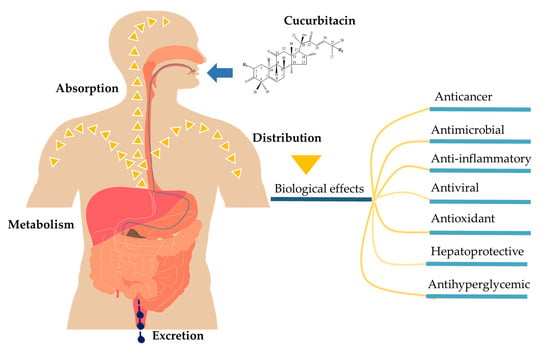
Pharmacokinetics and Biological Activity of Cucurbitacins
Abstract
1. Introduction
2. Pharmacokinetic Properties of Cucurbitacins
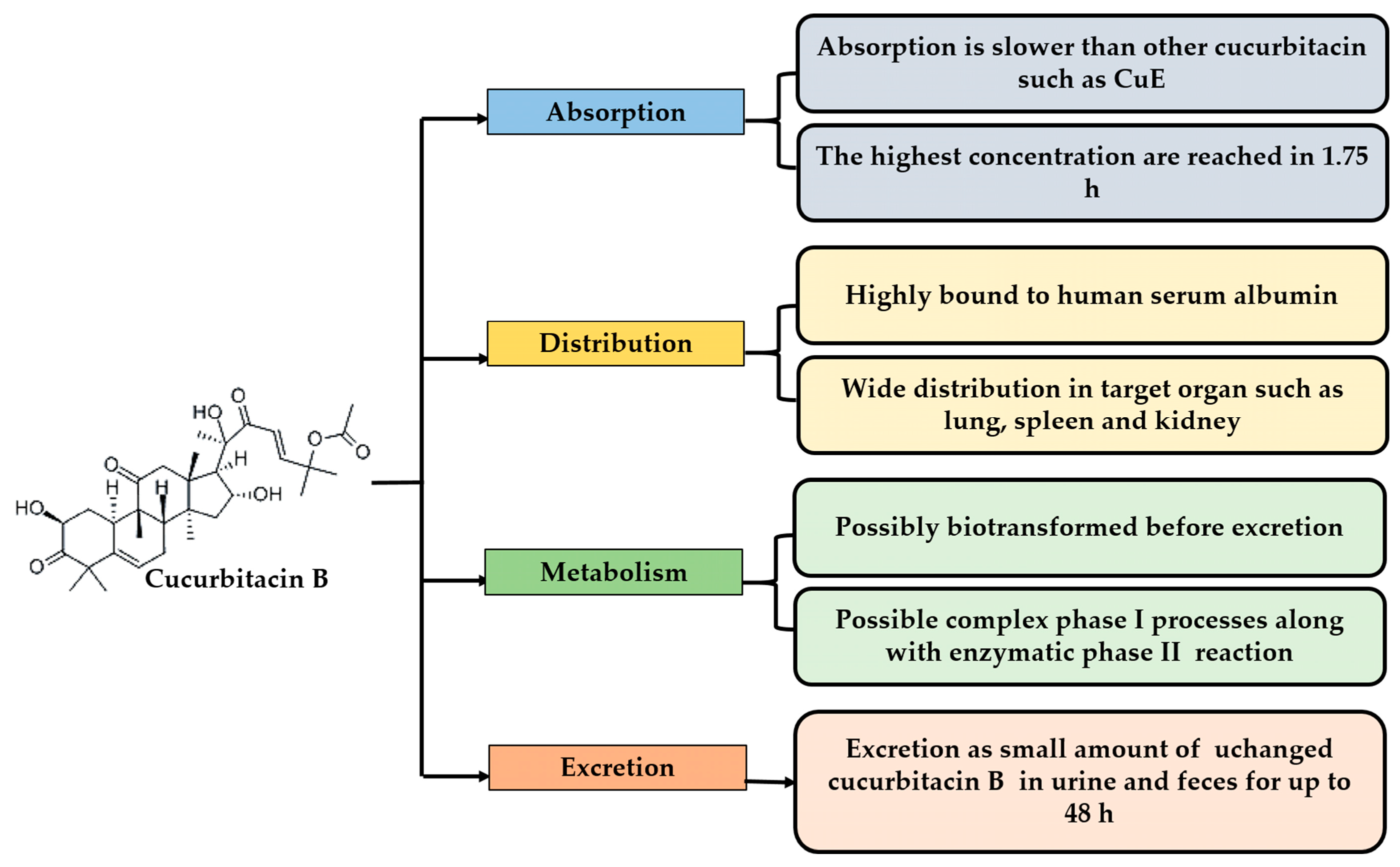
2.1. Absorption
2.2. Distribution
2.3. Metabolism and Excretion
3. Therapeutic Efficacy of Cucurbitacin
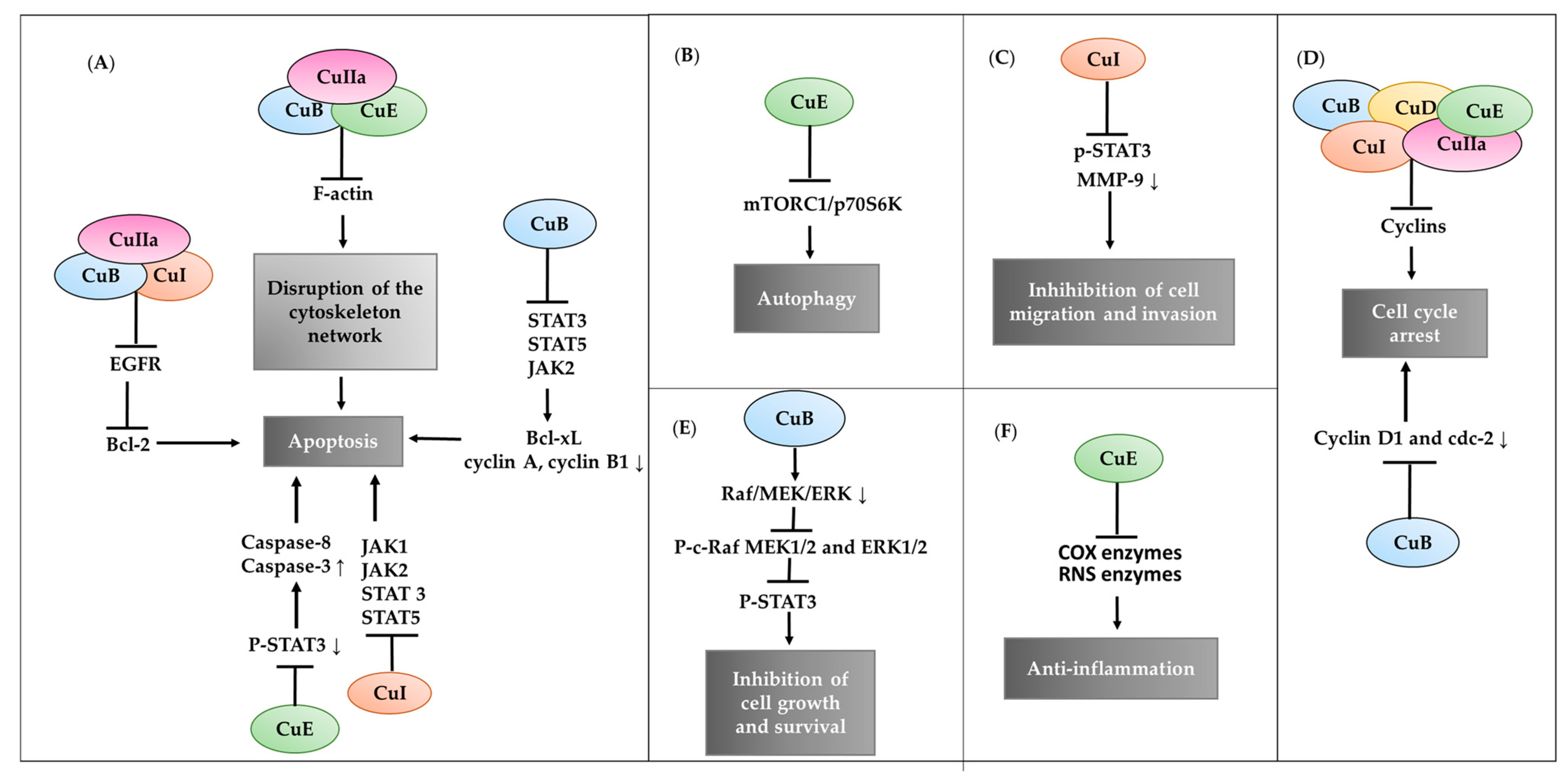
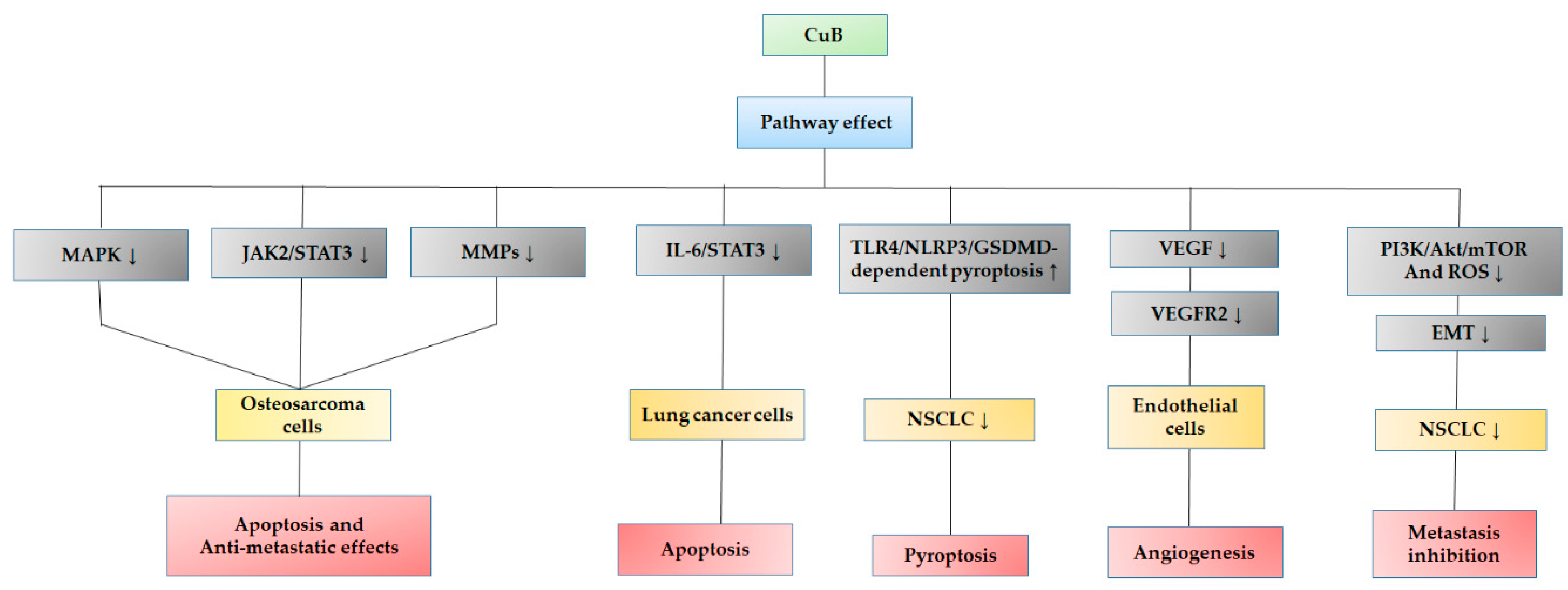
3.1. Biological Action of Cucurbitacin B
3.2. Biological Action of Cucurbitacin D
3.3. Biological Action of Cucurbitacin E
3.4. Biological Action of Cucurbitacin I
3.5. Biological Action of Cucurbitacin IIa
3.6. Biological Action of Cucurbitacin IIb
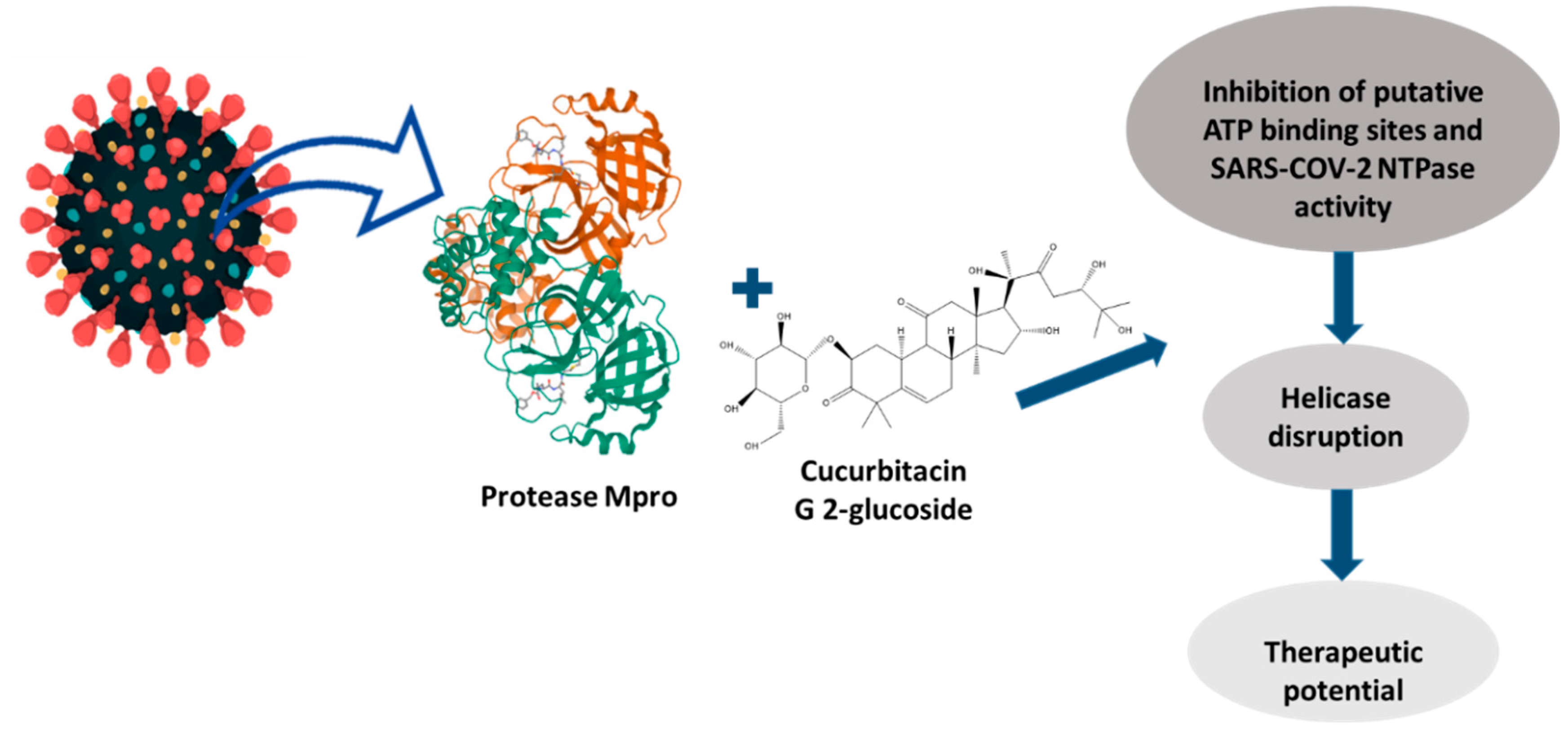
3.7. Cucurbitacin Derivatives and Their Biological Activity
4. Screening and Methodologies for the Identification of Cucurbitacins
5. Perspectives of Cucurbitacins from Plants of Mesoamerican Origin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordel, G.A.; Qiuz, S.X. Cucurbitacins and Cucurbitane Glycosides: Structures and Biological Activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Soto-Hernández, M.; Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Ruíz-Posadas, L.; del Mar Ruíz-Posadas, L. Lead Compounds from Cucurbitaceae for the Treatment of Cancer; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Lee, D.H.; Iwanski, G.B.; Thoennissen, N.H. Cucurbitacin: Ancient Compound Shedding New Light on Cancer Treatment. Sci. World J. 