Simvastatin Attenuated Tumor Growth in Different Pancreatic Tumor Animal Models
Abstract
1. Introduction
2. Results
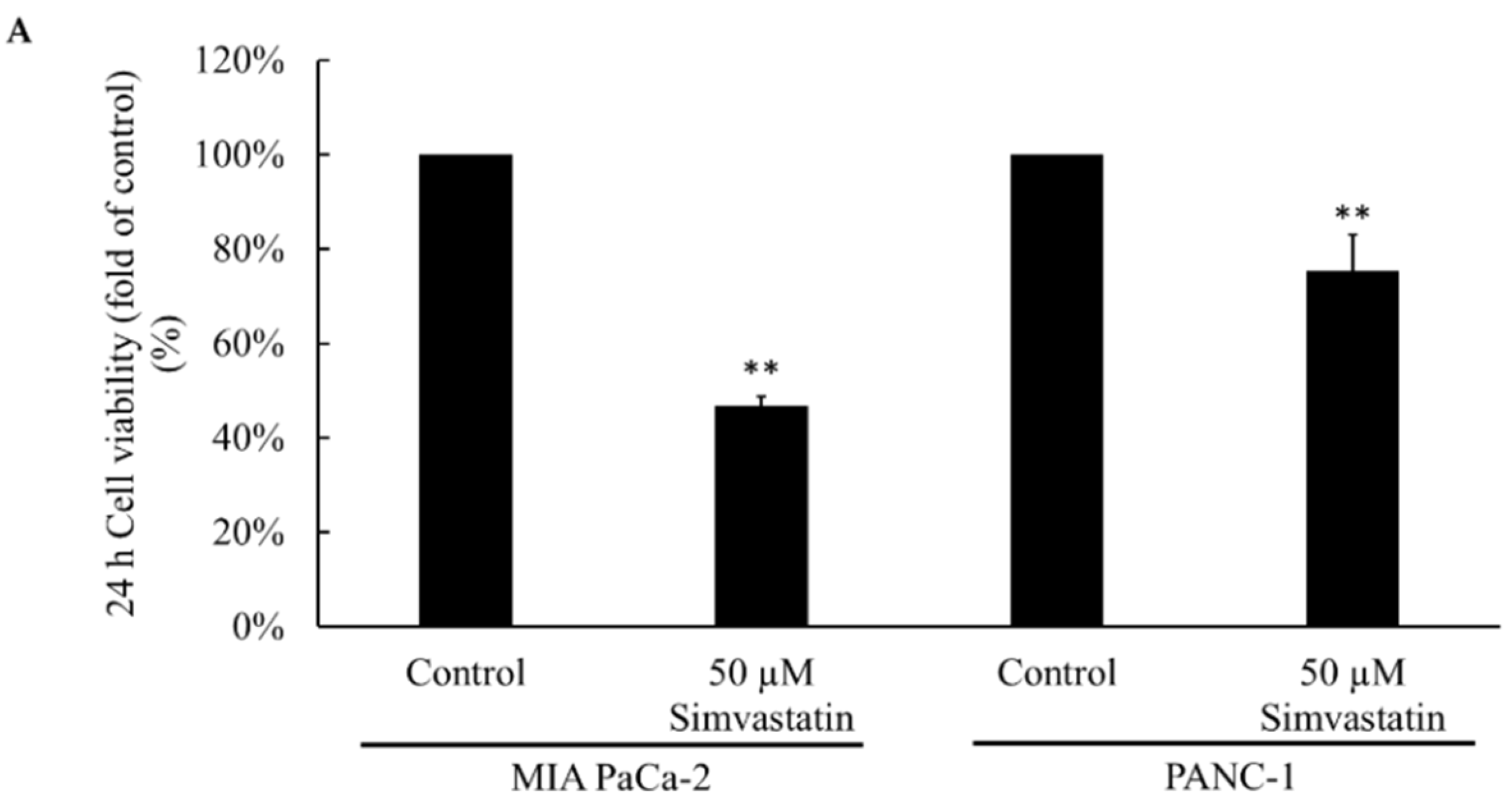
2.1. Cell Viability after Simvastatin Treatment
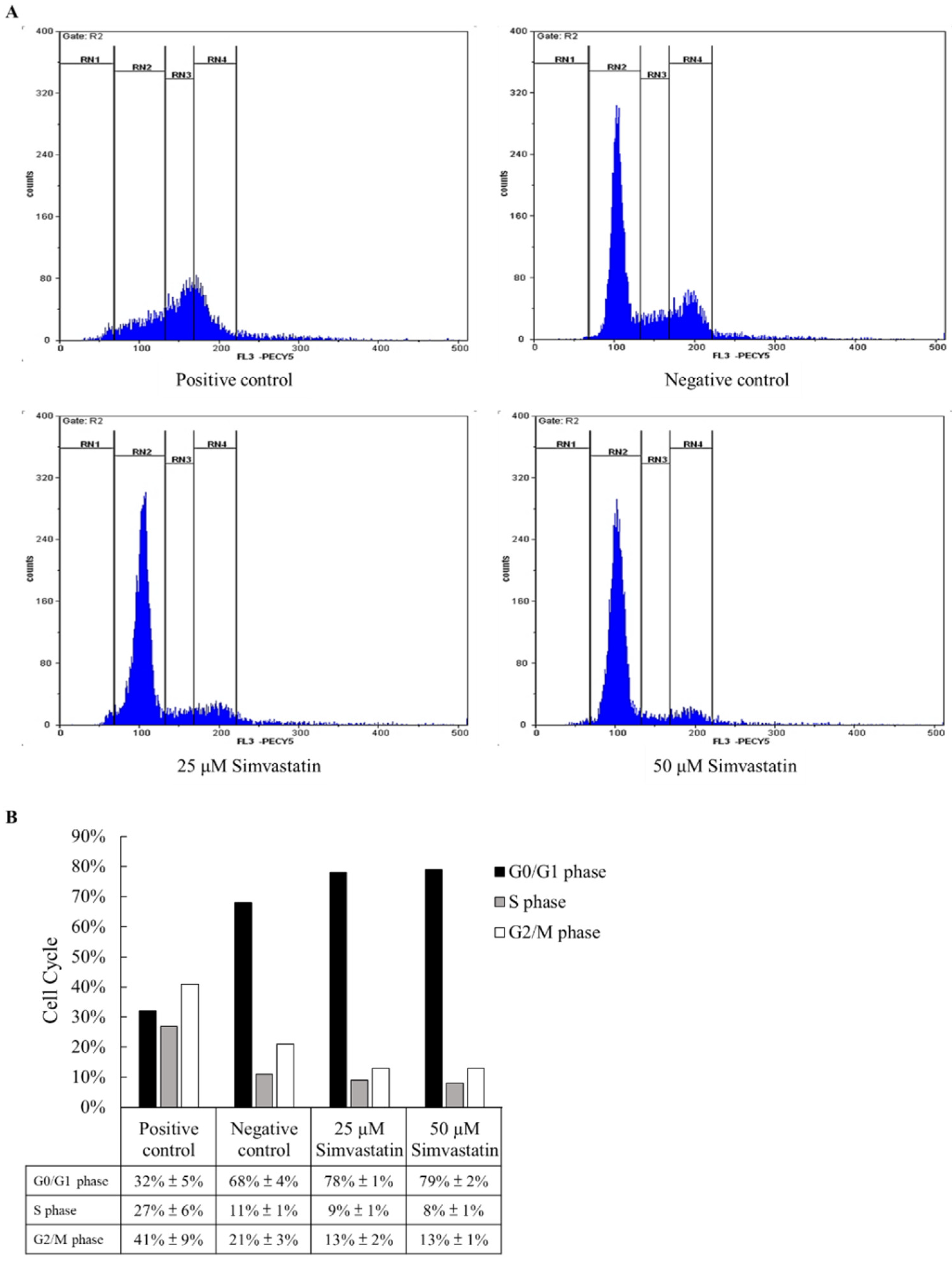
2.2. Cell Cycle Study
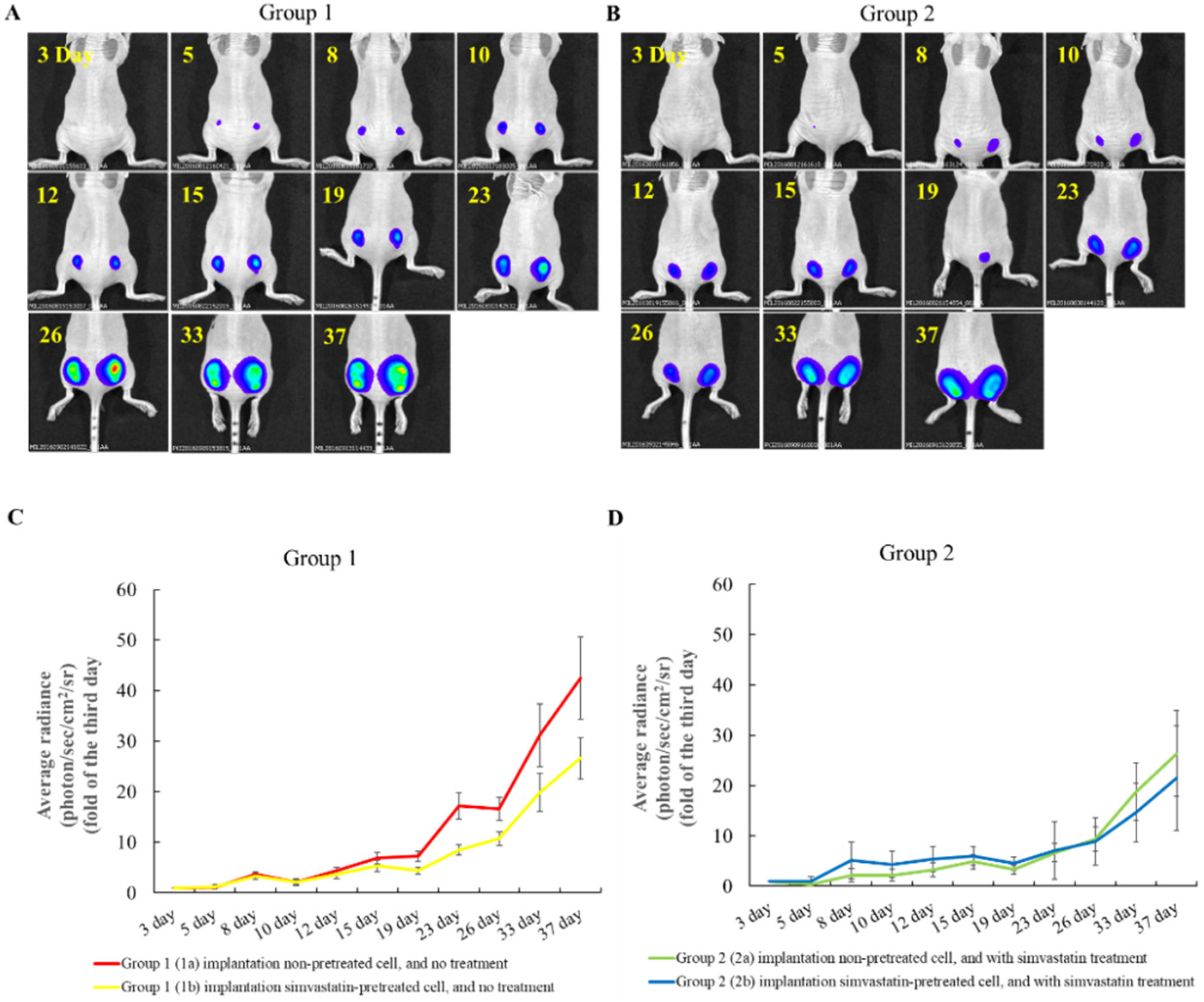
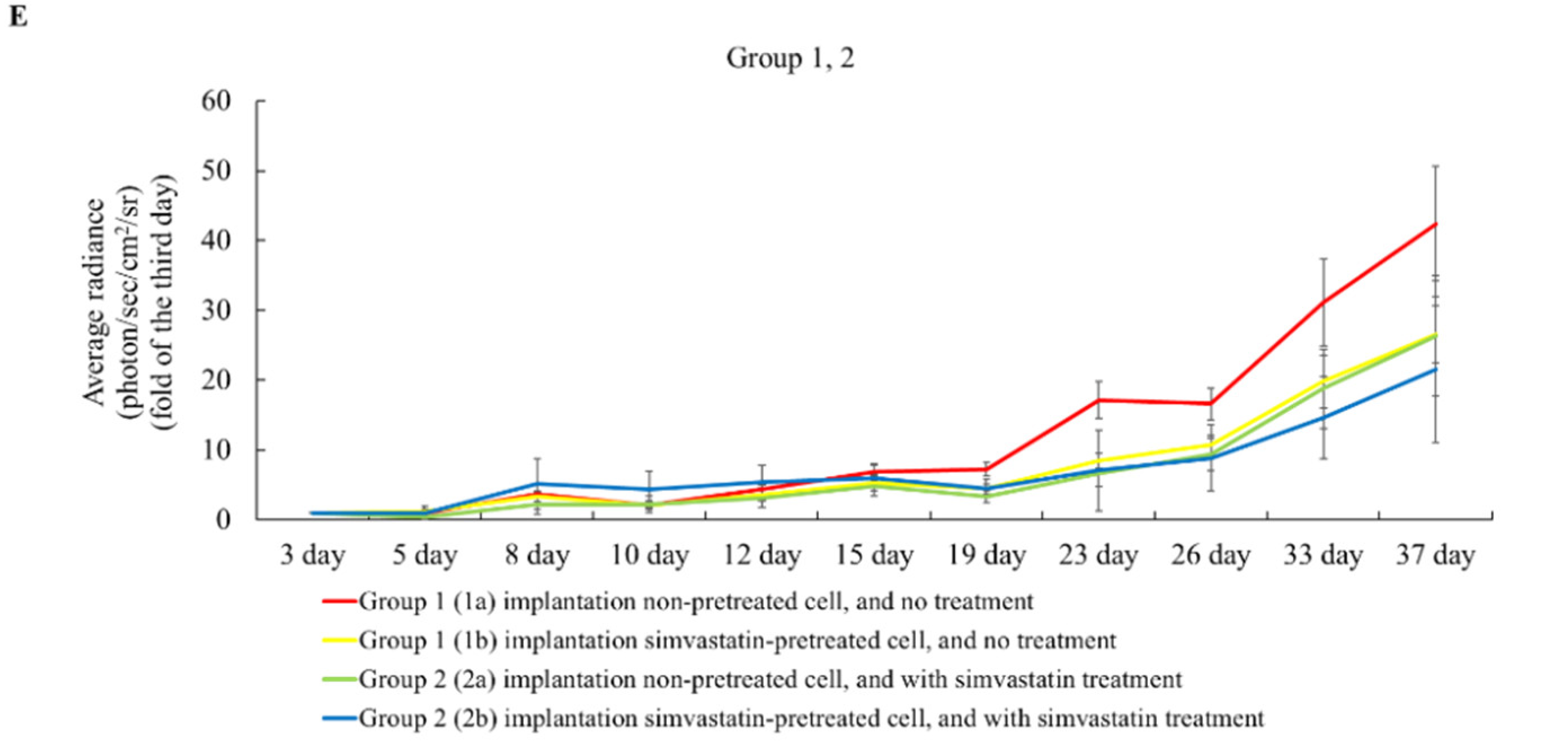
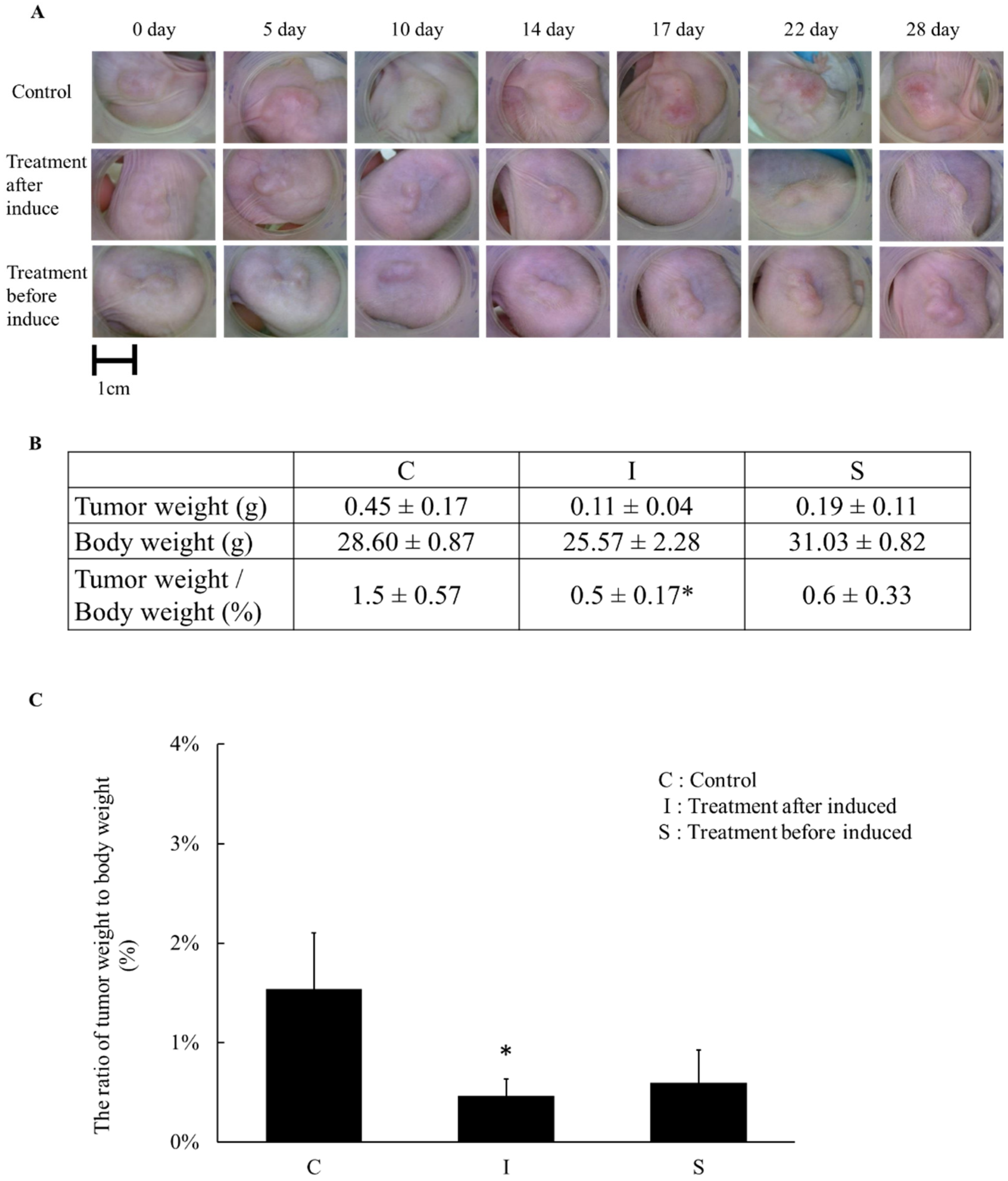
2.3. The Effects of Simvastatin Treatment in Subcutaneous Induced Animal Model
