Chemical Composition and Antimicrobial Potential of Essential Oil of Acritopappus confertus (Gardner) R.M.King & H.Rob. (Asteraceae)
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
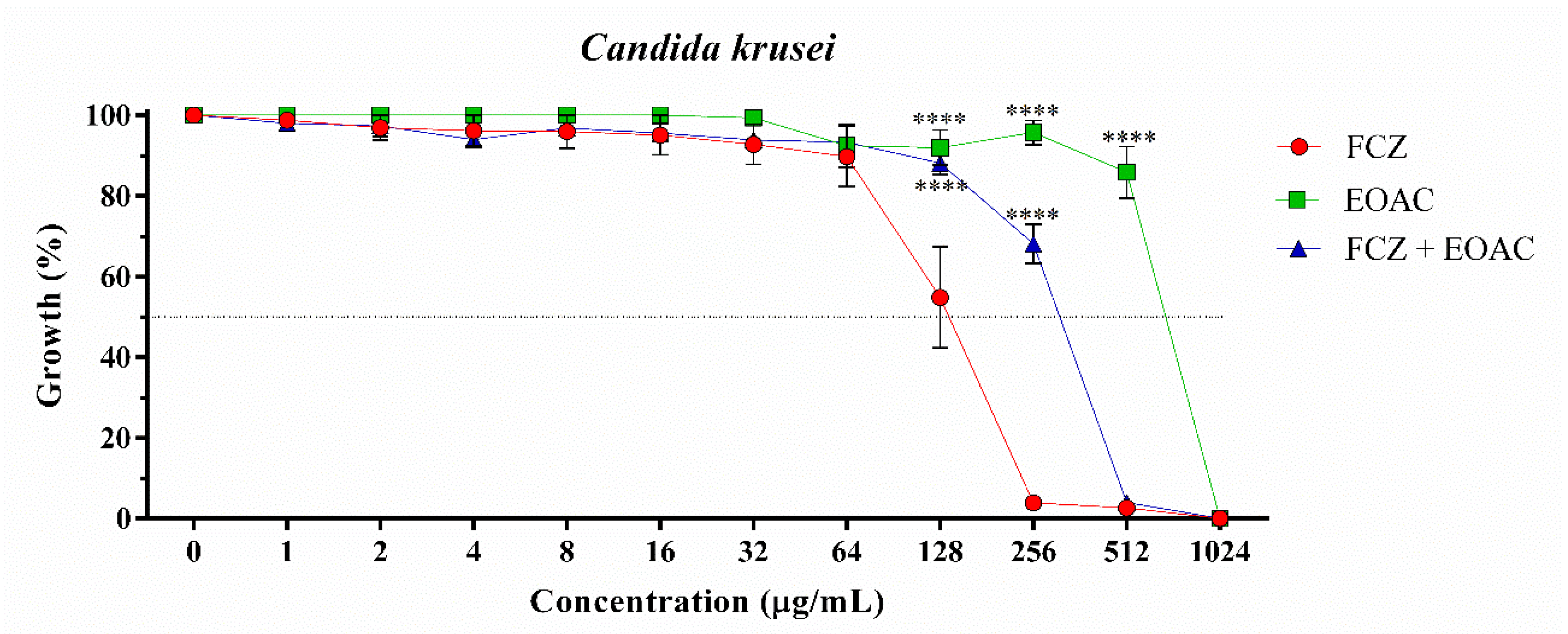
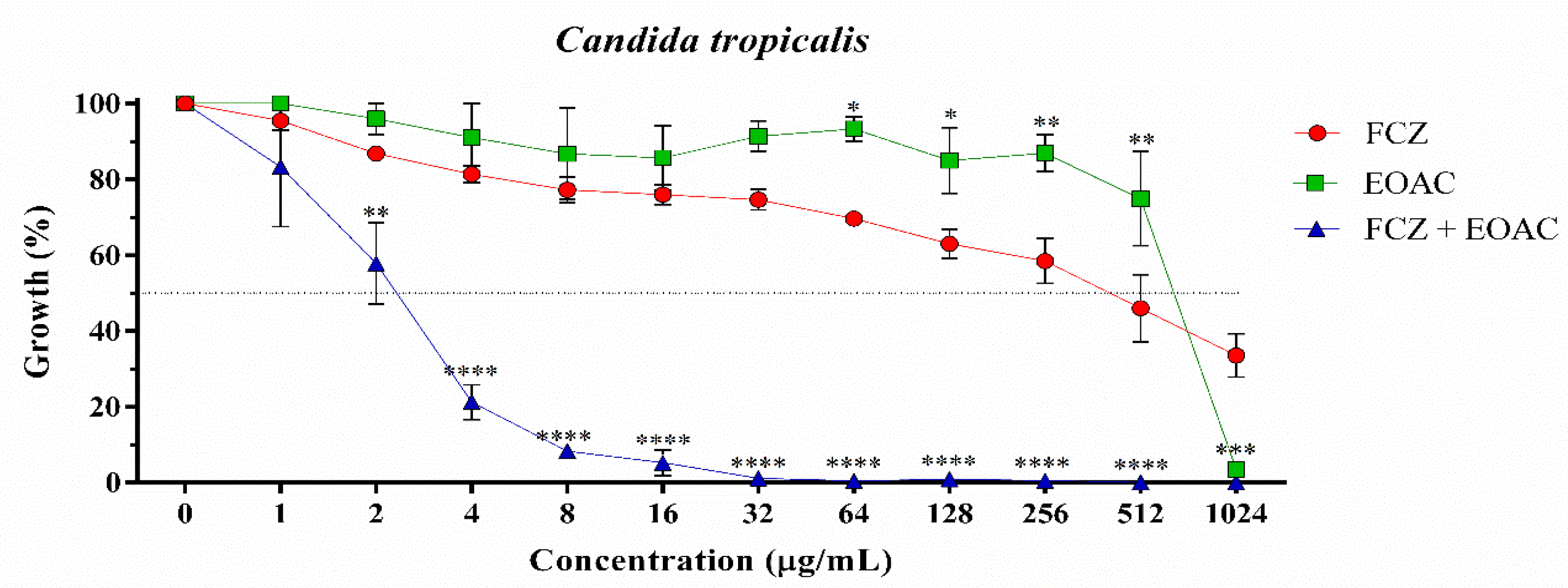
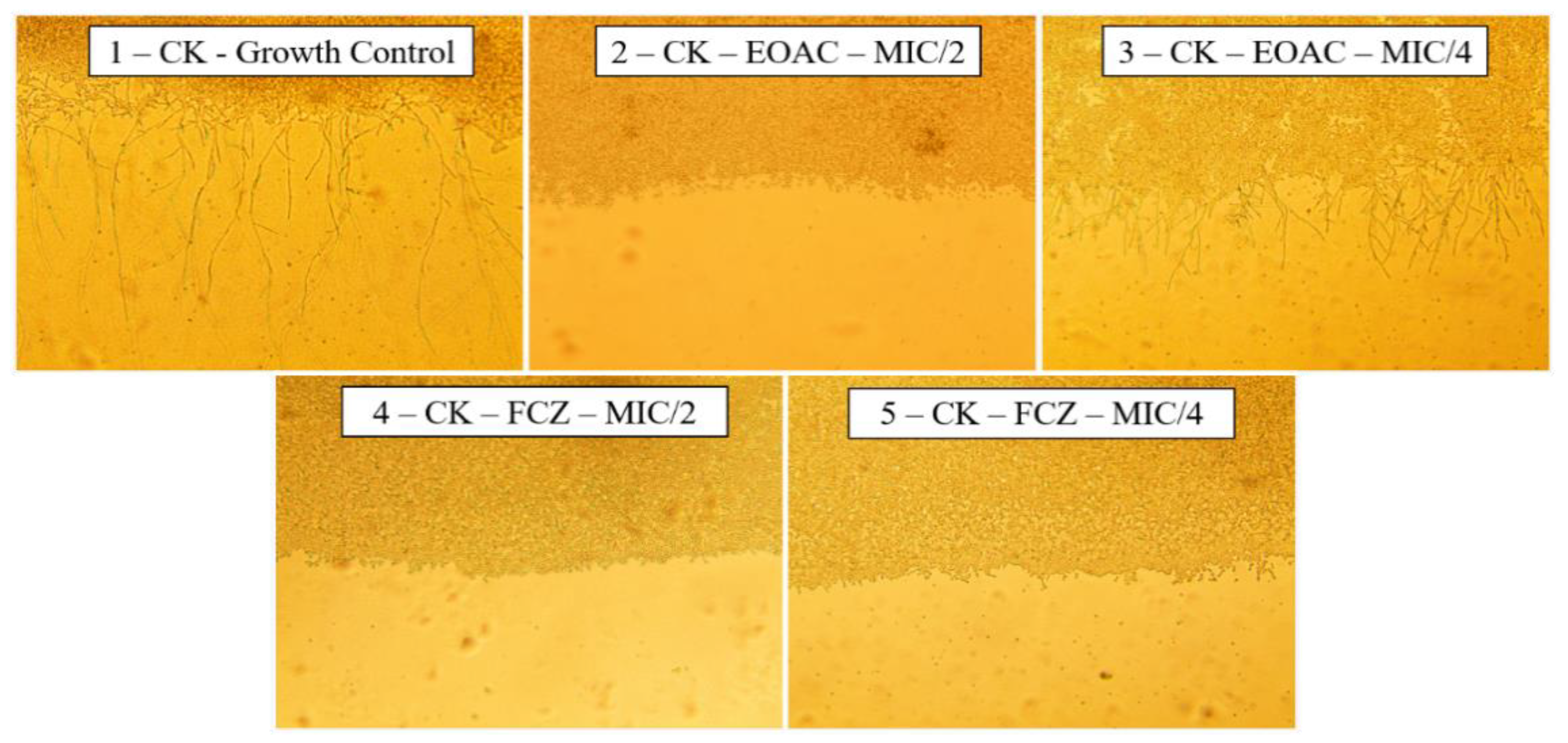
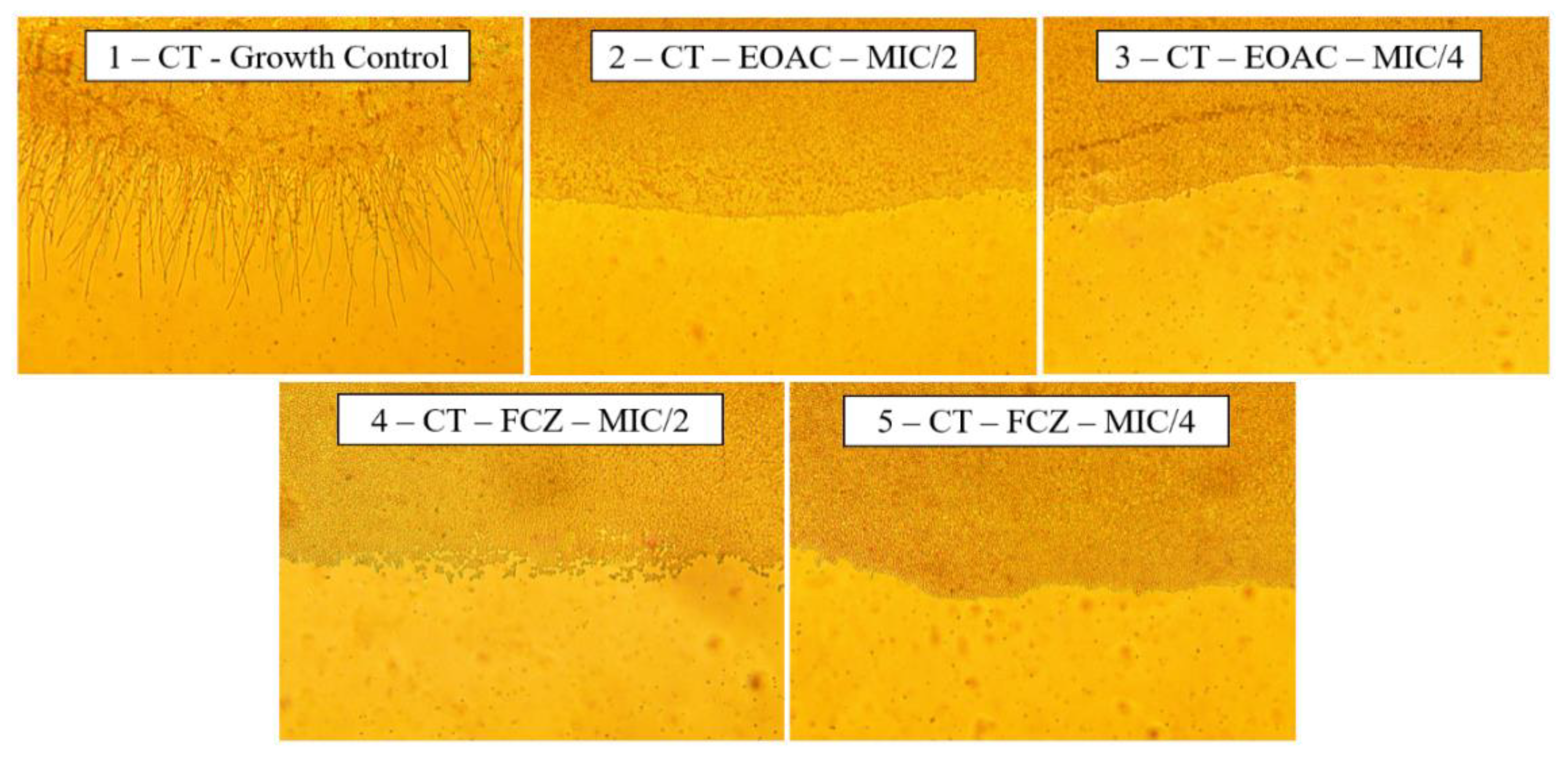
2.2. Antifungal and Fluconazole Modifying Activity
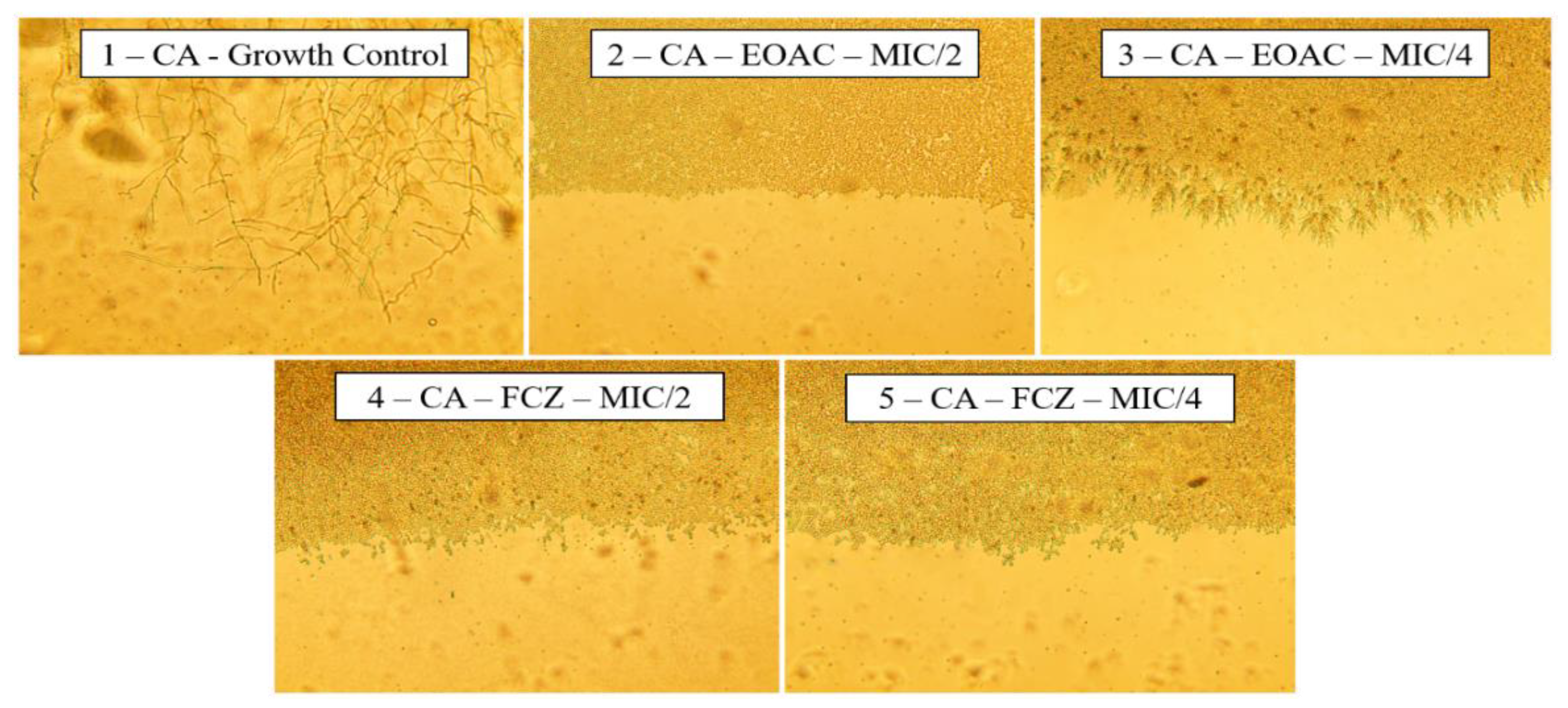
2.3. Anti-Virulence Potential
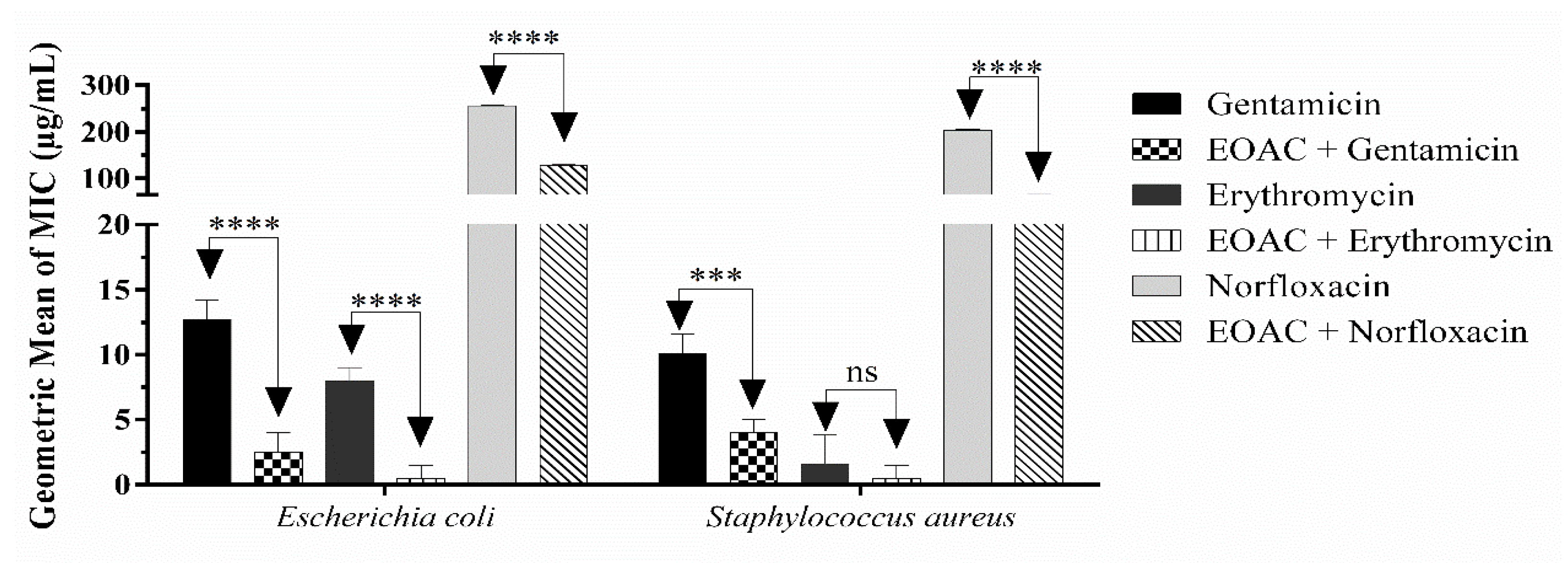
2.4. Antibacterial and Antibiotic Modifying Activity
3. Discussion
4. Materials and Methods
4.1. Collection of Botanical Material
4.2. Essential Oil Extraction
4.3. Analysis of the Essential Oil’s Chemical Composition
Essential Oil Analysis
4.4. Antifungal Activity
4.4.1. Fungal Strains
4.4.2. Fungal Culture
4.4.3. Determination of IC50 and Cell Viability Curve
4.4.4. Assessment of Fluconazole Modifying Activity
4.4.5. Evaluation of Fungal Virulence Inhibition
4.5. Antibacterial Activity
4.5.1. Bacterial Strains
4.5.2. Bacterial Culture
4.5.3. Determination of the Minimum Inhibitory Concentration (MIC)
4.5.4. Antibiotic Modifying Effect Test
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Baky, R.M.A.; Masoud, S.M.; Mohamed, D.S.; Waly, N.G.; Shafik, E.A.; Mohamed, D.A.; Elkady, A.; Elbadr, M.M.; Hetta, H.F. Prevalence and some possible mechanisms of colistin resistance among multidrug-resistant and extensively drug-resisant Pseudomonas aeruginosa infect. Drug Resist. 2020, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 19, 2016–2059. [Google Scholar] [CrossRef]
- Vieira, A.J.H.; Santos, J.I. Mechanisms of Candida albicans resistance to the antifungals amphotericin B, fluconazole and caspofungin. RBAC 2017, 49, 235–239. [Google Scholar] [CrossRef]
- Ellah, N.H.A.; Abdel-Aleem, J.A.; Abdo, M.N.; Abou-Ghadir, O.F.; Zahran, K.M.; Hetta, H.F. Efficacy of ketoconazole gel-flakes in the treatment of vaginal candidiasis: Formulation, in vitro and clinical evaluation. Int. J. Pharm. 2019, 567. [Google Scholar] [CrossRef]
- Khan, A.; Miller, W.R.; Arias, C.A. Mechanisms of antimicrobial resistance among hospital-associated pathogens. Expert Rev. Anti. Infect. Ther. 2018, 16, 269–287. [Google Scholar] [CrossRef]
