem. 2019, 26, 1270–1307. [Google Scholar] [PubMed]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [PubMed]
- El-Sheikh, A.A.K.; Masereeuw, R.; Russel, F.G.M. Mechanisms of renal anionic drug transport. Eur. J. Pharmacol. 2008, 585, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Verroust, P.J.; Nielsen, R. Receptor-mediated endocytosis in renal proximal tubule. Pflug. Arch. 2009, 458, 1039–1048. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 256–266. [Google Scholar] [PubMed]
- Coudroy, G.; Gburek, J.; Kozyraki, R.; Madsen, M.; Trugnan, G.; Moestrup, S.K.; Verroust, P.J.; Maurice, M. Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex. J. Am. Soc. Nephrol. 2005, 16, 2330–2337. [Google Scholar] [CrossRef]
- Fyfe, J.C.; Madsen, M.; Højrup, P.; Christensen, E.I.; Tanner, S.M.; de la Chapelle, A.; He, Q.; Moestrup, S.K. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 2004, 103, 1573–1579. [Google Scholar] [CrossRef]
- Carney, E.F. Endocytosis in the proximal tubule. Nat. Rev. Nephrol. 2019, 15, 2. [Google Scholar] [CrossRef]
- Hall, A.M.; Polesel, M.; Berquez, M. The proximal tubule, protein uptake, and the riddle of the segments. Kidney Int. 2021, 99, 803–805. [Google Scholar] [CrossRef]
- Leheste, J.R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Aucouturier, P.; Moskaug, J.O.; Otto, A.; Christensen, E.I.; et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 1999, 155, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Hao, C.; Zhai, R.; Yao, W. Folate and macrophage folate receptor-β in idiopathic pulmonary fibrosis disease: The potential therapeutic target? Biomed. Pharmacother. 2020, 131, 110711. [Google Scholar] [PubMed]
- Liu, J.; Chen, H.; Liu, Y.; Shen, Y.; Meng, F.; Kaniskan, H.Ü.; Jin, J.; Wei, W. Cancer Selective Target Degradation by Folate-Caged PROTACs. J. Am. Chem. Soc. 2021, 143, 7380–7387. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Kluppel, A.C.; Wartna, E.S.; Moolenaar, F.; Meijer, D.K.; de Jong, P.E.; de Zeeuw, D. Drug-targeting to the kidney: Renal delivery and degradation of a naproxen-lysozyme conjugate In Vivo. Kidney Int. 1997, 52, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Dolman, M.E.M.; Harmsen, S.; Pieters, E.H.E.; Sparidans, R.W.; Lacombe, M.; Szokol, B.; Orfi, L.; Kéri, G.; Storm, G.; Hennink, W.E.; et al. Targeting of a platinum-bound sunitinib analog to renal proximal tubular cells. Int. J. Nanomed. 2012, 7, 417–433. [Google Scholar]
- Haverdings, R.F.; Haas, M.; Greupink, A.R.; de Vries, P.A.; Moolenaar, F.; de Zeeuw, D.; Meijer, D.K. Potentials and limitations of the low-molecular-weight protein lysozyme as a carrier for renal drug targeting. Ren. Fail. 2001, 23, 397–409. [Google Scholar] [CrossRef]
- Vegt, E.; Van Eerd, J.E.M.; Eek, A.; Oyen, W.J.G.; Wetzels, J.F.M.; de Jong, M.; Russel, F.G.M.; Masereeuw, R.; Gotthardt, M.; Boerman, O.C. Reducing renal uptake of radiolabeled peptides using albumin fragments. J. Nucl. Med. 2008, 49, 1506–1511. [Google Scholar] [CrossRef]
- Yuan, Z.-X.; He, X.-K.; Wu, X.-J.; Gao, Y.; Fan, M.; Song, L.-Q.; Xu, C.-Q. Peptide fragments of human serum albumin as novel renal targeting carriers. Int. J. Pharm. 2014, 460, 196–204. [Google Scholar] [CrossRef]
- Yuan, Z.-X.; Wu, X.-J.; Mo, J.; Wang, Y.-l.; Xu, C.-q.; Lim, L.Y. Renal targeted delivery of triptolide by conjugation to the fragment peptide of human serum albumin. Eur. J. Pharm. Biopharm. 2015, 94, 363–371. [Google Scholar] [CrossRef]