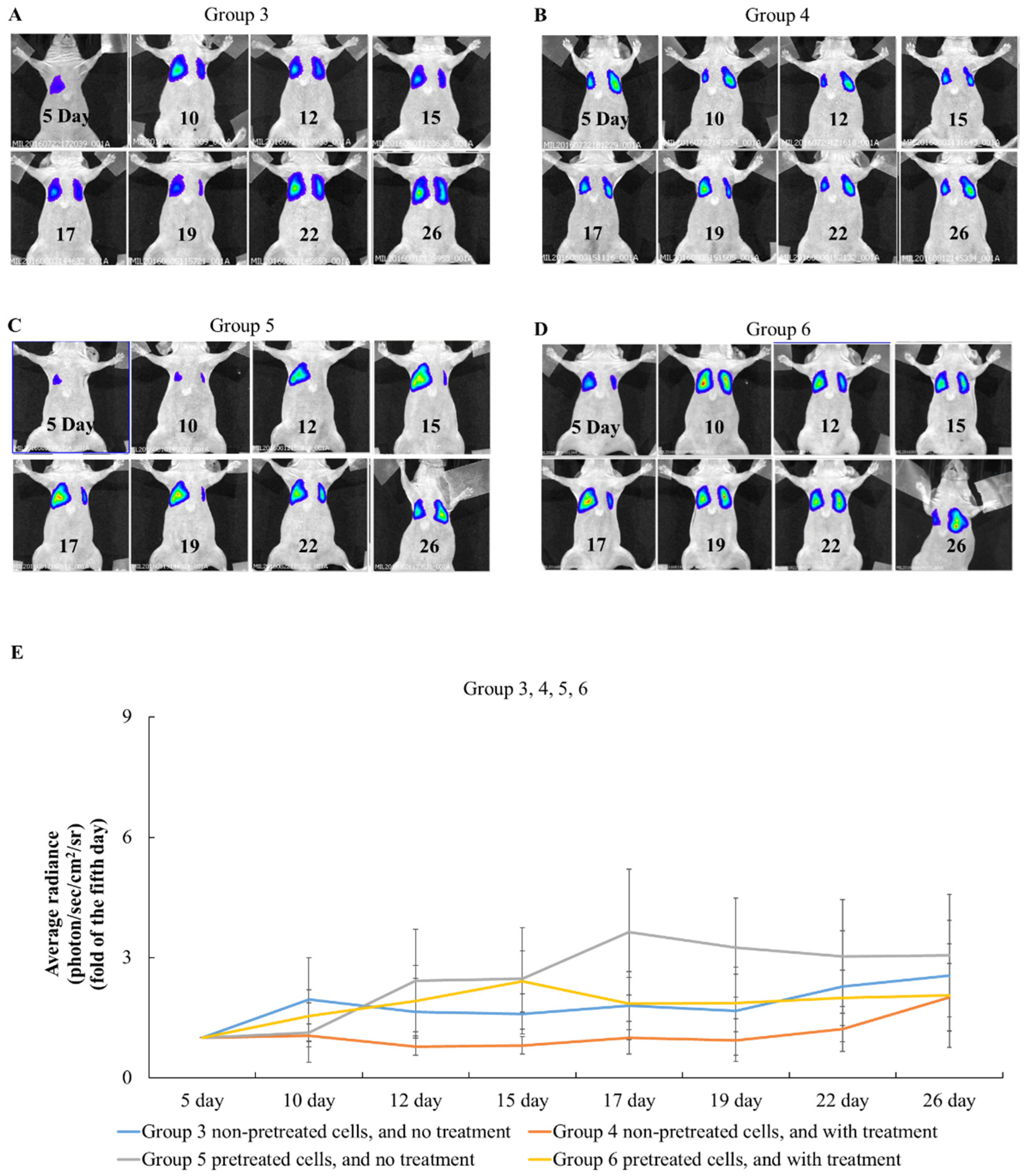
2.4. The Effects of Simvastatin Treatment in Intravenous Induced Animal Metastasis Model
2.5. The Tumor Weight Changed after Simvastatin Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Cell Viability Assay
4.3. Cell Proliferation
4.4. Cell Cycle Assay
4.5. Inhibitory Effects of Simvastatin on Subcutaneous and Lung Metastatic Tumor Growth In Vivo
4.6. In Vitro and In Vivo Bioluminescent Image
4.7. Xenograft Tumor Animal Model by PANC-1 Cell Line
