1. Introduction
The search persists for a safe and efficacious treatment for arterial thrombosis in the event of acute myocardial infarction or ischemic stroke. In the United States (US), approximately 795,000 people per year experience a stroke, with the vast majority (87%) being ischemic. Stroke is the most common cause of severe long-term disability. The total cost of stroke in the US is estimated to be over
$50 billion annually [
1].
The only drug currently approved to treat acute ischemic stroke (AIS) caused by pathological thrombus formation is recombinant tissue plasminogen activator (rtPA), which targets lysis of fibrin-rich clots via conversion of plasminogen into its active form, plasmin. Plasmin cleaves fibrin, thereby breaking down occlusive clot structure. rtPA has several limitations that impede its safety and efficacy in treating patients who present with AIS: (i) it has a narrow temporal window of use that excludes >90% of ischemic stroke patients [
2], (ii) treatment results in symptomatic intracranial hemorrhage (sICH) in ~6% of patients, resulting in significant morbidity and mortality, (iii) it achieves recanalization in only around 10% of patients who present with large vessel occlusion (LVO) stroke (15% of AIS patients) [
3] and 30% of all AIS patients, and (iv) re-occlusion often occurs after treatment [
4]. Endovascular thrombectomy (EVT) is a minimally invasive means of removing a thrombus blocking the intracranial internal carotid artery, middle cerebral artery (MCA) or anterior cerebral artery (ACA), but can only be performed in patients who present with LVO stroke. LVO stroke disproportionately results in poor outcomes compared to other stroke etiologies, and up to 90% of stroke-induced mortality occurs in patients with LVO stroke [
5]. Intravenous thrombolysis therapy (IVT) with tPA can be used in isolation or as a bridge to EVT [
6].
The mechanism of thrombosis responsible for thromboembolic stroke comprises elongation of von Willebrand Factor (VWF) on an extravascular surface (i.e., subendothelial collagen), resulting in pathological capture of platelets at high shear [
7]. Progressive vessel occlusion causes stagnation and potentiates coagulation. While cardioembolic strokes are canonically fibrinous due to formation in zones of stagnation in the left atrium, they may develop a VWF-platelet-rich outer shell once situated in the arterial setting post-embolization [
8]. The thrombi responsible for vessel occlusion in stroke of both thromboembolic and cardioembolic stroke may therefore be resistant to rtPA-mediated lysis. Attacking these targets on outer the shell of the thrombus may facilitate recanalization of the vessel.
BB-031 is a ribonucleic acid (RNA) aptamer that binds to the VWF A1 domain (note: in previous publications, BB-031 is called DTRI-031, and these two names refer to the same aptamer) [
