Analysis of the Anti-Inflammatory and Anti-Osteoarthritic Potential of Flonat Fast®, a Combination of Harpagophytum Procumbens DC. ex Meisn., Boswellia Serrata Roxb., Curcuma longa L., Bromelain and Escin (Aesculus hippocastanum), Evaluated in In Vitro Models of Inflammation Relevant to Osteoarthritis
Abstract
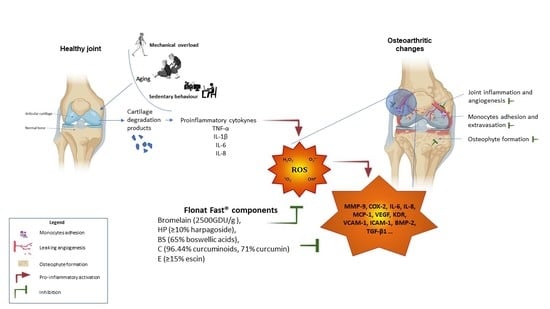
1. Introduction
2. Results
2.1. Total Polyphenol Content and Radical Scavenging Effects
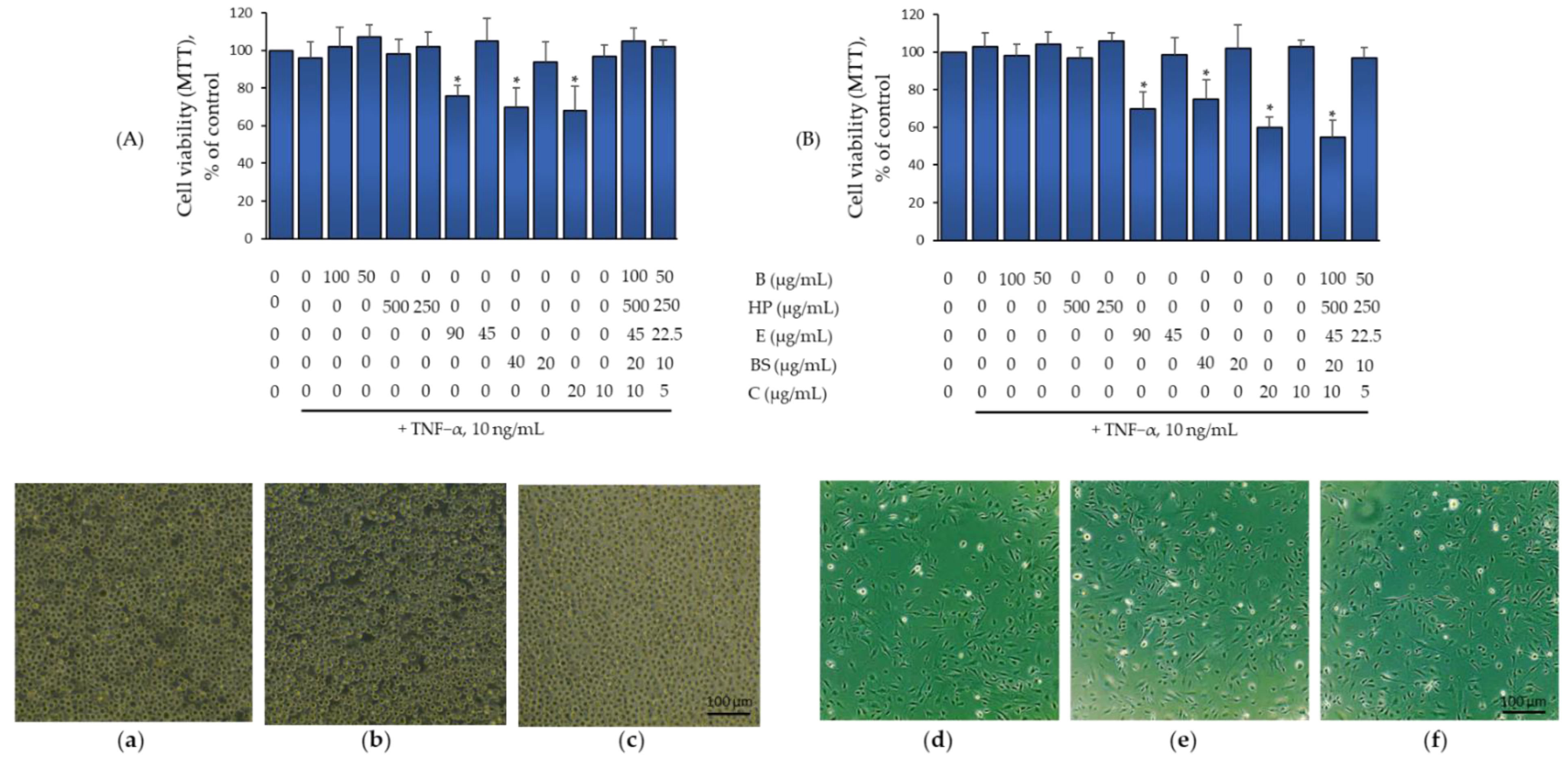
2.2. Effect of B, HP, BS, C, and E Alone and in Combination on Monocytes and Endothelial Cell Viability