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.M.; Mills, K.; Mendonca, S.C.; Abel, G.A.; Basu, B.; Carroll, N.; Ballard, S.; Lancaster, J.; Hamilton, W.; Rubin, G.P.; et al. Symptoms and patient factors associated with diagnostic intervals for pancreatic cancer (SYMPTOM pancreatic study): A prospective cohort study. Lancet Gastroenterol. Hepatol. 2016, 1, 298–306. [Google Scholar] [CrossRef]
- ACS. Lifetime Risk of Developing or Dying From Cancer. Available online: https://www.cancer.org/cancer/cancer-basics/lifetime-probability-of-developing-or-dying-from-cancer.html (accessed on 17 June 2021).
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- PDQ® Adult Treatment Editorial Board. PDQ Pancreatic Neuroendocrine Tumors (Islet Cell Tumors) Treatment. Bethesda, MD: National Cancer Institute. Updated <10/07/2022>. PMID: 26389340. Available online: https://www.cancer.gov/types/pancreatic/patient/pnet-treatment-pdq (accessed on 13 November 2022).
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Bond-Smith, G.; Banga, N.; Hammond, T.M.; Imber, C.J. Pancreatic adenocarcinoma. BMJ 2012, 344, e2476. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Iacobuzio-Donahue, C.A. The Pathology and Genetics of Metastatic Pancreatic Cancer. Arch. Pathol. Lab. Med. 2009, 133, 413–422. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Hariharan, D.; Saied, A.; Kocher, H.M. Analysis of mortality rates for pancreatic cancer across the world. HPB 2008, 10, 58–62. [Google Scholar] [CrossRef]
- ACS. Cancer Facts & Figures. 2010. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures.html (accessed on 17 June 2021).
- PDQ® Adult Treatment Editorial Board. PDQ Pancreatic Neuroendocrine Tumors (Islet Cell Tumors) Treatment. Bethesda, MD: National Cancer Institute. Updated <8/19/2022>. PMID: 26389309. Available online: https://www.cancer.gov/types/pancreatic/hp/pnet-treatment-pdq (accessed on 13 November 2022).