- Schechter, B.; Arnon, R.; Colas, C.; Burakova, T.; Wilchek, M. Renal accumulation of streptavidin: Potential use for targeted therapy to the kidney. Kidney Int. 1995, 47, 1327–1335. [Google Scholar] [CrossRef]
- Reubi, J.C.; Horisberger, U.; Studer, U.E.; Waser, B.; Laissue, J.A. Human kidney as target for somatostatin: High affinity receptors in tubules and vasa recta. J. Clin. Endocrinol. Metab. 1993, 77, 1323–1328. [Google Scholar] [PubMed]
- Kodaira, H.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Kaneda, Y.; Yamamoto, Y.; Tsunoda, S.-i.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The targeting of anionized polyvinylpyrrolidone to the renal system. Biomaterials 2004, 25, 4309–4315. [Google Scholar] [CrossRef] [PubMed]
- Kamada, H.; Tsutsumi, Y.; Sato-Kamada, K.; Yamamoto, Y.; Yoshioka, Y.; Okamoto, T.; Nakagawa, S.; Nagata, S.; Mayumi, T. Synthesis of a poly(vinylpyrrolidone-co-dimethyl maleic anhydride) co-polymer and its application for renal drug targeting. Nat. Biotechnol. 2003, 21, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Sato-Kamada, K.; Okamoto, T.; Mukai, Y.; Shibata, H.; Nakagawa, S.; Mayumi, T. Poly(vinylpyrrolidone-co-dimethyl maleic acid) as a novel renal targeting carrier. J. Control. Release 2004, 95, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-X.; Sun, X.; Gong, T.; Ding, H.; Fu, Y.; Zhang, Z.-R. Randomly 50% N-acetylated low molecular weight chitosan as a novel renal targeting carrier. J. Drug. Target 2007, 15, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Li, S.-J.; Tan, X.-Y.; Wang, J.-H.; Hu, Y.; Tan, Z.; Liang, J.; Hu, J.-B.; Li, Y.-G.; Zhao, Y.-F. Engineering of stepwise-targeting chitosan oligosaccharide conjugate for the treatment of acute kidney injury. Carbohydr. Polym. 2021, 256, 117556. [Google Scholar] [CrossRef] [PubMed]
- Kandav, G.; Bhatt, D.C.; Singh, S.K. Effect of Different Molecular Weights of Chitosan on Formulation and Evaluation of Allopurinol-Loaded Nanoparticles for Kidney Targeting and in Management of Hyperuricemic Nephrolithiasis. AAPS PharmSciTech 2022, 23, 144. [Google Scholar] [CrossRef]
- Ren, M.; Li, Y.; Zhang, H.; Li, L.; He, P.; Ji, P.; Yang, S. An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone. J. Mater. Chem. B 2021, 9, 2831–2844. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Y.; Xu, L.; Chen, D.; Cheng, L. Transferrin Conjugated pH- and Redox-Responsive Poly(Amidoamine) Dendrimer Conjugate as an Efficient Drug Delivery Carrier for Cancer Therapy. Int. J. Nanomed. 2020, 15, 2751–2764. [Google Scholar] [CrossRef]
- Matsuura, S.; Katsumi, H.; Suzuki, H.; Hirai, N.; Hayashi, H.; Koshino, K.; Higuchi, T.; Yagi, Y.; Kimura, H.; Sakane, T.; et al. l-Serine-modified polyamidoamine dendrimer as a highly potent renal targeting drug carrier. Proc. Natl. Acad. Sci. USA 2018, 115, 10511–10516. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, H.; Chen, B.; Wang, Y. Rethinking nanoparticulate polymer-drug conjugates for cancer theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, e1828. [Google Scholar] [CrossRef]
- Duan, Z.; Luo, Q.; Dai, X.; Li, X.; Gu, L.; Zhu, H.; Tian, X.; Zhang, H.; Gong, Q.; Gu, Z.; et al. Synergistic Therapy of a Naturally Inspired Glycopolymer-Based Biomimetic Nanomedicine Harnessing Tumor Genomic Instability. Adv. Mater. 2021, 33, e2104594. [Google Scholar] [CrossRef] [PubMed]
