The MEK Inhibitor Trametinib Improves Outcomes following Subarachnoid Haemorrhage in Female Rats
Abstract
1. Introduction
2. Results
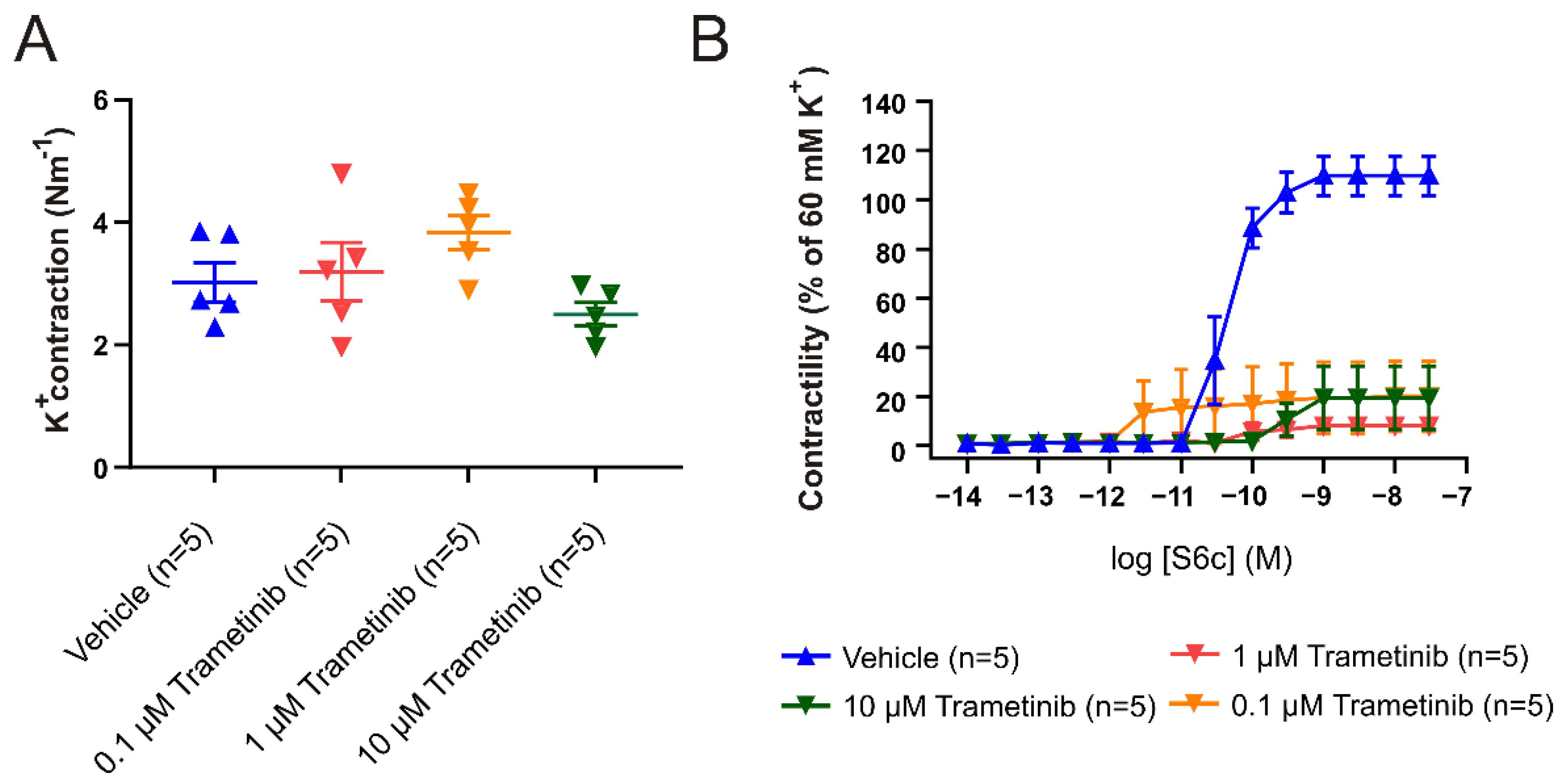
2.1. Organ Culture
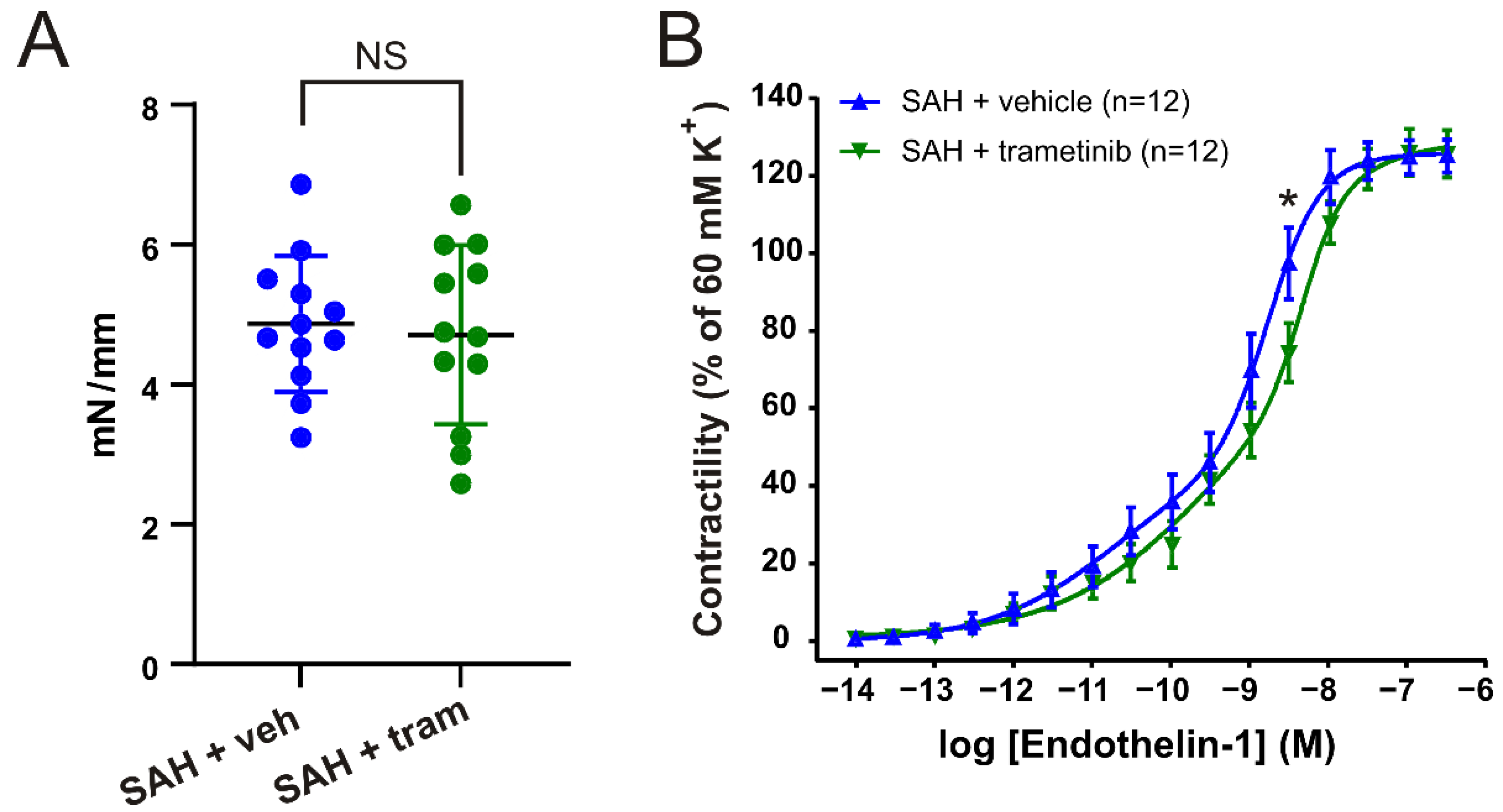
2.2. Experimental SAH
2.3. Standard Clinical Parameters
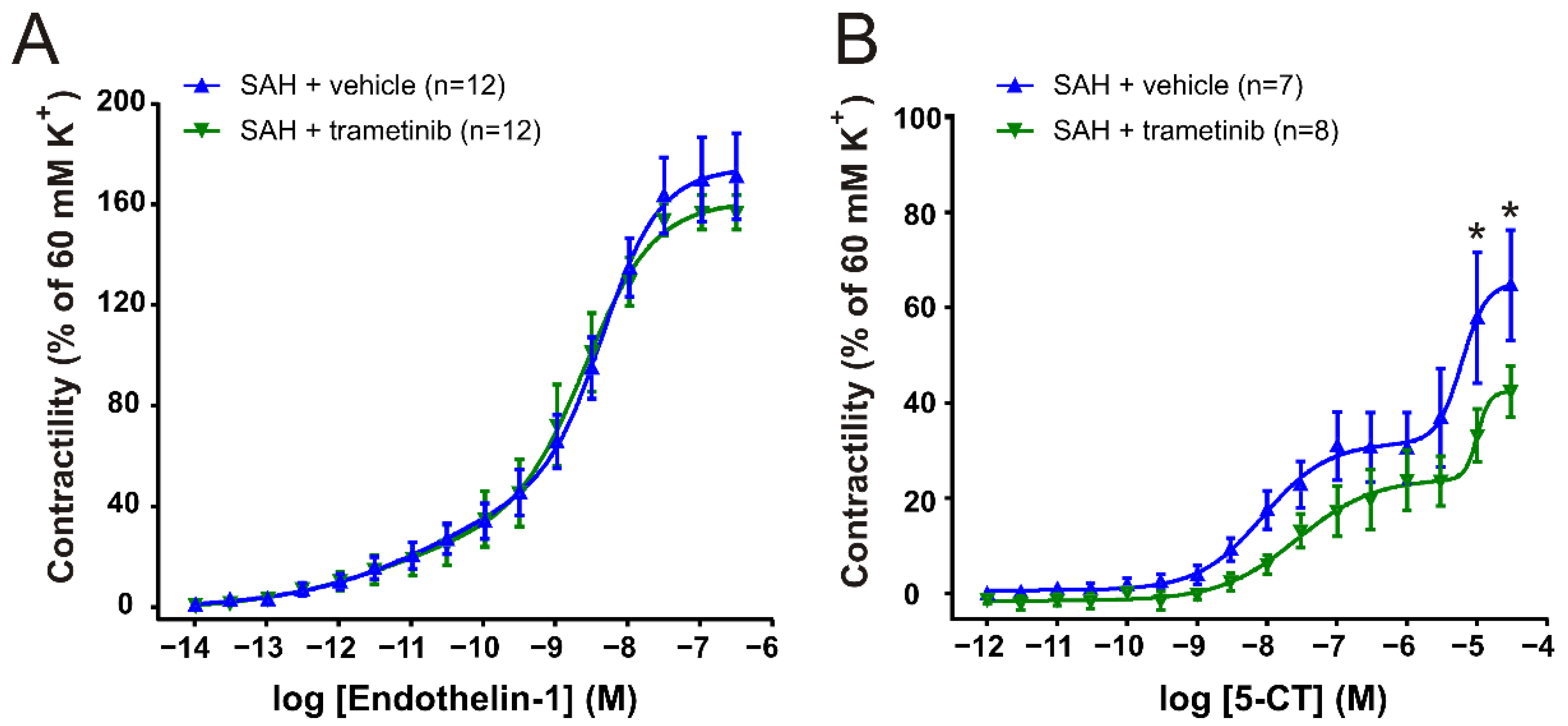
2.4. Effect of Trametinib Treatment on Vascular Contractile Responses
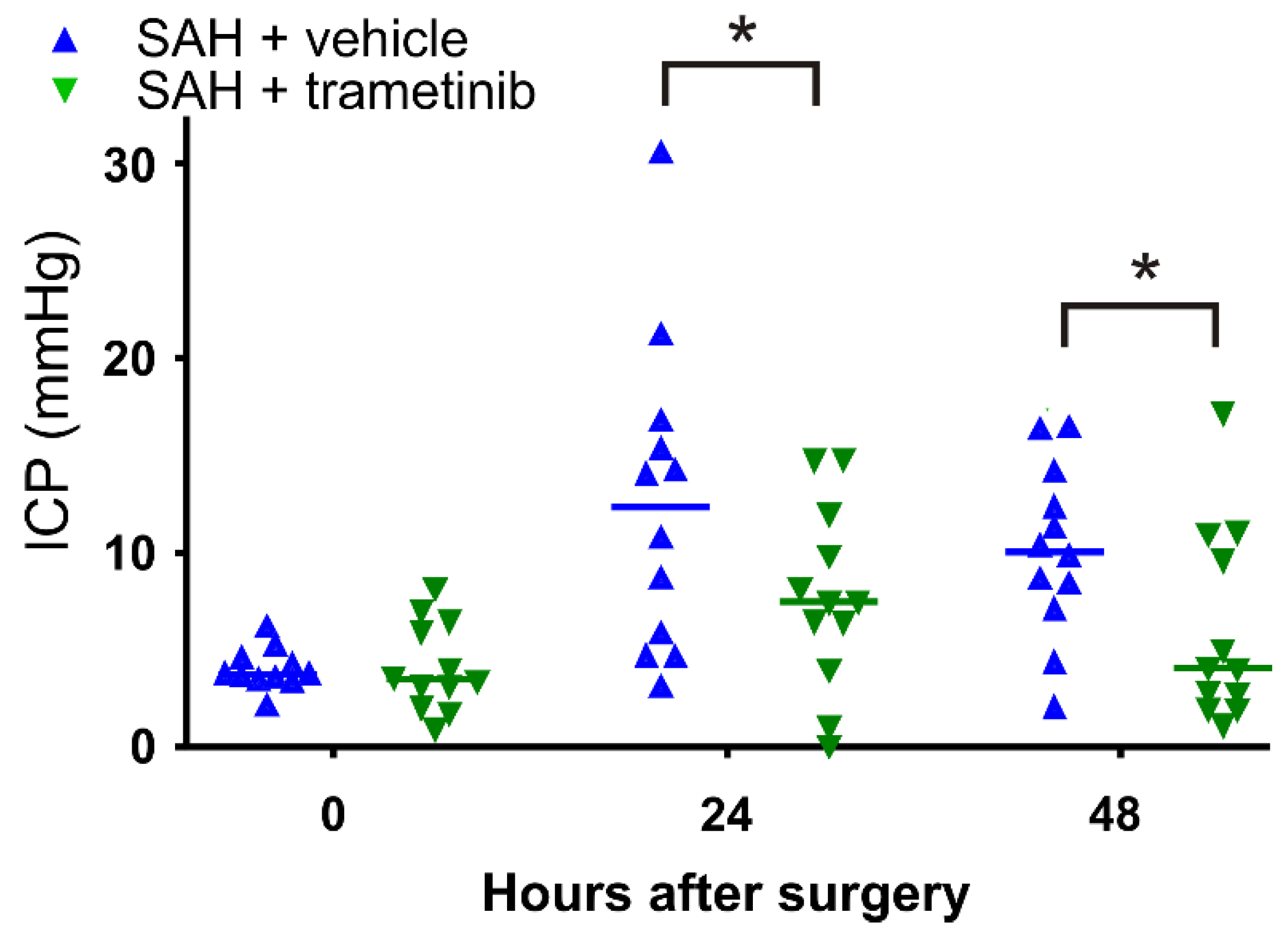
2.5. Elevated Intracranial Pressure in the Subacute Phase after SAH in Female Rats
2.6. Weight Loss and General Well-Being
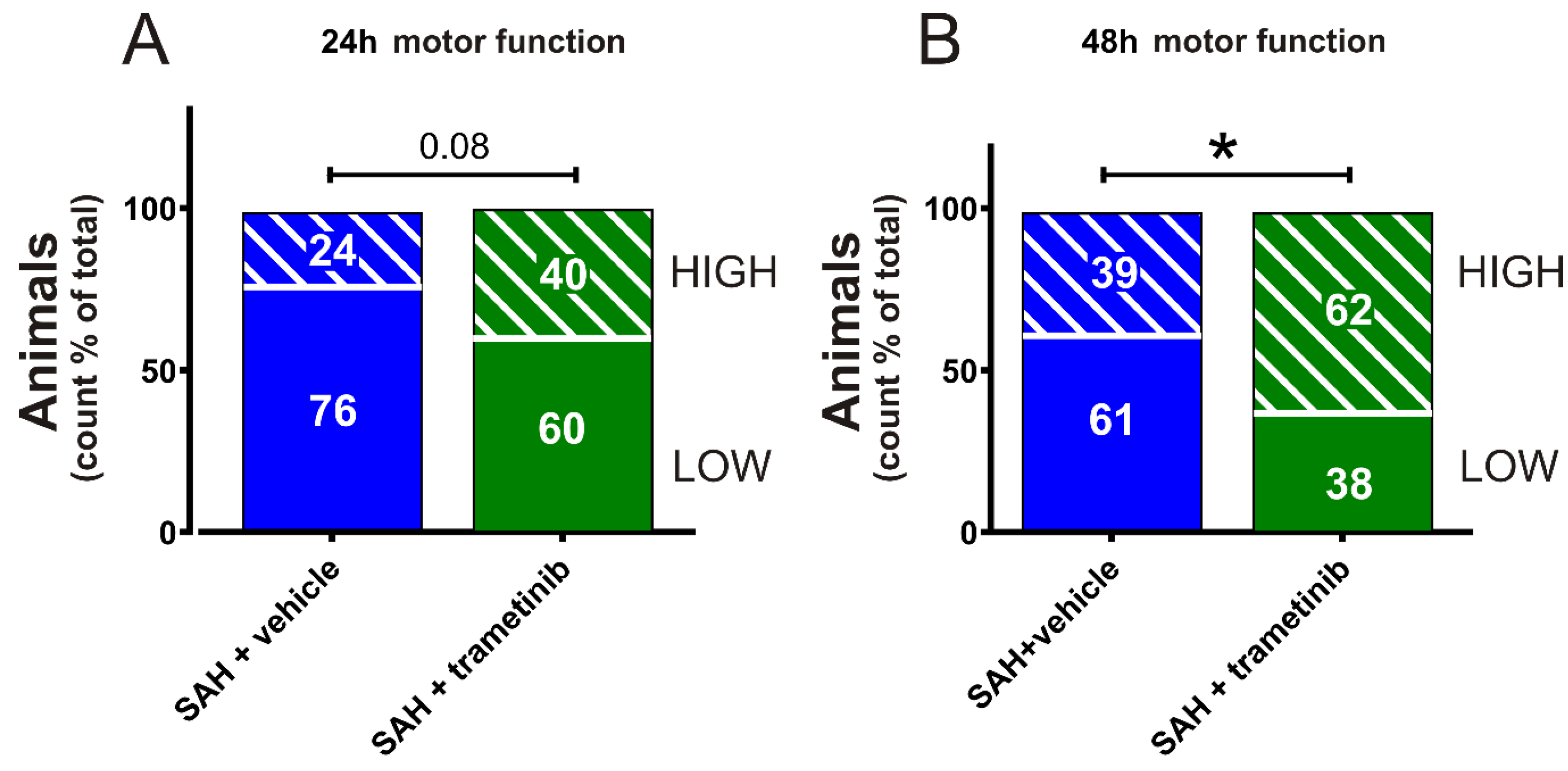
2.7. Sensorimotor Cognition after SAH in Female Rats
3. Discussion
3.1. Contribution to the Field
3.2. Clinical Relevance
3.3. Limitations
4. Materials and Methods
4.1. Animals
4.2. Preparation of Trametinib Solution
4.3. Artery Harvest and Organ Culture
4.4. Vaginal Smears—Estrous Cycle Determination
4.5. Experimental Model of SAH
4.6. Neurological Tests
4.6.1. Rotating Pole Test
4.6.2. Behavioural Observation
4.7. Harvest of Cerebral Arteries
4.8. In Vitro Pharmacology
4.9. Intracranial Pressure Measurements
4.10. Weight
4.11. Statistics and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAH | Subarachnoid haemorrhage |
| DCI | Delayed cerebral ischemia |
| ET-1 | Endothelin-1 |
| 5-CT | 5-carboxamidotryptamine |
| 5-HT | 5-Hydroxytryptamine |
| ICP | Intracranial pressure |
| IP | Intraperitoneal |
| CBF | Cerebral blood flow |
| ETA | Endothelin receptor A |
| ETB | Endothelin receptor B |
| MABP | Mean arterial blood pressure |
| MEK | Mitogen-activated protein kinase kinase |
| PKC | Protein kinase C |
| BBB | Blood–brain barrier |
| BA | Basilar artery |
| MCA | Middle cerebral artery |
References
- Mahlamaki, K.; Rautalin, I.; Korja, M. Case Fatality Rates of Subarachnoid Hemorrhage Are Decreasing with Substantial between-Country Variation: A Systematic Review of Population-Based Studies between 1980 and 2020. Neuroepidemiology 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Lee, M.; Rana, J.; Virani, S.S. Epidemiology and risk factors for stroke in young individuals: Implications for prevention. Curr. Opin. Cardiol. 2021, 36, 565–571. [Google Scholar] [CrossRef]