9]. Aptamers are single-stranded oligonucleotides that have high target binding affinity and can be rapidly reversed by their antisense counterpart, making them well suited for anticoagulant and antithrombotic applications [
10]. Pre-clinical development of BB-031 has demonstrated it to be a novel, rapid-onset, and rapidly reversible antithrombotic agent in both in vitro assays and in vivo animal models [
9]. Interestingly, BB-031 also has thrombolytic properties, and resulted in recanalization post-occlusion in a canine model of stroke [
9]. As BB-031 targets VWF A1 domain-platelet Glycoprotein Ib (GPIb) interactions, and not systemic induction of fibrinolysis as does rtPA, this limits the mechanism of treatment to that of arterial thrombosis and therefore would be a highly desirable alternative to current therapeutic approaches. Furthermore, rapid reversibility allows the treatment to be arrested, which is impossible with rtPA. BB-031 was tested in a recently completed Phase I clinical trial (NCT05005520) to assess safety in healthy volunteers.
The objectives of this study were to determine (i) optimal in vitro BB-031 dosing in a microfluidic model of thrombolytic efficacy, (ii) how thrombolytic efficacy correlates with measures of inhibition in clinical assays that are readily available for therapeutic direction, and (iii) which currently commercially available assay best capitulates dose-dependent functional effects of BB-031.
3. Discussion
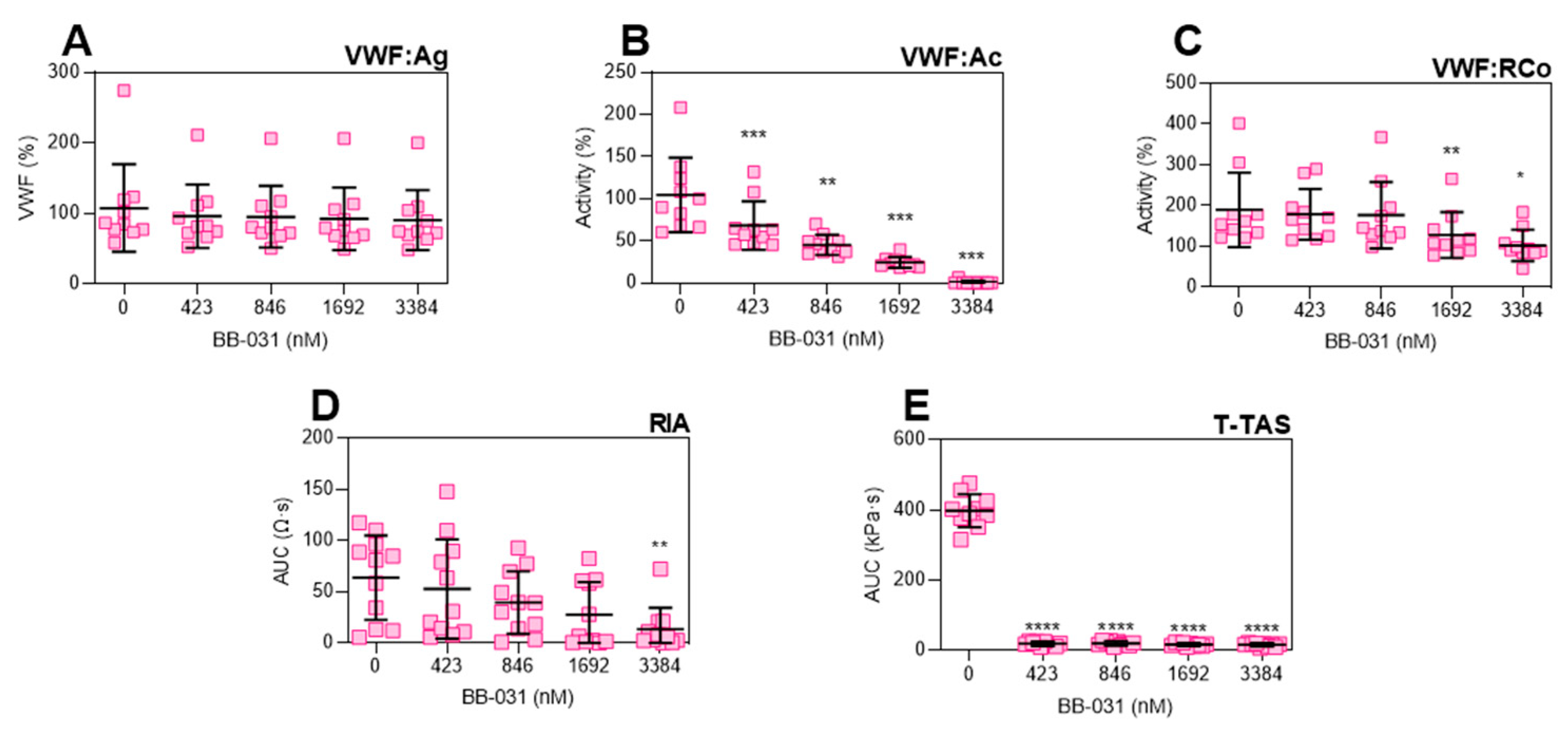
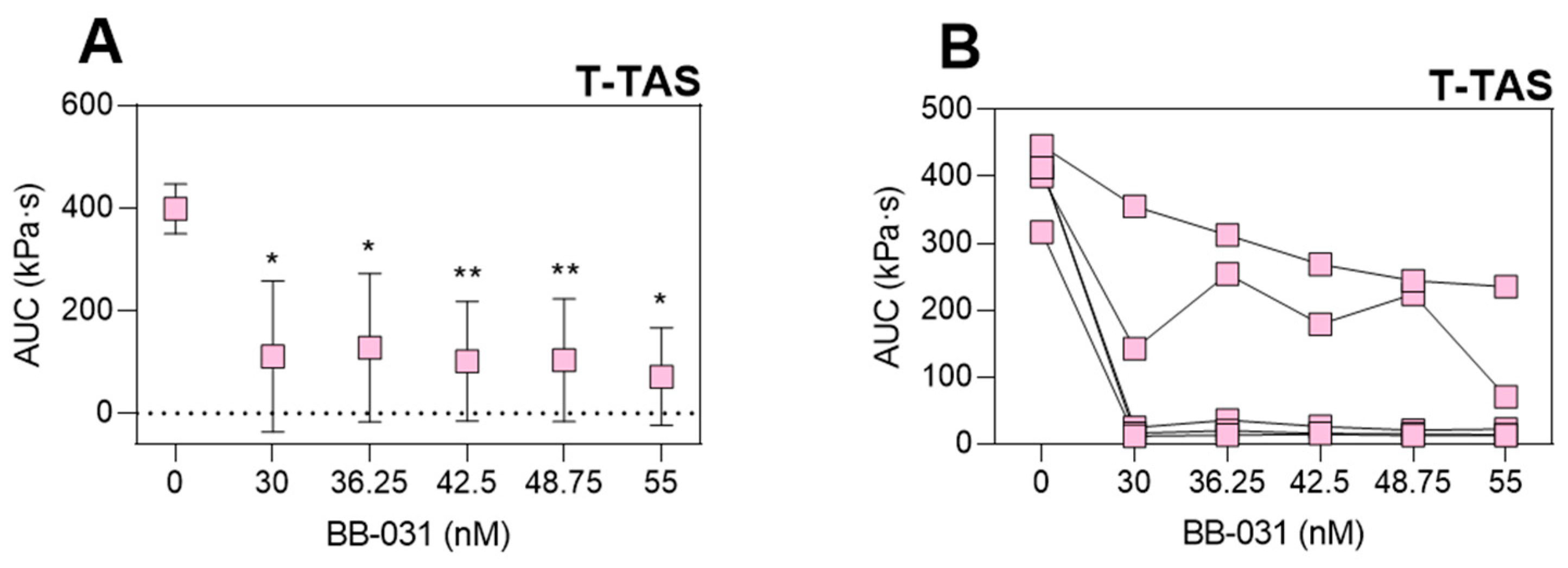
Treatment with BB-031 in healthy human whole blood samples in vitro resulted in inhibition of VWF function detectable by various clinical assays: Siemens VWF:Ac, DiaPharma T-TAS, Chrono-Log VWF:RCo, and Chrono-Log RIA (
Figure 1). Specifically, BB-031 inhibition of platelet GPIbα binding is dose-dependent and measurable on clinically available assays. Dosing with BB-031 did not alter VWF antigen levels, as expected (
Figure 1A). Furthermore, inhibitory effects were well preserved during sample handling and processing, including sample storage at −80 °C, shipment, and thawing. Additionally, of note, function was preserved during drug preparation using 0.45% saline alone at room temperature (no heat nor buffers were applied, as they had been for pre-clinical formulations of BB-031). Interestingly, sensitivity varied across assays, with the T-TAS assay being remarkably sensitive to inhibition by BB-031 at very low doses. As this is the only assay in our clinical panel that incorporates flow, we hypothesize that this sensitivity is due to the both the shear-induced exposure of additional A1 domains that are then inhibited by available aptamer, as well as the mechanistically necessary release of additional VWF from platelet alpha granules that participate in thrombosis [
12], which would also be inhibited by available aptamer.
