Safety, Tolerability, and Serum/Tear Pharmacokinetics of Human Recombinant Epidermal Growth Factor Eyedrops in Healthy Subjects
Abstract
1. Introduction
2. Results
2.1. Demography
2.2. Safety
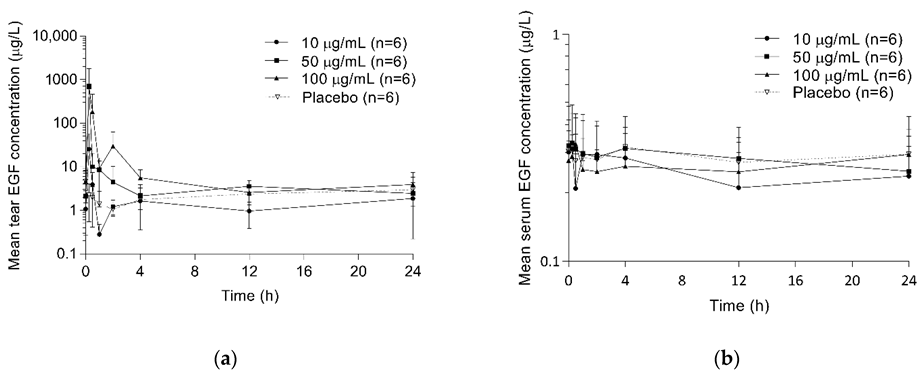
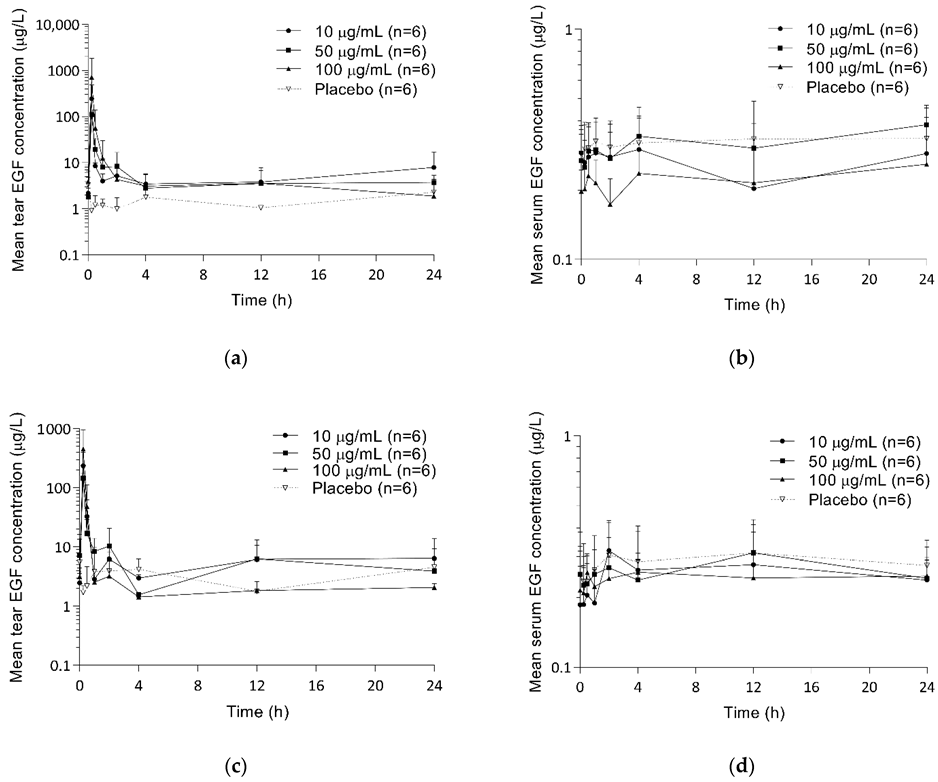
2.3. Pharmacokinetics
2.4. Immunogenicity
3. Discussion
4. Materials and Methods
4.1. Study Design and Subjects
4.2. Pharmacokinetic Sampling and Bioanalysis
4.3. Pharmacokinetic Analysis
4.4. Immunogenicity Evaluations
4.5. Safety/Tolerability Evaluations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willmann, D.; Fu, L.; Melanson, S.W. Corneal Injury; StatPearls: St. Petersburg, FL, USA, 2020. [Google Scholar]
- Wilson, S.E.; Medeiros, C.S.; Santhiago, M.R. Pathophysiology of corneal scarring in persistent epithelial defects after PRK and other corneal injuries. J. Refract. Surg. 2018, 34, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Progress Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Trosan, P.; Svobodova, E.; Chudickova, M.; Krulova, M.; Zajicova, A.; Holan, V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 2012, 21, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul. Surf. 2004, 2, 76–91. [Google Scholar] [CrossRef]
- Ke, Y.; Wu, Y.; Cui, X.; Liu, X.; Yu, M.; Yang, C.; Li, X. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS ONE 2015, 10, e0119725. [Google Scholar] [CrossRef]
- Yan, L.; Wu, W.; Wang, Z.; Li, C.; Lu, X.; Duan, H.; Zhou, J.; Wang, X.; Wan, P.; Song, Y. Comparative study of the effects of recombinant human epidermal growth factor and basic fibroblast growth factor on corneal epithelial wound healing and neovascularization in vivo and in vitro. Ophthalmic Res. 2013, 49, 150–160. [Google Scholar] [CrossRef]
- D’Souza, S.; Tong, L. Practical issues concerning tear protein assays in dry eye. Eye Vis. 2014, 1, 1–12. [Google Scholar] [CrossRef]
- Versura, P.; Profazio, V.; Buzzi, M.; Stancari, A.; Arpinati, M.; Malavolta, N.; Campos, E.C. Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea 2013, 32, 412–418. [Google Scholar] [CrossRef]
- Salman, I.A.; Gündoğdu, C. Epithelial healing in experimental corneal alkali wounds with nondiluted autologous serum eye drops. Cutan. Ocul. Toxicol. 2010, 29, 116–121. [Google Scholar] [CrossRef]
- Murri, M.S.; Moshirfar, M.; Birdsong, O.C.; Ronquillo, Y.C.; Ding, Y.; Hoopes, P.C. Amniotic membrane extract and eye drops: A review of literature and clinical application. Clin. Ophthalmol. 2018, 12, 1105. [Google Scholar] [CrossRef]