- Lai, P.M.R.; Gormley, W.B.; Patel, N.; Frerichs, K.U.; Aziz-Sultan, M.A.; Du, R. Age-Dependent Radiographic Vasospasm and Delayed Cerebral Ischemia in Women After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019, 130, e230–e235. [Google Scholar] [CrossRef]
- Fuentes, A.M.; Stone McGuire, L.; Amin-Hanjani, S. Sex Differences in Cerebral Aneurysms and Subarachnoid Hemorrhage. Stroke 2022, 53, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, S. Relationship between Postmenopausal Estrogen Deficiency and Aneurysmal Subarachnoid Hemorrhage. Behav. Neurol. 2015, 2015, 720141. [Google Scholar] [CrossRef] [PubMed]
- Carcel, C.; Harris, K.; Peters, S.A.E.; Sandset, E.C.; Balicki, G.; Bushnell, C.D.; Howard, V.J.; Reeves, M.J.; Anderson, C.S.; Kelly, P.J.; et al. Representation of Women in Stroke Clinical Trials: A Review of 281 Trials Involving More Than 500,000 Participants. Neurology 2021, 97, e1768–e1774. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, S.D.; Haanes, K.A.; Warfvinge, K.; Edvinsson, L. Perivascular neurotransmitters: Regulation of cerebral blood flow and role in primary headaches. J. Cereb. Blood Flow Metab. 2019, 39, 610–632. [Google Scholar] [CrossRef]
- Geraghty, J.R.; Testai, F.D. Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: Beyond Vasospasm and Towards a Multifactorial Pathophysiology. Curr. Atheroscler. Rep. 2017, 19, 50. [Google Scholar] [CrossRef]
- Viderman, D.; Tapinova, K.; Abdildin, Y.G. Mechanisms of cerebral vasospasm and cerebral ischaemia in subarachnoid haemorrhage. Clin. Physiol. Funct. Imaging 2022. ahead of print. [Google Scholar] [CrossRef]
- Chaudhry, S.R.; Shafique, S.; Sajjad, S.; Hanggi, D.; Muhammad, S. Janus Faced HMGB1 and Post-Aneurysmal Subarachnoid Hemorrhage (aSAH) Inflammation. Int. J. Mol. Sci. 2022, 23, 11216. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Huang, L.; Wang, X.; Si, X.; Lenahan, C.; Shi, H.; Shao, A.; Tang, J.; Chen, S.; Zhang, J.; et al. A new perspective on cerebrospinal fluid dynamics after subarachnoid hemorrhage: From normal physiology to pathophysiological changes. J. Cereb. Blood Flow Metab. 2022, 42, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Stokum, J.A.; Gerzanich, V.; Simard, J.M. Molecular pathophysiology of cerebral edema. J. Cereb. Blood Flow Metab. 2016, 36, 513–538. [Google Scholar] [CrossRef]