2010, 10, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.I.; Hassan, L.E.A.; Sirat, H.M.; Yagi, S.M.A.; Koko, W.S.; Mohan, S.; Taha, M.M.E.; Ahmad, S.; Chuen, C.S.; Narrima, P.; et al. Anti-Inflammatory Activities of Cucurbitacin E Isolated from Citrullus Lanatus Var. Citroides: Role of Reactive Nitrogen Species and Cyclooxygenase Enzyme Inhibition. Fitoterapia 2011, 82, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Huang, Y.; Deng, X.; Liu, M.; Luo, W. Cucurbitacin B Supplementation Reduces Inflammatory Responses and Alveolar Bone Loss via Regulating MPO, COX-2 and RANK/RANKL/OPG Signals in a Rodent Model of Ligature-Induced Periodontitis. J. King Saud Univ. Sci. 2020, 32, 1889–1895. [Google Scholar] [CrossRef]
- Arjaibi, H.M.; Ahmed, M.S.; Halaweish, F.T. Mechanistic Investigation of Hepato-Protective Potential for Cucurbitacins. Med. Chem. Res. 2017, 26, 1567–1573. [Google Scholar] [CrossRef]
- Shawkey, A.M.; Rabeh, M.A.; Abdellatif, A.O. Biofunctional Molecules from Citrullus Colocynthis: An HPLC/MS Analysis in Correlation to Antimicrobial and Anticancer Activities. Adv. Life Sci. Technol. 2014, 17, 51. [Google Scholar]
- Silvestre, G.F.G.; de Lucena, R.P.; da Silva Alves, H. Cucurbitacins and the Immune System: Update in Research on Anti-Inflammatory, Antioxidant, and Immunomodulatory Mechanisms. Available online: https://www.ingentaconnect.com/content/ben/cmc/pre-prints/content-34994307 (accessed on 11 May 2022).