- Kissel, M.; Peschke, P.; Subr, V.; Ulbrich, K.; Strunz, A.M.; Kühnlein, R.; Debus, J.; Friedrich, E. Detection and cellular localisation of the synthetic soluble macromolecular drug carrier pHPMA. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1055–1062. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, N.; Wang, Y.; Li, M.; Zhang, W.; Fan, L.; Liu, L.; Tang, Z.; Chen, X. Cisplatin nanoparticles boost abscopal effect of radiation plus anti-PD1 therapy. Biomater. Sci. 2021, 9, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Cheah, H.Y.; Gallon, E.; Dumoulin, F.; Hoe, S.Z.; Japundžić-Žigon, N.; Glumac, S.; Lee, H.B.; Anand, P.; Chung, L.Y.; Vicent, M.J.; et al. Near-Infrared Activatable Phthalocyanine-Poly-L-Glutamic Acid Conjugate: Enhanced In Vivo Safety and Antitumor Efficacy toward an Effective Photodynamic Cancer Therapy. Mol. Pharm. 2018, 15, 2594–2605. [Google Scholar] [CrossRef]
- Chai, H.-J.; Kiew, L.-V.; Chin, Y.; Norazit, A.; Mohd Noor, S.; Lo, Y.-L.; Looi, C.-Y.; Lau, Y.-S.; Lim, T.-M.; Wong, W.-F.; et al. Renal targeting potential of a polymeric drug carrier, poly-l-glutamic acid, in normal and diabetic rats. Int. J. Nanomed. 2017, 12, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.P.; Chandra, S.; Tiwari, R.; Srivastava, A.; Tiwari, G. Therapeutic Potential of Prodrugs Towards Targeted Drug Delivery. Open Med. Chem. J. 2018, 12, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, W.; Li, Y.; Wei, C.; Zhong, P.; He, D.; Liu, H.; Wang, P.; Huang, Z.; Zhu, W.; et al. Folic Acid-Modified Erythrocyte Membrane Loading Dual Drug for Targeted and Chemo-Photothermal Synergistic Cancer Therapy. Mol. Pharm. 2021, 18, 386–402. [Google Scholar] [CrossRef]
- Qiu, L.; Dong, C.; Kan, X. Lymphoma-targeted treatment using a folic acid-decorated vincristine-loaded drug delivery system. Drug Des. Dev. Ther. 2018, 12, 863–872. [Google Scholar] [CrossRef]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.P.; Mathias, C.J.; Yang, Z.; Low, P.S.; Marmion, M.; Green, M.A. Synthesis and evaluation of 99mTc(CO)(3)-DTPA-folate as a folate-receptor-targeted radiopharmaceutical. Nucl. Med. Biol. 2002, 29, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, L.; Wang, Z.; Liu, P.; Liu, X.; Ding, J.; Zhou, W. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and Re-polarization. Biomaterials 2021, 264, 120390. [Google Scholar] [CrossRef]
- Hu, J.-B.; Li, S.-J.; Kang, X.-Q.; Qi, J.; Wu, J.-H.; Wang, X.-J.; Xu, X.-L.; Ying, X.-Y.; Jiang, S.-P.; You, J.; et al. CD44-targeted hyaluronic acid-curcumin prodrug protects renal tubular epithelial cell survival from oxidative stress damage. Carbohydr. Polym. 2018, 193, 268–280. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Wang, X.; Gong, T.; Zhang, L.; Sun, X. Targeted drug delivery to renal proximal tubule epithelial cells mediated by 2-glucosamine. J. Control. Release 2013, 167, 148–156. [Google Scholar] [CrossRef]
- Yuan, Z.-X.; Shang, Z.; Gu, J.; He, L. Renal targeting delivery systems. Future Med. Chem. 2019, 11, 2237–2240. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R. Solid lipid nanocarriers in drug delivery: Characterization and design. Expert Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar]
- Xu, S.; Chang, L.; Zhao, X.; Hu, Y.; Lin, Y.; Chen, Z.; Ren, X.; Mei, X. Preparation of epigallocatechin gallate decorated Au-Ag nano-heterostructures as NIR-sensitive nano-enzymes for the treatment of osteoarthritis through mitochondrial repair and cartilage protection. Acta Biomater. 2022, 144, 168–182. [Google Scholar] [CrossRef]