- Edvinsson, L.I.; Povlsen, G.K. Vascular plasticity in cerebrovascular disorders. J. Cereb. Blood Flow Metab. 2011, 31, 1554–1571. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.; Heider, R.A.; Zaharieva, D.; Ahmad, F.U.; Barrow, D.L.; Pradilla, G. Sex Differences in the Formation of Intracranial Aneurysms and Incidence and Outcome of Subarachnoid Hemorrhage: Review of Experimental and Human Studies. Transl. Stroke Res. 2016, 7, 12–19. [Google Scholar] [CrossRef]
- Edvinsson, L.; Larsen, S.S.; Maddahi, A.; Nielsen, J. Plasticity of cerebrovascular smooth muscle cells after subarachnoid hemorrhage. Transl. Stroke Res. 2014, 5, 365–376. [Google Scholar] [CrossRef]
- Ansar, S.; Edvinsson, L. Subtype activation and interaction of protein kinase C and mitogen-activated protein kinase controlling receptor expression in cerebral arteries and microvessels after subarachnoid hemorrhage. Stroke 2008, 39, 185–190. [Google Scholar] [CrossRef]
- Ansar, S.; Edvinsson, L. Equal contribution of increased intracranial pressure and subarachnoid blood to cerebral blood flow reduction and receptor upregulation after subarachnoid hemorrhage. Laboratory investigation. J. Neurosurg. 2009, 111, 978–987. [Google Scholar] [CrossRef]
- You, Y.; Niu, Y.; Zhang, J.; Huang, S.; Ding, P.; Sun, F.; Wang, X. U0126: Not only a MAPK kinase inhibitor. Front. Pharmacol. 2022, 13, 927083. [Google Scholar] [CrossRef]
- Christensen, S.T.; Haanes, K.A.; Spray, S.; Grell, A.S.; Warfvinge, K.; Edvinsson, L.; Johansson, S.E. Pre-clinical effects of highly potent MEK1/2 inhibitors on rat cerebral vasculature after organ culture and subarachnoid haemorrhage. Clin. Sci. 2019, 133, 1797–1811. [Google Scholar] [CrossRef]
- Zeiser, R.; Andrlova, H.; Meiss, F. Trametinib (GSK1120212). Recent Results Cancer Res. 2018, 211, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Kim, M.Y.; Lee, S.Y.; Kim, M.J.; Hong, T.J.; Jeon, J.K.; Ganbat, D.; Kim, H.T.; Kim, S.S.; Kam, T.I.; et al. MEK1/2 inhibition rescues neurodegeneration by TFEB-mediated activation of autophagic lysosomal