- Patilaya, P.; Husori, D. Preliminary Study on the Anthelmintic Activity of the Leaf Ethanolic Extract of Indonesian Curanga Fel-Terrae (Lour.) Merr. Int. J. Pharmtech Res. 2015, 8, 347–351. [Google Scholar]
- Kapoor, N.; Ghorai, S.M.; Kushwaha, P.K.; Shukla, R.; Aggarwal, C.; Bandichhor, R. Plausible Mechanisms Explaining the Role of Cucurbitacins as Potential Therapeutic Drugs against Coronavirus 2019. Inform. Med. Unlocked 2020, 21, 100484. [Google Scholar] [CrossRef]
- Hernández Navia, S.E.; Figueroa-Hernández, J.L.; Rodríguez-Zavala, J.S.; Rodriguez-Sosa, M.; Martínez-Vázquez, M. Anti-Diabetic Effects of Cucurbitacins from Ibervillea lindheimeri on Induced Mouse Diabetes. J. Chem. 2022, 2022, e3379557. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Z.; Wu, Q.-Q.; Jiang, X.-H.; Yuan, Y.; Chang, W.; Bian, Z.Y.; Zhu, J.X.; Tang, Q.-Z. Cucurbitacin B Protects Against Pressure Overload Induced Cardiac Hypertrophy. J. Cell. Biochem. 2017, 118, 3899–3910. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Park, K.A.; Byun, H.S.; Won, M.; Jeon, J.; Lee, Y.; Seok, J.H.; Choi, S.-W.; Lee, S.-H.; et al. Cucurbitacin induces Autophagy through Mitochondrial ROS Production Which Counteracts to Limit Caspase-Dependent Apoptosis. Autophagy 2012, 8, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-L.; Tao, W.-H.; Yang, T.-X.; Qiao, J.-G. Anticancer Effect of Cucurbitacin B on MKN-45 Cells via Inhibition of the JAK2/STAT3 Signaling Pathway. Exp. Ther. Med. 2016, 12, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth Inhibitory Activity of Cucurbitacin Glucosides Isolated from Citrullus Colocynthis on Human Breast Cancer Cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S. Cucurbitacin Glucosides: Antioxidant and Free-Radical Scavenging Activities. Biochem. Biophys. Res. Commun. 2007, 364, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Shi, Y.; Han, L.; Li, P.; Zha, Z.; Liu, C.; Liu, X.; Huang, P.; Liu, Y.; Tang, Q.; et al. Identification of Seven Undescribed Cucurbitacins in Cucumis Sativus (Cucumber) and Their Cytotoxic Activity. Phytochemistry 2022, 197, 113123. [Google Scholar] [CrossRef] [PubMed]
- Turfus, S.C.; Delgoda, R.; Picking, D.; Gurley, B.J. Chapter 25—Pharmacokinetics. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 495–512. [Google Scholar] [CrossRef]
- Rao, T.; Tan, Z.; Peng, J.; Guo, Y.; Chen, Y.; Zhou, H.; Ouyang, D. The Pharmacogenetics of Natural Products: A Pharmacokinetic and Pharmacodynamic Perspective. Pharmacol. Res. 2019, 146, 104283. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, D.; Yan, W.; Wang, Y.; Zhang, N. Development and Validation of a UPLC-MS/MS Method for the Determination of Cucurbitacin B in Rat Plasma and Application to a Pharmacokinetic Study. Biomed. Chromatogr. 2016, 30, 503–507. [Google Scholar] [CrossRef]
- Hunsakunachai, N.; Nuengchamnong, N.; Jiratchariyakul, W.; Kummalue, T.; Khemawoot, P. Pharmacokinetics of Cucurbitacin B from Trichosanthes cucumerina L. in Rats. BMC Complement. Altern. Med. 2019, 19, 157. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, J.; Huang, Q.; Ren, Y.; Li, T.; Zhang, X.; Yao, R.; Sun, J. Cucurbitacin IIa: A Review of Phytochemistry and Pharmacology. Phytother. Res. 2021, 35, 4155–4170. [Google Scholar] [CrossRef]
- Fiori, G.M.L.; D’Agate, S.; Rocha, A.; Pereira, A.M.S.; Della Pasqua, O.; Lopes, N.P. Development and Validation of a Quantification Method for Cucurbitacins E and I in Rat Plasma: Application to Population Pharmacokinetic Studies. J. Pharm. Biomed. Anal. 2017, 144, 99–105. [Google Scholar] [CrossRef]
- Wang, S.; Guan, X.; Zhong, X.; Yang, Z.; Huang, W.; Jia, B.; Cui, T. Simultaneous Determination of Cucurbitacin IIa and Cucurbitacin IIb of Hemsleya amabilis by HPLC–MS/MS and Their Pharmacokinetic Study in Normal and Indomethacin-Induced Rats. Biomed. Chromatogr. 2016, 30, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Li, H.-L.; He, J.-C.; He, G.-H.; Feng, E.-F.; Liu, Y.-Q.; Shi, P.-P.; Xu, G.-L. Development and Validation of an LC-ESI-MS/MS Method for the Quantitation of Hemslecin A in Rhesus Monkey Plasma and Its Application in Pharmacokinetics. Biomed. Chromatogr. 2014, 28, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Gao, M.; Wu, C.; Yang, C.; Yang, J.; Wu, G.; Yang, B.; Kuang, H. Simultaneous Determination of Cucurbitacin B and Cucurbitacin E in Rat Plasma by UHPLC-MS/MS: A Pharmacokinetics Study after Oral Administration of Cucurbitacin Tablets. J. Chromatogr. B 2017, 1065–1066, 63–69. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, Q.; Wu, Q.; Chang, J.; Xue, H.; Liu, C.; Liu, X. A New Sensitive UPLC-MS/MS Method for the Determination of Cucurbitacin B in Rat Plasma: Application to an Absolute Bioavailability Study. RSC Adv. 2018, 8, 30978–30985. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Cai, Q.; Li, S. Determination of Cucurbitacin IIa in Rat Plasma by HPLC and its Pharmacokinetic Study. China Pharm. 2015, 11, 1873–1875+1878. [Google Scholar]
- Abou-Khalil, R.; Jraij, A.; Magdalou, J.; Ouaini, N.; Tome, D.; Greige-Gerges, H. Interaction of Cucurbitacins with Human Serum Albumin: Thermodynamic Characteristics and Influence on the Binding of Site Specific Ligands. J. Photochem. Photobiol. B 2009, 95, 189–195. [Google Scholar] [CrossRef]
- Bartalis, J. Hepatoprotective Activity of Cucurbitacin. Ph.D. Thesis, South Dakota State University, Brookings, SD, USA, 2005. [Google Scholar]
- Wang, W.; Zhao, X.; Hu, H.; Chen, D.; Gu, J.; Deng, Y.; Sun, J. Galactosylated Solid Lipid Nanoparticles with Cucurbitacin B Improves the Liver Targetability. Drug Deliv. 2010, 17, 114–122. [Google Scholar] [CrossRef]
- Abbas, S.; Vincourt, J.-B.; Habib, L.; Netter, P.; Greige-Gerges, H.; Magdalou, J. The Cucurbitacins E, D and I: Investigation of Their Cytotoxicity toward Human Chondrosarcoma SW 1353 Cell Line and Their Biotransformation in Man Liver. Toxicol. Lett. 2013, 216, 189–199. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Fu, R.; Ding, G.; Amin, S.; Li, Z. Cucurbitacin E Chemosensitizes Colorectal Cancer Cells via Mitigating TFAP4/Wnt/β-Catenin Signaling. J. Agric. Food Chem. 2020, 68, 14148–14160. [Google Scholar] [CrossRef]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 287. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial–Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef]
- Samuel, A. Cucurbitacins and Its Anticancer Property: A Review. Himal. J. Health Sci. 2019, 4, 17–23. [Google Scholar] [CrossRef]
- Klampfer, L. Signal Transducers and Activators of Transcription (STATs): Novel Targets of Chemopreventive and Chemotherapeutic Drugs. Curr. Cancer Drug Targets 2006, 6, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Benerini Gatta, L.; Melocchi, L.; Bugatti, M.; Missale, F.; Lonardi, S.; Zanetti, B.; Cristinelli, L.; Belotti, S.; Simeone, C.; Ronca, R.; et al. Hyper-Activation of STAT3 Sustains Progression of Non-Papillary Basal-Type Bladder Cancer via FOSL1 Regulome. Cancers 2019, 11, 1219. [Google Scholar] [CrossRef]
- Ferrajoli, A.; Faderl, S.; Ravandi, F.; Estrov, Z. The JAK-STAT Pathway: A Therapeutic Target in Hematological Malignancies. Curr. Cancer Drug Targets 2006, 6, 671–679. [Google Scholar] [CrossRef]
