Association between Statin Use and Survival in Cancer Patients with Brain Metastasis: Retrospective Analysis from the Chinese Population
Abstract
1. Introduction
2. Results
2.1. Demographic Clinical Characteristics of the Study Population
2.2. Univariate Cox Analysis
2.3. Multivariate Cox Analysis
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Patient Selection
4.3. Clinical Variables Selection
4.4. Cancer-Related Factors
4.5. Study Outcome
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Statement
References
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro-Oncology 2021, 23, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Valiente, M.; Ahluwalia, M.S.; Boire, A.; Brastianos, P.K.; Goldberg, S.B.; Lee, E.Q.; Le Rhun, E.; Preusser, M.; Winkler, F.; Soffietti, R. The Evolving Landscape of Brain Metastasis. Trends Cancer 2018, 4, 176–196. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, M.H.; Seong, M.; Kim, S.T.; Kang, J.H.; Cho, B.C.; Lee, K.; Cho, E.; Sun, J.-M.; Lee, S.-H.; et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann. Oncol. 2020, 31, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Schalper, K.A.; Gettinger, S.N.; Mahajan, A.; Herbst, R.S.; Chiang, A.C.; Lilenbaum, R.; Wilson, F.H.; Omay, S.B.; Yu, J.B.; et al. Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 655–663. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bull, C.J.; Vincent, E.E.; Robinson, J.; Walther, A.; Smith, G.D.; Lewis, S.J.; Relton, C.L.; Martin, R.M. Association between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer. JAMA 2020, 323, 646–655. [Google Scholar] [CrossRef]
- El-Hamamsy, M.; Elwakil, H.; Saad, A.S.; Shawki, M.A. A Randomized Controlled Open-Label Pilot Study of Simvastatin Addition to Whole-Brain Radiation Therapy in Patients with Brain Metastases. Oncol. Res. 2016, 24, 521–528. [Google Scholar] [CrossRef]
- Luttman, J.H.; Hoj, J.P.; Lin, K.H.; Lin, J.; Gu, J.J.; Rouse, C.; Nichols, A.G.; MacIver, N.J.; Wood, K.C.; Pendergast, A.M. ABL allosteric inhibitors synergize with statins to enhance apoptosis of metastatic lung cancer cells. Cell. Rep. 2021, 37, 109880. [Google Scholar] [CrossRef]