- Kadri, S.S. Key takeaways from the US CDC’s 2019 antibiotic resistance threats report for frontline providers. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence strategies for the treatment of Staphylococcus aureus infections: A mini review. Microbiol. Front. 2021, 11, 632706. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, F.; Saikia, D. Exploration of Medicinal Plants as Sources of Novel Anticandidal Drugs. Curr. Top. Med. Chem 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 2, 79. [Google Scholar] [CrossRef]
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; Garcia-Carnero, L.C.; Amezcua-Hernández, D.G.; Lozoya-Pérez, N.E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794. [Google Scholar] [CrossRef]
- Kołaczkowska, A.; Kołaczkowski, M. Drug resistance mechanisms and their regulation in non-albicans Candida species. J. Antimicrob. Chemother. 2016, 71, 1438–1450. [Google Scholar] [CrossRef]
- Cid-Chevecich, C.; Müller-Sepúlveda, A.; Jara, J.A.; López-Muñoz, R.; Santander, R.; Budini, M.; Escobar, A.; Quijada, R.; Criollo, A.; Díaz-Dosque, M.; et al. Origanum vulgare L. essential oil inhibits virulence patterns of Candida spp. and potentiates the effects of fluconazole and nystatin in vitro. BMC Complement. Med. Ther. 2022, 1, 39. [Google Scholar] [CrossRef]
- Sánchez-Martínez, C.; Pérez-Martín, J. Dimorphism in fungal pathogens: Candida albicans and Ustilago maydis—Similar inputs, different outputs. Curr. Opin. Microbiol. 2001, 4, 214–221. [Google Scholar] [CrossRef]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A.; Cullen, P.J.; Edgerton, M. Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. NPJ Biofilms Microbiomes 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Sharma, J.; Rosiana, S.; Razzaq, I.; Shapiro, R.S. Linking cellular morphogenesis with antifungal treatment and susceptibility in candida pathogens. J. Fungi 2019, 5, 17. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Alshehrei, F.; Al-Ghamdi, S.B.; Bamaga, M.A.; Al-Thubiani, A.S.; Alam, M.Z. Virulence and biofilms as promising targets in developing antipathogenic drugs against candidiasis. Future Sci. OA. 2020, 6, FSO440. [Google Scholar] [CrossRef]
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnopharmacol. 2020, 262, 113135. [Google Scholar] [CrossRef]
- Braga, A.L.; Cruz, R.P.; Carneiro, J.N.P.; Santos, A.T.L.; Sales, D.L.; Bezerra, C.F.; Fonseca, V.J.A.; Rocha, J.E.; Freitas, T.S.; Campina, F.F.; et al. Piper regnellii (Miq.) C. DC.: Chemical composition, antimicrobial effects, and modulation of antimicrobial resistance. S. Afr. J. Bot. 2021, 142, 495–501. [Google Scholar] [CrossRef]