- Navas, A.; Magaña-Guerrero, F.S.; Domínguez-López, A.; Chávez-García, C.; Partido, G.; Graue-Hernández, E.O.; Sánchez-García, F.J.; Garfias, Y. Anti-inflammatory and anti-fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.; Shi, T.; Lu, L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor κB subtype-regulated CCCTC binding factor (CTCF) activation. J. Biol. Chem. 2013, 288, 24363–24371. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Bonafonte-Marquez, E.; Bonafonte-Royo, S.; Martínez-Carpio, P.A. Posology, efficacy, and safety of epidermal growth factor eye drops in 305 patients: Logistic regression and group-wise odds of published data. J. Ocul. Pharmacol. Ther. 2012, 28, 467–472. [Google Scholar] [CrossRef]
- Haus, E.; Haus, E.; Dumitriu, L.; Nicolau, G.; Bologa, S.; Sackett-Lundeen, L. Circadian rhythms of basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), cortisol, and melatonin in women with breast cancer. Chronobiol. Int. 2001, 18, 709–727. [Google Scholar] [CrossRef] [PubMed]
- Idania, G.; Hassiul, C.; Adriana, C.; Kalet, L. Measurement of Serum EGF Levels, a Methodological Approach: Learning What Means Low-/High-Concentration of EGF In Serum. Some Clinical Implications. J. Mol. Biomark. Diagn. 2017, 8, 2. [Google Scholar]
- Lanuti, M.; Liu, G.; Goodwin, J.M.; Zhai, R.; Fuchs, B.C.; Asomaning, K.; Su, L.; Nishioka, N.S.; Tanabe, K.K.; Christiani, D.C. A functional epidermal growth factor (EGF) polymorphism, EGF serum levels, and esophageal adenocarcinoma risk and outcome. Clin. Cancer Res. 2008, 14, 3216–3222. [Google Scholar] [CrossRef][Green Version]
- da Silveira, F.d.C.A.; de Almeida Lopes, B.; da Fonseca, C.O.; Quirico-Santos, T.; de Palmer Paixão, I.C.N.; de Amorim, L.M.d.F. Analysis of EGF+ 61A> G polymorphism and EGF serum levels in Brazilian glioma patients treated with perillyl alcohol-based therapy. J. Cancer Res. Clin. Oncol. 2012, 138, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, W.; Xu, A.; Zhang, L.; Yan, T.; Li, Z.; Wu, X.; Zhu, X.; Ma, J.; Li, K. Association of epidermal growth factor and epidermal growth factor receptor polymorphisms with the risk of hepatitis B virus-related hepatocellular carcinoma in the population of North China. Genet. Test. Mol. Biomark. 2013, 17, 595–600. [Google Scholar] [CrossRef]
