A Systematic Review and Meta-Analysis of Premenstrual Syndrome with Special Emphasis on Herbal Medicine and Nutritional Supplements
Abstract
1. Introduction
1.1. Study Aim and Research Question
- (i)
- What is the role of a systematic review with risk assessment on PMS?
- (ii)
- How to design the meta-analysis of RCTs based on high-quality studies related to PMS with herbal medicine and nutritional supplements?
- (iii)
- What is the comprehensive presentation of the mechanism of action in plant metabolites and bioactive molecules?
- (iv)
- How to design a database based on network visualization, world cloud, and previously published articles?
- (v)
- What is the main research gap and what is the future in the area of PMS regarding herbal medicine and nutritional supplements?
1.2. Main Contributions of This Study
- (i)
- To design an up-to-date systematic review and meta-analysis of RCTs to determine the efficacy and safety of herbal medicines and nutritional supplements with their mechanism of action on premenstrual somatic and psycho-behavioural symptoms.
- (ii)
- To determine the risk of bias in randomized controlled trials.
- (iii)
- To design a database such as the number of authors, university/institution, research area-wise and country-wise on previously published publications.
- (iv)
- To design a comprehensive picture based on previous studies and present a study using network visualization and word cloud.
- (v)
- To explore the research breaches and prospects.
1.3. Paper Structure
2. Methods
2.1. Eligibility Criteria, Study Selection, and Participants
2.2. Information Data Source and Search Strategies
2.3. Data Extraction
2.4. Outcomes
2.5. Risk of Bias (RoB) and Quality Assessment (QA)
2.6. Statistical Methods
3. Results
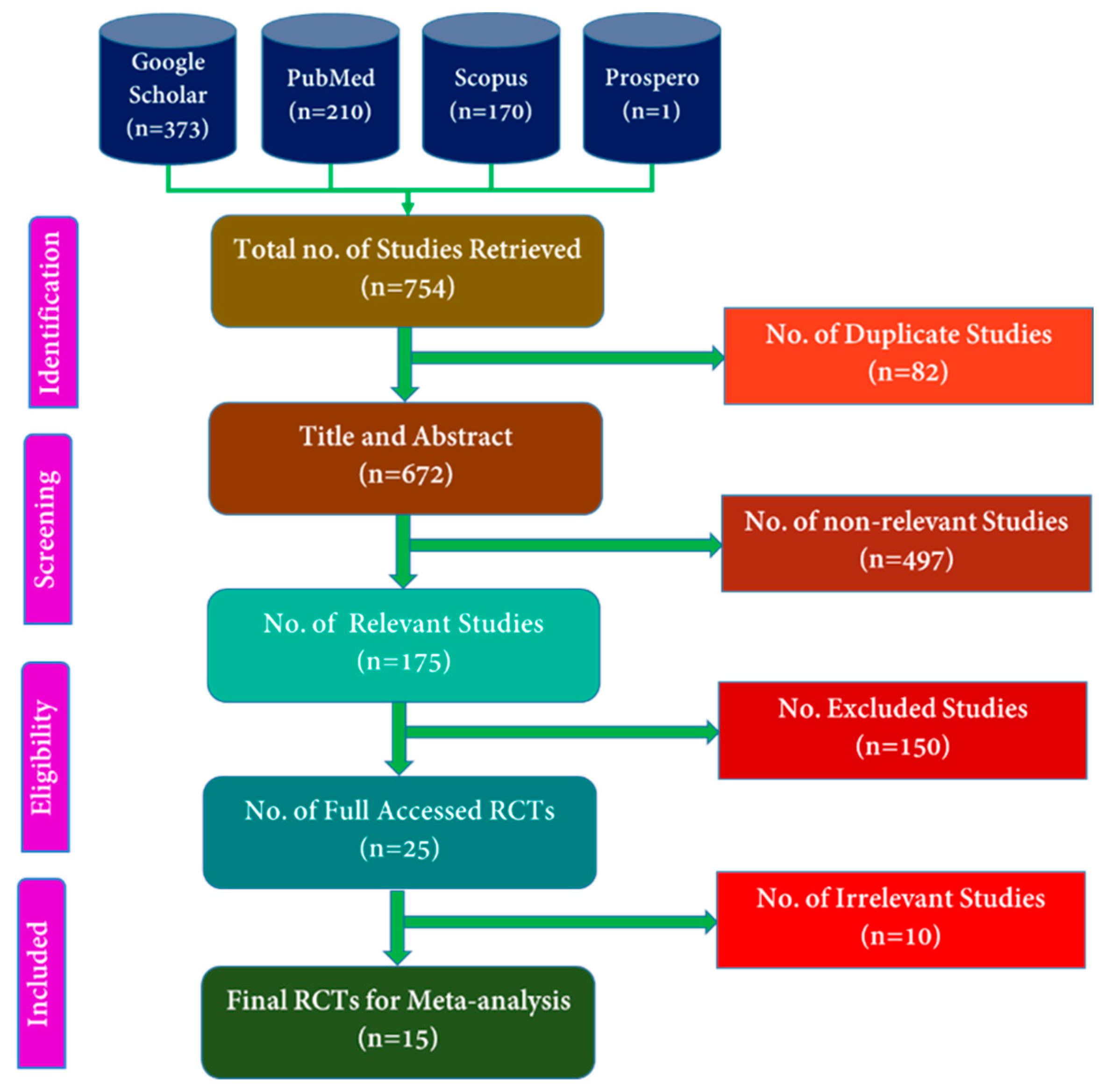
3.1. Literature Review of the Randomized Controlled Trials Based on PRISMA Guideline
3.2. Characteristics of the Included RCT Studies and Patients
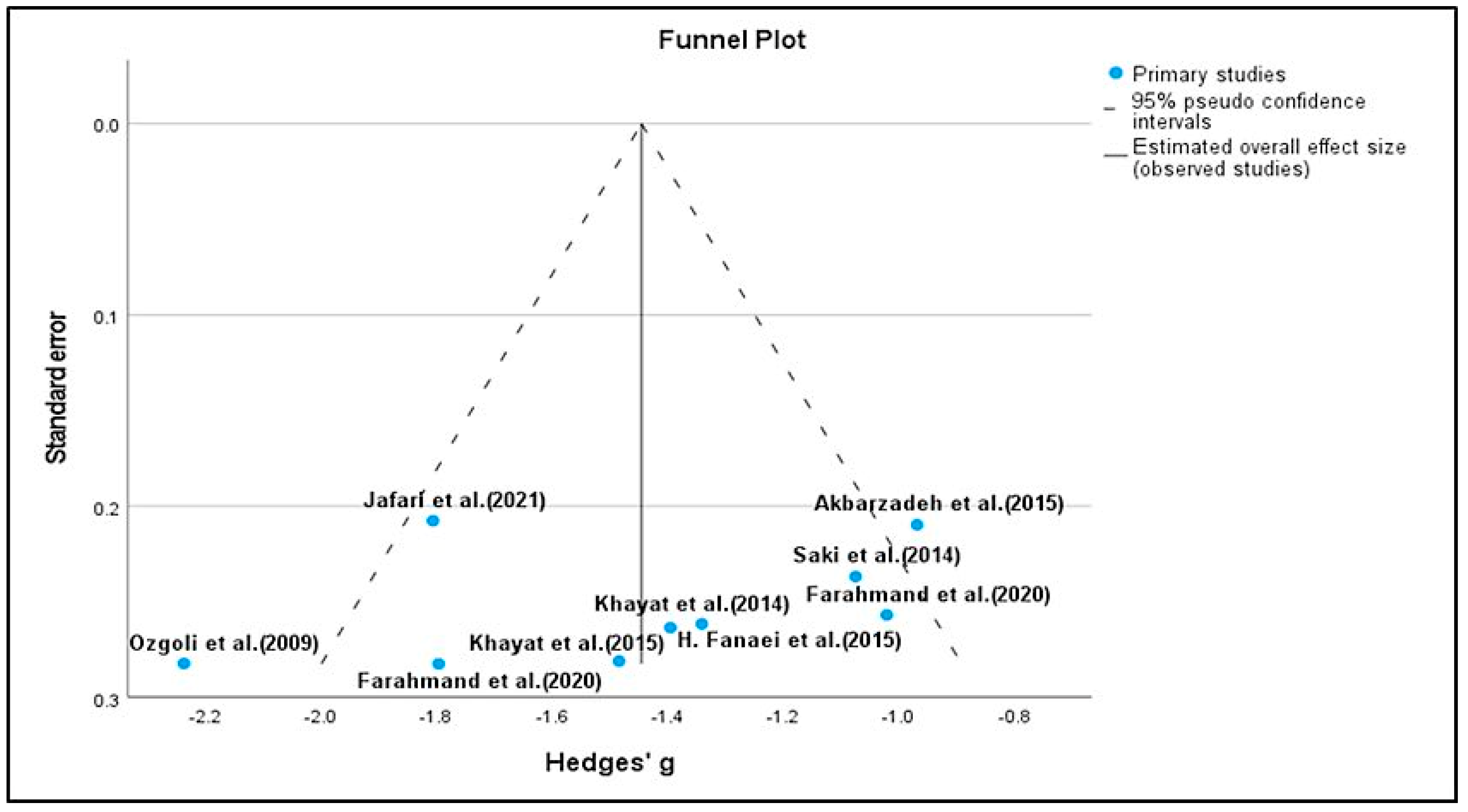
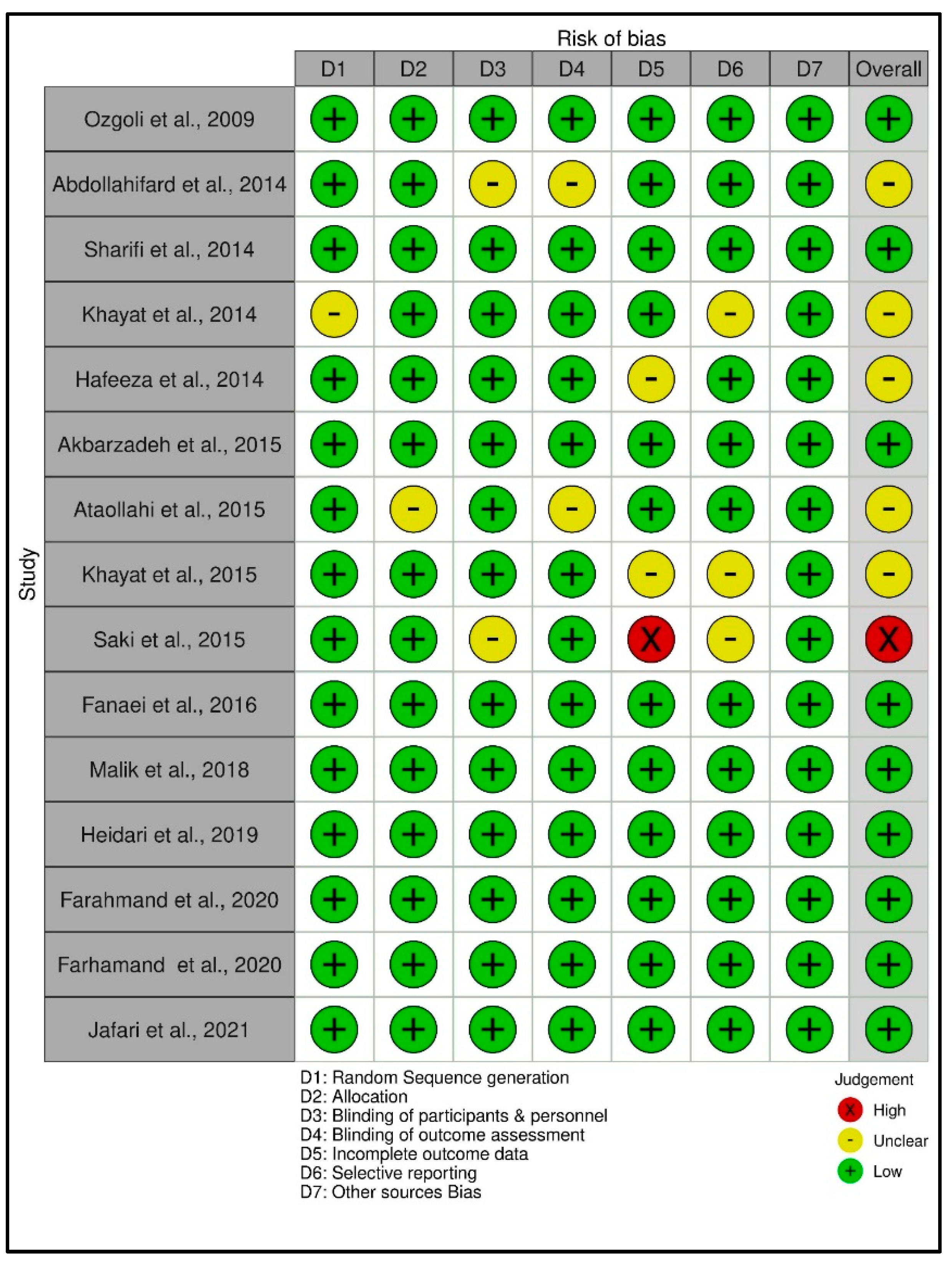
3.3. Risk of Bias Assessment of the Data
3.4. Efficacy of Nutritional Supplements and Herbal Medicine on PMS
3.4.1. Primary Parameters
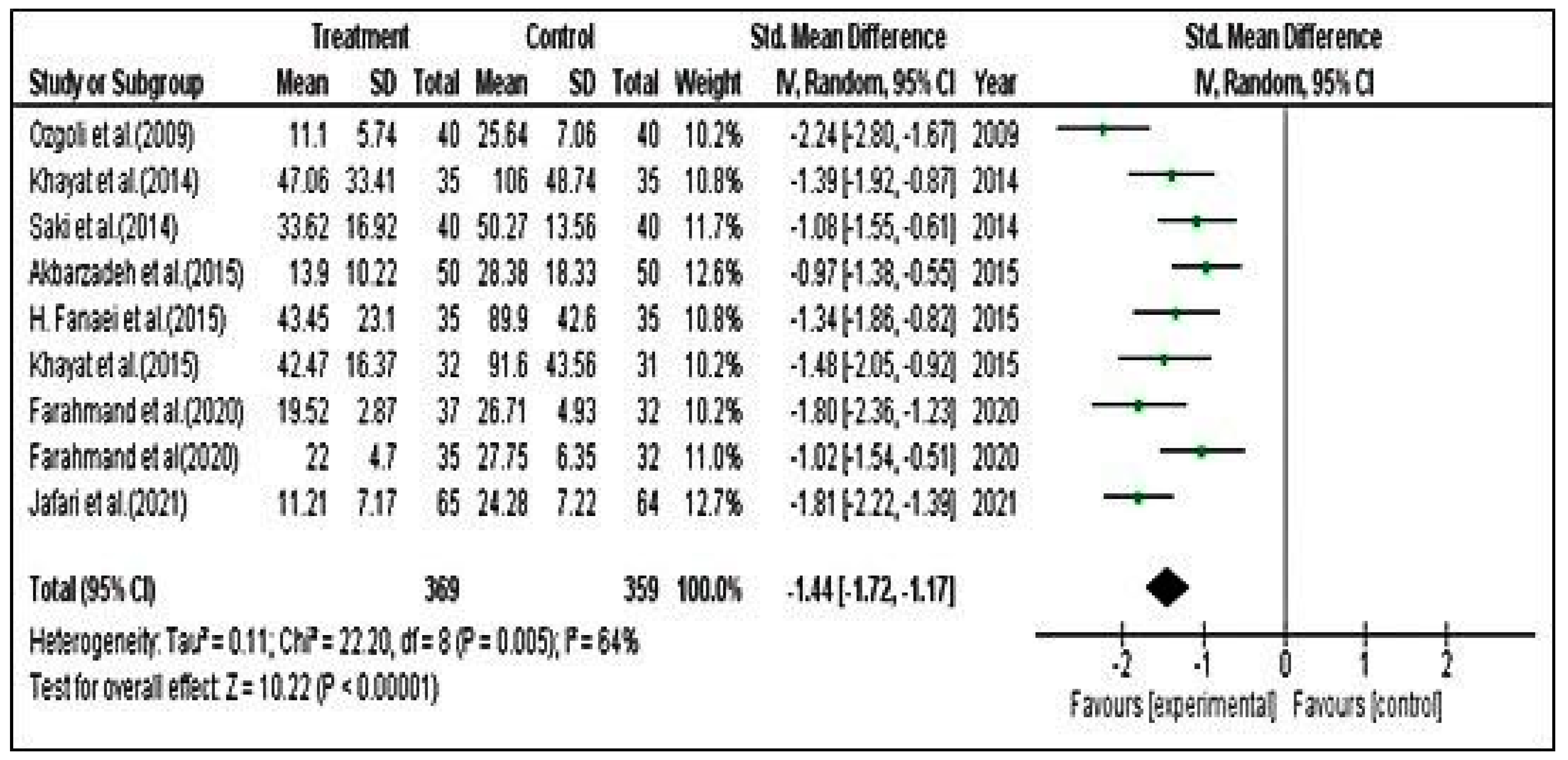
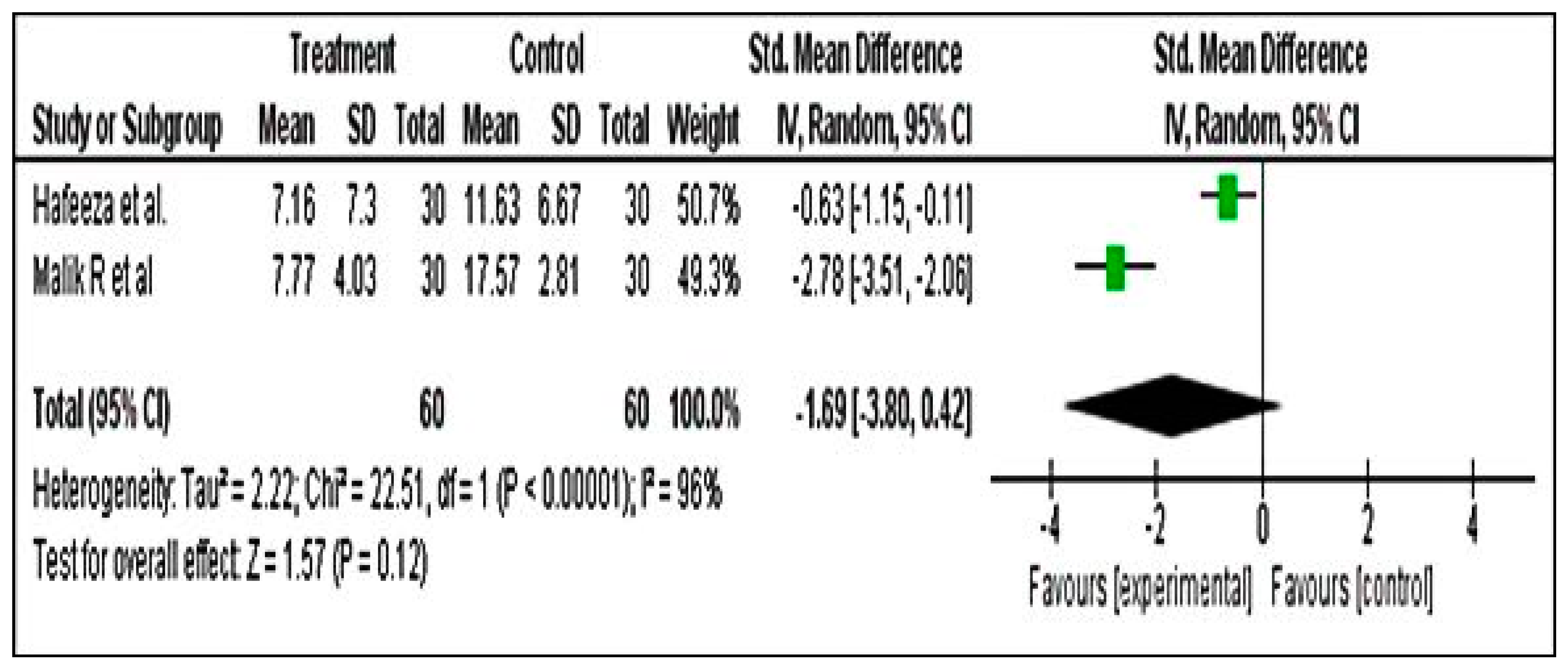
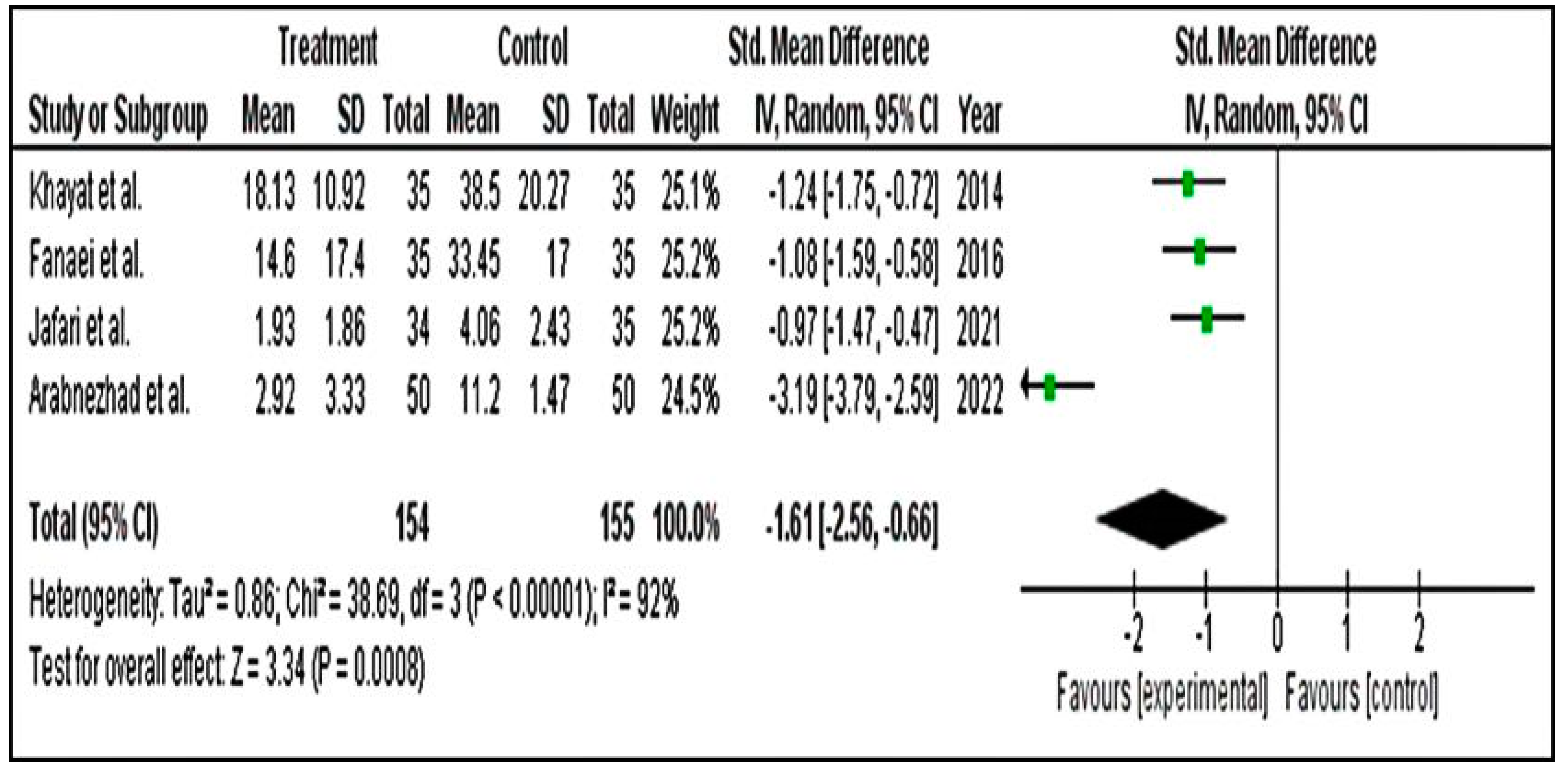
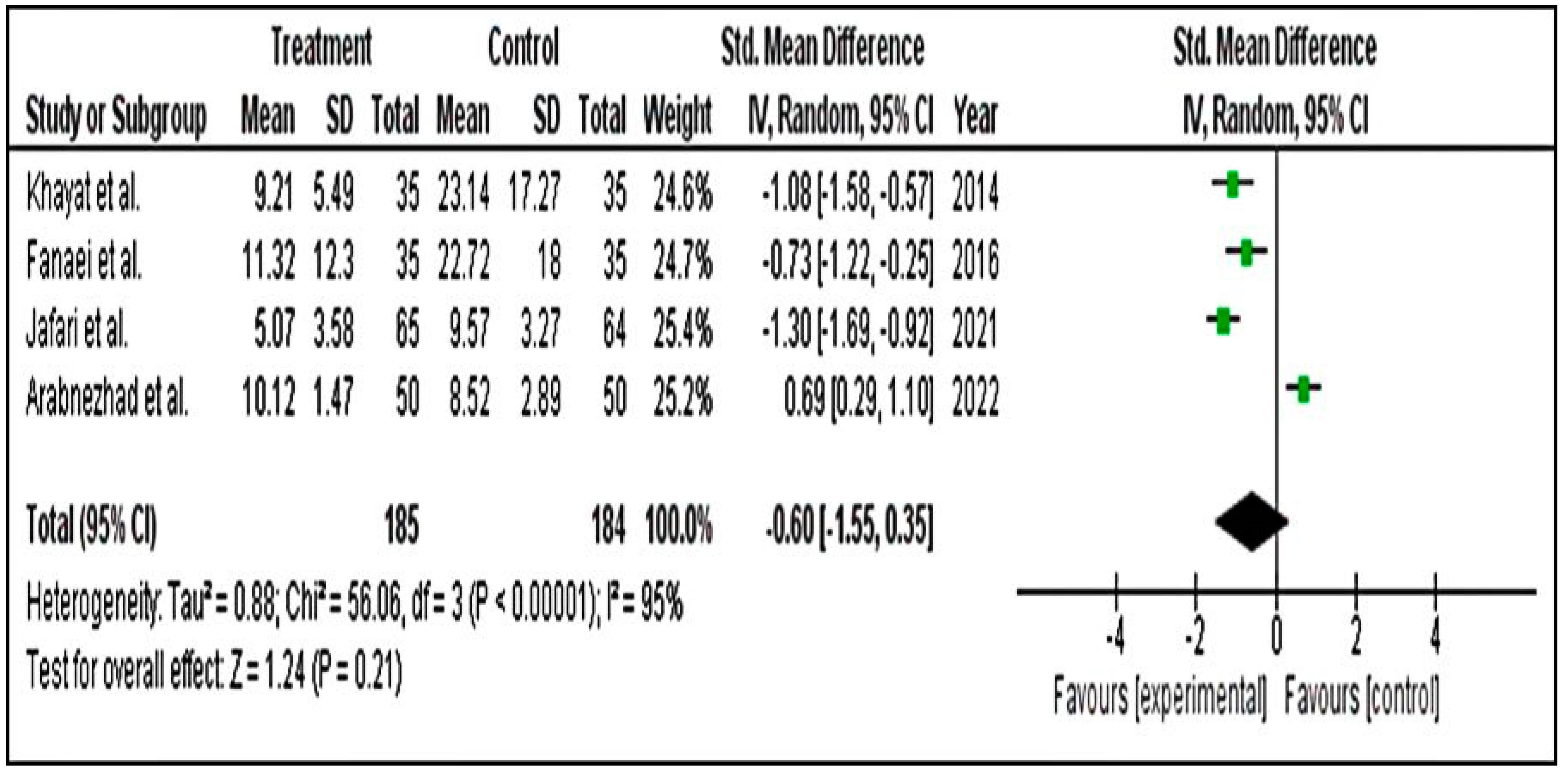
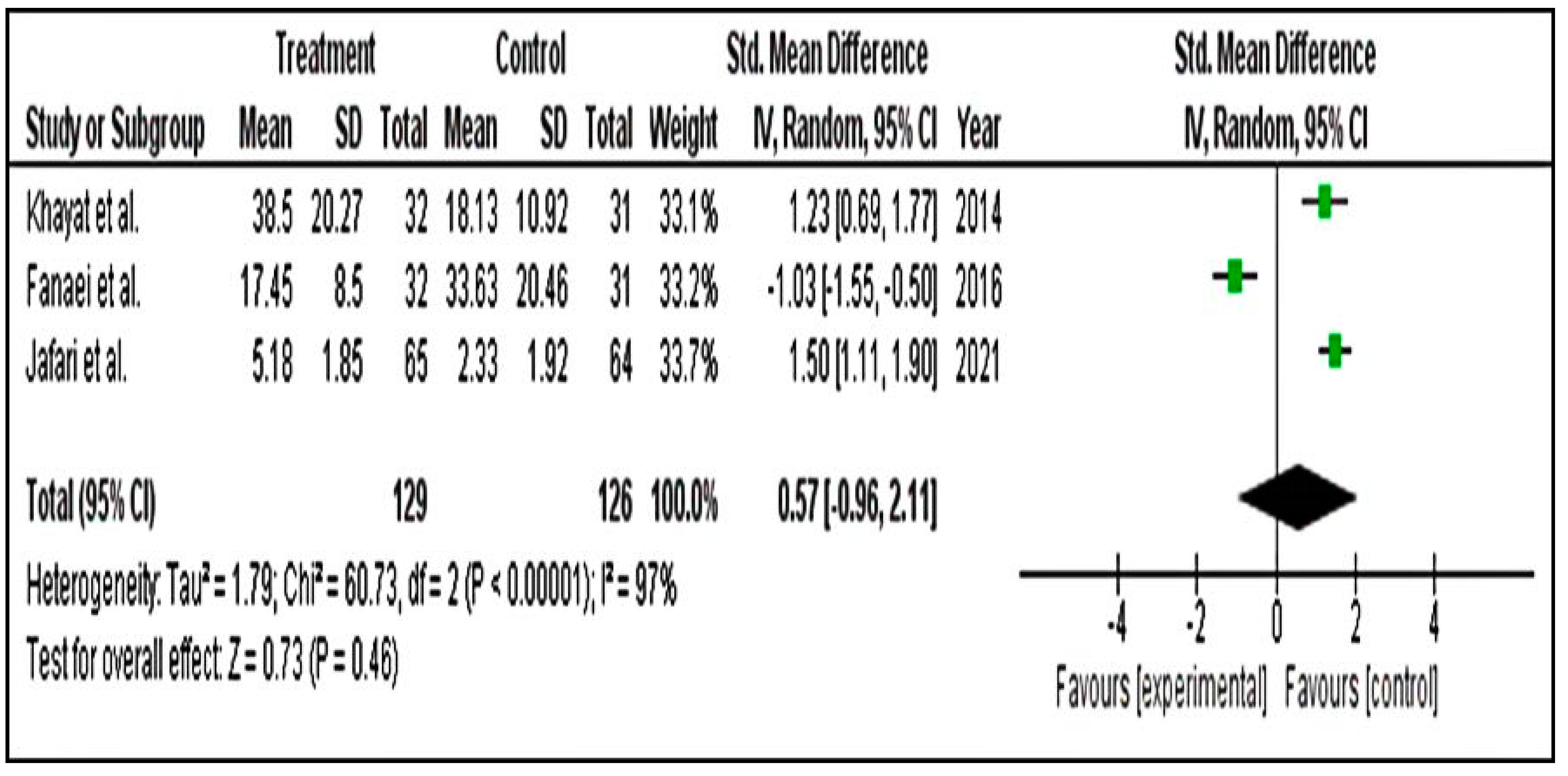
Meta-Analysis for PSST Scores
Meta-Analysis for DSR Scores
Meta-Analysis for PMTS Scores
3.4.2. Secondary Parameters
3.5. Safety of Nutritional Supplements and Herbal Medicine
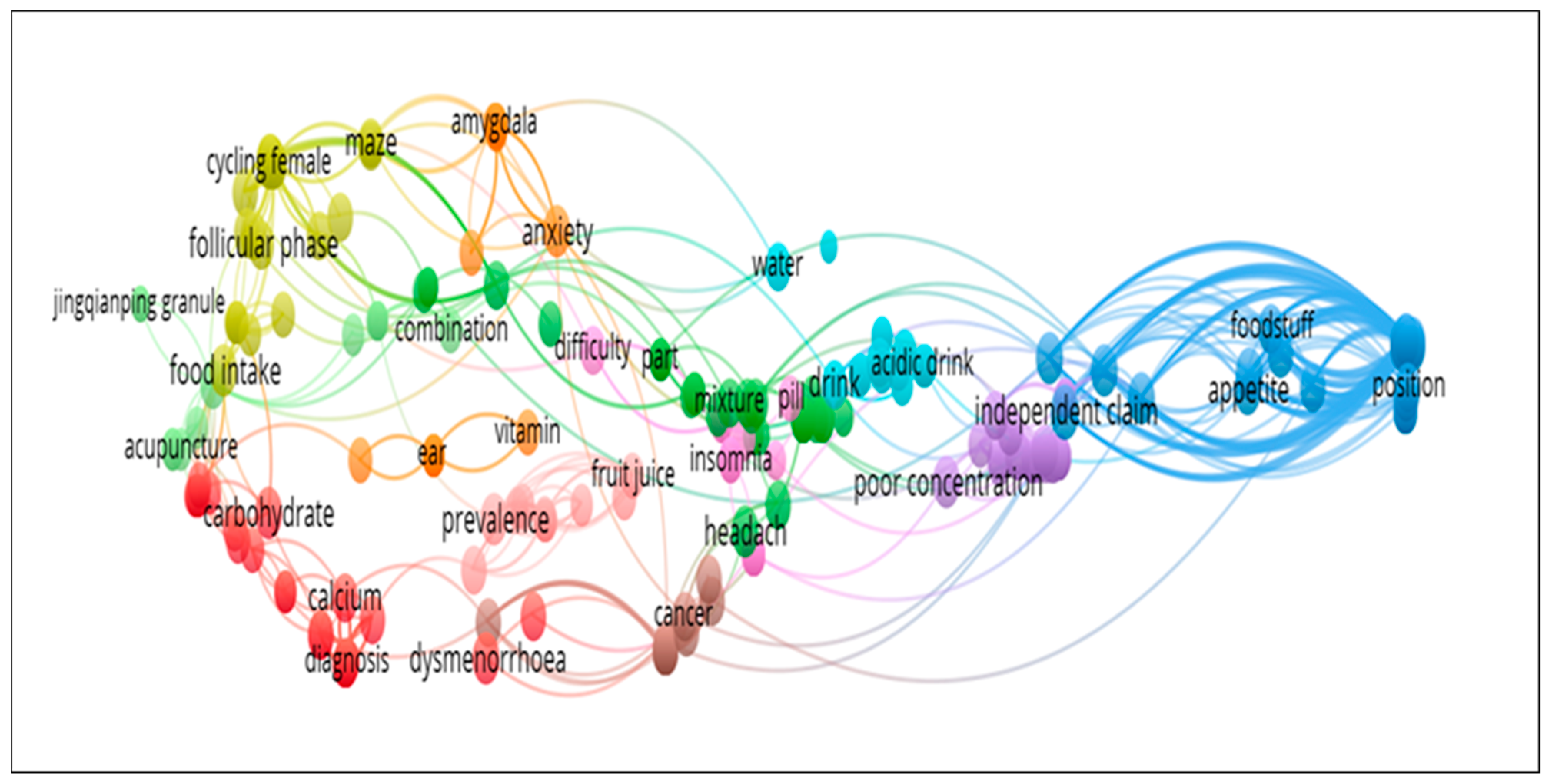
3.6. Synthesis and Analysis of Previous Studies Related to Herbal Medicine and Nutritional Supplements Related to Premenstrual Syndrome
4. Discussion
4.1. Major Findings
4.2. Comparison with Previously Published Articles
4.3. Mechanism of Action of Plant Products
4.4. Strength of the Study
4.5. Research Gaps, Implications, and Practices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arabnezhad, L.; Mohammadifard, M.; Rahmani, L.; Majidi, Z.; Ferns, G.A.; Bahrami, A. Effects of curcumin supplementation on vitamin D levels in women with premenstrual syndrome and dysmenorrhea: A randomized controlled study. BMC Complement. Med. Ther. 2022, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Purnawati, J.; Sinrang, A.W.; Jusuf, E.C.; Limoa, E.; Ahmad, M.; Usman, A.N. Nutrition, mental status and level of 8-hydroxy-2-deoxyguanosine (OHDG) urine as predictors of premenstrual syndrome (PMS) in adolescent girls. Int. J. Curr. Res. Rev. 2020, 12, 7–13. [Google Scholar] [CrossRef]