- Linkoln, A.; Leal, A.L.A.B.; Bezerra, C.F.; Rocha, J.E.; Santos, A.T.L.; Cruz, R.P.; Carneiro, J.N.P.; Sales, D.L.; Freitas, T.S.; Tintino, S.R.; et al. Piper cernuum Vell.: Chemical profile and antimicrobial potential evaluation. Ind. Crops Prod. 2019, 140, 111577. [Google Scholar] [CrossRef]
- Córdoba, S.; Vivot, W.; Szusz, W.; Albo, G. Antifungal activity of essential oils against Candida species isolated from clinical samples. Mycopathologia 2019, 184, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Baj, T.; Biernasiuk, A.; Wróbel, R.; Malm, A. Chemical composition and in vitro activity of Origanum vulgare L. Satureja hortensis L. Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata. Open Chem. J. 2020, 18, 108–118. [Google Scholar] [CrossRef]
- Salkar, K.; Suthar, A.; Chauhan, V.; Naik, V. Evaluation of selected medicinal plants for anticandida potential. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1229–1235. [Google Scholar]
- Ferronatto, R.; Marchesan, E.D.; Pezenti, E.; Bednarski, F.; Onofre, S.B. Atividade antimicrobiana de óleos essenciais produzidos por Baccharis dracunculifolia D.C. e Baccharis uncinella D.C. (Asteraceae). Rev. Bras. Farmacogn. 2007, 17, 224–230. [Google Scholar] [CrossRef]
- Funch, L.S.; Hayley, R.; Funch, R.; Giuletti, A.M.; Melo, E. Chapadada Diamantina a Useful Plants; RiMa: São Carlos, Brazil, 2004; p. 206. [Google Scholar]
- Lima, M.A.S.; Barros, M.C.P.; Pinheiro, S.M.; Nascimento, R.F.; Abreu Matos, F.J.; Silveira, E.R. Volatile compositions of two asteraceae from the north-east of Brazil: Ageratum conyzoides and Acritopappus confertus (Eupatorieae). Flavour Fragr. J. 2005, 20, 559–561. [Google Scholar] [CrossRef]
- Sousa, J.D.; Leite, T.R.; Linhares, K.V.; Sousa, J.D.; Bezerra, J.W.A.; Santos, M.A.F.; Torquato, I.H.S.; Boligon, A.A.; Bezerra, J.S.; Campos, N.B.; et al. Chromatographic profile and allelopathic potential of the essential oil of Acritopappus confertus (Gardner) RM King & H. Rob. (Asteraceae). Res. Soc. Dev. 2020, 9, e1991210450. [Google Scholar] [CrossRef]
- Hanbali, F.E.; Mellouki, F.; Akssira, M.; El Hassani, B.; Blázquez, M.A.; Boira, H. Composition and antibacterial activity of essential oils of Cladanthus arabicus Cass. (Asteraceae). J. Essent. Oil-Bear. Plants 2005, 8, 213–217. [Google Scholar] [CrossRef]