- Yao, X.; Xie, R.; Cao, Y.; Tang, J.; Men, Y.; Peng, H.; Yang, W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J. Nanobiotechnol. 2021, 19, 311. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhang, R.; Xia, Y.; Shao, Z.; Mei, Z. Effects of statin exposure and lung cancer survival: A meta-analysis of observational studies. Pharmacol. Res. 2019, 141, 357–365. [Google Scholar] [CrossRef]
- Lee, Y.R.; Oh, S.S.; Jang, S.I.; Park, E.C. Statin adherence and risk of all-cause, cancer, and cardiovascular mortality among dyslipidemia patients: A time-dependent analysis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2207–2214. [Google Scholar] [CrossRef]
- Afshari, A.R.; Mollazadeh, H.; Henney, N.C.; Jamialahmad, T.; Sahebkar, A. Effects of statins on brain tumors: A review. Semin. Cancer Biol. 2021, 73, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Y.; Zhao, F.; Chen, J.; He, L.; Liu, Z.; Pei, Y.; Wei, Z.; Li, R.; Ai, P.; et al. Standard or extended STUPP? Optimal duration of temozolomide for patients with high-grade gliomas: A retrospective analysis. J. Neuro-Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.G.; Hu, X.L.; He, Y.; Guan, H.; Wang, J.J.; He, L.; Mu, X.; Liu, Z.; Li, R.; Peng, X. Clinical and survival analysis of nasopharyngeal carcinoma with consistently negative Epstein-Barr virus DNA. Head Neck 2021, 43, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Langen, K.J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro-Oncology 2019, 21, 585–595. [Google Scholar] [CrossRef]
- Genre, L.; Roché, H.; Varela, L.; Kanoun, D.; Ouali, M.; Filleron, T.; Dalenc, F. External validation of a published nomogram for prediction of brain metastasis in patients with extra-cerebral metastatic breast cancer and risk regression analysis. Eur. J. Cancer 2017, 72, 200–209. [Google Scholar] [CrossRef]
- Videtic, G.M.; Reddy, C.A.; Chao, S.T.; Rice, T.W.; Adelstein, D.J.; Barnett, G.H.; Mekhail, T.M.; Vogelbaum, M.A.; Suh, J.H. Gender, race, and survival: A study in non-small-cell lung cancer brain metastases patients utilizing the radiation therapy oncology group recursive partitioning analysis classification. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1141–1147. [Google Scholar] [CrossRef]