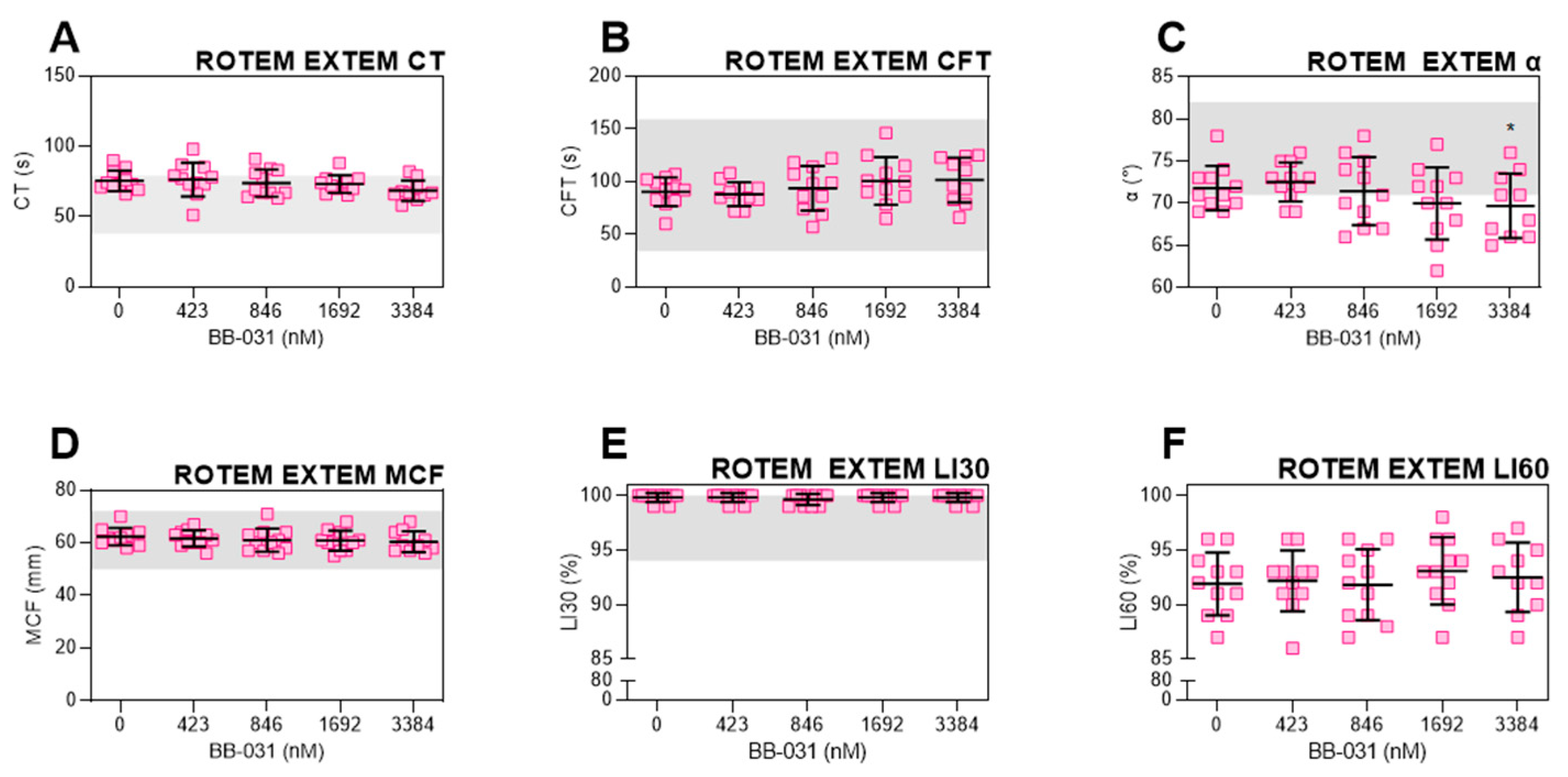
BB-031 did not induce fibrinolysis, as expected (
Figure 3E,F). This is supportive of its advantageous use over rtPA. Interestingly, only α angle was statistically significantly different from baseline at the highest dose (
Figure 3D), demonstrating a very slight decrease in clot formation kinetics as measured by viscoelastic testing. CT trended down, which may indicate faster thrombin generation and initial fibrin formation, yet CFT trended upwards. Both remain within the healthy reference range and are not clinically-relevant changes. The mechanism of these slight effects may be due to off-target inhibition of either platelets mediated by VWF A1, or inhibition of other VWF binding sites, such as the D’-D3 region, which binds FVIII. However, as ROTEM parameters have yet to be fully translated to rtPA-treated patients, we cannot make outcome predictions.