- Romero-Ventosa, E.Y.; Blanco-Prieto, S.; González-Piñeiro, A.L.; Rodríguez-Berrocal, F.J.; Piñeiro-Corrales, G.; de la Cadena, M.P. Pretreatment levels of the serum biomarkers CEA, CYFRA 21–1, SCC and the soluble EGFR and its ligands EGF, TGF-alpha, HB-EGF in the prediction of outcome in erlotinib treated non-small-cell lung cancer patients. Springerplus 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Márquez, E.B.; Ortueta, D.D.; Royo, S.B.; Martínez-Carpio, P.A. Epidermal growth factor receptor in corneal damage: Update and new insights from recent reports. Cutan. Ocul. Toxicol. 2011, 30, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Jun, R.-M.; Kim, W.-K.; Hann, H.-J.; Chong, Y.H.; Park, H.-Y.; Chung, J.-H. Optimal concentration of human epidermal growth factor (hEGF) for epithelial healing in experimental corneal alkali wounds. Curr. Eye Res. 2001, 22, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kandarakis, A.S.; Page, C.; Kaufman, H.E. The effect of epidermal growth factor on epithelial healing after penetrating keratoplasty in human eyes. Am. J. Ophthalmol. 1984, 98, 411–415. [Google Scholar] [CrossRef]
- Arkhipov, A.; Shan, Y.; Das, R.; Endres, N.F.; Eastwood, M.P.; Wemmer, D.E.; Kuriyan, J.; Shaw, D.E. Architecture and membrane interactions of the EGF receptor. Cell 2013, 152, 557–569. [Google Scholar] [CrossRef]
- Baron, A.T.; Lafky, J.M.; Suman, V.J.; Hillman, D.W.; Buenafe, M.C.; Boardman, C.H.; Podratz, K.C.; Perez, E.A.; Maihle, N.J. A preliminary study of serum concentrations of soluble epidermal growth factor receptor (sErbB1), gonadotropins, and steroid hormones in healthy men and women. Cancer Epidemiol. Prev. Biomark. 2001, 10, 1175–1185. [Google Scholar]
- Al-Khafaji, Q.; Harris, M.; Tombelli, S.; Laschi, S.; Turner, A.; Mascini, M.; Marrazza, G. An electrochemical immunoassay for HER2 detection. Electroanalysis 2012, 24, 735–742. [Google Scholar] [CrossRef]
- Lembach, K.J. Induction of human fibroblast proliferation by epidermal growth factor (EGF): Enhancement by an EGF-binding arginine esterase and by ascorbate. Proc. Natl. Acad. Sci. USA 1976, 73, 183–187. [Google Scholar] [CrossRef]
| Dose Group | |||||
|---|---|---|---|---|---|
| 10 μg/mL (n = 6) | 50 μg/mL (n = 6) | 100 μg/mL (n = 6) | Placebo (n = 6) | Total (n = 24) | |
| SAD study | |||||
| Total | 3 (50) [3] | 1 (16.7) [2] | 1 (16.7) [1] | 2 (33.3) [2] | 7 (29.2) [8] |
| Eye disorders | 3 (50) [3] | 1 (16.7) [2] | 1 (16.7) [1] | 2 (33.3) [2] | 7 (29.2) [8] |
| Corneal erosion | 3 (50) [3] | 1 (16.7) [1] | 2 (33.3) [2] | 6 (25) [6] | |
| Dry eye | 1 (16.7) [1] | 1 (4.2) [1] | |||
| Eye pain | 1 (16.7) [1] | 1 (4.2) [1] | |||
| MAD study | |||||