- De Vin, T.; Engels, B.; Gevaert, T.; Storme, G.; De Ridder, M. Stereotactic radiotherapy for oligometastatic cancer: A prognostic model for survival. Ann. Oncol. 2014, 25, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, E. Designing complex group sequential survival trials. Stat. Med. 2002, 21, 1969–1989. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics 1988, 44, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Ho, S.C.; Chen, C.C.; Yang, C.Y. Statin use and the risk of liver cancer: A population-based case–control study. Am. J. Gastroenterol. 2011, 106, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.; Liao, K.F.; Lai, H.C.; Muo, C.H.; Sung, F.C.; Chen, P.C. Statin use and risk of hepatocellular carcinoma. Eur. J. Epidemiol. 2013, 28, 485–492. [Google Scholar] [CrossRef]
- Liu, Z.; Alsaggaf, R.; McGlynn, K.A.; Anderson, L.A.; Tsai, H.T.; Zhu, B.; Zhu, Y.; Mbulaiteye, S.M.; Gadalla, S.M.; Koshiol, J. Statin use and reduced risk of biliary tract cancers in the UK Clinical Practice Research Datalink. Gut 2019, 68, 1458–1464. [Google Scholar] [CrossRef]
- Raymakers, A.; Sin, D.D.; Sadatsafavi, M.; FitzGerald, J.M.; Marra, C.A.; Lynd, L.D. Statin use and lung cancer risk in chronic obstructive pulmonary disease patients: A population-based cohort study. Respir. Res. 2020, 21, 118. [Google Scholar] [CrossRef]
- Lin, J.J.; Ezer, N.; Sigel, K.; Mhango, G.; Wisnivesky, J.P. The effect of statins on survival in patients with stage IV lung cancer. Lung Cancer 2016, 99, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Aragaki, A.K.; Tang, J.Y.; Kurian, A.W.; Manson, J.E.; Chlebowski, R.T.; Simon, M.; Desai, P.; Wassertheil-Smoller, S.; Liu, S.; et al. Statin use and all-cancer survival: Prospective results from the Women’s Health Initiative. Br. J. Cancer 2016, 115, 129–135. [Google Scholar] [CrossRef]
- Madison, C.J.; Heinrich, M.C.; Thompson, R.F.; Yu, W.Y. Statin use is associated with improved overall survival in patients with melanoma. Melanoma Res. 2022, 32, 291–294. [Google Scholar] [CrossRef]
- Yu, W.Y.; Hill, S.T.; Chan, E.R.; Pink, J.J.; Cooper, K.; Leachman, S.; Lund, A.W.; Kulkarni, R.; Bordeaux, J.S. Computational Drug Repositioning Identifies Statins as Modifiers of Prognostic Genetic Expression Signatures and Metastatic Behavior in Melanoma. J. Investig. Dermatol. 2021, 141, 1802–1809. [Google Scholar] [CrossRef]