- Mei, X.; Hu, T.; Wang, H.; Liang, R.; Bu, W.; Wei, M. Highly dispersed nano-enzyme triggered intracellular catalytic reaction toward cancer specific therapy. Biomaterials 2020, 258, 120257. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-M.; Yan, C.; Feng, Y.-M. Nanomedicine for the treatment of diabetes-associated cardiovascular diseases and fibrosis. Adv. Drug Deliv. Rev. 2021, 172, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.V.; Chang, H.M.; Yanagawa, N.; Hamon, M. Nanotechnology, Nanomedicine, and the Kidney. Appl. Sci. 2021, 11, 7187. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [PubMed]
- Paluszkiewicz, P.; Martuszewski, A.; Zaręba, N.; Wala, K.; Banasik, M.; Kepinska, M. The Application of Nanoparticles in Diagnosis and Treatment of Kidney Diseases. Int. J. Mol. Sci. 2021, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Weng, T.-H.; Chuang, C.-H.; Huang, K.-Y.; Huang, S.-C.; Chen, P.-R.; Huang, H.-H.; Huang, L.-Y.; Shen, P.-C.; Chuang, P.-Y.; et al. Transdermal nanolipoplex simultaneously inhibits subcutaneous melanoma growth and suppresses systemically metastatic melanoma by activating host immunity. Nanomedicine 2022, 47, 102628. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Kumar, H.; Kommineni, N.; Benival, D. Anabolic Peptide-Enriched Stealth Nanoliposomes for Effective Anti-Osteoporotic Therapy. Pharmaceutics 2022, 14, 2417. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar]
- Huang, C.; Xue, L.-F.; Hu, B.; Liu, H.-H.; Huang, S.-B.; Khan, S.; Meng, Y. Calycosin-loaded nanoliposomes as potential nanoplatforms for treatment of diabetic nephropathy through regulation of mitochondrial respiratory function. J. Nanobiotechnol. 2021, 19, 178. [Google Scholar] [CrossRef]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Subroto, E.; Andoyo, R.; Indiarto, R.; Wulandari, E.; Wadhiah, E.F.N. Preparation of Solid Lipid Nanoparticle-Ferrous Sulfate by Double Emulsion Method Based on Fat Rich in Monolaurin and Stearic Acid. Nanomaterials 2022, 12, 3054. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Solid Lipid Nanoparticles of Myricitrin Have Antioxidant and Antidiabetic Effects on Streptozotocin-Nicotinamide-Induced Diabetic Model and Myotube Cell of Male Mouse. Oxid. Med. Cell. Longev. 2018, 2018, 7496936. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Antioxidant, anti-apoptotic, and protective effects of myricitrin and its solid lipid nanoparticle on streptozotocin-nicotinamide-induced diabetic nephropathy in type 2 diabetic male mice. Iran. J. Basic Med. Sci. 2019, 22, 1424–1431. [Google Scholar]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Hyperglycemia-induced oxidative stress in isolated proximal tubules of mouse: The in vitro effects of myricitrin and its solid lipid nanoparticle. Arch. Physiol. Biochem. 2021, 127, 422–428. [Google Scholar] [CrossRef]
- Asfour, M.H.; Salama, A.A.A.; Mohsen, A.M. Fabrication of All-Trans Retinoic Acid loaded Chitosan/Tripolyphosphate Lipid Hybrid Nanoparticles as a Novel Oral Delivery Approach for Management of Diabetic Nephropathy in Rats. J. Pharm. Sci. 2021, 110, 3208–3220. [Google Scholar] [CrossRef]
- Sierra-Mondragon, E.; Rodríguez-Muñoz, R.; Namorado-Tonix, C.; Molina-Jijon, E.; Romero-Trejo, D.; Pedraza-Chaverri, J.; Reyes, J.L. All-Trans Retinoic Acid Attenuates Fibrotic Processes by Downregulating TGF-β1/Smad3 in Early Diabetic Nephropathy. Biomolecules 2019, 9, 525. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Kan, M.; Yang, J.; Gong, Q.; Jin, R.; Dai, Y.; Jin, J.; Zang, H. Gypenoside XLIX loaded nanoparticles targeting therapy for renal fibrosis and its mechanism. Eur. J. Pharmacol. 2021, 910, 174501. [Google Scholar] [CrossRef]