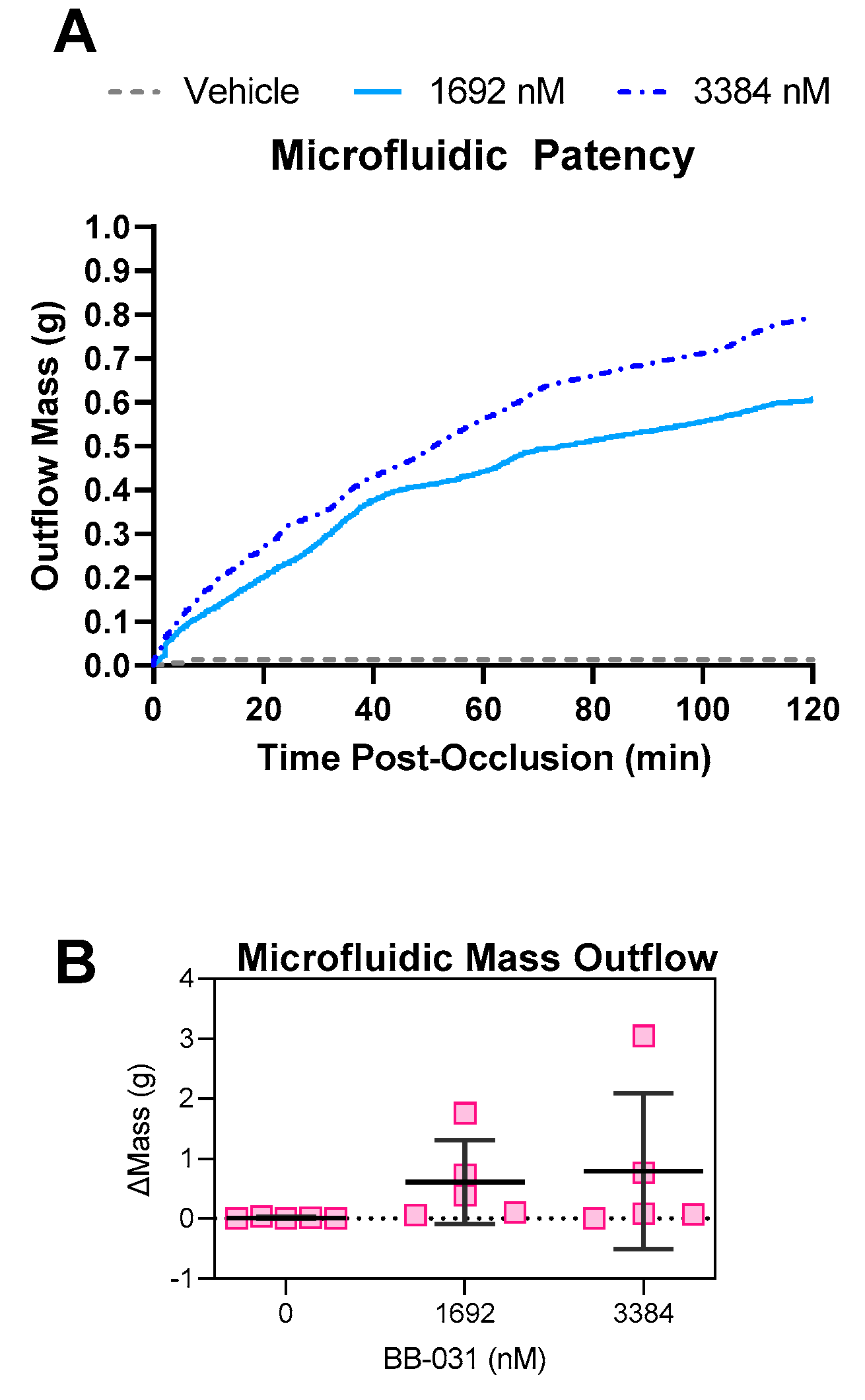
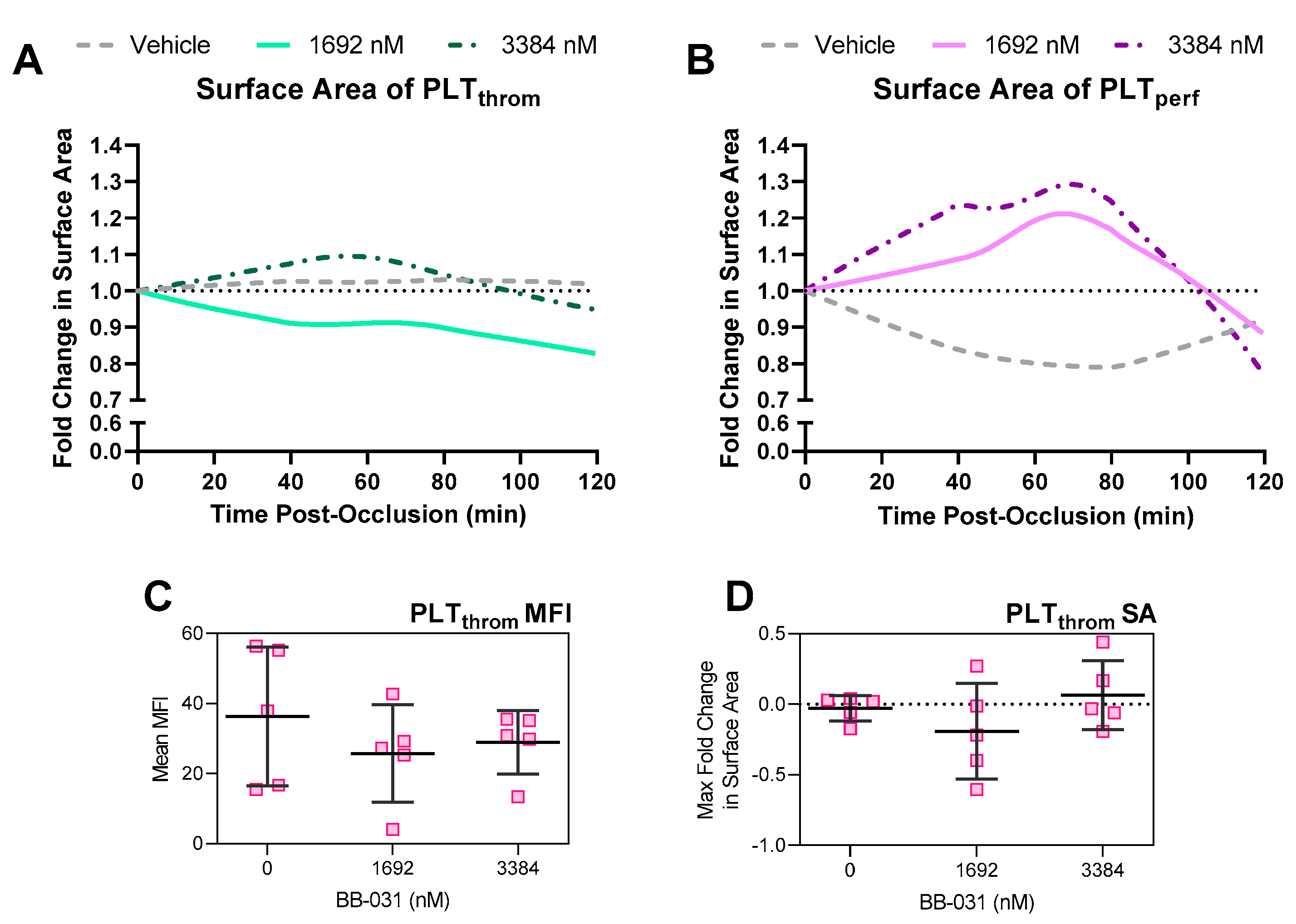
BB-031 induced thrombolysis in a microfluidic model of reperfusion of a nearly occlusive arterial thrombus, as shown in both trends in patency (
Figure 5) and in reductions in thrombus surface area (
Figure 6) relative to treatment with vehicle. The microfluidic reperfusion system has high (patho)physiological fidelity, as thrombosis is induced in an arterial setting on a collagen-coated stenosis, simulating the post-plaque rupture environment and inducing the resulting catastrophic acute event of vessel occlusion. To our knowledge, this system is the closest in vitro microfluidic model approximating thrombotic occlusion and treatment. Loyau et al. took a similar approach to study rtPA-induced fibrinolysis under flow, but focused on the induction of coagulation to form fibrin-rich clots, perfused them at venous and a lower arterial shear rate (1500 s
−1) for only 10 min [
13]. Wang et al. also studied urokinase-type plasminogen activator (uPA) thrombolysis in vitro in an endothelialized system, but again focused on non-occlusive fibrin-rich clots and utilized shorter timescales (~35 min) than our study [
14]. The thrombi in our system are mechanistically VWF-dependent and platelet-rich (
Figure 4) as they are formed at an initial wall shear rate of 3500 s
−1, and our post-occlusion reperfusion time was much longer (120 min), selected based on timescales seen in animal models where BB-031 induced recanalization [
9]. Kim and Shea et al. previously published a macro-scale glass capillary tube to induce high shear thrombosis, and reperfused thrombi with potential lytic agents [
15]. In this system, we advance our approach via (i) perfusing post-occlusion with autologous dosed blood instead of saline, (ii) a microfluidic model to reduce volume needs allowing higher throughput, (iii) quantification of thrombi using fluorescent microscopy, and (iv) reperfusion with a gravity pressure head instead of a constant flowrate. Reperfusion post-occlusion with constant pressure better recapitulates systemic administration of drug that is delivered in vivo to a site of ischemia. While BB-031 has previously been shown to inhibit thrombosis in vitro in canine whole blood using the T-TAS, we for the first time demonstrate inhibition in human samples in various assays, demonstrate that inhibition of BB-031 survives a freeze–thaw cycle in plasma, and demonstrate microfluidic thrombolysis, both informing on clinical use of BB-031 and recapitulating recanalization observed by Nimjee et al. in vivo in canines [