- Abay, H.; Kaplan, S. Current approaches in premenstrual syndrome management. Bezmialem Sci. 2019, 7, 150–156. [Google Scholar] [CrossRef]
- Masoumi, S.Z.; Ataollahi, M.; Oshvandi, K. Effect of combined use of calcium and vitamin B6 on premenstrual syndrome symptoms: A randomized clinical trial. J. Caring Sci. 2016, 5, 67–73. [Google Scholar] [CrossRef]
- Jafari, F.; Tabarrai, M.; Abbassian, A.; Jafari, F.; Ayati, M.H. Effect of garlic (Allium sativum) supplementation on premenstrual disorders: A randomized, double-blind, placebo-controlled trial. Evid. Based Complement. Altern. Med. 2021, 2021, 9965064. [Google Scholar] [CrossRef]
- Khalesi, Z.B.; Beiranvand, S.P.; Bokaie, M. Efficacy of chamomile in the treatment of premenstrual syndrome: A systematic review. J. Pharmacopunct. 2019, 22, 204–209. [Google Scholar] [CrossRef]
- Ozgoli, G.; Selselei, E.A.; Mojab, F.; Majd, H.A. A randomized, placebo-controlled trial of Ginkgo biloba L. in treatment of premenstrual syndrome. J. Altern. Complement. Med. 2009, 15, 845–851. [Google Scholar] [CrossRef]
- Pearce, E.; Jolly, K.; Jones, L.L.; Matthewman, G.; Zanganeh, M.; Daley, A. Exercise for premenstrual syndrome: A systematic review and meta-analysis of randomised controlled trials. BJGP Open 2020, 4, bjgpopen20X101032. [Google Scholar] [CrossRef]
- Kang, W.; Ishida, E.; Amita, M.; Tatsumi, K.; Yonezawa, H.; Yohtsu, M.; Katano, D.; Onozawa, K.; Kaneko, E.; Iwasaki, W.; et al. Trehalose suppresses lysosomal anomalies in supporting cells of oocytes and maintains female fertility. Nutrients 2022, 14, 2156. [Google Scholar] [CrossRef]
- Frankel, R.A.; Michels, K.A.; Kim, K.; Kuhr, D.L.; Omosigho, U.R.; Wactawski-Wende, J.; Levine, L.; Perkins, N.J.; Mumford, S.L. Serum antioxidant vitamin concentrations and oxidative stress markers associated with symptoms and severity of premenstrual syndrome: A prospective cohort study. BMC Womens Health 2021, 21, 49. [Google Scholar] [CrossRef]
- Saeedi, R.; Sultana, A.; Rahman, K.; Heyat, B.B.M.; Kamal, M.A.; Ishawu, M. Efficacy of Acacia nilotica Linn. Pod’s Sitz Bath plus vaginal pessary in syndromic management of abnormal vaginal discharge: A randomized controlled trial. Evid. Based Complement. Altern. Med. 2022, 2022, 5769555. [Google Scholar] [CrossRef]
- Fazmiya, M.J.A.; Sultana, A.; Rahman, K.; Heyat, M.B.B.; Sumbul; Akhtar, F.; Khan, S.; Appiah, S.C.Y. Current Insights on bioactive molecules, antioxidant, anti-inflammatory, and other pharmacological activities of Cinnamomum camphora Linn. Oxid. Med. Cell. Longev. 2022, 2022, 9354555. [Google Scholar] [CrossRef]
- Sultana, A.; Rahman, K.; Heyat, M.B.B.; Sumbul; Akhtar, F.; Muaad, A.Y. Role of inflammation, oxidative stress, and mitochondrial changes in premenstrual psychosomatic behavioral symptoms with anti-inflammatory, antioxidant herbs, and nutritional supplements. Oxid. Med. Cell. Longev. 2022, 2022, 3599246. [Google Scholar] [CrossRef]
- Naoi, M.; Shamoto-Nagai, M.; Maruyama, W. Neuroprotection of multifunctional phytochemicals as novel therapeutic strategy for neurodegenerative disorders: Antiapoptotic and antiamyloidogenic activities by modulation of cellular signal pathways. Future Neurol. 2019, 14, FNL9. [Google Scholar] [CrossRef]
- Dante, G.; Facchinetti, F. Herbal treatments for alleviating premenstrual symptoms: A systematic review. J. Psychosom. Obstet. Gynecol. 2011, 32, 42–51. [Google Scholar] [CrossRef]