function in a model of Alzheimer’s Disease. Mol. Psychiatry 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.R.; Stanford, S.C.; Panattieri, R.A.; Alexander, S.P.H.; Cirino, G.; George, C.H.; Hoyer, D.; Izzo, A.A.; Ji, Y.; Lilley, E.; et al. Sex: A change in our guidelines to authors to ensure that this is no longer an ignored experimental variable. Br. J. Pharmacol. 2019, 176, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Rehnstrom, M.; Frederiksen, S.D.; Ansar, S.; Edvinsson, L. Transcriptome profiling revealed early vascular smooth muscle cell gene activation following focal ischemic stroke in female rats—Comparisons with males. BMC Genomics 2020, 21, 883. [Google Scholar] [CrossRef]
- Rehnstrom, M.; Ahnstedt, H.; Krause, D.N.; Edvinsson, M.L.; Haanes, K.A.; Edvinsson, L. Ovariectomy Reduces Vasocontractile Responses of Rat Middle Cerebral Arteries After Focal Cerebral Ischemia. J. Cardiovasc. Pharmacol. 2022, 79, e122–e128. [Google Scholar] [CrossRef]
- Mostajeran, M.; Edvinsson, L.; Ahnstedt, H.; Arkelius, K.; Ansar, S. Repair-related molecular changes during recovery phase of ischemic stroke in female rats. BMC Neurosci. 2022, 23, 23. [Google Scholar] [CrossRef]
- Ahnstedt, H.; Mostajeran, M.; Blixt, F.W.; Warfvinge, K.; Ansar, S.; Krause, D.N.; Edvinsson, L. U0126 attenuates cerebral vasoconstriction and improves long-term neurologic outcome after stroke in female rats. J. Cereb. Blood Flow Metab. 2015, 35, 454–460. [Google Scholar] [CrossRef]
- Bomers, J.P.; Johansson, S.E.; Edvinsson, L.; Mathiesen, T.I.; Haanes, K.A. Pre-Chiasmatic, Single Injection of Autologous Blood to Induce Experimental Subarachnoid Hemorrhage in a Rat Model. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Jonsson, A.C.; Lindgren, I.; Norrving, B.; Lindgren, A. Weight loss after stroke: A population-based study from the Lund Stroke Register. Stroke 2008, 39, 918–923. [Google Scholar] [CrossRef]
- Jeon, H.; Ai, J.; Sabri, M.; Tariq, A.; Shang, X.; Chen, G.; Macdonald, R.L. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009, 10, 103. [Google Scholar] [CrossRef]