| Total | 1 (16.7) [1] | 2 (33.3) [3] | 2 (33.3) [2] | 4 (66.7) [5] | 9 (37.5) [11] |
| Eye disorders | 1 (16.7) [1] | 1 (16.7) [1] | 2 (33.3) [2] | 4 (66.7) [5] | 8 (33.3) [9] |
| Corneal erosion | 1 (16.7) [1] | 1 (16.7) [1] | 2 (33.3) [2] | 3 (50) [4] | 7 (29.2) [8] |
| Punctate keratitis | 1 (16.7) [1] | 1 (4.2) [1] | |||
| Gastrointestinal disorders | 1 (16.7) [1] | 1 (4.2) [1] | |||
| Stomatitis | 1 (16.7) [1] | 1 (4.2) [1] | |||
| 1 (16.7) [1] | 1 (4.2) [1] | ||||
| Headache | 1 (16.7) [1] | 1 (4.2) [1] | |||
| Dose Group | ||||
|---|---|---|---|---|
| 10 μg/mL (n = 6) | 50 μg/mL (n = 6) | 100 μg/mL (n = 6) | Placebo (n = 6) | |
| SAD study | ||||
| Cmax (μg/L) | ||||
| day −1 | 0.37 ± 0.08 | 0.37 ± 0.12 | 0.35 ± 0.07 | 0.39 ± 0.08 |
| day 1 | 0.38 ± 0.10 | 0.36 ± 0.14 | 0.35 ± 0.07 | 0.34 ± 0.06 |
| C12h (μg/L) | ||||
| day −1 | 0.28 ± 0.12 | 0.26 ± 0.09 | 0.21 ± 0.06 | 0.23 ± 0.09 |
| day 1 | 0.30 ± 0.12 | 0.32 ± 0.16 | 0.28 ± 0.06 | 0.30 ± 0.07 |
| AUC0-12h (μg∙h/L) | ||||
| day −1 | 3.94 ± 1.05 | 3.21 ± 1.89 | 2.42 ± 0.57 | 3.60 ± 0.73 |
| day 1 | 3.06 ± 1.04 | 3.56 ± 1.34 | 3.03 ± 0.62 | 3.49 ± 0.56 |
| Tmax (h) | ||||
| day −1 | 3 (2–11.9) | 7 (0.5–12) | 2 (0–12) | 2 (0.5–11.95) |
| day 1 | 0.25 (0–1) | 0.38 (0.25–3.97) | 0.48 (0–0.5) | 3.98 (0–11.82) |
| MAD study | ||||
| Cmax (μg/L) | ||||
| day −1 | 0.39 ± 0.14 | 0.33 ± 0.09 | 0.28 ± 0.08 | 0.32 ± 0.07 |
| day 1 | 0.32 ± 0.11 | 0.36 ± 0.10 | 0.28 ± 0.08 | 0.38 ± 0.12 |
| day 14 | 0.33 ± 0.11 | 0.33 ± 0.10 | 0.29 ± 0.06 | 0.37 ± 0.09 |
| Ctrough (μg/L) | ||||
| day −1 | 0.25 ± 0.11 | 0.22 ± 0.09 | 0.16 ± 0.04 | 0.24 ± 0.08 |
| day 1 | 0.29 ± 0.09 | 0.27 ± 0.08 | 0.2 ± 0.05 | 0.29 ± 0.08 |
| day 14 | 0.19 ± 0.09 | 0.25 ± 0.08 | 0.22 ± 0.1 | 0.25 ± 0.13 |
| AUC0-12h (μg∙h/L) | ||||
| day −1 | 3.76 ± 1.34 | 3.24 ± 0.72 | 2.50 ± 0.68 | 3.23 ± 0.92 |
| day 1 | 3.07 ± 1.29 | 3.70 ± 1.05 | 2.59 ± 0.88 | 3.80 ± 1.18 |
| day 14 | 3.13 ± 1.27 | 3.17 ± 0.87 | 2.94 ± 0.60 | 3.45 ± 1.25 |
| Tmax (h) | ||||
| day −1 | 11.68 (0.47–11.7) | 11.72 (0.5–11.93) | 7.93 (1–12) | 2 (0.5–12) |
| day 1 | 1 (0–4) | 4 (0.25–11.78) | 6.23 (0–11.95) | 3.01 (0.5–12) |
| day 14 | 2.01 (2–11.75) | 6.9 (0–11.92) | 0.5 (0–4) | 7.87 (0–11.9) |
| Dose Group | ||||
|---|---|---|---|---|
| 10 μg/mL (n = 6) | 50 μg/mL (n = 6) | 100 μg/mL (n = 6) | Placebo (n = 6) | |
| SAD study | ||||
| Cmax (μg/L) | ||||
| day −1 | 2.45 1 | 3.42 1 | 6.09 1 | NA |
| day 1 | 3.24 ± 0.97 2 | 1004.59 ± 1450.07 3 | 678.37 ± 138.92 2 | 4.11 ± 2.71 2 |
| C12h (μg/L) | ||||
| day −1 | 2.08 ± 0.48 2 | 1.43 1 | 5.36 ± 1.00 2 | 8.28 1 |