- Feng, J.L.; Qin, X. Does adherence to lipid-lowering medications improve cancer survival? A nationwide study of breast and colorectal cancer, and melanoma. Br. J. Clin. Pharmacol. 2021, 87, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Kidera, Y.; Tsubaki, M.; Yamazoe, Y.; Shoji, K.; Nakamura, H.; Ogaki, M.; Satou, T.; Itoh, T.; Isozaki, M.; Kaneko, J.; et al. Reduction of lung metastasis, cell invasion, and adhesion in mouse melanoma by statin-induced blockade of the Rho/Rho-associated coiled-coil-containing protein kinase pathway. J. Exp. Clin. Cancer Res. 2010, 29, 127. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Takeda, T.; Obata, N.; Kawashima, K.; Tabata, M.; Imano, M.; Satou, T.; Nishida, S. Combination therapy with dacarbazine and statins improved the survival rate in mice with metastatic melanoma. J. Cell. Physiol. 2019, 234, 17975–17989. [Google Scholar] [CrossRef] [PubMed]
- Nübel, T.; Dippold, W.; Kleinert, H.; Kaina, B.; Fritz, G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004, 18, 140–142. [Google Scholar] [CrossRef]
- Marcianò, G.; Palleria, C.; Casarella, A.; Rania, V.; Basile, E.; Catarisano, L.; Vocca, C.; Bianco, L.; Pelaia, C.; Cione, E.; et al. Effect of Statins on Lung Cancer Molecular Pathways: A Possible Therapeutic Role. Pharmaceuticals 2022, 15, 589. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Li, Y.; Li, W.; Chen, Y. Simvastatin prevents proliferation and bone metastases of lung adenocarcinoma in vitro and in vivo. Neoplasma 2013, 60, 240–246. [Google Scholar] [CrossRef]
- Amin, F.; Fathi, F.; Reiner, Ž.; Banach, M.; Sahebkar, A. The role of statins in lung cancer. Arch. Med. Sci. 2022, 18, 141–152. [Google Scholar] [CrossRef]
- Wang, I.K.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of invasion and MMP-9 expression in NIH 3T3 and v-H-Ras 3T3 fibroblasts by lovastatin through inhibition of ras isoprenylation. Oncology 2000, 59, 245–254. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef] [PubMed]
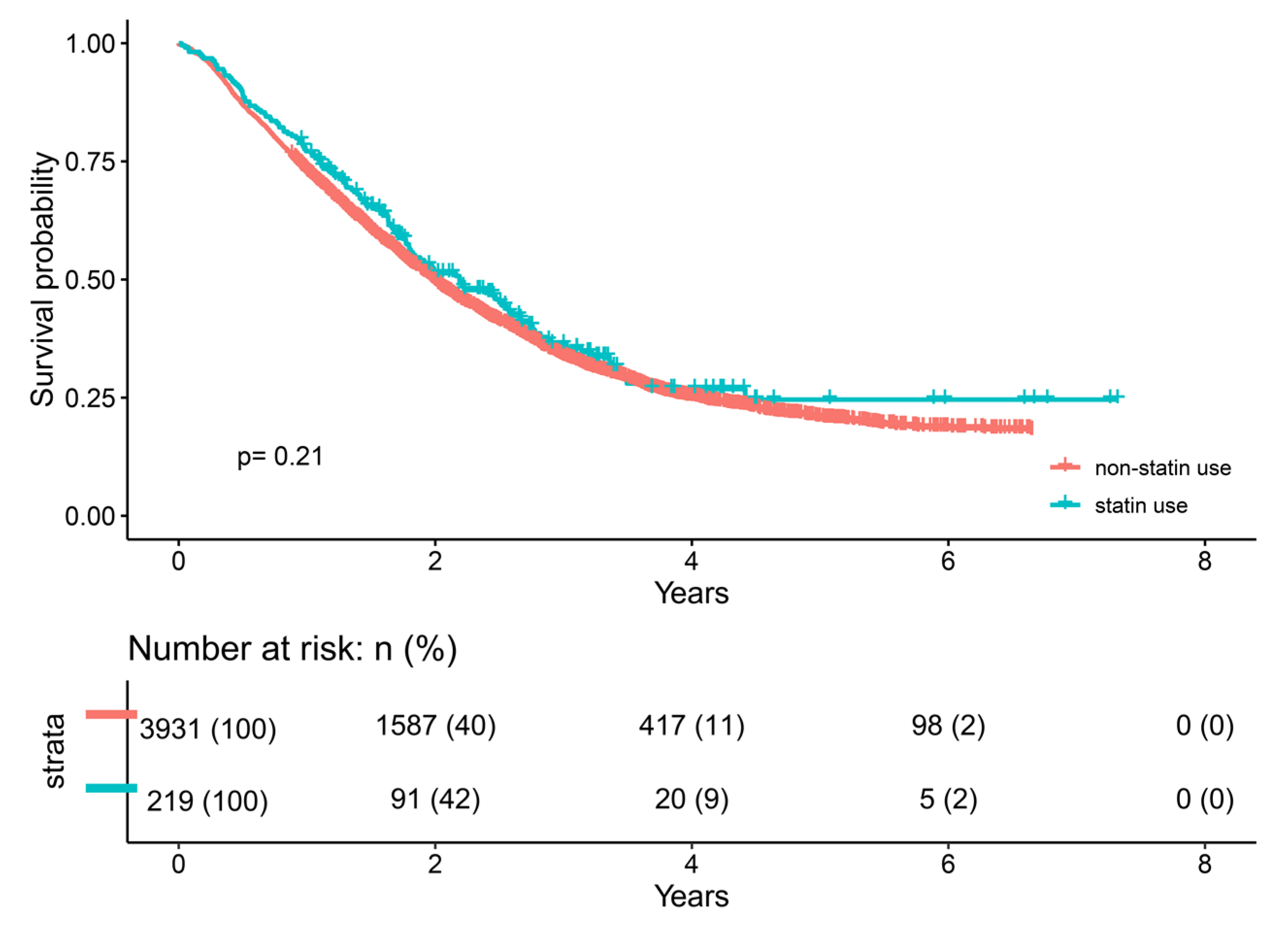
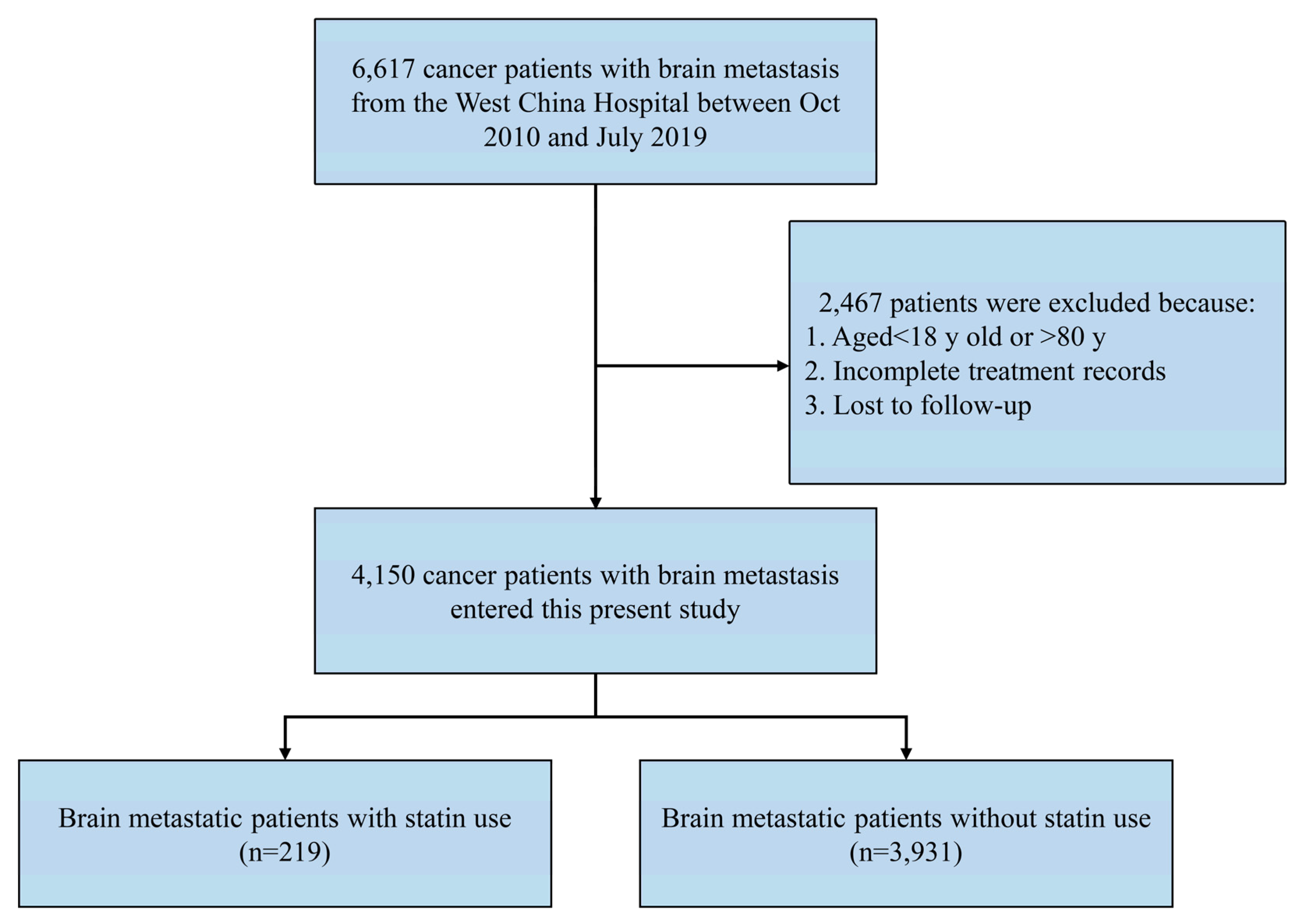
| Variables | Subgroup | Overall (n = 4150) | Non-Statin Use (n = 3931) | Statin Use (n = 219) | p |