- Boudreau, D.M.; Yu, O.; Johnson, J. Statin use and cancer risk: A comprehensive review. Expert Opin. Drug Saf. 2010, 9, 603–621. [Google Scholar] [CrossRef]
- Gong, J.; Sachdev, E.; Robbins, L.A.; Lin, E.; Hendifar, A.E.; Mita, M.M. Statins and pancreatic cancer. Oncol. Lett. 2017, 13, 1035–1040. [Google Scholar] [CrossRef]
- Katz, M.S. Therapy insight: Potential of statins for cancer chemoprevention and therapy. Nat. Clin. Pract. Oncol. 2005, 2, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Z.; Chang, J.I.; Li, E.; Xiang, A.H.; Wu, B.U. Influence of Statins and Cholesterol on Mortality Among Patients With Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109, djw275. [Google Scholar] [CrossRef] [PubMed]
- Fendrich, V.; Sparn, M.; Lauth, M.; Knoop, R.; Plassmeier, L.; Bartsch, D.K.; Waldmann, J. Simvastatin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Pancreatology 2013, 13, 502–507. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Ho, S.C.; Chen, C.C.; Yang, C.Y. Statin use and the risk of liver cancer: A population-based case-control study. Am. J. Gastroenterol. 2011, 106, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Nam, E.M.; Lee, J.; Park, J.O.; Lee, S.C.; Song, S.Y.; Choi, S.H.; Heo, J.S.; Park, S.H.; Lim, H.Y.; et al. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother. Pharmacol. 2014, 73, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Jian-Yu, E.; Graber, J.M.; Lu, S.E.; Lin, Y.; Lu-Yao, G.; Tan, X.L. Effect of Metformin and Statin Use on Survival in Pancreatic Cancer Patients: A Systematic Literature Review and Meta-analysis. Curr. Med. Chem. 2018, 25, 2595–2607. [Google Scholar] [CrossRef]
- Gbelcova, H.; Rimpelova, S.; Ruml, T.; Fenclova, M.; Kosek, V.; Hajslova, J.; Strnad, H.; Kolar, M.; Vitek, L. Variability in statin-induced changes in gene expression profiles of pancreatic cancer. Sci. Rep. 2017, 7, 44219. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, J.S.; Kim, J.M.; Lee, J.Y.; Jung, H.C.; Song, I.S. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int. J. Cancer 2008, 123, 951–957. [Google Scholar] [CrossRef]
- Liu, S.; Uppal, H.; Demaria, M.; Desprez, P.Y.; Campisi, J.; Kapahi, P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci. Rep. 2015, 5, 17895. [Google Scholar] [CrossRef]
- Hoque, A.; Chen, H.; Xu, X.C. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 2008, 17, 88–94. [Google Scholar] [CrossRef]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The potential use of simvastatin for cancer treatment: A review. Biomed. Pharmacother. 2021, 141, 111858. [Google Scholar] [CrossRef]
- Fujiwara, D.; Tsubaki, M.; Takeda, T.; Tomonari, Y.; Koumoto, Y.I.; Sakaguchi, K.; Nishida, S. Statins induce apoptosis through inhibition of Ras signaling pathways and enhancement of Bim and p27 expression in human hematopoietic tumor cells. Tumour. Biol. 2017, 39, 1010428317734947. [Google Scholar] [CrossRef] [PubMed]
- Sumi, S.; Beauchamp, R.D.; Townsend, C.M., Jr.; Uchida, T.; Murakami, M.; Rajaraman, S.; Ishizuka, J.; Thompson, J.C. Inhibition of pancreatic adenocarcinoma cell growth by lovastatin. Gastroenterology 1992, 103, 982–989. [Google Scholar] [CrossRef]
- Issat, T.; Nowis, D.; Bil, J.; Winiarska, M.; Jakobisiak, M.; Golab, J. Antitumor effects of the combination of cholesterol reducing drugs. Oncol. Rep. 2011, 26, 169–176. [Google Scholar] [CrossRef]
- Paskeviciute, M.; Petrikaite, V. Differences of statin activity in 2D and 3D pancreatic cancer cell cultures. Drug Des. Devel. Ther. 2017, 11, 3273–3280. [Google Scholar] [CrossRef] [PubMed]
- You, H.Y.; Zhang, W.J.; Xie, X.M.; Zheng, Z.H.; Zhu, H.L.; Jiang, F.Z. Pitavastatin suppressed liver cancer cells in vitro and in vivo. Onco Targets Ther. 2016, 9, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Lee, S.H.; Kim, J.Y.; Ahn, J.S.; Park, Y.H.; Im, Y.H. Statins affect ETS1-overexpressing triple-negative breast cancer cells by restoring DUSP4 deficiency. Sci. Rep. 2016, 6, 33035. [Google Scholar] [CrossRef]