- DeCoster, R.C.; Clemens, M.W.; Di Napoli, A.; Lynch, E.B.; Bonaroti, A.R.; Rinker, B.D.; Butterfield, T.A.; Vasconez, H.C. Cellular and Molecular Mechanisms of Breast Implant–Associated Anaplastic Large Cell Lymphoma. Plast. Reconstr. Surg. 2021, 147, 30e. [Google Scholar] [CrossRef]
- Alghasham, A.A. Cucurbitacins—A Promising Target for Cancer Therapy. Int. J. Health Sci. 2013, 7, 77–89. [Google Scholar] [CrossRef]
- Ríos, J.L.; Andújar, I.; Escandell, J.M.; Giner, R.; Recio, M.C. Cucurbitacins as Inducers of Cell Death and a Rich Source of Potential Anticancer Compounds. Curr. Pharm. Des. 2012, 18, 1663–1676. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, T.; Ma, L.; Liang, M.; Guo, Y.-J.; Zhao, L.-M. Cucurbitacin B and SCH772984 Exhibit Synergistic Anti-Pancreatic Cancer Activities by Suppressing EGFR, PI3K/Akt/MTOR, STAT3 and ERK Signaling. Oncotarget 2017, 8, 103167–103181. [Google Scholar] [CrossRef]
- Huang, W.-W.; Yang, J.-S.; Lin, M.-W.; Chen, P.-Y.; Chiou, S.-M.; Chueh, F.-S.; Lan, Y.-H.; Pai, S.-J.; Tsuzuki, M.; Ho, W.-J.; et al. Cucurbitacin E Induces G2/M Phase Arrest through STAT3/P53/P21 Signaling and Provokes Apoptosis via Fas/CD95 and Mitochondria-Dependent Pathways in Human Bladder Cancer T24 Cells. Evid. Based Complement. Alternat. Med. 2012, 2012, e952762. [Google Scholar] [CrossRef] [PubMed]
- Thoennissen, N.; Iwanski, G.; Doan, N.; Okamoto, R.; Lin, P.; Abbassi, S.; Song, J.H.; Yin, D.; Toh, M.; Xie, W.; et al. Cucurbitacin B Induces Apoptosis by Inhibition of the JAK/STAT Pathway and Potentiates Antiproliferative Effects of Gemcitabine on Pancreatic Cancer Cells. Cancer Res. 2009, 69, 5876–5884. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.L.K.; Duncan, M.D.; Alley, M.C.; Sausville, E.A. Cucurbitacin E-Induced Disruption of the Actin and Vimentin Cytoskeleton in Prostate Carcinoma Cells. Biochem. Pharmacol. 1996, 52, 1553–1560. [Google Scholar] [CrossRef]
- Zha, Q.-B.; Zhang, X.-Y.; Lin, Q.-R.; Xu, L.-H.; Zhao, G.-X.; Pan, H.; Zhou, D.; Ouyang, D.-Y.; Liu, Z.-H.; He, X.-H. Cucurbitacin E Induces Autophagy via Downregulating MTORC1 Signaling and Upregulating AMPK Activity. PLoS ONE 2015, 10, e0124355. [Google Scholar] [CrossRef]
- Song, J.; Liu, H.; Li, Z.; Yang, C.; Wang, C. Cucurbitacin I Inhibits Cell Migration and Invasion and Enhances Chemosensitivity in Colon Cancer. Oncol. Rep. 2015, 33, 1867–1871. [Google Scholar] [CrossRef]
- Chan, K.T.; Li, K.; Liu, S.L.; Chu, K.H.; Toh, M.; Xie, W.D. Cucurbitacin B Inhibits STAT3 and the Raf/MEK/ERK Pathway in Leukemia Cell Line K562. Cancer Lett. 2010, 289, 46–52. [Google Scholar] [CrossRef]
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and Cancer Intervention: Chemistry, Biology and Mechanisms (Review). Int. J. Oncol. 2018, 52, 19–37. [Google Scholar] [CrossRef]
- Dandawate, P.; Subramaniam, D.; Panovich, P.; Standing, D.; Krishnamachary, B.; Kaushik, G.; Thomas, S.M.; Dhar, A.; Weir, S.J.; Jensen, R.A.; et al. Cucurbitacin B and I Inhibits Colon Cancer Growth by Targeting the Notch Signaling Pathway. Sci. Rep. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Dittharot, K.; Dakeng, S.; Suebsakwong, P.; Suksamrarn, A.; Patmasiriwat, P.; Promkan, M. Cucurbitacin B Induces Hypermethylation of Oncogenes in Breast Cancer Cells. Planta Med. 2019, 85, 370–378. [Google Scholar] [CrossRef]
- Xue, Y.; Li, R.; Fang, P.; Ye, Z.-Q.; Zhao, Y.; Zhou, Y.; Zhang, K.-Q.; Li, L. NLRP3 Inflammasome Inhibitor Cucurbitacin B Suppresses Gout Arthritis in Mice. J. Mol. Endocrinol. 2021, 67, 27–40. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B Interacts Synergistically with Antibiotics against Staphylococcus Aureus Clinical Isolates and Exhibits Antiviral Activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Lin, Y.; Kotakeyama, Y.; Li, J.; Pan, Y.; Matsuura, A.; Ohya, Y.; Yoshida, M.; Xiang, L.; Qi, J. Cucurbitacin B Exerts Antiaging Effects in Yeast by Regulating Autophagy and Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, e4517091. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, I.-S.; Park, J.Y.; Kim, Y.; An, E.-J.; Jang, H.-J. Cucurbitacin B Induces Hypoglycemic Effect in Diabetic Mice by Regulation of AMP-Activated Protein Kinase Alpha and Glucagon-Like Peptide-1 via Bitter Taste Receptor Signaling. Front. Pharmacol. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, K.; Muroi, M.; Gao, L.; Chang, Y.-T.; Osada, H.; Xiang, L.; Qi, J. Cucurbitacin B Induces Neurogenesis in PC12 Cells and Protects Memory in APP/PS1 Mice. J. Cell. Mol. Med. 2019, 23, 6283–6294. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, M.; Zhang, H.; Sun, C.; Deng, Y. Inhibitory Effects of Cucurbitacin B on Laryngeal Squamous Cell Carcinoma. Eur. Arch. Otorhinolaryngol. 2008, 265, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Haritunians, T.; Gueller, S.; Zhang, L.; Badr, R.; Yin, D.; Xing, H.; Fung, M.C.; Koeffler, H.P. Cucurbitacin B Induces Differentiation, Cell Cycle Arrest, and Actin Cytoskeletal Alterations in Myeloid Leukemia Cells. Leuk. Res. 2008, 32, 1366–1373. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.-L.; Yuan, J.-W.; Zhang, H.-R.; Liu, D.; Hao, J.; Ji, W.; Wu, X.-Z.; Chen, D. Cucurbitacin B Inhibits the Migration and Invasion of Breast Cancer Cells by Altering the Biomechanical Properties of Cells. Phytother. Res. 2019, 33, 618–630. [Google Scholar] [CrossRef]
- Liu, J.-H.; Li, C.; Cao, L.; Zhang, C.-H.; Zhang, Z.-H. Cucurbitacin B Regulates Lung Cancer Cell Proliferation and Apoptosis via Inhibiting the IL-6/STAT3 Pathway through the LncRNA XIST/MiR-Let-7c Axis. Pharm. Biol. 2022, 60, 154–162. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, G.; Liu, Y. Long Non-Coding RNA XIST Sponges MiR-34a to Promotes Colon Cancer Progression via Wnt/β-Catenin Signaling Pathway. Gene 2018, 665, 141–148. [Google Scholar] [CrossRef]
- Zheng, R.; Lin, S.; Guan, L.; Yuan, H.; Liu, K.; Liu, C.; Ye, W.; Liao, Y.; Jia, J.; Zhang, R. Long Non-Coding RNA XIST Inhibited Breast Cancer Cell Growth, Migration, and Invasion via MiR-155/CDX1 Axis. Biochem. Biophys. Res. Commun. 2018, 498, 1002–1008. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Gao, M.-X.; Yang, K. Cucurbitacin B Inhibits Cell Proliferation and Induces Apoptosis in Human Osteosarcoma Cells via Modulation of the JAK2/STAT3 and MAPK Pathways. Exp. Ther. Med. 2017, 14, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Thayanithy, V.; Park, C.; Sarver, A.L.; Kartha, R.V.; Korpela, D.M.; Graef, A.J.; Steer, C.J.; Modiano, J.F.; Subramanian, S. Combinatorial Treatment of DNA and Chromatin-Modifying Drugs Cause Cell Death in Human and Canine Osteosarcoma Cell Lines. PLoS ONE 2012, 7, e43720. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Li, J.; Si, Y.; He, Z.; Zhang, T.; Wang, D.; Liu, X.; Guo, Y.; Zhang, L.; Li, S.; et al. Cucurbitacin B Induces Inhibitory Effects via CIP2A/PP2A/Akt Pathway in Glioblastoma Multiforme. Mol. Carcinog. 2018, 57, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Kusagawa, E.; Okuda, C.; Yamaguchi, R.; Nakano, K.; Miyake, Y.; Kataoka, T. Cucurbitacin B Down-Regulates TNF Receptor 1 Expression and Inhibits the TNF-α-Dependent Nuclear Factor ΚB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells. Int. J. Mol. Sci. 2022, 23, 7130. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Khan, S.; Kumar, S.; Sinha, S.; Farhan, M.; Bora, H.; Maurya, R.; Meeran, S.M. Cucurbitacin B Alters the Expression of Tumor-Related Genes by Epigenetic Modifications in NSCLC and Inhibits NNK-Induced Lung Tumorigenesis. Cancer Prev. Res. 2015, 8, 552–562. [Google Scholar] [CrossRef]