| day 1 | 1.07 ± 0.80 4 | 2.14 ± 0.67 4 | 4.61 ± 2.53 3 | 3.25 ± 2.00 3 |
| AUC0-12h (μg∙h/L) | ||||
| day −1 | 7.35 1 | 20.33 1 | 44.42 1 | NA |
| day 1 | 8.89 ± 4.42 2 | 187.89 ± 233.79 3 | 225.66 ± 45.55 2 | 32.34 ± 15.88 2 |
| Tmax (h) | ||||
| day −1 | 0.43 1 | 3.92 1 | 11.68 1 | NA |
| day 1 | 0.18 (0.17–0.18) 2 | 0.18 (0.17–0.2) 3 | 0.2 (0.18–0.22) 2 | 7 (2–12) 2 |
| MAD study | ||||
| Cmax (μg/L) | ||||
| day −1 | 5.05 ± 4.60 2 | NA | 1.94 1 | NA |
| day 1 | 154.91 1 | 112.00 1 | 53.70 ± 53.09 2 | 2.43 ± 0.24 2 |
| day 14 | 193.66 ± 88.04 2 | 156.21 1 | 803.53 1 | 6.21 1 |
| Ctrough (μg/L) | ||||
| day −1 | 7.75 ± 7.03 | NA | 3.60 ± 2.38 | 1.30 ± 0.36 |
| day 1 | 2.18 ± 0.59 | 1.82 ± 0.31 | 3.87 ± 2.75 | 3.32 ± 1.56 |
| day 14 | 1.30 ± 0.36 | 3.32 ± 1.56 | 8.29 ± 9.10 | 8.29 ± 9.10 |
| AUC0-12h (μg∙h/L) | ||||
| day −1 | 27.98 ± 17.56 2 | NA | 16.22 1 | NA |
| day 1 | 70.65 1 | 69.86 1 | 30.91 ± 15.86 2 | 14.82 ± 2.62 2 |
| day 14 | 85.2 ± 45.61 2 | 49.19 1 | 148.35 1 | 42.35 1 |
| Tmax (h) | ||||
| day −1 | 2.96 [2–3.92] 2 | NA | 0 1 | NA |
| day 1 | 0.18 1 | 0.27 1 | 0.19 [0.18–0.2] 2 | 0 [0–0] 2 |
| day 14 | 0.22 [0.22–0.22] 2 | 0.23 1 | 0.18 1 | 4 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.; Yoon, S.; Jang, I.-J.; Yu, K.-S.; Hyon, J.Y.; Hwang, J.; Hwang, I.; Sunwoo, J.; Chung, J.-Y. Safety, Tolerability, and Serum/Tear Pharmacokinetics of Human Recombinant Epidermal Growth Factor Eyedrops in Healthy Subjects. Pharmaceuticals 2022, 15, 1312. https://doi.org/10.3390/ph15111312
Yoo H, Yoon S, Jang I-J, Yu K-S, Hyon JY, Hwang J, Hwang I, Sunwoo J, Chung J-Y. Safety, Tolerability, and Serum/Tear Pharmacokinetics of Human Recombinant Epidermal Growth Factor Eyedrops in Healthy Subjects. Pharmaceuticals. 2022; 15(11):1312. https://doi.org/10.3390/ph15111312
Chicago/Turabian StyleYoo, Hyounggyoon, Seonghae Yoon, In-Jin Jang, Kyung-Sang Yu, Joon Young Hyon, Jungi Hwang, Inyoung Hwang, Jung Sunwoo, and Jae-Yong Chung. 2022. "Safety, Tolerability, and Serum/Tear Pharmacokinetics of Human Recombinant Epidermal Growth Factor Eyedrops in Healthy Subjects" Pharmaceuticals 15, no. 11: 1312. https://doi.org/10.3390/ph15111312
APA StyleYoo, H., Yoon, S., Jang, I.-J., Yu, K.-S., Hyon, J. Y., Hwang, J., Hwang, I., Sunwoo, J., & Chung, J.-Y. (2022). Safety, Tolerability, and Serum/Tear Pharmacokinetics of Human Recombinant Epidermal Growth Factor Eyedrops in Healthy Subjects. Pharmaceuticals, 15(11), 1312. https://doi.org/10.3390/ph15111312