- Verkaik, S.; Kamperman, A.M.; van Westrhenen, R.; Schulte, P.F.J. The treatment of premenstrual syndrome with preparations of Vitex agnus castus: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 217, 150–166. [Google Scholar] [CrossRef]
- Canning, S.; Waterman, M.; Orsi, N.; Ayres, J.; Simpson, N.; Dye, L. The efficacy of Hypericum perforatum (St. John’s wort) for the treatment of premenstrual syndrome: A randomized, double-blind, placebo-controlled trial. CNS Drugs 2010, 24, 207–225. [Google Scholar] [CrossRef]
- Koohpayeh, S.; Hosseini, M.; Nasiri, M.; Rezaei, M. Effects of Rosa damascena (Damask rose) on menstruation-related pain, headache, fatigue, anxiety, and bloating: A systematic review and meta-analysis of randomized controlled trials. J. Educ. Health Promot. 2021, 10, 272. [Google Scholar] [CrossRef]
- Csupor, D.; Lantos, T.; Hegyi, P.; Benkő, R.; Viola, R.; Gyöngyi, Z.; Csécsei, P.; Tóth, B.; Vasas, A.; Márta, K.; et al. Vitex agnus-castus in premenstrual syndrome: A meta-analysis of double-blind randomised controlled trials. Complement. Ther. Med. 2019, 47, 102190. [Google Scholar] [CrossRef]
- Maleki-Saghooni, N.; Karimi, F.Z.; Behboodi Moghadam, Z.; Mirzaii Najmabadi, K. The effectiveness and safety of Iranian herbal medicines for treatment of premenstrual syndrome: A systematic review. Avicenna J. Phytomed. 2018, 8, 96–113. [Google Scholar]
- Tu, W.J.; He, J.; Chen, H.; Shi, X.D.; Li, Y. Psychological effects of false-positive results in expanded newborn screening in China. PLoS ONE 2012, 7, e0036235. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Teelhawod, B.N.; Akhtar, F.; Heyat, M.B.B.; Tripathi, P.; Mehrotra, R.; Asfaw, A.B.; Shorman, O.A.; Masadeh, M. Machine Learning in E-health: A Comprehensive Survey of Anxiety. In Proceedings of the 2021 International Conference on Data Analytics for Business and Industry, Sakheer, Bahrain, 25–26 October 2021; pp. 167–172. [Google Scholar]
- Heyat, M.B.B.; Akhtar, F.; Ansari, M.A.; Khan, A.; Alkahtani, F.; Khan, H.; Lai, D. Progress in detection of insomnia sleep disorder: A comprehensive review. Curr. Drug Targets 2020, 22, 672–684. [Google Scholar] [CrossRef]
- Heyat, M.B.B.; Akhtar, F.; Khan, M.H.; Ullah, N.; Gul, I.; Khan, H.; Lai, D. Detection, treatment planning, and genetic predisposition of bruxism: A systematic mapping process and network visualization technique. CNS Neurol. Disord. Drug Targets 2020, 20, 755–775. [Google Scholar] [CrossRef]
- Akhtar, F.; Patel, P.K.; Heyat, M.B.B.; Yousaf, S.; Baig, A.A.; Mohona, R.A.; Mutoffar, M.M.; Bhattacharya, T.; Teelhawod, B.N.; Li, J.P.; et al. Smartphone addiction among students and its harmful effects on mental health, oxidative stress, and neurodegeneration towards future modulation of anti-addiction therapies: A comprehensive survey based on SLR, research questions, and network visualization. CNS Neurol. Disord. Drug Targets 2022. [Google Scholar] [CrossRef]
- Hussain, K.; Mohd Salleh, M.N.; Cheng, S.; Shi, Y. Metaheuristic research: A comprehensive survey. Artif. Intell. Rev. 2019, 52, 2191–2233. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Abelha, M.; Fernandes, S.; Mesquita, D.; Seabra, F.; Ferreira-Oliveira, A.T. Graduate employability and competence development in higher education-A systematic literature review using PRISMA. Sustainability 2020, 12, 5900. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, K. Review of literature of lean construction and lean tools using systematic literature review technique (2008–2018). Ain Shams Eng. J. 2020, 11, 465–471. [Google Scholar] [CrossRef]