- Christensen, S.T.; Johansson, S.E.; Radziwon-Balicka, A.; Warfvinge, K.; Haanes, K.A.; Edvinsson, L. MEK1/2 inhibitor U0126, but not nimodipine, reduces upregulation of cerebrovascular contractile receptors after subarachnoid haemorrhage in rats. PLoS ONE 2019, 14, e0215398. [Google Scholar] [CrossRef]
- Povlsen, G.K.; Edvinsson, L. MEK1/2 inhibitor U0126 but not endothelin receptor antagonist clazosentan reduces upregulation of cerebrovascular contractile receptors and delayed cerebral ischemia, and improves outcome after subarachnoid hemorrhage in rats. J. Cereb. Blood Flow Metab. 2015, 35, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Povlsen, G.K. Late cerebral ischaemia after subarachnoid haemorrhage: Is cerebrovascular receptor upregulation the mechanism behind? Acta Physiol. 2011, 203, 209–224. [Google Scholar] [CrossRef]
- Spray, S.; Johansson, S.E.; Radziwon-Balicka, A.; Haanes, K.A.; Warfvinge, K.; Povlsen, G.K.; Kelly, P.A.T.; Edvinsson, L. Enhanced contractility of intraparenchymal arterioles after global cerebral ischaemia in rat—New insights into the development of delayed cerebral hypoperfusion. Acta Physiol. 2017, 220, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Spray, S.; Haanes, K.A.; Edvinsson, L.; Johansson, S.E. Subacute phase of subarachnoid haemorrhage in female rats: Increased intracranial pressure, vascular changes and impaired sensorimotor function. Microvasc. Res. 2021, 135, 104127. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gordon, W.P.; Richmond, W.; Groessl, T.; Tuntland, T. Use of solubilizers in preclinical formulations: Effect of Cremophor EL on the pharmacokinetic properties on early discovery compounds. Eur. J. Pharm. Sci. 2016, 87, 52–57. [Google Scholar] [CrossRef]
- Christensen, S.T.; Grell, A.S.; Johansson, S.E.; Andersson, C.M.; Edvinsson, L.; Haanes, K.A. Synergistic effects of a cremophor EL drug delivery system and its U0126 cargo in an ex vivo model. Drug Deliv. 2019, 26, 680–688. [Google Scholar] [CrossRef]
- Zhao, F.K.; Chuang, L.F.; Israel, M.; Chuang, R.Y. Cremophor EL, a widely used parenteral vehicle, is a potent inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1989, 159, 1359–1367. [Google Scholar] [CrossRef]