- Oroojalian, F.; Rezayan, A.H.; Mehrnejad, F.; Nia, A.H.; Shier, W.T.; Abnous, K.; Ramezani, M. Efficient megalin targeted delivery to renal proximal tubular cells mediated by modified-polymyxin B-polyethylenimine based nano-gene-carriers. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 770–782. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Q.; Zhang, L.; Sun, H.; Ding, F.; Li, Y.; Chen, P. FeO magnetic nanoparticles ameliorate albumin-induced tubulointerstitial fibrosis by autophagy related to Rab7. Colloids Surf. B Biointerfaces 2021, 198, 111470. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Luo, S.; Du, Z.; Zhou, M.; Li, P.; Fu, Y.; Sun, X.; Huang, Y.; Zhang, Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017, 8, 878. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Poon, C.; Chin, D.; Milkowski, S.; Lu, V.; Hallows, K.R.; Chung, E.J. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 2018, 11, 5584–5595. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Ng, B.D.; Minton, D.R.; Gudas, L.J.; Park, C.Y.; Heller, D.A. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015, 15, 2358–2364. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Tian, H.S.; Chen, X.; Geissmann, F.; Jaimes, E.A.; Heller, D.A. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension 2018, 71, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Williams, R.M.; D'agati, V.; Jaimes, E.A.; Heller, D.A.; Lee, H.T. Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int. 2020, 98, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bellini, S.; Kimura, S.; Hase, K.; Corbetta, B.; Corbelli, A.; Fiordaliso, F.; Bruno, S.; Biancone, L.; Barreca, A.; et al. Protective effect of the tunneling nanotube-TNFAIP2/M-sec system on podocyte autophagy in diabetic nephropathy. Autophagy 2022, 1–20. [Google Scholar] [CrossRef]
- Yu, M.; Wang, D.; Zhong, D.; Xie, W.; Luo, J. Adropin Carried by Reactive Oxygen Species-Responsive Nanocapsules Ameliorates Renal Lipid Toxicity in Diabetic Mice. ACS Appl. Mater. Interfaces 2022, 14, 37330–37344. [Google Scholar] [CrossRef]
| Carriers | Applications | Limitations |
|---|---|---|
| Protein-based and peptide-based carriers | Lysozyme | Systemic hypotension and nephrotoxicity |
| Albumin fragment | ||
| Streptavidin | ||
| Somatostatin | ||
| Polymeric carriers | PVP | Inferior biodegradability |
| PVD | ||
| LMWC | ||
| PAMAM | ||
| pHPMA | ||
| PG | ||
| Small-molecule prodrugs | Folic acid | Low targeting efficiency, retentiveness, and poor cell permeability |
| Hyaluronic acid | ||
| PCG | ||
| Nanoparticles | Nanoliposomes | Difficulty in mass production and lack of technology |
| Solid lipid nanoparticles | ||
| Organic nanoparticles | ||
| Inorganic nanoparticles | ||
| Micelles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Dai, W.; Liu, Z.; He, L. Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease. Pharmaceuticals 2022, 15, 1494. https://doi.org/10.3390/ph15121494
Li H, Dai W, Liu Z, He L. Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease. Pharmaceuticals. 2022; 15(12):1494. https://doi.org/10.3390/ph15121494
Chicago/Turabian StyleLi, Hao, Wenni Dai, Zhiwen Liu, and Liyu He. 2022. "Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease" Pharmaceuticals 15, no. 12: 1494. https://doi.org/10.3390/ph15121494
APA StyleLi, H., Dai, W., Liu, Z., & He, L. (2022). Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease. Pharmaceuticals, 15(12), 1494. https://doi.org/10.3390/ph15121494