9]. Other VWF A1 domain-targeting aptamers have been developed, such as ARC 1779 [
16] and TAGX-004 [
17], and have been evaluated both in thrombo-prevention and thrombocytopenia purpura (TTP). In this study, we tested the hypothesis that BB-031 is a VWF-platelet-specific thrombolytic agent, furthering the study of the previous in vivo recanalization findings [
9]. Of note, ARC 1779 and TAGX-004 have not been evaluated as recanalization agents.
In our microfluidic system, surprisingly, treatment with 1692 nM BB-031 was more efficacious than treatment with 3384 nM BB-031. Treatment with the lower dose resulted in greater lysis of the original thrombus (reduction in PLT
throm SA), as well as better inhibition of deposition of new thrombus (reduced PLT
perf fold change;
Figure 6). Interestingly, inhibition correlated with dose as expected in non-flow based assays (e.g., VWF:Ac, which was maximally inhibited at the highest dose). Treatment with vehicle resulted in a stable, reinforced thrombus that did not change throughout reperfusion, as represented by complete stagnation throughout the reperfusion period (no patency;
Figure 5) and the accompanying expected lack of surface area reduction and lack of significant deposition of new thrombus due to lack of flow (
Figure 6). The surface area of new deposition decreased from baseline in the setting of reperfusion with vehicle, which is attributable to retraction in the setting of stagnation. While surface area reduction was not extreme, clear differences in patency were observed. Reductions in the height of the thrombus were not captured, a limitation of 2D image acquisition, and are likely occurring and responsible for the improvements in flow. Off-target effects (e.g., inflammatory pathways [
18]) may be responsible for the surprising reduction in thrombolytic and inhibitory efficacy observed at the highest dose, as well as the effects seen on ROTEM. However, the highest dose selected in this study was chosen intentionally to be at the maximal potential clinical dose. Further exploration of the mechanism of these effects may help inform on clinical safety.