|---|---|---|---|---|---|
| Age a | / | 56.83 (11.77) | 56.40 (11.67) | 64.53 (10.87) | <0.001b |
| Sex | Female | 1817 (43.8) | 1726 (43.9) | 91 (41.6) | 0.539 c |
| KPS a | / | 79.04 (10.57) | 79.18 (10.49) | 76.48 (11.52) | <0.001b |
| No.MT a | / | 4.99 (3.89) | 5.00 (3.89) | 4.87 (3.94) | 0.664 b |
| Secondary Tumor | Yes | 2865 (69.0) | 2711 (69.0) | 154 (70.3) | 0.729 c |
| BMI a | / | 22.34 (3.19) | 22.30 (3.18) | 23.09 (3.29) | 0.002b |
| HDL a | / | 1.25 (0.39) | 1.25 (0.39) | 1.19 (0.39) | 0.020b |
| LDL a | / | 2.59 (0.81) | 2.58 (0.80) | 2.65 (1.02) | 0.232 b |
| TG a | / | 1.45 (0.89) | 1.43 (0.86) | 1.73 (1.30) | <0.001b |
| Smoking | Never | 2764 (66.6) | 2632 (67.0) | 132 (60.3) | 0.228 c |
| Ever | 928 (22.4) | 870 (22.1) | 58 (26.5) | ||
| Current | 456 (11.0) | 427 (10.9) | 29 (13.2) | ||
| NA | 2 (0.0) | 2 (0.1) | 0 (0.0) | ||
| Alcohol | Yes | 876 (21.1) | 825 (21.0) | 51 (23.3) | 0.467 c |
| Hypertension | Yes | 659 (15.9) | 554 (14.1) | 105 (47.9) | <0.001c |
| Diabetes | Yes | 345 (8.3) | 293 (7.5) | 52 (23.7) | <0.001c |
| Hyperlipidemia | Yes | 101(2.4) | 55 (1.3) | 46 (21.0) | <0.001c |
| Craniotomy | Performed | 470 (11.3) | 454 (11.5) | 16 (7.3) | 0.157 c |
| Radiotherapy | Performed | 1812 (43.6) | 1706 (43.3) | 106 (48.4) | 0.146 c |
| Chemotherapy | Performed | 2587 (62.3) | 2454 (62.4) | 133 (60.7) | 0.614 c |
| Targeted Therapy | Performed | 1239 (29.8) | 1175 (29.8) | 64 (29.2) | 0.834 c |
| Variables | Subgroup | HR (95%CI) | p | HR (95%CI) | p |
|---|---|---|---|---|---|
| Statin use | No | Reference | 0.213 | Reference | 0.034 |
| Yes | 0.90 (0.73–1.07) | 0.82 (0.69–0.99) | |||
| Age | / | 1.01 (1.00–1.01) | <0.001 | 1.01 (1.00–1.01) | 0.014 |
| Sex | male | Reference | <0.001 | Reference | 0.951 |
| female | 0.81 (0.75–0.87) | 1.00 (0.90–1.11) | |||
| KPS | / | 0.98 (0.98–0.99) | <0.001 | 0.99 (0.98–0.99) | <0.001 |
| No. MT | / | 1.03 (1.01–1.04) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Secondary malignancy | No | Reference | 0.002 | Reference | <0.001 |
| Yes | 1.14 (1.05–1.24) | 1.23 (1.13–1.35) | |||
| BMI | / | 0.97 (0.95–0.98) | <0.001 | 0.97 (0.96–0.98) | <0.001 |
| HDL | / | 0.76 (0.69–0.85) | <0.001 | 0.78 (0.70–0.87) | <0.001 |