- Mayekiso, B.; Magwa, M.L.; Coopoosamy, R.M. Variation in the essential oil constituents of Pteronia incana (Asteraceae). Afr. J. Biotechnol. 2006, 5, 1220–1226. [Google Scholar]
- Hulley, I.M.; Viljoen, A.M.; Tilney, P.M.; Van Vuuren, S.F.; Kamatou, G.P.P.; Van Wyk, B.E. The ethnobotany, leaf anatomy, essential oil variation and biological activity of Pteronia incana (Asteraceae). S. Afr. J. Bot. 2010, 76, 668–675. [Google Scholar] [CrossRef][Green Version]
- Hulley, I.M.; Viljoen, A.M.; Tilney, P.M.; Vuuren, S.F.V.; Kamatou, G.P.P.; Van Wyk, B.E. Ethnobotany, leaf anatomy, essential oil composition and antibacterial activity of Pteronia onobromoides (Asteraceae). S. Afr. J. Bot. 2010, 76, 43–48. [Google Scholar] [CrossRef]
- Haouas, D.; Cioni, P.L.; Flamini, G.; Halima-Kamel, M.B.; Hamouda, M.H.B. Variation of chemical composition in flowers and leaves essential oils among natural population of Tunisian Glebionis coronaria (L.) Tzvelev (Asteraceae). Chem. Biodivers. 2016, 13, 1251–1261. [Google Scholar] [CrossRef]
- Moreira, R.R.D.; Martins, G.Z.; Varandas, R.; Cogo, J.; Perego, C.H.; Roncoli, G.; Sousa, M.C.; Nakamura, C.V.; Salgueiro, L.; Cavaleiro, C. Composition and leishmanicidal activity of the essential oil of Vernonia polyanthes Less (Asteraceae). Nat. Prod. Res. 2017, 31, 2905–2908. [Google Scholar] [CrossRef]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Gilardoni, G. Chemical, enantioselective, and sensory analysis of a cholinesterase inhibitor essential oil from Coreopsis triloba SF Blake (Asteraceae). Plants 2019, 8, 448. [Google Scholar] [CrossRef]
- Rojas, J.; Ntoutoume, G.M.A.N.; Martin, P.; Morillo, M. Antibacterial Activity and Reversal of Multidrug Resistance of Tumor Cells by Essential Oils from Fresh Leaves, Flowers, and Stems of Montanoa quadrangularis Schultz Bipontinus (Asteraceae) Collected in Mérida—Venezuela. Biomolecules 2021, 11, 605. [Google Scholar] [CrossRef]
- Tavares, A.C.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Lopes, M.C.; Canhoto, J.; Salgueiro, L.R. Essential oils from Distichoselinum tenuifolium: Chemical composition, cytotoxicity, antifungal and anti-inflammatory properties. J. Ethnopharmacol. 2010, 130, 593–598. [Google Scholar] [CrossRef]
- Taweechaisupapong, S.; Aieamsaard, J.; Chitropas, P.; Khunkitti, W. Inhibitory effect of lemongrass oil and its major constituents on Candida biofilm and germ tube formation. S. Afr. J. Bot. 2012, 81, 95–102. [Google Scholar] [CrossRef]