- Maskani, S.; Tafazoli, M.; Rakhshandeh, H.; Esmaily, H. Effect of nigella sativa seeds on the severity of symptoms of premenstrual syndrome: A randomized clinical trial. Koomesh 2020, 22, 33–40. [Google Scholar] [CrossRef]
- Ozgoli, G.; Shahveh, M.; Esmaielli, S.; Nassiri, N. Essential oil of citrus sinensis for the treatment of premenstrual syndrome; a randomized double-blind placebo-controlled trial. J. Reprod. Infertil. 2011, 12, 123–129. [Google Scholar]
- Zamani, M.; Neghab, N.; Torabian, S. Therapeutic effect of Vitex agnus castus in patients with premenstrual syndrome. Acta Med. Iran. 2012, 50, 101–106. [Google Scholar]
- Retallick-Brown, H.; Blampied, N.; Rucklidge, J.J. A pilot randomized treatment-controlled trial comparing vitamin B6 with broad-spectrum micronutrients for premenstrual syndrome. J. Altern. Complement. Med. 2020, 26, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Amani, R.; Tarrahi, M.J. Effect of zinc supplementation on physical and psychological symptoms, biomarkers of inflammation, oxidative stress, and brain-derived neurotrophic factor in young women with premenstrual syndrome: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2020, 194, 89–95. [Google Scholar] [CrossRef]
- Winther, K.; Campbell-Tofte, J.; Motawei, A.M.; Pedersen, F.; Roos, S.B.; Hansen, A.S.V.; Fornitz, G.G.; Killi, M.; Gerhardsen, G. A double-blinded, randomized, placebo controlled, parallel study of pollen pistil extract (Sèrèlys) on women reporting irritability as predominant PMS symptom. J. Herb. Med. 2018, 12, 23–32. [Google Scholar] [CrossRef]
- Delaram, M.; Heydarnejad, M.S. Herbal remedy for premenstrual syndrome with fennel (Foeniculum vulgare)—Randomized, placebo-controlled study. Adv. Clin. Exp. Med. 2011, 20, 509–512. [Google Scholar]
- Gerhardsen, G.; Hansen, A.V.; Killi, M.; Fornitz, G.G.; Pedersen, F.; Roos, S.B. The efficacy of Femal in women with premenstrual syndrome: A randomised, double-blind, parallel-group, placebo-controlled, multicentre study. Adv. Ther. 2008, 25, 595–607. [Google Scholar] [CrossRef]
- Agha-Hosseini, M.; Kashani, L.; Aleyaseen, A.; Ghoreishi, A.; Rahmanpour, H.; Zarrinara, A.R.; Akhondzadeh, S. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: A double-blind, randomised and placebo-controlled trial. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Bahrami-Taghanaki, H.; Khorasanchi, Z.; Timar, A.; Jaberi, N.; Azaryan, E.; Tayefi, M.; Ferns, G.A.; Sadeghnia, H.R.; Ghayour-Mobarhan, M. Menstrual problems in adolescence: Relationship to serum vitamins A and E, and systemic inflammation. Arch. Gynecol. Obstet. 2020, 301, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, M.; Ghasemian, S.; Alizadeh, M. Diets enriched with whole grains reduce premenstrual syndrome scores in nurses: An open-label parallel randomised controlled trial. Br. J. Nutr. 2019, 121, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, Z.; Haghollahi, F.; Shariat, M.; Foroshani, A.R.; Ashrafi, M. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan. J. Obstet. Gynecol. 2009, 48, 124–129. [Google Scholar] [CrossRef]
- Abdollahifard, S.; Rahmanian Koshkaki, A.; Moazamiyanfar, R. The effects of vitamin B1 on ameliorating the premenstrual syndrome symptoms. Glob. J. Health Sci. 2014, 6, 144–153. [Google Scholar] [CrossRef][Green Version]