- Klungsaeng, S.; Kukongviriyapan, V.; Prawan, A.; Kongpetch, S.; Senggunprai, L. Targeted Modulation of FAK/PI3K/PDK1/AKT and FAK/P53 Pathways by Cucurbitacin B for the Antiproliferation Effect Against Human Cholangiocarcinoma Cells. Am. J. Chin. Med. 2020, 48, 1475–1489. [Google Scholar] [CrossRef]
- Yuan, R.; Zhao, W.; Wang, Q.-Q.; He, J.; Han, S.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B Inhibits Non-Small Cell Lung Cancer in Vivo and in Vitro by Triggering TLR4/NLRP3/GSDMD-Dependent Pyroptosis. Pharmacol. Res. 2021, 170, 105748. [Google Scholar] [CrossRef]
- Piao, X.-M.; Gao, F.; Zhu, J.-X.; Wang, L.-J.; Zhao, X.; Li, X.; Sheng, M.-M. Cucurbitacin B Inhibits Tumor Angiogenesis by Triggering the Mitochondrial Signaling Pathway in Endothelial Cells. Int. J. Mol. Med. 2018, 42, 1018. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, Q.; Liang, X.; Han, S.; He, J.; Wang, Q.-Q.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B Inhibits TGF-Β1-Induced Epithelial–Mesenchymal Transition (EMT) in NSCLC through Regulating ROS and PI3K/Akt/MTOR Pathways. Chin. Med. 2022, 17, 24. [Google Scholar] [CrossRef]
- Yesilada, E.; Tanaka, S.; Sezik, E.; Tabata, M. Isolation of an Anti-Inflammatory Principle from the Fruit Juice of Ecballium elaterium. J. Nat. Prod. 1988, 51, 504–508. [Google Scholar] [CrossRef]
- Toker, G.; Memişoğlu, M.; Toker, M.C.; Yeşilada, E. Callus Formation and Cucurbitacin B Accumulation in Ecballium elaterium Callus Cultures. Fitoterapia 2003, 74, 618–623. [Google Scholar] [CrossRef]
- Touihri-Barakati, I.; Kallech-Ziri, O.; Ayadi, W.; Kovacic, H.; Hanchi, B.; Hosni, K.; Luis, J. Cucurbitacin B Purified from Ecballium elaterium (L.) A. Rich from Tunisia Inhibits A5β1 Integrin-Mediated Adhesion, Migration, Proliferation of Human Glioblastoma Cell Line and Angiogenesis. Eur. J. Pharmacol. 2017, 797, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kongtun, S.; Jiratchariyakul, W.; Kummalue, T.; Tan-ariya, P.; Kunnachak, S.; Frahm, A.W. Cytotoxic Properties of Root Extract and Fruit Juice of Trichosanthes Cucumerina. Planta Med. 2009, 75, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, S.-E.N.; Basaif, S.A.; Abdel-Lateff, A.; Shier, T. Cucurbitacins-Type Triterpene with Potent Activity on Mouse Embryonic Fibroblast from Cucumis Prophetarum, Cucurbitaceae. Pharmacogn. Res. 2011, 3, 189–193. [Google Scholar] [CrossRef]
- Galucio, N.C.D.R.; Moysés, D.D.A.; Pina, J.R.S.; Marinho, P.S.B.; Júnior, P.C.G.; Cruz, J.N.; Vale, V.V.; Khayat, A.S.; Marinho, A.M.D.R. Antiproliferative, Genotoxic Activities and Quantification of Extracts and Cucurbitacin B Obtained from Luffa operculata (L.) Cogn. Arab. J. Chem. 2022, 15, 103589. [Google Scholar] [CrossRef]
- Clericuzio, M.; Tabasso, S.; Bianco, M.A.; Pratesi, G.; Beretta, G.; Tinelli, S.; Zunino, F.; Vidari, G. Cucurbitane Triterpenes from the Fruiting Bodies and Cultivated Mycelia of Leucopaxillus gentianeus. J. Nat. Prod. 2006, 69, 1796–1799. [Google Scholar] [CrossRef]
- Sharma, D.; Rawat, I.; Goel, H. Anticancer and Anti-Inflammatory Activities of Some Dietary Cucurbits. Indian J. Exp. Biol. 2015, 53, 216–221. [Google Scholar]
- Duangmano, S.; Dakeng, S.; Jiratchariyakul, W.; Suksamrarn, A.; Smith, D.R.; Patmasiriwat, P. Antiproliferative Effects of Cucurbitacin B in Breast Cancer Cells: Down-Regulation of the c-Myc/HTERT/Telomerase Pathway and Obstruction of the Cell Cycle. Int. J. Mol. Sci. 2010, 11, 5323–5338. [Google Scholar] [CrossRef]
- El Naggar, E.M.B.; Chalupová, M.; Pražanová, G.; Parák, T.; Švajdlenka, E.; Žemlička, M.; Suchý, P. Hepatoprotective and Proapoptotic Effect of Ecballium elaterium on CCl4-Induced Hepatotoxicity in Rats. Asian Pac. J. Trop. Med. 2015, 8, 526–531. [Google Scholar] [CrossRef]
- Wu, P.-L.; Lin, F.-W.; Wu, T.-S.; Kuoh, C.-S.; Lee, K.-H.; Lee, S.-J. Cytotoxic and Anti-HIV Principles from the Rhizomes of Begonia Nantoensis. Chem. Pharm. Bull. 2004, 52, 345–349. [Google Scholar] [CrossRef]
- Panda, S.P.; Sarangi, A.K.; Panigrahy, U.P. Isolation of cucurbitacin-b from Cucumis callosus and its hypoglycemic effect in isolated rat enterocytes. Int. J. Pharm. Pharm. Sci. 2018, 10, 123. [Google Scholar] [CrossRef]
- Dat, N.T.; Jin, X.; Hong, Y.-S.; Lee, J.J. An Isoaurone and Other Constituents from Trichosanthes kirilowii Seeds Inhibit Hypoxia-Inducible Factor-1 and Nuclear Factor-ΚB. J. Nat. Prod. 2010, 73, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Dwijayanti, D.R.; Shimada, T.; Ishii, T.; Okuyama, T.; Ikeya, Y.; Mukai, E.; Nishizawa, M. Bitter Melon Fruit Extract Has a Hypoglycemic Effect and Reduces Hepatic Lipid Accumulation in Ob/Ob Mice. Phytother. Res. 2020, 34, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, D.-B.; Xia, M.-Y.; Luo, J.-F.; Li, X.-Y.; Wang, Y.-H. Bioassay-Guided Isolation of Cytotoxic Constituents from the Flowers of Aquilaria Sinensis. Nat. Prod. Bioprospect. 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Yamashita, U.; Matsuoka, H.; Sugiura, T.; Tsukada, J.; Noguchi, J.; Yoshida, Y. Apoptosis Induction through Proteasome Inhibitory Activity of Cucurbitacin D in Human T-Cell Leukemia. Cancer 2011, 117, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Song, Y.; He, C.; Wang, D.; Morita, K.; Tsukada, J.; Kanazawa, T.; Yoshida, Y. Autophagy Is Associated with Cucurbitacin D-Induced Apoptosis in Human T Cell Leukemia Cells. Med. Oncol. Northwood Lond. Engl. 2016, 33, 30. [Google Scholar] [CrossRef]
- Ishii, T.; Kira, N.; Yoshida, T.; Narahara, H. Cucurbitacin D Induces Growth Inhibition, Cell Cycle Arrest, and Apoptosis in Human Endometrial and Ovarian Cancer Cells. Tumor Biol. 2013, 34, 285–291. [Google Scholar] [CrossRef]
- Sikander, M.; Malik, S.; Chauhan, N.; Khan, P.; Kumari, S.; Kashyap, V.K.; Khan, S.; Ganju, A.; Halaweish, F.T.; Yallapu, M.M.; et al. Cucurbitacin D Reprograms Glucose Metabolic Network in Prostate Cancer. Cancers 2019, 11, 364. [Google Scholar] [CrossRef]
- Song, Y.; Ding, N.; Kanazawa, T.; Yamashita, U.; Yoshida, Y. Cucurbitacin D Is a New Inflammasome Activator in Macrophages. Int. Immunopharmacol. 2013, 17, 1044–1050. [Google Scholar] [CrossRef]