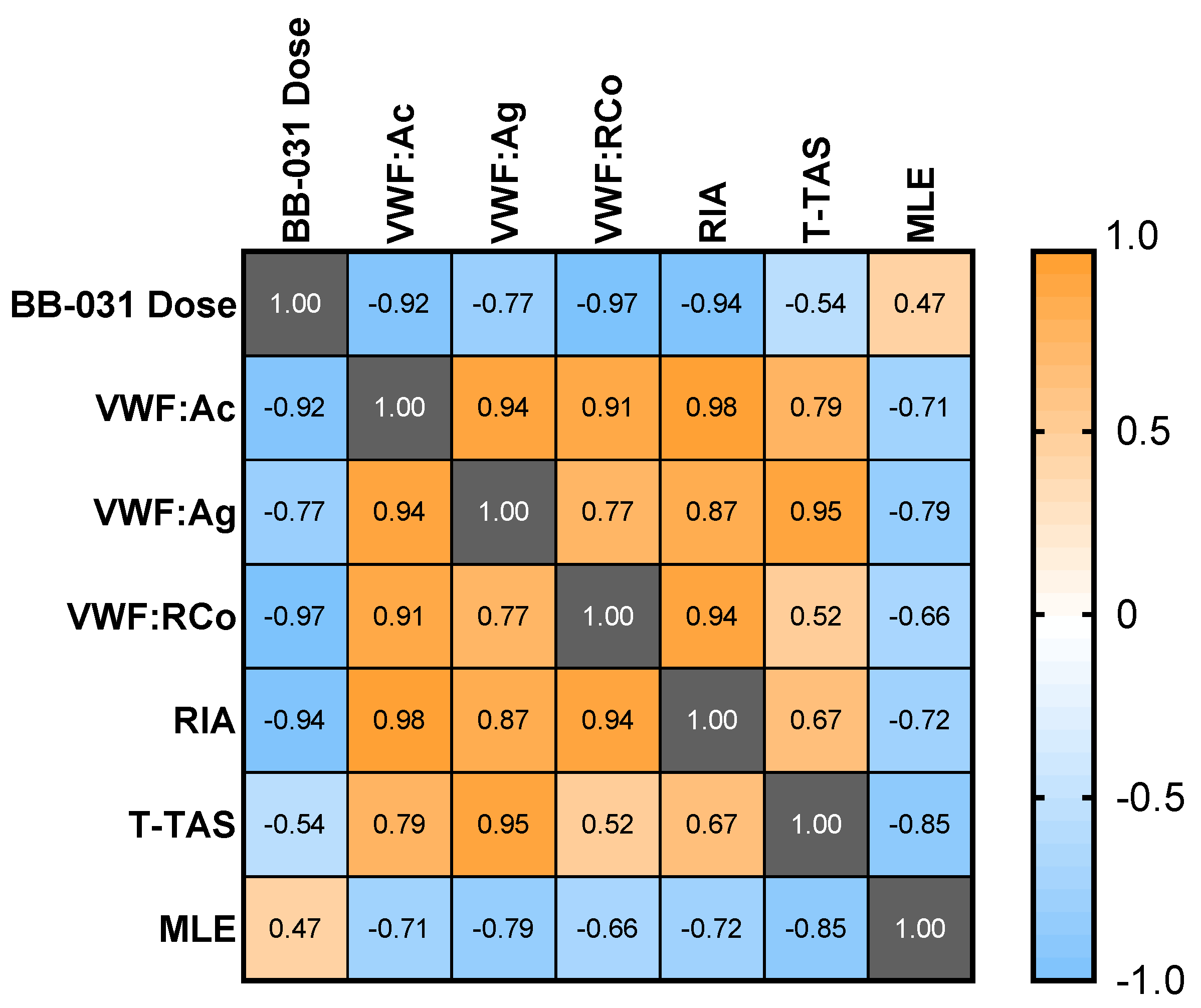
While dose best correlated with VWF:RCo (ρ = −0.97) and ristocetin-induced whole blood impedance aggregometry (ρ = −0.97), VWF:Ac had a high correlation coefficient (ρ = −0.92), a log-linear dose response, and was maximally inhibited at the highest dose. It may therefore be an ideal clinical measure of inhibition, however this assay has limitations: (i) it requires processing of the sample into platelet-poor plasma (PPP), which may increase turnaround times relative to a whole blood assay, and (ii) it is neither FDA-approved nor yet available in the US at the time of this writing. Incredibly low doses of drug (≤30 nM, two orders of magnitude less than an equivalent 0.25 mg/kg in vitro dose) completely inhibited the T-TAS assay. While this potentially indicates complete inhibition of VWF-platelet-mediated thrombosis under flow, other assays such as VWF:Ac may better provide information regarding the level of molecular VWF inhibition, especially if and when BB-031 is administered clinically as a thrombolytic agent. However, T-TAS could still be of potential clinical utility for BB-031 monitoring as it most directly represents thrombotic function of the assays used in this study. MLE correlated weakly with dose, due to the highest dose being less efficacious than the lower dose in the model of reperfusion in vitro. However, MLE still correlated well with the other assays of inhibition. The negative correlation coefficients indicate that enhanced thrombolysis corresponds to greater inhibition of VWF, suggesting that the thrombolytic efficacy of the drug may also be captured clinically in an assay of VWF functional inhibition.
There are a handful of limitations to this study. First, as these are healthy volunteer samples dosed with drug ex vivo, they likely do not fully encompass the effects of in vivo intravenous administration, nor the blood state or local milieu in a patient experiencing a stroke. Furthermore, while cardioembolic strokes may have VWF-platelet rich structure in addition to a fibrin-rich core presumed to be due to the origin of the thrombus [
8], this system is more directly applicable to thromboembolic stroke. Chamber rigidity, perfusion immediately post thrombus formation, along with other in vitro factors may also hinder the physiological relevance of the assay. As the primary goal of this study was to determine dose–response of BB-031 and assess its efficacy in the microfluidic model, we did not compare head-to-head with current clinically used formulations of rtPA. Given the promising findings in this study, studies comparing lytic efficacy of alteplase and tenecteplase with BB-031 and other design modifications to address the above limitations would be worthwhile future endeavors.