- Sharifi, F.; Simbar, M.; Mojab, F.; Majd, H.A. Comparison of the effects of Matricaria chamomila (Chamomile) extract and mefenamic acid on the intensity of premenstrual syndrome. Complement. Ther. Clin. Pract. 2014, 20, 81–88. [Google Scholar] [CrossRef]
- Khayat, S.; Kheirkhah, M.; Behboodi Moghadam, Z.; Fanaei, H.; Kasaeian, A.; Javadimehr, M. Effect of treatment with ginger on the severity of premenstrual syndrome symptoms. ISRN Obstet. Gynecol. 2014, 2014, 792708. [Google Scholar] [CrossRef]
- Naveed, W.; Shameem, I.; Tabassum, K. Clinical study of Mutlazima Qabl Haiz (Premenstrual Syndrome) and its management with unani formulation—A randomized controlled trial. Int. J. Cur. Res. Rev. 2014, 6, 51–57. [Google Scholar]
- Akbarzadeh, M.; Dehghani, M.; Moshfeghy, Z.; Emamghoreishi, M.; Tavakoli, P.; Zare, N. Effect of Melissa officinalis capsule on the intensity of premenstrual syndrome symptoms in high school girl students. Nurs. Midwifery Stud. 2015, 4, e27001. [Google Scholar] [CrossRef]
- Ataollahi, M.; Ali Akbari, S.A.; Mojab, F.; Majd, H.A. The effect of wheat germ extract on premenstrual syndrome symptoms. Iran. J. Pharm. Res. 2015, 14, 159–166. [Google Scholar]
- Khayat, S.; Fanaei, H.; Kheirkhah, M.; Moghadam, Z.B.; Kasaeian, A.; Javadimehr, M. Curcumin attenuates severity of premenstrual syndrome symptoms: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2015, 23, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Saki, M.; Akbari, S.; Saki, M.; Tarrahi, M.; Gholami, M.; Pirdadeh, S. The effect of primrose oil on the premenstrual syndrome among the female students in Lorestan University of Medical Sciences: A triple blind study. J. Nurs. Midwifery Sci. 2015, 2, 20. [Google Scholar] [CrossRef]
- Fanaei, H.; Khayat, S.; Kasaeian, A.; Javadimehr, M. Effect of curcumin on serum brain-derived neurotrophic factor levels in women with premenstrual syndrome: A randomized, double-blind, placebo-controlled trial. Neuropeptides 2016, 56, 25–31. [Google Scholar] [CrossRef]
- Malik, R.; Firdose, K.F.; Bhat, M.D.A. Efficacy of Nardostachys jatamansi DC. in the management of premenstrual syndrome: A randomized controlled study. J. Herb. Med. 2018, 14, 17–21. [Google Scholar] [CrossRef]
- Heidari, H.; Amani, R.; Feizi, A.; Askari, G.; Kohan, S.; Tavasoli, P. Vitamin D Supplementation for Premenstrual Syndrome-Related inflammation and antioxidant markers in students with vitamin D deficient: A randomized clinical trial. Sci. Rep. 2019, 9, 14939. [Google Scholar] [CrossRef]
- Farahmand, M.; Khalili, D.; Ramezani Tehrani, F.; Amin, G.; Negarandeh, R. Effectiveness of Echium amoenum on premenstrual syndrome: A randomized, double-blind, controlled trial. BMC Complement. Med. Ther. 2020, 20, 295. [Google Scholar] [CrossRef]
- Farahmand, M.; Khalili, D.; Ramezani Tehrani, F.; Amin, G.; Negarandeh, R. Could Anise decrease the intensity of premenstrual syndrome symptoms in comparison to placebo? A double-blind randomized clinical trial. J. Complement. Integr. Med. 2021, 17, 20190077. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact; Springer: Cham, Switzerland, 2014; pp. 285–320. [Google Scholar] [CrossRef]
- Sheikh, S.; Heyat, M.B.B.; AlShorman, O.; Masadeh, M.; Alkahatni, F. A review of usability evaluation techniques for augmented reality systems in education. In Proceedings of the 2021 Innovation and New Trends in Engineering, Technology and Science Education Conference (IETSEC), Amman, Jordan, 16–18 May 2021; pp. 1–6. [Google Scholar]