- Ku, J.M.; Hong, S.H.; Kim, H.I.; Lim, Y.S.; Lee, S.J.; Kim, M.; Seo, H.S.; Shin, Y.C.; Ko, S.-G. Cucurbitacin D Exhibits Its Anti-Cancer Effect in Human Breast Cancer Cells by Inhibiting Stat3 and Akt Signaling. Eur. J. Inflamm. 2018, 16, 1721727X17751809. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, C.F.; Zhang, L.F. Cucurbitacin D Impedes Gastric Cancer Cell Survival via Activation of the INOS/NO and Inhibition of the Akt Signalling Pathway. Oncol. Rep. 2018, 39, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Kodidela, S.; Sinha, N.; Kumar, A.; Kumar, S. Anti-HIV Activity of Cucurbitacin-D against Cigarette Smoke Condensate-Induced HIV Replication in the U1 Macrophages. Viruses 2021, 13, 1004. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, Q.; Qiang, Y.; Guo, W.; Ma, Y. Cucurbitacin E Induces Apoptosis of Human Prostate Cancer Cells via Cofilin-1 and MTORC1. Oncol. Lett. 2017, 13, 4905–4910. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, L.; Yang, Q. Infection of Porcine Circovirus 2 (PCV2) in Intestinal Porcine Epithelial Cell Line (IPEC-J2) and Interaction between PCV2 and IPEC-J2 Microfilaments. Virol. J. 2014, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; El-Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Koshak, A.E.; Elhady, S.S. Cucurbitacin E Glucoside Alleviates Concanavalin A-Induced Hepatitis through Enhancing SIRT1/Nrf2/HO-1 and Inhibiting NF-ĸB/NLRP3 Signaling Pathways. J. Ethnopharmacol. 2022, 292, 115223. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.E.M.; Boulos, J.C.; Elhaboub, G.; Rigano, D.; Saab, A.; Loizzo, M.R.; Hassan, L.E.A.; Sugimoto, Y.; Piacente, S.; Tundis, R.; et al. Cytotoxicity of Cucurbitacin E from Citrullus colocynthis against Multidrug-Resistant Cancer Cells. Phytomedicine 2019, 62, 152945. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Liu, W.; Yin, C.; Chu, H.; Zhang, M. Cucurbitacin E Ameliorates Lipopolysaccharide-Evoked Injury, Inflammation and MUC5AC Expression in Bronchial Epithelial Cells by Restraining the HMGB1-TLR4-NF-ΚB Signaling. Mol. Immunol. 2019, 114, 571–577. [Google Scholar] [CrossRef]
- Attard, E.; Brincat, M.P.; Cuschieri, A. Immunomodulatory Activity of Cucurbitacin E Isolated from Ecballium elaterium. Fitoterapia 2005, 76, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Arel-Dubeau, A.-M.; Longpré, F.; Bournival, J.; Tremblay, C.; Demers-Lamarche, J.; Haskova, P.; Attard, E.; Germain, M.; Martinoli, M.-G. Cucurbitacin E Has Neuroprotective Properties and Autophagic Modulating Activities on Dopaminergic Neurons. Oxid. Med. Cell. Longev. 2014, 2014, e425496. [Google Scholar] [CrossRef]
- Jafargholizadeh, N.; Zargar, S.J.; Yassa, N.; Tavakoli, S. Purification of Cucurbitacins D, E, and I from Ecballium elaterium (L.) A. Rich Fruits and Study of Their Cytotoxic Effects on the AGS Cell Line. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 4631–4635. [Google Scholar] [CrossRef]
- Attard, E.; Cuschieri, A.; Brincat, M.P. Morphological Effects Induced by Cucurbitacin E on Ovarian Cancer Cells in Vitro. J. Nat. Remedies 2005, 5, 70–74. [Google Scholar]
- Blaskovich, M.A.; Sun, J.; Cantor, A.; Turkson, J.; Jove, R.; Sebti, S.M. Discovery of JSI-124 (Cucurbitacin I), a Selective Janus Kinase/Signal Transducer and Activator of Transcription 3 Signaling Pathway Inhibitor with Potent Antitumor Activity against Human and Murine Cancer Cells in Mice1. Cancer Res. 2003, 63, 1270–1279. [Google Scholar] [PubMed]
- van Kester, M.S.; Out-Luiting, J.J.; von dem Borne, P.A.; Willemze, R.; Tensen, C.P.; Vermeer, M.H. Cucurbitacin I Inhibits Stat3 and Induces Apoptosis in Sézary Cells. J. Investig. Dermatol. 2008, 128, 1691–1695. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Li, R.; Xin, J.; Wu, S.; Lan, J.; Xue, K.; Li, X.; Zuo, C.; Jiang, W.; et al. Cucurbitacin I Induces Cancer Cell Death through the Endoplasmic Reticulum Stress Pathway. J. Cell. Biochem. 2019, 120, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wu, S.; Wang, X.; Zhu, G.; Chen, X.; Ding, Y.; Jiang, W. Cucurbitacin I Induces Pro-Death Autophagy in A549 Cells via the ERK-MTOR-STAT3 Signaling Pathway. J. Cell. Biochem. 2018, 119, 6104–6112. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Yang, X.; Li, Y.; Cui, X.; Liu, B.; Yao, Q. Synthesis of Cucurbitacin IIa Derivatives with Apoptosis-Inducing Capabilities in Human Cancer Cells. RSC Adv. 2020, 10, 3872–3881. [Google Scholar] [CrossRef] [PubMed]
- Boykin, C.; Zhang, G.; Chen, Y.-H.; Zhang, R.-W.; Fan, X.-E.; Yang, W.-M.; Lu, Q. Cucurbitacin IIa: A Novel Class of Anti-Cancer Drug Inducing Non-Reversible Actin Aggregation and Inhibiting Survivin Independent of JAK2/STAT3 Phosphorylation. Br. J. Cancer 2011, 104, 781–789. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, T.; Luo, L.; Li, L.; Cao, W.; Xu, X.; Zhang, Y.; Yue, P.; Dai, X.; Ji, Z.; et al. Isoforskolin and Cucurbitacin IIa Promote the Expression of Anti-Inflammatory Regulatory Factor SIGIRR in Human Macrophages Stimulated with Borrelia Burgdorferi Basic Membrane Protein A. Int. Immunopharmacol. 2020, 88, 106914. [Google Scholar] [CrossRef]
- Guo, R.-H.; Geng, C.-A.; Huang, X.-Y.; Ma, Y.-B.; Zhang, Q.; Wang, L.-J.; Zhang, X.-M.; Zhang, R.-P.; Chen, J.-J. Synthesis of Hemslecin A Derivatives: A New Class of Hepatitis B Virus Inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 1201–1205. [Google Scholar] [CrossRef]
- Zhou, S.-M.; Guan, S.-Y.; Yang, L.; Yang, L.-K.; Wang, L.; Nie, H.-F.; Li, X.; Zhao, M.-G.; Yang, Q.; Wu, H. Cucurbitacin IIa Exerts Antidepressant-like Effects on Mice Exposed to Chronic Unpredictable Mild Stress. NeuroReport 2017, 28, 259–267. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Y.; Liang, Y.; Zou, H.; Zuo, P.; Yan, M.; Jing, S.; Li, T.; Wang, Y.; Li, D.; et al. Cucurbitacin IIa Interferes with EGFR-MAPK Signaling Pathway Leads to Proliferation Inhibition in A549 cells. Food Chem. Toxicol. 2019, 132, 110654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xian, Q.; Xiao, C.; Zhong, Y.; Su, X.; Xu, L.; Luo, Q.; Cheng, P.; Wang, T.; Liu, J.; et al. Effects of cucurbitacin IIa on apoptosis of humanlung cancer cell lines NCI-H460 and A549 and its mechanism. Chin. Pharmacol. Bull. 2017, 33, 922–927. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.-X.; Xu, L.-H.; Liu, K.-P.; Pan, H.; He, J.; Cai, J.-Y.; Ouyang, D.-Y.; He, X.-H. Cucurbitacin IIb Exhibits Anti-Inflammatory Activity through Modulating Multiple Cellular Behaviors of Mouse Lymphocytes. PLoS ONE 2014, 9, e89751. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, T.; Ren, L.; Jing, S.; Li, Z.; Zuo, P.; Li, T.; Wang, Y.; Zhang, J.; Wei, Z. Cucurbitacin IIb Induces Apoptosis and Cell Cycle Arrest through Regulating EGFR/MAPK Pathway. Environ. Toxicol. Pharmacol. 2021, 81, 103542. [Google Scholar] [CrossRef]
- Torres-Moreno, H.; Marcotullio, M.C.; Velázquez, C.; Ianni, F.; Garibay-Escobar, A.; Robles-Zepeda, R.E. Cucurbitacin IIb, a Steroidal Triterpene from Ibervillea Sonorae Induces Antiproliferative and Apoptotic Effects on Cervical and Lung Cancer Cells. Steroids 2020, 157, 108597. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Dong, D.; Bi, X.; Liu, Y.; Ma, Y. Cucurbitacin IIb Improved Active Chromatin-Induced Systemic Lupus Erythematosus via Balancing the Percentage of Th17 and Treg Cells. Clin. Exp. Pharmacol. Physiol. 2021, 48, 329–336. [Google Scholar] [CrossRef]
- Ge, W.; Chen, X.; Han, F.; Liu, Z.; Wang, T.; Wang, M.; Chen, Y.; Ding, Y.; Zhang, Q. Synthesis of Cucurbitacin B Derivatives as Potential Anti-Hepatocellular Carcinoma Agents. Molecules 2018, 23, 3345. [Google Scholar] [CrossRef]
- Cai, Y.; Fang, X.; He, C.; Li, P.; Xiao, F.; Wang, Y.; Chen, M. Cucurbitacins: A Systematic Review of the Phytochemistry and Anticancer Activity. Am. J. Chin. Med. 2015, 43, 1331–1350. [Google Scholar] [CrossRef]
- Escandell, J.M.; Recio, M.-C.; Máñez, S.; Giner, R.-M.; Cerdá-Nicolás, M.; Ríos, J.-L. Dihydrocucurbitacin B, Isolated from Cayaponia Tayuya, Reduces Damage in Adjuvant-Induced Arthritis. Eur. J. Pharmacol. 2006, 532, 145–154. [Google Scholar] [CrossRef]