- Donati, M.; Mondin, A.; Chen, Z.; Miranda, F.M.; Nascimento, B.B., Jr.; Schirato, G.; Pastore, P.; Froldi, G. Radical scavenging and antimicrobial activities of Croton zehntneri, Pterodon emarginatus and Schinopsis brasiliensis essential oils and their major constituents: Estragole, trans-anethole, β-caryophyllene and myrcene. Nat. Prod. Res. 2014, 29, 939–946. [Google Scholar] [CrossRef]
- Jain, N.; Sharma, M. Inhibitory effect of some selected essential oil terpenes on fungi causing superficial infection in human beings. J. Essent. Oil Bear. Plants 2020, 23, 862–869. [Google Scholar] [CrossRef]
- Silva, A.C.R.D.; Lopes, P.M.; Azevedo, M.M.B.D.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.B.; Karuppayil, S.M. Small molecules inhibit growth, viability and ergosterol biosynthesis in Candida albicans. Springerplus 2013, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.M.; Rosalen, P.L.; Freires, I.A.; Scotti, L.; Scotti, M.T.; Aquino, S.G.; Castro, R.D. Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-β-pinene enantiomers against Candida spp. Curr. Top. Med. Chem. 2018, 18, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K.; et al. Limonene inhibits Candida albicans growth by inducing apoptosis. Med. Mycol. J. 2018, 56, 565–578. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Rossi, D.C.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.; Nunes, L.R.; Arruda, D.C.; Taborda, C.P. In Vitro and In Vivo Inhibitory Activity of Limonene against Different Isolates of Candida spp. J. Fungi 2020, 6, 183. [Google Scholar] [CrossRef]
- Prabajati, R.; Hernawan, I.; Hendarti, H.T. Effects of Citrus limon essential oil (Citrus limon L.) on cytomorphometric changes of Candida albicans. DJMKG 2017, 50, 43–48. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Rezende, C.M.; Fraga, S.R.; Svidzinski, T.I.E.; Cortez, D.A.G. Antifungal activity of the essential oil from Calendula officinalis L. (Asteraceae) growing in Brazil. Braz. J. Microbiol. 2008, 39, 61–63. [Google Scholar] [CrossRef]
- Zapata, B.; Duran, C.; Stashenko, E.; Betancur-Galvis, L.; Mesa-Arango, A.C. Antifungal activity, cytotoxicity and composition of essential oils from the Asteraceae plant family. Rev. Iberoam. Micol. 2010, 27, 101–103. [Google Scholar] [CrossRef]
- Silvério, M.S.; Del-Vechio-Vieira, G.; Pinto, M.A.; Alves, M.S.; Sousa, O.V. Chemical composition and biological activities of essential oils of Eremanthus erythropappus (DC) McLeisch (Asteraceae). Molecules 2013, 18, 9785–9796. [Google Scholar] [CrossRef]