- Akhtar, F.; Bin Heyat, M.B.; Li, J.P.; Patel, P.K.; Rishipal; Guragai, B. Role of machine learning in human stress: A review. In Proceedings of the 2020 17th International Computer Conference on Wavelet Active Media Technology and Information Processing (ICCWAMTIP), Chengdu, China, 18–20 December 2020; pp. 170–174. [Google Scholar]
- Akhtar, F.; Li, J.P.; Heyat, M.B.B.; Quadri, S.L.; Ahmed, S.S.; Yun, X.; Haq, A.U. Potential of Blockchain technology in digital currency: A review. In Proceedings of the 2019 16th International Computer Conference on Wavelet Active Media Technology and Information Processing (ICCWAMTIP), Chengdu, China, 14–15 December 2019; pp. 85–91. [Google Scholar]
- Guragai, B.; Alshorman, O.; Masadeh, M.; Heyat, M.B.B. A survey on deep learning classification algorithms for motor imagery. In Proceedings of the 2020 32nd International Conference on Microelectronics (ICM), Aqaba, Jordan, 14–17 December 2020; Volume 2020, pp. 1–4. [Google Scholar]
- Granda, D.; Szmidt, M.K.; Kaluza, J. Is premenstrual syndrome associated with inflammation, oxidative stress and antioxidant status? A systematic review of case-control and cross-sectional studies. Antioxidants 2021, 10, 604. [Google Scholar] [CrossRef]
- Chen, S.; Gao, L.; Li, X.; Ye, Y. Allopregnanolone in mood disorders: Mechanism and therapeutic development. Pharmacol. Res. 2021, 169, 105682. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Ronnenberg, A.G.; Houghton, S.C.; Nobles, C.; Zagarins, S.E.; Takashima-Uebelhoer, B.B.; Faraj, J.L.; Whitcomb, B.W. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum. Reprod. 2014, 29, 1987–1994. [Google Scholar] [CrossRef]
- Mannan, A.; Khan, R.A.; Asif, M. Pharmacodynamic studies on Polypodium vulgare (Linn.). Indian J. Exp. Biol. 1989, 27, 556–560. [Google Scholar]
- Saeedi, M.; Babaie, K.; Karimpour-Razkenari, E.; Vazirian, M.; Akbarzadeh, T.; Khanavi, M.; Hajimahmoodi, M.; Shams Ardekani, M.R. In vitro cholinesterase inhibitory activity of some plants used in Iranian traditional medicine. Nat. Prod. Res. 2017, 31, 2690–2694. [Google Scholar] [CrossRef]
- Sofiane, G.; Wafa, N.; Ouarda, D. Antioxidant, antimicrobial and anti-inflammatory activities of flavonoids and tannins extracted from Polypodium vulgare L. Asian J. Biochem. Pharm. Res. 2015, 5, 114–122. [Google Scholar]
- Naz, S.B.; Chaudhry, M.A.; Rahaman, M.S.U. Dual receptors blocked mechanism arbitrates smooth muscles relaxant effect of Polypodium vulgare. Bangladesh J. Pharmacol. 2016, 11, 414–420. [Google Scholar] [CrossRef]
- Farràs, A.; Mitjans, M.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; López, V. Polypodium vulgare L. (Polypodiaceae) as a source of bioactive compounds: Polyphenolic profile, cytotoxicity and cytoprotective properties in different cell lines. Front. Pharmacol. 2021, 12, 727528. [Google Scholar] [CrossRef] [PubMed]
- Kīrtikara, K.R.; Basu, B.D. Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Mousavi, S.M.; Hajishafiee, M.; Clark, C.C.T.; do Nascimento, I.J.B.; Milajerdi, A.; Amini, M.R.; Esmaillzadeh, A. Clinical effectiveness of zinc supplementation on the biomarkers of oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105166. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Evening Primrose (Oenothera biennis) Oil in Management of Female Ailments. J. Menopausal Med. 2019, 25, 74. [Google Scholar] [CrossRef]
- Duvan, C.I.; Cumaoglu, A.; Turhan, N.O.; Karasu, C.; Kafali, H. Oxidant/antioxidant status in premenstrual syndrome. Arch. Gynecol. Obstet. 2011, 283, 299–304. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. Multiple antidepressant potential modes of action of curcumin: A review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharmacol. 2012, 26, 1512–1524. [Google Scholar] [CrossRef]