4. Materials and Methods
4.1. Sample Collection
Whole blood was collected from healthy volunteer donors (N = 10) in anticoagulated collection tubes (sodium citrate or Benzylsulfonyl-D-Arg-Pro-4-amidinobenzylamide (BAPA; a synthetic inhibitor of FXa and thrombin), depending on assay instructions for use (IFU)), or green-top heparin vacutainers for use in microfluidic models (Washington University in St. Louis (WUSTL) Institutional Review Board (IRB) Approved, Study #202105078). Donors returned for a second collection to allow for paired analyses; all assays were run using samples from the same donor pool. Immediately post collection, a CBC was performed using a hematology analyzer (Advia 2120i; Siemens, Munich, Germany). Samples were either used immediately for whole blood functional assays, or further processed into PPP and stored at −80 °C for batched analyses, per assay IFU. Prior to assay or the above mentioned further processing, samples were aliquoted and dosed with 423 nM, 846 nM, 1692 nM, or 3384 nM of BB-031, which correspond to 0.25 mg/kg, 0.5 mg/kg, 1 mg/kg, and 2 mg/kg, respectively, in vivo. Vehicle controls (addition of same volume of normal saline) were also run. Dosed samples were allowed to incubate for 5 min before further handling.
4.2. VWF:Ag
Frozen citrated dosed PPP samples (N = 10) were thawed in a 37 °C water bath for 5 min, and then run on a CompactMax (Diagnostica Stago, Parisppany, NJ, USA) using STA®-Liatest® VWF:Ag, an immuno-turbidimetric assay for measuring VWF antigen levels. Antigen levels are reported as a percentage (%) relative to a control level as determined by the test-provided controls and CompactMax software.
4.3. VWF:Ac
Frozen citrated dosed PPP samples (N = 10) were shipped on dry ice to Dr. David Lillicrap’s lab at Queen’s University, Kingston, Ontario, Canada. The Innovance® “VWF:Ac” assay (Siemens) measures GPIb binding via the addition of recombinant GPIb and anti-GPIb polystyrene particles. VWF binding to particles results in agglutination which is measured turbidimetrically. The assay was run using a BCSXP 1500 (Siemens). Data are reported as a percentage (%) of activity.
4.4. T-TAS
The T-TAS is a commercialized microfluidic device comprising an automated microfluidic constant-flow rate driven system [
19]. Whole blood samples (
N = 10) were collected into 3 mL BAPA tubes, then dosed with BB-031. Dosed samples were run on PL-Chips (DiaPharma, West Chester Township, OH, USA) per manufacturer’s instructions. In brief, samples were loaded into reservoirs which were then capped, inverted, attached to the chip, and perfused using the accompanying software for up to 10 min (assay time limit). Data are reported as AUC of the pressure in the channel over time.
4.5. VWF:RCo
VWF-Ristocetin cofactor assay (VWF:RCo) was performed using a kit containing lyophilized platelets and reagents (Chrono-Log, Havertown, PA, USA) via light transmission aggregometry (LTA). Frozen citrated dosed PPP samples (N = 10) were thawed as above and run per manufacturer’s instructions. In brief, VWF:RCo activity is determined by agglutination of standard suspensions of lyophilized human platelets with sample plasma in the presence of ristocetin. Both normal and deficient reference human plasma are provided for generation of a standard curve and quality control, respectively. Data are reported as a percentage (%) of activity as determined relative to the standard curve, calculated by the Chrono-Log software.
4.6. Ristocetin Induced Whole Blood Aggregometry (RIA)
Citrated dosed whole blood (N = 10) was used for ristocetin-induced whole blood impedance aggregometry. The assay was run per manufacturer’s instructions. In brief, dosed whole blood samples were mixed with pre-warmed sodium chloride. Ristocetin (Chrono-Log) was added such that the final concentration was 1 mg/mL, and aggregation recorded using the accompanying software. Data are reported as AUC of the aggregation trace.
4.7. ROTEM
Citrated dosed whole blood (N = 10 was used for ROTEM (Werfen Worldwide, Bedford, MA, USA). The assay was performed using the EXTEM (extrinsic pathway) agonist per manufacturer’s instructions. Data reported from this assay include clotting time (CT, s), clot formation time (CFT, s), maximum clot formation (MCF, mm), alpha angle (α, °), lysis index at 30 min (LI30, %), and lysis index at 60 min (LI60, %).