- Henriksson, M.; Stenman, E.; Vikman, P.; Edvinsson, L. MEK1/2 inhibition attenuates vascular ETA and ETB receptor alterations after cerebral ischaemia. Exp. Brain Res. 2007, 178, 470–476. [Google Scholar] [CrossRef][Green Version]
- Beg, S.S.; Hansen-Schwartz, J.A.; Vikman, P.J.; Xu, C.B.; Edvinsson, L.I. Protein kinase C inhibition prevents upregulation of vascular ET(B) and 5-HT(1B) receptors and reverses cerebral blood flow reduction after subarachnoid haemorrhage in rats. J. Cereb. Blood Flow Metab. 2007, 27, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Mascia, L.; Fedorko, L.; Stewart, D.J.; Mohamed, F.; terBrugge, K.; Ranieri, V.M.; Wallace, M.C. Temporal relationship between endothelin-1 concentrations and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Stroke 2001, 32, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Voldby, B.; Engbaek, F.; Enevoldsen, E.M. CSF serotonin concentrations and cerebral arterial spasm in patients with ruptured intracranial aneurysm. Stroke 1982, 13, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Laban, K.G.; Vergouwen, M.D.; Dijkhuizen, R.M.; Sena, E.S.; Macleod, M.R.; Rinkel, G.J.; van der Worp, H.B. Effect of endothelin receptor antagonists on clinically relevant outcomes after experimental subarachnoid hemorrhage: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2015, 35, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, N.M.; Wang, J.Z.; Pasarikovski, C.R.; Guha, D.; Al-Mufti, F.; Mamdani, M.; Saposnik, G.; Schweizer, T.A.; Macdonald, R.L. Management of raised intracranial pressure in aneurysmal subarachnoid hemorrhage: Time for a consensus? Neurosurg. Focus 2017, 43, E13. [Google Scholar] [CrossRef]
- Wettervik, T.S.; Hanell, A.; Howells, T.; Engstrom, E.R.; Lewen, A.; Enblad, P. ICP, CPP, and PRx in traumatic brain injury and aneurysmal subarachnoid hemorrhage: Association of insult intensity and duration with clinical outcome. J. Neurosurg. 2022, 1–8. [Google Scholar] [CrossRef]
- Friedrich, V.; Bederson, J.B.; Sehba, F.A. Gender influences the initial impact of subarachnoid hemorrhage: An experimental investigation. PLoS ONE 2013, 8, e80101. [Google Scholar] [CrossRef]
- Friedrich, V.; Bi, W.; Sehba, F.A. Sexual dimorphism in gene expression after aneurysmal subarachnoid hemorrhage. Neurol. Res. 2015, 37, 1054–1059. [Google Scholar] [CrossRef]