- Rodrigues, F.F.G.; Colares, A.V.; Nonato, C.D.F.A.; Galvão-Rodrigues, F.F.; Mota, M.L.; Braga, M.F.B.M.; Costa, J.G.M. In vitro antimicrobial activity of the essential oil from Vanillosmopsis arborea Barker (Asteraceae) and its major constituent, α-bisabolol. Micróbio. Pathog. 2018, 125, 144–149. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Dimitrijević, M.; Genčić, M.; Pejčić, M.; Radulović, N. Anticandidal activity of Inula helenium root essential oil: Synergistic potential, anti-virulence efficacy and mechanism of action. Ind. Crops Prod. 2020, 149, 112373. [Google Scholar] [CrossRef]
- Silva, T.G.; Silva, J.C.P.; Carneiro, J.N.P.; Amaral, W.; Deschamps, C.; Araújo, J.P.; Costa, J.G.M.; Almeida, W.; Silva, L.E.; Coutinho, H.D.M.; et al. Phytochemical characterization and inhibition of Candida sp. by the essential oil of Baccharis trimera (Less.) DC. Arch. Microbiol. 2021, 203, 3077–3087. [Google Scholar] [CrossRef]
- Keereedach, P.; Hrimpeng, K.; Boonbumrung, K. Antifungal activity of Thai cajuput oil and its effect on efflux-pump gene expression in fluconazole-resistant Candida albicans clinical isolates. Int. J. Microbiol. 2020, 2020, 5989206. [Google Scholar] [CrossRef]
- Fernandes, E.; Sousa, M.J.; Dias, A. Evaluation of antimicrobial activity of essential oils from the Brazilian plants Acritopappus confertus, Cuphea carthagenensis and Poiretia bahiana. Planta Med. 2014, 80, P2O65. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Mohanty, P. Bacterial efflux pumps involved in multidrug resistance and their inhibitors: Rejuvinating the antimicrobial chemotherapy. Recente Pat. Antiinfect. Drug Discov. 2012, 7, 73–89. [Google Scholar] [CrossRef]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hamashima, H.; Shimada, J. The antibacterial effects of myrcene on Staphylococcus aureus and its role in the essential oil of the tea tree (Melaleuca alternifolia). Nat. Med. 2004, 58, 10–14. [Google Scholar]
- Gallucci, N.; Casero, C.; Oliva, M.; Zygadlo, J.; Demo, M. Interaction between terpenes and penicillin on bacterial strains resistant to beta-lactam antibiotics. Mol. Med. Chem. 2006, 10, 30–32. [Google Scholar]