- Ren, S.; Ouyang, D.-Y.; Saltis, M.; Xu, L.-H.; Zha, Q.-B.; Cai, J.-Y.; He, X.-H. Anti-Proliferative Effect of 23,24-Dihydrocucurbitacin F on Human Prostate Cancer Cells through Induction of Actin Aggregation and Cofilin-Actin Rod Formation. Cancer Chemother. Pharmacol. 2012, 70, 415–424. [Google Scholar] [CrossRef]
- Attar, U.A.; Ghane, S.G. Optimized Extraction of Anti-Cancer Compound—Cucurbitacin I and LC–MS Identification of Major Metabolites from Wild Bottle Gourd (Lagenaria Siceraria (Molina) Standl.). S. Afr. J. Bot. 2018, 119, 181–187. [Google Scholar] [CrossRef]
- Aiswarya, S.U.D.; Vikas, G.; Haritha, N.H.; Liju, V.B.; Shabna, A.; Swetha, M.; Rayginia, T.P.; Keerthana, C.K.; Nath, L.R.; Reshma, M.V.; et al. Purified and Characterized From the Rhizome of Corallocarpus epigaeus Exhibits Anti-Melanoma Potential. Front. Oncol. 2022, 12, 903832. [Google Scholar] [CrossRef] [PubMed]
- Attar, U.A.; Ghane, S.G.; Chavan, N.S.; Shiragave, P.D. Simultaneous Detection of Anticancer Compounds (Cucurbitacin I, B and E) and Some Pharmacological Properties of Indian Blastania Species. South Afr. J. Bot. 2022, 147, 871–881. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hasan, M.K.; Shoeb, M.; Mamun, M.I.R.; Nahar, N.; Mosihuzzaman, M. Isolation and Characterization of Two Cucurbitane Type Triterpenoid Glycocide from 1-Butanol Soluble Part of Momordica Charantia Fruit Pulp Juice. Dhaka Univ. J. Sci. 2018, 66, 145–149. [Google Scholar] [CrossRef]
- Tosun, E.; Baysar, A. Simultaneous Isolation and Purification of Cucurbitacin D and I from Ecballium elaterium (l.) A. Rich Fruit Juice. Maced. J. Chem. Chem. Eng. 2019, 38, 171–182. [Google Scholar] [CrossRef]
- Riviello-Flores, M.D.L.L.; Arévalo-Galarza, M.D.L.; Cadena-Iñiguez, J.; Soto-Hernández, R.M.; Ruiz-Posadas, L.D.M.; Gómez-Merino, F.C. Nutraceutic Characteristics of the Extracts and Juice of Chayote (Sechium edule (Jacq.) Sw.) Fruits. Beverages 2018, 4, 37. [Google Scholar] [CrossRef]
- De Monte, C.; Carradori, S.; Granese, A.; Di Pierro, G.B.; Leonardo, C.; De Nunzio, C. Modern Extraction Techniques and Their Impact on the Pharmacological Profile of Serenoa Repens Extracts for the Treatment of Lower Urinary Tract Symptoms. BMC Urol. 2014, 14, 63. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An Insight into Medicinal Leads from Nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar] [CrossRef]
- Lira, R.; Rodriguez-Jimenez, C.; Alvarado, J.L.; Rodriguez, I.; Castrejon, J.; Dominguez-Marian, A. Diversidad e importancia de la familia Cucurbitaceae en México. Acta Bot. Mex. 1998, 42, 43–77. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; et al. Cucurbita Plants: From Farm to Industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef]
- Rehm, S.; Wessels, J.H. Bitter Principles of the Cucurbitaceae. VIII.—Cucurbitacins in Seedlings—Occurrence, Biochemistry and Genetical Aspects. J. Sci. Food Agric. 1957, 8, 687–691. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Rhodes, A.M.; Metcalf, R.A.; Ferguson, J.; Metcalf, E.R.; Lu, P.-Y. Cucurbitacin Contents and Diabroticite (Coleoptera: Chrysomelidae) Feeding Upon Cucurbita pp. Environ. Entomol. 1982, 11, 931–937. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Aguiñiga-Sánchez, I.; Uriostegui-Arias, M.T.; Santiago-Osorio, E.; Ruiz-Posadas, L.D.M.; Soto-Hernández, M. Antiproliferative Effect of Sechium edule (Jacq.) Sw., Cv. Madre Negra Extracts on Breast Cancer In Vitro. Separations 2022, 9, 230. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Ruíz-Posadas, L.D.M.; Cadena-Zamudio, J.D.; González-Ugarte, A.K.; Steider, B.W.; Santiago-Osorio, E. Fruit Extract from A Sechium edule Hybrid Induce Apoptosis in Leukaemic Cell Lines but Not in Normal Cells. Nutr. Cancer 2015, 67, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.H.; Whelpton, R. Introduction to Drug Disposition and Pharmacokinetics; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Ding, T.; Zhang, Y.; Chen, A.; Tang, Y.; Liu, M.; Wang, X. Effects of Cucurbitacin E, a Tetracyclic Triterpene Compound from Cucurbitaceae, on the Pharmacokinetics and Pharmacodynamics of Warfarin in Rats. Basic Clin. Pharmacol. Toxicol. 2015, 116, 385–389. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Sun, M.; Liu, M.; Wang, X. Comprehensive Assessment of Cucurbitacin E Related Hepatotoxicity and Drug-Drug Interactions Involving CYP3A and P-Glycoprotein. Phytomedicine 2017, 26, 1–10. [Google Scholar] [CrossRef]
- Dominguez More, G.P.; Cardenas, P.A.; Costa, G.M.; Simoes, C.M.O.; Aragon, D.M. Pharmacokinetics of Botanical Drugs and Plant Extracts. Mini Rev. Med. Chem. 2017, 17, 1646–1664. [Google Scholar] [CrossRef]
- Cadena Iñiguez, J.; Soto Hernández, M.; del Mar Ruiz Posadas, L.; Avendaño Arrazate, C.H.; Aguirre Medina, J.F.; Ruiz Posadas, L. del M. Caracterización bioquímica de variedades domesticadas de chayote Sechium edule (Jacq.) Sw. comparadas con parientes silvestres. Rev. Chapingo Ser. Hortic. 2011, 17, 45–55. [Google Scholar] [CrossRef]
- Jaworski, A.; Gorski, P.M.; Shannon, S.; Robinson, R.W. Cucurbitacin Concentrations in Different Plant Parts of Cucurbita Species as a Function of Age. Rep. Cucurbit Genet. Coop. USA 1985, 8, 71–73. [Google Scholar]
- Aguiñiga Sánchez, I. Potencial antileucémico in vitro de extractos de cuatro genotipos de Sechium spp. (Cucurbitaceae). Doctoral Thesis, Colegio de Postgraduados, Estado de México, México, 2013. [Google Scholar]
- Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Soto-hernandez, M.; Avendaño-Arrazate, C.; Ruiz-Posadas, L.; Santiago-Osorio, E.; Ramos, M.; Cisneros, V.; Aguirre Medina, J. Production, Genetics, Postharvest Management and Pharmacological Characteristics of Sechium edule (Jacq) Sw. Fresh Produce 2007, 1, 41–53. [Google Scholar]

| Compound | Source | Model/Mode of Administration | Dose | Pharmacokinetic Parameters | Reference |
|---|---|---|---|---|---|
| CuIIa | Hemsleya amabilis extract | Normal rats/Oral administration | 1.36 g/kg | Cmax = 0.021 ± 0.0057 mg/L | [24] |
| Tmax = 0.333 ± 0.183 h | |||||
| AUC(0-t) = 21.36 ± 5.60 ng/h/mL | |||||
| AUC(0-∞) = 21.76 ± 5.60 ng/h/mL | |||||
| MRT(0–t) = 1.01 ± 0.23 h | |||||
| MRT(0–∞) = 1.08 ± 0.23 h | |||||
| T1/2 = 0.678 ± 0.219 h | |||||
| CLz = 7209.68 ± 1805.38 L/h/kg | |||||
| CuIIa | Commercial sources | Rhesus monkeys /Intravenous injection | 0.18 mg/kg | Cmax = 1.3565 ± 0.12868 mg/L | [25] |
| T1/2Z = 0.455 ± 0.117 h | |||||
| VZ = 0.844 ± 0.299 L/kg | |||||
| CLz = 1.265 ± 0.149 L/h/kg | |||||
| AUC(0–∞) = 142.328 ± 16.392 μg/h/L | |||||
| MRT = 0.338 ± 0.040 h | |||||
| CuIIa | Commercial sources | Wistar rats/Intravenous injection | 1.0 mg/kg | Cmax = 3.537 ± 0.278 mg/L T1/2α = 0.073 ± 0.042 h T1/2β = 0.732 ± 0.151 h T1/2Z = 1.168 ± 0.415 h Vd = 0.147 ± 0.089 L/kg CLz = 0.287 ± 0.031 L/h/kg AUC(0–t) = 2.824 ± 0.578 mg/h/L AUC(0–∞) = 3.646 ± 1.124 mg/h/L MRT = 0.479 ± 0.038 h | [28] |
| CuIIa | Commercial sources | Wistar rats /Intravenous injection | 2.0 mg/kg | Cmax = 6.452 ± 0.867 mg/L T1/2α = 0.068 ± 0.031 h T1/2β = 0.681 ± 0.055 h T1/2Z = 0.985 ± 0.351 h Vd = 0.131 ± 0.095 L/kg CLz = 0.304 ± 0.063 L/h/kg AUC(0–t) = 4.133 ± 0.829 mg/h/L AUC(0–∞) = 4.916 ± 1.227 mg/h/L MRT = 0.553 ± 0.054 h | [28] |