| LDL | / | 0.93 (0.88–0.97) | 0.002 | 0.97 (0.92–1.02) | 0.181 |
| TG | / | 0.93 (0.89–0.98) | 0.005 | 0.95 (0.90–1.00) | 0.034 |
| Smoking | never | Reference | Reference | ||
| ever | 1.30 (1.19–1.42) | <0.001 | 1.25 (1.11–1.41) | <0.001 | |
| current | 1.27 (1.13–1.42) | <0.001 | 1.15 (0.99–1.32) | 0.061 | |
| Alcohol | No | Reference | <0.001 | Reference | 0.489 |
| Yes | 1.21 (1.11–1.32) | 1.04 (0.93–1.16) | |||
| Hypertension | No | Reference | 1.000 | ||
| Yes | 1.00 (0.90–1.11) | ||||
| Diabetes | No | Reference | 0.899 | ||
| Yes | 1.01 (0.88–1.16) | ||||
| Hyperlipidemia | No | Reference | 0.151 | ||
| Yes | 0.83 (0.64–1.07) | ||||
| Craniotomy | No | Reference | 0.761 | ||
| Performed | 0.94 (0.62–1.43) | ||||
| Radiotherapy | No | Reference | 0.001 | Reference | 0.192 |
| Performed | 0.88 (0.82–0.95) | 0.94 (0.86–1.03) | |||
| Chemotherapy | No | Reference | <0.001 | Reference | 0.071 |
| Performed | 0.84 (0.78–0.91) | 0.92 (0.83–1.01) | |||
| Targeted Therapy | No | Reference | <0.001 | Reference | <0.001 |
| Performed | 0.72 (0.66–0.78) | 0.76 (0.69–0.83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Y.; Liu, Z.; Wei, Z.; Li, R.; Jin, J.; Zhang, Y.; Peng, X. Association between Statin Use and Survival in Cancer Patients with Brain Metastasis: Retrospective Analysis from the Chinese Population. Pharmaceuticals 2022, 15, 1474. https://doi.org/10.3390/ph15121474
Min Y, Liu Z, Wei Z, Li R, Jin J, Zhang Y, Peng X. Association between Statin Use and Survival in Cancer Patients with Brain Metastasis: Retrospective Analysis from the Chinese Population. Pharmaceuticals. 2022; 15(12):1474. https://doi.org/10.3390/ph15121474
Chicago/Turabian StyleMin, Yu, Zheran Liu, Zhigong Wei, Ruidan Li, Jing Jin, Yu Zhang, and Xingchen Peng. 2022. "Association between Statin Use and Survival in Cancer Patients with Brain Metastasis: Retrospective Analysis from the Chinese Population" Pharmaceuticals 15, no. 12: 1474. https://doi.org/10.3390/ph15121474
APA StyleMin, Y., Liu, Z., Wei, Z., Li, R., Jin, J., Zhang, Y., & Peng, X. (2022). Association between Statin Use and Survival in Cancer Patients with Brain Metastasis: Retrospective Analysis from the Chinese Population. Pharmaceuticals, 15(12), 1474. https://doi.org/10.3390/ph15121474