- Sieniawska, E.; Swatko-Ossor, M.; Sawicki, R.; Skalicka-Woźniak, K.; Ginalska, G. Natural terpenes influence the activity of antibiotics against isolated Mycobacterium tuberculosis. Clínica Med. Princ. 2017, 26, 108–112. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.D.O.; Souza, E.L.D.; Diniz, M.D.F.F.M.; Trajano, V.N.; Medeiros, I.A.D. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev. Bras. Cienc. Farm. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Araújo, A.C.J.; Freitas, P.R.; Barbosa, C.R.S.; Muniz, D.F.; Almeida, R.S.; Menezes, I.R.A.; Ribeiro-Filho, J.; Tintino, S.R.; Coutinho, H.D.M. In vitro and in silico inhibition of Staphylococcus aureus efflux pump NorA by α-pinene and limonene. Curr. Microbiol. 2021, 78, 3388–3393. [Google Scholar] [CrossRef]
- Freitas, P.R.; Araújo, A.C.J.; Barbosa, C.R.; Muniz, D.F.; Tintino, S.R.; Ribeiro-Filho, J.; Júnior, J.P.S.; Filho, J.M.B.; Sousa, G.R.; Coutinho, H.D. Inhibition of Efflux Pumps by Monoterpene (α-Pinene) and impact on Staphylococcus aureus Resistance to Tetracycline and Erythromycin. Curr. Drug Metab. 2021, 22, 123–126. [Google Scholar] [CrossRef]
- Azevedo, I.L.; Almeida, A.C.; Martins, E.R.; Nogueira, W.C.L.; Faria Filho, D.E.; Oliveira, S.P.; Prates, J.P.B.; Souza, C.N. Eficácia in vitro do óleo essencial de capim-limão (cymbopogon flexuosus steud. wats.) frente a bactérias entéricas de origem avícola. Acta Vet. Bras. 2016, 10, 25–31. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils--a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Mollica, A.; Macedonio, G.; Stefanucci, A.; Costante, R.; Carradori, S.; Cataldi, V.; Giulio, M.D.; Cellini, L.; Silvestri, R.; Giordano, C.; et al. Arginine-and lysine-rich peptides: Synthesis, characterization and antimicrobial activity. Lett. Drug Des. Discov. 2018, 15, 220–226. [Google Scholar] [CrossRef]
- Matos, F.J.A. Introdução à Fitoquímica Experimental; Edições UFC: Fortaleza, Brasil, 1997. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Coutinho, H.D.M.; Costa, J.G.M.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira- Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Sidrim, J.J.C.; Rocha, M.F.G. Micologia Médica à Luz de Autores Contemporâneos; Guanabara Koogan: Rio de Janeiro, Brazil, 2010; p. 388. [Google Scholar]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef]

| Compounds | RI a | RI b | Essential Oil |
|---|---|---|---|
| α-Pinene | 937 | 935 | 3.24 |
| Sabinene | 978 | 978 | 0.19 |
| β-Pinene | 983 | 981 | 18.2 |
| Myrcene | 987 | 989 | 54.71 |
| Limonene | 1029 | 1031 | 6.52 |
| β-Cubebene | 1390 | 1391 | 4.81 |