| CuIIa | Commercial sources | Wistar rats/Intravenous injection | 4.0 mg/kg | Cmax = 12.231 ± 2.77 mg/L T1/2α = 0.074 ± 0.052 h T1/2β = 0.667 ± 0.064 h T1/2Z = 1.127 ± 0.614 h Vd = 0.153 ± 0.047 L/kg CLz = 0.318 ± 0.029 L/h/kg AUC(0–t) = 7.916 ± 0.582 mg/h/L AUC(0–∞) = 9.385 ± 1.419 mg/h/L MRT = 0.517 ± 0.067 h | [28] |
| CuIIb | Hemsleya amabilis extract | Normal rats/Oral administration | 1.36 g/kg | Cmax = 0.02103 ± 0.00672 mg/L Tmax = 0.667 ± 0.211 h AUC(0–t) = 37.63 ± 13.01 ng/h/L AUC(0–∞) = 38.54 ± 13.05 mg/h/L MRT(0–t) = 1.35 ± 0.20 h MRT(0–∞) = 1.48 ± 0.23 h T1/2 = 0.907 ± 0.349 h CLz = 1553.35 ± 489.41 L/h/kg | [24] |
| CuB | Trichosanthes Cucumerina extract | Wistar rats/Oral administration | 2.0 mg/kg | Cmax = 0.0097 ± 0.0039 mg/L Tmax = 0.50 ± 0.00 h AUC0–t = 15.10 ± 3.57 μg/h/L AUC0–inf = 25.33 ± 12.13 μg/h/L Vd = N/A L/kg CL = N/A L/h/kg T1/2 = N/A h MRT = 9.95 ± 12.27 h | [21] |
| CuB | Trichosanthes cucumerina extract | Wistar rats/Intravenous injection | 0.1 mg/kg | Cmax = N/A Tmax = N/A AUC(0–t) = 13.92 ± 11.11 μg/h/L AUC0–inf = 17.95 ± 13.21 μg/h/L Vd = 51.65 ± 39.16 L/kg CL = 7.24 ± 2.92 L/h/kg T1/2 = 5.08 ± 2.87 h MRT = 6.03 ± 2.93 h | [21] |
| CuB | Trichosanthes cucumerina extract | Wistar rats/Oral administration | 4.0 mg/kg | Cmax = 0.03124 ± 0.0105 mg/L Tmax = 0.60 ± 0.22 h AUC(0–t) = 45.22 ± 10.14 μg/h/L AUC0–inf = 52.42 ± 29.58 μg/h/L Vd = N/A L/kg CL = N/A L/h/kg T1/2 = N/A h MRT = 5.50 ± 2.28 h | [21] |
| CuB | Commercial sources | Wistar rats/Oral administration | 20 mg/kg | Cmax = 0.0059 ± 0.00101 mg/L Tmax = 1.75 ± 0.88 h T1/2 = 2.50 ± 0.58 h AUClast = 0.022 ± 0.005 mg/h/L AUCinf = 0.024 ± 0.005 mg/h/L CL = 845.99 ± 183.70 L/h/kg | [20] |
| CuB | Commercial sources (Tablets) | Sprague Dawley rats/Oral administration | 0.09 mg/kg | Cmax = 0.030 ± 0.007 mg/L Tmax = 2.41 ± 0.42 h Ke = 0.22 ± 0.04 T1/2 = 3.19 ± 0.54 h AUC(0–t) = 140.4 = 2 ± 31.35 ng/h/L AUC(0–∞) = 152.01 ± 35.02 ng/h/L | [26] |
| CuB | Commercial sources (Tablets) | Wistar rats/Oral administration | 8 mg/kg | Cmax = 3.41 × 10−5 ± 0.0029 mg/L Tmax = 3 h T1/2z = 4.129 ± 0.54 h AUC = (0–t) = 183.28 ± 10.24 ng/L/h AUC(0–∞) = 187.41 ± 10.41 ng/L/h Vz/F = 2.55 × 108 ± 3.62 × 107 CLz/F = 4.28 × 107 ± 2.47 × 106 MRT(0–t) = 6.49 ± 0.18 h MRT(0–∞) = 7.02 ± 0.29 h | [27] |
| CuE | Commercial sources (Tablets) | Sprague Dawley rats/Oral administration | 0.09 mg/kg | Cmax = 0.009 ± 0.0026 mg/L Tmax = 2.10 ± 0.21 h Ke = 0.23 ± 0.05 T1/2 = 2.58 ± 0.66 h AUC(0–t) = 63.56 ± 11.92 ng/h/L AUC(0–∞) = 67.27 ± 11.31 ng/h/L | [26] |
| Source | Biological Activity | Reference |
|---|---|---|
| Ecballium elaterium | Anti-inflammatory | [74] |
| Citrullus colocynthis | Cytotoxic in breast cancer | [15] |
| Ecballium elaterium | Anti-inflammatory | [75] |
| Ecballium elaterium | Antiproliferative | [76] |
| Trichosanthes cucumerina | Cytotoxic in breast cancer cells | [77] |
| Cucumis prophetarum | Cytotoxic in embryonal cancer | [78] |
| Luffa operculata | Antiproliferative, genotoxic activities in human breast cancer cells | [79] |
| Leucopaxillus gentianeus | Cytotoxic in breast cancer cells | [80] |
| Cucurbita pepo | Anti-inflammatory | [81] |
| Trichosanthes cucumerina | Cytotoxic in breast cancer | [82] |
| Ecballium elaterium | Hepatoprotective | [83] |
| Begonia nantoensis | Cytotoxic in multiple cancers | [84] |
| Luffa graveolense | Cytotoxic in lung cancer | [69] |
| Cucumis callous | Hypoglycemic | [85] |
| Trichosanthes kirilowii | Cytotoxic in liver cancer | [86] |
| Momordica charantia | Anti-inflammatory and antidiabetic | [87] |
| Luffa operculata | Antiproliferative in gastric adenocarcinoma cell line | [79] |
| Aquilaria sinensis | Cytotoxic | [88] |
| Source | Biological Effect | Reference |
|---|---|---|
| Ecballium elaterium | Immunomodulatory | [102] |
| Commercial source | Cytotoxic enchondrosarcoma | [32] |
| Citrullus lanatus | Anti-inflammatory | [4] |
| Ecballium elaterium | Neuroprotective | [103] |
| Ecballium elaterium | Cytotoxic in human stomach adenocarcinoma cell line | [104] |
| Ecballium elaterium | Cytotoxic in ovarian cancer | [105] |
| Species | Parts of the Plant | CuB | CuD | CuE | CuI | Reference |
|---|---|---|---|---|---|---|
| Cucurbita maxima | Radicle | 0.1–1 | Trace | 0.01–0.1 | ND | [135,136] |
| cotyledons | 0.1–1 | 0.1–1 | 0.01–0.1 | ND | ||
| leaf, fruit, root | >0.02 | >0.02 | >0.02 | >0.02 | ||
| Cucurbita andreana | Sheet | 0.15 | 0.12 | ND | ND | [136] |
| Fruit | 2.78 | 2.78 | ND | ND | ||
| Root | 0.58 | 0.58 | ND | ND | ||
| Cucurbita pepo | Radicle | Trace | ND | 0.1–1 | Trace | [135] |
| cotyledons | 0.1–1 | 0.01–0.1 | 0.01–0.1 | NI | ||
| Fruit | ND | ND | 3.1 | NI | ||
| Cucurbita martinezii | Sheet | ND | ND | 0.42 | 0.25 | [136] |
| Fruit | ND | ND | 0.36 | 0.45 | ||
| Root | ND | ND | 0.23 | 0.65 | ||
| Cucurbita lundelliana | Sheet | 0.47 | 0.12 | ND | NI | [136] |
| Fruit | 0.63 | 0.15 | ND | NI | ||
| Root | 0.53 | 0.29 | ND | NI | ||
| Cucurbita foetidissima | Root | ND | ND | 0.28 | 1.72 | [136] |
| Cucurbita equadorensis | Placenta | 0.14 | 0.5 | 0.03 | 0.06 | [144] |
| Pulp | 0.006 | 0.01 | Trace | Trace | ||
| Cortex | 0.02 | 0.02 | Trace | Trace | ||
| Sechium edule var. virens levis | Fruit | ND | 3.534 | 0.003 | ND | [130] |
| Sechium edule var. virens levis | Fruit | 0.0016 | 3.95 | 0.03 | 0.003 | [130] |
| Sechium edule var. nigrum spinosum | Fruit | 0.24 | ND | 0.05 | ND | [130] |
| Sechium edule var. nigrum spinosum | Fruit | 0.001 | 1.34 | 0.02 | 0.002 | [130] |
| Sechium hybrid H387 | Fruit | 1.63 | 1.62 | 0.42 | 0.088 | [145] |
| Species | Parts of the Plant | Total Content of Cucurbitacins | Reference |
|---|---|---|---|
| Sechium edule var. nigrum xalapensis | Fruit | 0.00195 | [146] |
| Sechium edule var. nigrum levis | Fruit | 0.00066 | [146] |
| Sechium edule var. amarus sylvestris | Fruit | 0.001456 | [146] |
| Sechium edule var. albus minor | Fruit | 3.9 × 10−5 | [146] |
| Sechium edule var. albus dulcis | Fruit | 2.7 × 10−5 | [146] |
| Sechium edule var. albus levis | Fruit | 8.8 × 10−5 | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Tiburcio, E.E.; Cadena-Iñiguez, J.; Santiago-Osorio, E.; Ruiz-Posadas, L.d.M.; Castillo-Juárez, I.; Aguiñiga-Sánchez, I.; Soto-Hernández, M. Pharmacokinetics and Biological Activity of Cucurbitacins. Pharmaceuticals 2022, 15, 1325. https://doi.org/10.3390/ph15111325
Delgado-Tiburcio EE, Cadena-Iñiguez J, Santiago-Osorio E, Ruiz-Posadas LdM, Castillo-Juárez I, Aguiñiga-Sánchez I, Soto-Hernández M. Pharmacokinetics and Biological Activity of Cucurbitacins. Pharmaceuticals. 2022; 15(11):1325. https://doi.org/10.3390/ph15111325
Chicago/Turabian StyleDelgado-Tiburcio, Eugenia Elisa, Jorge Cadena-Iñiguez, Edelmiro Santiago-Osorio, Lucero del Mar Ruiz-Posadas, Israel Castillo-Juárez, Itzen Aguiñiga-Sánchez, and Marcos Soto-Hernández. 2022. "Pharmacokinetics and Biological Activity of Cucurbitacins" Pharmaceuticals 15, no. 11: 1325. https://doi.org/10.3390/ph15111325
APA StyleDelgado-Tiburcio, E. E., Cadena-Iñiguez, J., Santiago-Osorio, E., Ruiz-Posadas, L. d. M., Castillo-Juárez, I., Aguiñiga-Sánchez, I., & Soto-Hernández, M. (2022). Pharmacokinetics and Biological Activity of Cucurbitacins. Pharmaceuticals, 15(11), 1325. https://doi.org/10.3390/ph15111325