4.8. Microfluidics
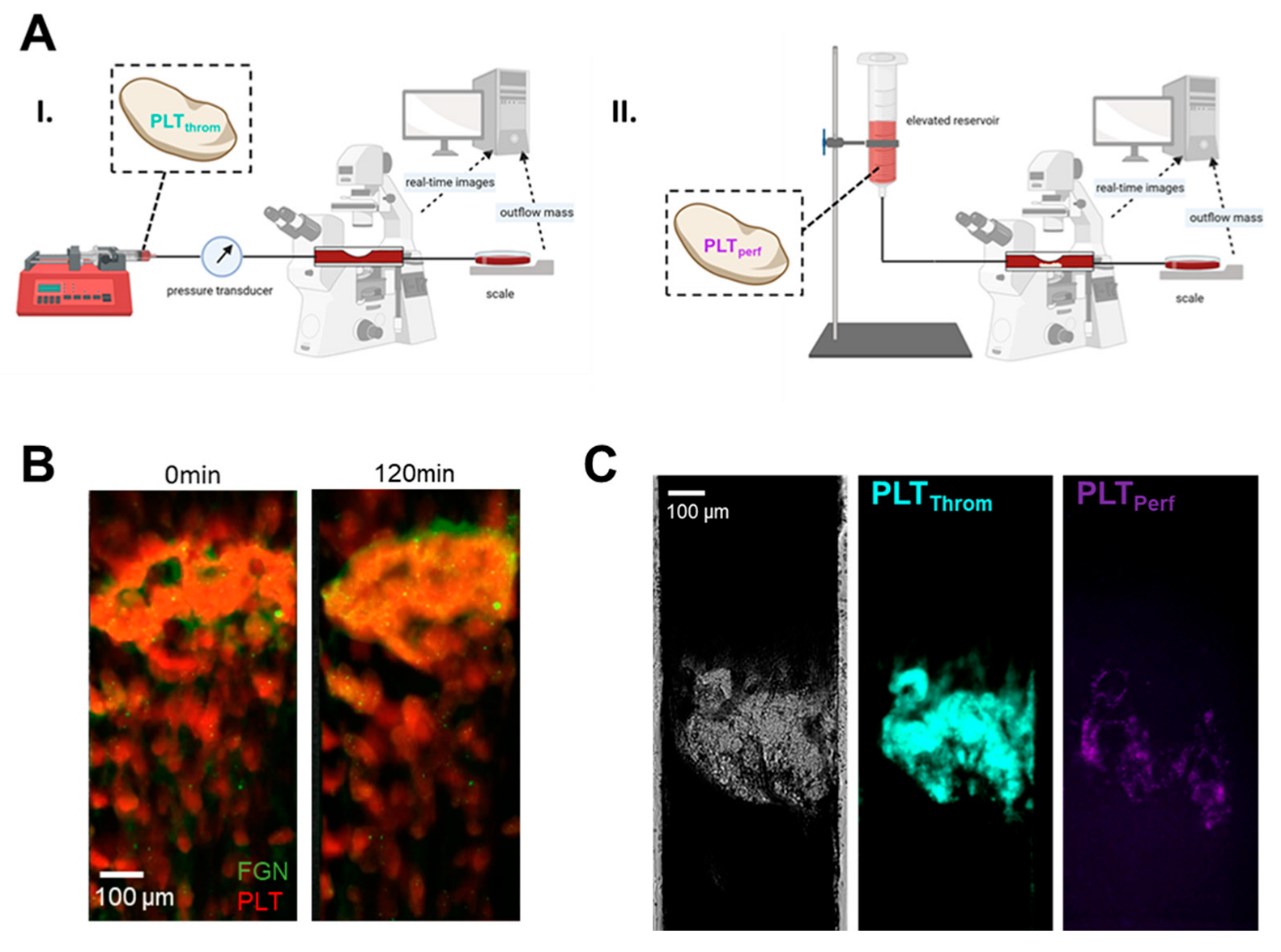
A micromachined mold comprising 8 flow chambers with stenotic regions of interest was used to fabricate polydimethyl siloxane (PDMS) negatives, which were bonded to glass slides using a plasma cleaner (Harrick Plasma, Ithaca, NY, USA). Individual flow chambers were 480 µm wide, and the height at the stenosis was 80 µm. Chambers were incubated with 9:1 0.9% sodium chloride:collagen (equine fibrillar type I, Chrono-Log) overnight and rinsed with phosphate-buffered saline (PBS) prior to perfusion. Whole blood (
N = 5) not treated with BB-031 nor vehicle was incubated with a monoclonal CD41 antibody conjugated to DyLight 350 (Novus Biologicals, Littleton, CO, USA; catalog no. NB100-2614UV; labeling original thrombus platelets, “PLT
throm”) and perfused through the stenotic microfluidic device to induce pathophysiologically-relevant arterial thrombosis (initial wall shear rate 3500 s
−1 (flowrate 0.108 mL/min); system previously described in [
20]). A separate aliquot of whole blood from the same donor was incubated with the same clone of CD41 conjugated to Janelia Fluor 646 (Novus Biologicals; catalog no. NB100-2614JF646; labeling new perfusion platelets, “PLT
perf”) and dosed with either vehicle (normal saline), 1692 nM BB-031, or 3384 nM BB-031. The second aliquot was delivered upstream without disturbing the original thrombus, gently perfused to the site of thrombus using a syringe pump, and then switched to constant-pressure perfusion using gravity and an arterial pressure head which would induce a shear rate of 3500 s
−1 in an unobstructed identical channel. A schematic of the approach is shown in
Figure 4. AF488 fibrinogen (Fisher Scientific, Pittsburgh, PA, USA; 0.01 mg/mL) was also added to a healthy control along with PLT
throm labelling as described above, perfused until occlusion, and imaged after two hours of retraction to visualize platelet and fibrinogen content in the thrombus (
Figure 4B). Outflow of blood through the flow chamber was measured using a downstream scale. Upon occlusion, there is no flow out of the chamber, and the mass slows to a stop, first remaining constant but then shortly resulting in evaporation of the outflow and a concomitant decrease in mass over the remaining duration of the experiment. A constant mass was assumed if mass was decreasing to approximate a correction for evaporation. Total change in corrected mass was reported as the end value (maximal total outflow).
Experiments were imaged in real time (framerate 2/s) using fluorescence microscopy. Images of reperfusion were thresholded using Python (version 3.10.0) to yield surface area over time of the original thrombus (PLTthrom) and any new deposition (PLTperf), and Loess curves were used to smooth variation in fluorescence data due to artifacts. Fold change was calculated by dividing surface area at each timepoint by the surface area at the first timepoint of drug or vehicle dosed WB perfusion, i.e., after thrombus had formed. MFI was also derived from unaltered (i.e., no changes in histogram curves applied) original data files and fold change was calculated in the same fashion. The average MFI across all biological replicates was derived to yield MLE values for use in correlation analyses.
4.9. Analysis
Samples from the same donor were assumed paired. Data within each assay were analyzed using a One-way Analysis of Variance (ANOVA) using the Geisser-Greenhouse correction, which corrects for violations of the assumption of sphericity (inherent to ANOVA, assumes variance among treatments is equal). If the ANOVA was statistically significant (p < 0.05), the analysis proceeded with multiple comparisons (Dunnett’s test) of each dose against the untreated measurement. Data are visualized as mean, with error bars representing SEM unless otherwise described. Correlations were also performed among assays and among individual doses using Pearson’s correlation coefficient (ρ) and visualized using a correlation matrix.