- Johansson, S.E.; Abdolalizadeh, B.; Sheykhzade, M.; Edvinsson, L.; Sams, A. Vascular pathology of large cerebral arteries in experimental subarachnoid hemorrhage: Vasoconstriction, functional CGRP depletion and maintained CGRP sensitivity. Eur. J. Pharmacol. 2019, 846, 109–118. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Lai, N.; Tong, J.; Wang, G.; Tang, D. Association between pro-inflammatory cytokines in cerebrospinal fluid and headache in patients with aneurysmal subarachnoid hemorrhage. J. Neuroimmunol. 2022, 366, 577841. [Google Scholar] [CrossRef]
- Ehrlichman, L.K.; Ford, J.W.; Roelofs, K.J.; Tedeschi-Filho, W.; Futchko, J.S.; Ramacciotti, E.; Eliason, J.L.; Henke, P.K.; Upchurch, G.R., Jr. Gender-dependent differential phosphorylation in the ERK signaling pathway is associated with increased MMP2 activity in rat aortic smooth muscle cells. J. Surg. Res. 2010, 160, 18–24. [Google Scholar] [CrossRef]
- Koss, W.A.; Haertel, J.M.; Philippi, S.M.; Frick, K.M. Sex Differences in the Rapid Cell Signaling Mechanisms Underlying the Memory-Enhancing Effects of 17beta-Estradiol. eNeuro 2018, 5, ENEURO.0267-18.2018. [Google Scholar] [CrossRef]
- Ahnstedt, H.; Cao, L.; Krause, D.N.; Warfvinge, K.; Saveland, H.; Nilsson, O.G.; Edvinsson, L. Male-female differences in upregulation of vasoconstrictor responses in human cerebral arteries. PLoS ONE 2013, 8, e62698. [Google Scholar] [CrossRef]
- Vaidhyanathan, S.; Mittapalli, R.K.; Sarkaria, J.N.; Elmquist, W.F. Factors influencing the CNS distribution of a novel MEK-1/2 inhibitor: Implications for combination therapy for melanoma brain metastases. Drug Metab. Dispos. 2014, 42, 1292–1300. [Google Scholar] [CrossRef]
- Gampa, G.; Kim, M.; Cook-Rostie, N.; Laramy, J.K.; Sarkaria, J.N.; Paradiso, L.; DePalatis, L.; Elmquist, W.F. Brain Distribution of a Novel MEK Inhibitor E6201: Implications in the Treatment of Melanoma Brain Metastases. Drug Metab. Dispos. 2018, 46, 658–666. [Google Scholar] [CrossRef]
- Kim, K.B.; Kefford, R.; Pavlick, A.C.; Infante, J.R.; Ribas, A.; Sosman, J.A.; Fecher, L.A.; Millward, M.; McArthur, G.A.; Hwu, P.; et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J. Clin. Oncol. 2013, 31, 482–489. [Google Scholar] [CrossRef]
- Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/mekinist-epar-product-information_en.pdf (accessed on 17 October 2022).
- Vikman, P.; Edvinsson, L. Gene expression profiling in the human middle cerebral artery after cerebral ischemia. Eur. J. Neurol. 2006, 13, 1324–1332. [Google Scholar] [CrossRef]
- Shaw, S.; Kumar, U.; Bhaumik, G.; Reddy, M.P.K.; Kumar, B.; Ghosh, D. Alterations of estrous cycle, 3beta hydroxysteroid dehydrogenase activity and progesterone synthesis in female rats after exposure to hypobaric hypoxia. Sci. Rep. 2020, 10, 3458. [Google Scholar] [CrossRef]
- Prunell, G.F.; Mathiesen, T.; Svendgaard, N.A. A new experimental model in rats for study of the pathophysiology of subarachnoid hemorrhage. Neuroreport 2002, 13, 2553–2556. [Google Scholar] [CrossRef]
- Johansson, S.E.; Larsen, S.S.; Povlsen, G.K.; Edvinsson, L. Early MEK1/2 inhibition after global cerebral ischemia in rats reduces brain damage and improves outcome by preventing delayed vasoconstrictor receptor upregulation. PLoS ONE 2014, 9, e92417. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bömers, J.P.; Grell, A.-S.; Edvinsson, L.; Johansson, S.E.; Haanes, K.A. The MEK Inhibitor Trametinib Improves Outcomes following Subarachnoid Haemorrhage in Female Rats. Pharmaceuticals 2022, 15, 1446. https://doi.org/10.3390/ph15121446
Bömers JP, Grell A-S, Edvinsson L, Johansson SE, Haanes KA. The MEK Inhibitor Trametinib Improves Outcomes following Subarachnoid Haemorrhage in Female Rats. Pharmaceuticals. 2022; 15(12):1446. https://doi.org/10.3390/ph15121446
Chicago/Turabian StyleBömers, Jesper Peter, Anne-Sofie Grell, Lars Edvinsson, Sara Ellinor Johansson, and Kristian Agmund Haanes. 2022. "The MEK Inhibitor Trametinib Improves Outcomes following Subarachnoid Haemorrhage in Female Rats" Pharmaceuticals 15, no. 12: 1446. https://doi.org/10.3390/ph15121446
APA StyleBömers, J. P., Grell, A.-S., Edvinsson, L., Johansson, S. E., & Haanes, K. A. (2022). The MEK Inhibitor Trametinib Improves Outcomes following Subarachnoid Haemorrhage in Female Rats. Pharmaceuticals, 15(12), 1446. https://doi.org/10.3390/ph15121446