- Parhizkar, S.; Latiff, L.A.; Rahman, S.A.; Dollah, M.A.; Parichehr, H. Assessing estrogenic activity of nigella sativa in ovariectomized rats using vaginal cornification assay. Afr. J. Pharm. Pharmacol. 2011, 5, 137–142. [Google Scholar] [CrossRef][Green Version]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]





| Authors | Study Design | Interven. | Control | Part. | Age (y) | Tools | Route of Admin., Durat. and Dosage | Durat. of Interven. (Cycles) | Result | Adv. Event | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ozgoli et al. (2009) | Single blind | Gingko biloba L. tablet | Placebo | 90 | 18–30 | PSST | One tablet (containing 40 mg leaf extracts) three times per day from the 16th day of the menstrual cycle to the 5th day of the next cycle | 2 | Severity of symptoms reduced significantly | Reported (Nausea and excessive sleep in intervention group) | [7] |
| Abdollahifard et al. (2014) | Double Blind | B1 (Thiamine) | Placebo (Starch powder) | 80 | 18–30 | DSR | Two pills of Vit B1 (each pill contains 100 mg) twice daily | 3 | Reduces mental and physical symptoms | Reported (No side effects) | [43] |
| Sharifi et al. (2014) | Double blind | M. chamomile extract | Mefenamic acid 250 mg TID | 90 | 18–35 | DSR | 100 mg capsules thrice daily from the 21st day until the next onset of menstruation period, three times daily for two cycles | 2 | Chamomile is more effective in relieving symptoms | Reported (Excessive bleeding in intervention group and GI complication in MA group) | [44] |
| Khayat et al. (2014) | Double blind | Z. officinale capsules | Placebo | 70 | 18–35 | PSST | Two capsules 250 mg/12 h (7 days) before menstruation to three days after menstruation | 3 | Reduction in mood, physical, and behavioural symptoms | Reported (Complaint of nausea in the intervention group) | [45] |
| Hafeeza et al. (2014) | Single blind | V. agnus castus seed and Mentha piperita Linndistillate (Arq Pudina) 72 mL | Placebo | 60 | 13–40 | PMTS-SR, PMTS-O | V. agnus castus seed 1 g and M. piperita distillate 36 mL were administered orally twice daily, 10 days before menstruation in every cycle | 3 | Significant reduction in PMTS score in the intervention group | Not reported | [46] |
| Akbarzadeh et al. (2015) | Double blind | Melissa. officinalis Linn (Badranjboya) essence capsules | Placebo (starch) | 100 | - | PSST | 2 capsules (1200 mg) daily from the first to the last day of their menstrualcycle | 3 | Effective in reduction of symptoms | Not reported | [47] |
| Ataollahi et al. (2015) | Triple blind | Triticum aestivum Linn (Wheat germ) extract | Placebo | 100 | 20–45 | DSR | I capsule (400 mg), three times per day between the 16th day of the menstrual cycle to the 5th day of the next menstrual period | 2 | Wheat germ significantly reduced physical (63.56%), psychological (66.30%), and the general score (64.99% | Reported (No side effects) | [48] |
| Khayat et al. (2015) | Double Blind | Curcumin from Curcuma longa Linn (haldi) | Placebo (brown sugar) | 70 | - | PSST | Two capsules (100 mg) BID daily for seven days before menstruation and three days after menstruation | 3 | Reduction in symptoms | Reported (No side effects) | [49] |
| Saki et al. (2015) | Triple blind | Oenothera biennis Linn (Primrose) oil | Placebo (n = 40) | 80 | 18–30 | PSST | 3 capsules (1500 mg) TID per day | 3 | Significant relief in symptoms | Not reported | [50] |