| β-Caryophyllene | 1418 | 1418 | 1.93 |
| α-Humulene | 1454 | 1459 | 0.80 |
| α-Eudesmol | 1631 | 1630 | 0.21 |
| β-Eudesmol | 1652 | 1652 | 5.72 |
| Total Identified (%) | 96.33 |
| Products Tested | Candida albicans | Candida krusei | Candida tropicalis |
|---|---|---|---|
| FCZ | 12.33 μg/mL | 131.6 μg/mL | 362.9 μg/mL |
| EOAC | 22.19 μg/mL | 592.6 μg/mL | 615.4 μg/mL |
| FCZ + EOAC | 6.53 μg/mL | 295.8 μg/mL | 2.25 μg/mL |
| Strain | EOAC |
|---|---|
| Staphylococcus aureus ATCC 25923 | 256 μg/mL |
| Staphylococcus aureus 10 | ≥512 μg/mL |
| Escherichia coli ATCC 25922 | ≥512 μg/mL |
| Escherichia coli 06 | ≥512 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cruz, R.P.; Castro, J.W.G.; Leite, D.O.D.; de Carvalho, N.K.G.; Almeida-Bezerra, J.W.; Pereira, R.L.S.; Rodrigues, F.F.G.; Bezerra, J.J.L.; Costa, A.R.; Mori, E.; et al. Chemical Composition and Antimicrobial Potential of Essential Oil of Acritopappus confertus (Gardner) R.M.King & H.Rob. (Asteraceae). Pharmaceuticals 2022, 15, 1275. https://doi.org/10.3390/ph15101275
da Cruz RP, Castro JWG, Leite DOD, de Carvalho NKG, Almeida-Bezerra JW, Pereira RLS, Rodrigues FFG, Bezerra JJL, Costa AR, Mori E, et al. Chemical Composition and Antimicrobial Potential of Essential Oil of Acritopappus confertus (Gardner) R.M.King & H.Rob. (Asteraceae). Pharmaceuticals. 2022; 15(10):1275. https://doi.org/10.3390/ph15101275
Chicago/Turabian Styleda Cruz, Rafael Pereira, José Walber Gonçalves Castro, Débora Odília Duarte Leite, Natália Kelly Gomes de Carvalho, José Weverton Almeida-Bezerra, Raimundo Luiz Silva Pereira, Fázia Fernandes Galvão Rodrigues, José Jailson Lima Bezerra, Adrielle Rodrigues Costa, Edna Mori, and et al. 2022. "Chemical Composition and Antimicrobial Potential of Essential Oil of Acritopappus confertus (Gardner) R.M.King & H.Rob. (Asteraceae)" Pharmaceuticals 15, no. 10: 1275. https://doi.org/10.3390/ph15101275
APA Styleda Cruz, R. P., Castro, J. W. G., Leite, D. O. D., de Carvalho, N. K. G., Almeida-Bezerra, J. W., Pereira, R. L. S., Rodrigues, F. F. G., Bezerra, J. J. L., Costa, A. R., Mori, E., de Farias, P. A. M., Coutinho, H. D. M., Morais-Braga, M. F. B., Iriti, M., da Costa, J. G. M., & Rodrigues, F. F. G. (2022). Chemical Composition and Antimicrobial Potential of Essential Oil of Acritopappus confertus (Gardner) R.M.King & H.Rob. (Asteraceae). Pharmaceuticals, 15(10), 1275. https://doi.org/10.3390/ph15101275