- Fujita, M.; Hasegawa, A.; Yamamori, M.; Okamura, N. In vitro and in vivo cytotoxicity of troglitazone in pancreatic cancer. J. Exp. Clin. Cancer Res. 2017, 36, 91. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Wang, J.F.; Chen, D.H.; Ma, X.S.; Wu, Y.; Tang, Z.; Dang, X.W. Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci. 2017, 7, 66. [Google Scholar] [CrossRef]
- Afzali, M.; Vatankhah, M.; Ostad, S.N. Investigation of simvastatin-induced apoptosis and cell cycle arrest in cancer stem cells of MCF-7. J. Cancer Res. Ther. 2016, 12, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Bhardwaj, A.; Srivastava, S.K.; Singh, S.; McClellan, S.; Wang, B.; Singh, A.P. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS ONE 2011, 6, e21573. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xia, X.; Yang, Z.; Tian, Y.; Di, J.; Guo, M. Silencing of TCTN1 inhibits proliferation, induces cell cycle arrest and apoptosis in human thyroid cancer. Exp. Ther. Med. 2017, 14, 3720–3726. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Hac, S.; Wyrzykowski, D.; Zauszkiewicz-Pawlak, A.; Inkielewicz-Stepniak, I. Selective cytotoxicity of vanadium complexes on human pancreatic ductal adenocarcinoma cell line by inducing necroptosis, apoptosis and mitotic catastrophe process. Oncotarget 2017, 8, 60324–60341. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Wang, P.C.; Sridhar, R. Evaluation of Tumor Cell Response to Hyperthermia with Bioluminescent Imaging. J. Basic Clin. Med. 2012, 1, 16–19. [Google Scholar] [PubMed]
- Shan, L.; Wang, S.; Korotcov, A.; Sridhar, R.; Wang, P.C. Bioluminescent animal models of human breast cancer for tumor biomass evaluation and metastasis detection. Ethn. Dis. 2008, 18, S2–S65. [Google Scholar]
- Zhu, Q.; Pan, X.; Sun, Y.; Wang, Z.; Liu, F.; Li, A.; Zhao, Z.; Wang, Y.; Li, K.; Mi, L. Biological nanoparticles carrying the Hmda-7 gene are effective in inhibiting pancreatic cancer in vitro and in vivo. PLoS ONE 2017, 12, e0185507. [Google Scholar] [CrossRef] [PubMed]

| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |||
|---|---|---|---|---|---|---|---|---|
| Pretreatment (cells) | Left (1a) | Right (Pretreatment Simvastatin) (1b) | Left (2a) | Right (Pretreatment Simvastatin) (2b) | Pretreatment Simvastatin | Pretreatment Simvastatin | ||
| Induce cancer cells | SC | SC | IV | IV | IV | IV | ||
| IP 10 mg/kg everyday | Simvastatin (after tumor cells implantation) | DMSO | Simvastatin (after tumor cells implantation) | DMSO | Simvastatin (after tumor cells implantation) | |||
| Animal number | 8 | 8 | 8 | 8 | 8 | 8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Yang, Y.-F.; Wang, P.C.; Shan, L.; Lin, S.; Chen, P.-J.; Chen, Y.-J.; Chiang, H.-S.; Lin, J.-T.; Hung, C.-F.; et al. Simvastatin Attenuated Tumor Growth in Different Pancreatic Tumor Animal Models. Pharmaceuticals 2022, 15, 1408. https://doi.org/10.3390/ph15111408
Chen C-Y, Yang Y-F, Wang PC, Shan L, Lin S, Chen P-J, Chen Y-J, Chiang H-S, Lin J-T, Hung C-F, et al. Simvastatin Attenuated Tumor Growth in Different Pancreatic Tumor Animal Models. Pharmaceuticals. 2022; 15(11):1408. https://doi.org/10.3390/ph15111408
Chicago/Turabian StyleChen, Chao-Yi, Yi-Feng Yang, Paul C. Wang, Liang Shan, Stephen Lin, Po-Jung Chen, Yi-Jung Chen, Han-Sun Chiang, Jaw-Town Lin, Chi-Feng Hung, and et al. 2022. "Simvastatin Attenuated Tumor Growth in Different Pancreatic Tumor Animal Models" Pharmaceuticals 15, no. 11: 1408. https://doi.org/10.3390/ph15111408
APA StyleChen, C.-Y., Yang, Y.-F., Wang, P. C., Shan, L., Lin, S., Chen, P.-J., Chen, Y.-J., Chiang, H.-S., Lin, J.-T., Hung, C.-F., & Liang, Y.-J. (2022). Simvastatin Attenuated Tumor Growth in Different Pancreatic Tumor Animal Models. Pharmaceuticals, 15(11), 1408. https://doi.org/10.3390/ph15111408