| Fanaei et al. (2016) | Double blind | Curcumin capsules | Placebo (Brown sugar) | 70 | - | DSR Fasting Serum BDNF level | 1 capsule of 100 mg/12 h was given for 10 days (in each menstrual cycle 7 days before and 3 days after onset of menstrual bleeding) | 3 | Significant relief in symptoms and increased level of BDNF in the intervention group | Not reported | [51] |
| Malik et al. (2018) | Single blind | Nardostachys jatamansi (D. Don) DC. (jatamansi) capsules | Placebo (Roasted wheat flour) | 60 | 18–45 | PMTS-O, PMTS-SR | 3 capsules orally, BD for the 15 days before the expected date of menstruation, up until the onset of the next menstrual cycle | 2 | PTMS and VAS scores were significantly reduced in the intervention group | Reported (No side effects) | [52] |
| Heidari et al. (2019) | Double blind | 50,000 IU of vitamin D3 | Placebo pearl fortnightly | 44 | 18–25 | PMS Daily Symptoms Rating form | 50,000 IU of vitamin D3 for fortnightly | 4 | Significant improvement in 25(OH) D, serum IL-12, and TAC levels. | Reported (No side effects) | [53] |
| Farahmand et al. (2020) | Double blind | Flowers of Echium amoenum Fisch. & C A Mey (Gole gauzaban) | Placebo | 84 | 20–35 | PSST | Capsules 450 mg of TID from the 21st day to the 3rd day of their next cycle | 2 | Improve PMS symptoms | Reported (No side effects) | [54] |
| Farahmand et al. (2021) | Double blind | P. anisum seed | Placebo (starch) | 84 | 18–35 | PSST | 110 mg capsules of Anise three times per day started 7 days before the start of the menstruation and continued until 3 days after menses | 2 | Significant relief of symptoms | Reported (No side effects) | [55] |
| Jafari et al. (2021) | Double blind | Allium sativum Linn (lahsun) tablet (1.1 mg allicin) | Placebo (Starch tablet) | 129 | 15–49 | PSST | One tablet (400 mg) daily | 3 | Significant reduction in symptoms | Reported (Mild complaints) | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, A.; Heyat, M.B.B.; Rahman, K.; Kunnavil, R.; Fazmiya, M.J.A.; Akhtar, F.; Sumbul; Vidal Mazón, J.L.; Rodríguez, C.L.; De La Torre Díez, I. A Systematic Review and Meta-Analysis of Premenstrual Syndrome with Special Emphasis on Herbal Medicine and Nutritional Supplements. Pharmaceuticals 2022, 15, 1371. https://doi.org/10.3390/ph15111371
Sultana A, Heyat MBB, Rahman K, Kunnavil R, Fazmiya MJA, Akhtar F, Sumbul, Vidal Mazón JL, Rodríguez CL, De La Torre Díez I. A Systematic Review and Meta-Analysis of Premenstrual Syndrome with Special Emphasis on Herbal Medicine and Nutritional Supplements. Pharmaceuticals. 2022; 15(11):1371. https://doi.org/10.3390/ph15111371
Chicago/Turabian StyleSultana, Arshiya, Md Belal Bin Heyat, Khaleequr Rahman, Radhika Kunnavil, Mohamed Joonus Aynul Fazmiya, Faijan Akhtar, Sumbul, Juan Luis Vidal Mazón, Carmen Lili Rodríguez, and Isabel De La Torre Díez. 2022. "A Systematic Review and Meta-Analysis of Premenstrual Syndrome with Special Emphasis on Herbal Medicine and Nutritional Supplements" Pharmaceuticals 15, no. 11: 1371. https://doi.org/10.3390/ph15111371
APA StyleSultana, A., Heyat, M. B. B., Rahman, K., Kunnavil, R., Fazmiya, M. J. A., Akhtar, F., Sumbul, Vidal Mazón, J. L., Rodríguez, C. L., & De La Torre Díez, I. (2022). A Systematic Review and Meta-Analysis of Premenstrual Syndrome with Special Emphasis on Herbal Medicine and Nutritional Supplements. Pharmaceuticals, 15(11), 1371. https://doi.org/10.3390/ph15111371