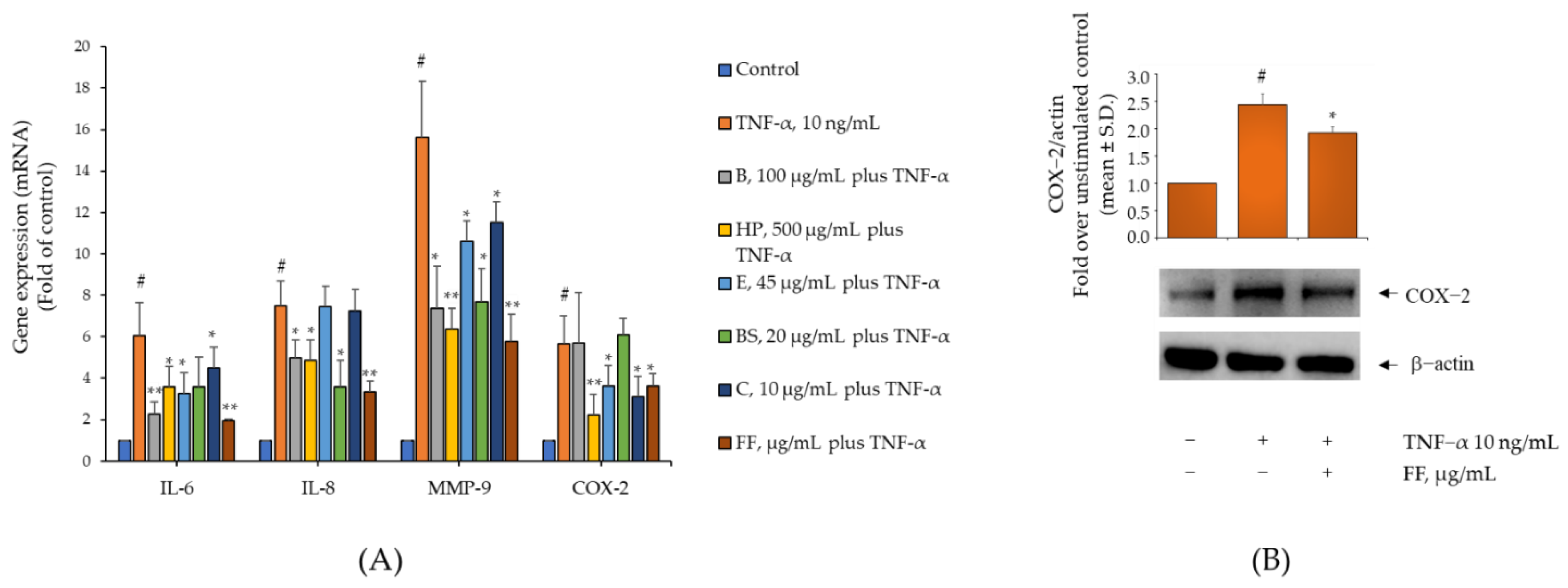
2.3. Comparison of the Activity of B, HP, BS, C, and E on Gene and Protein Expression of IL-6, IL-8, MMP-9, and COX-2 in TNF-α-Stimulated Human Monocytes
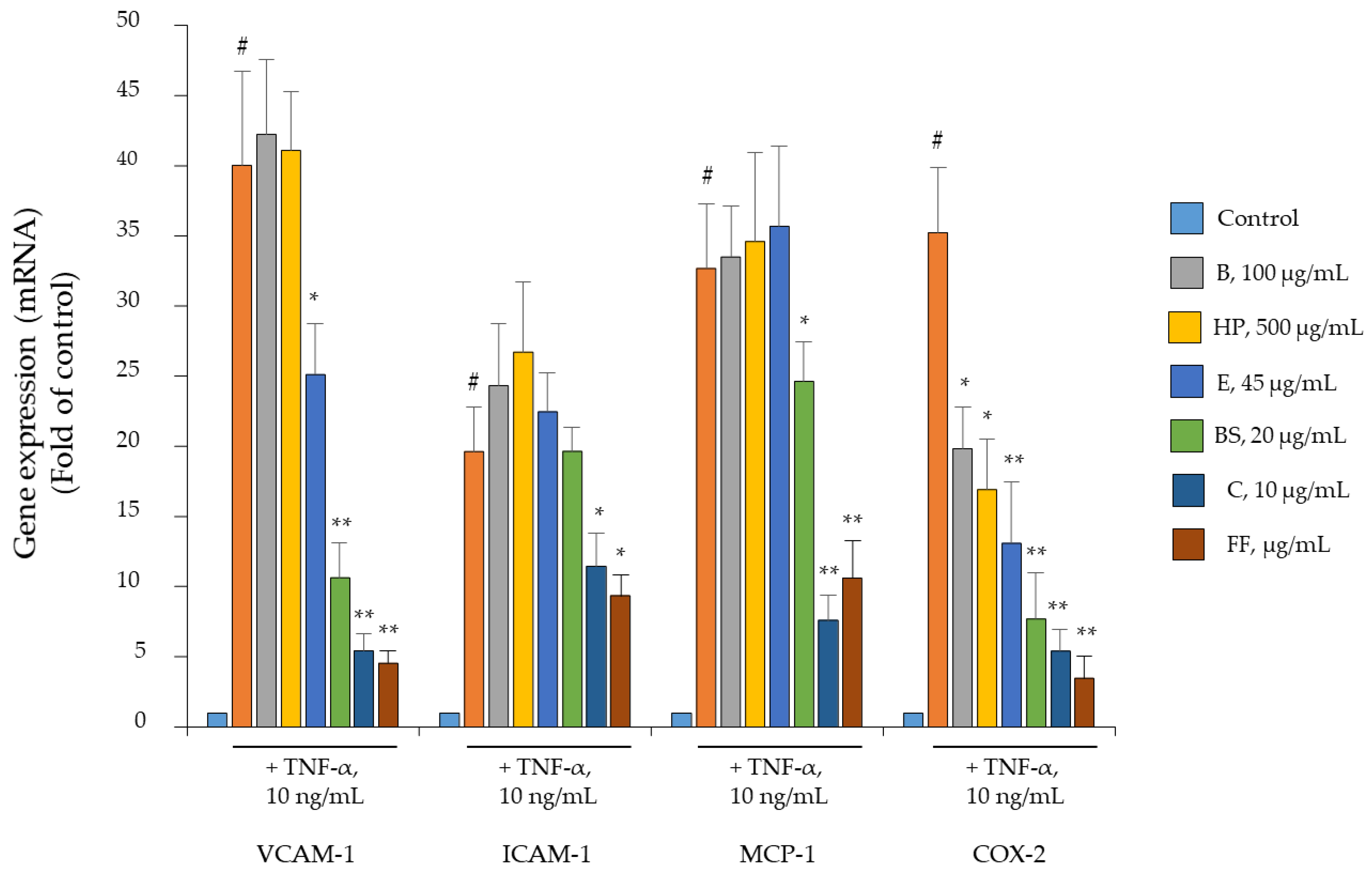
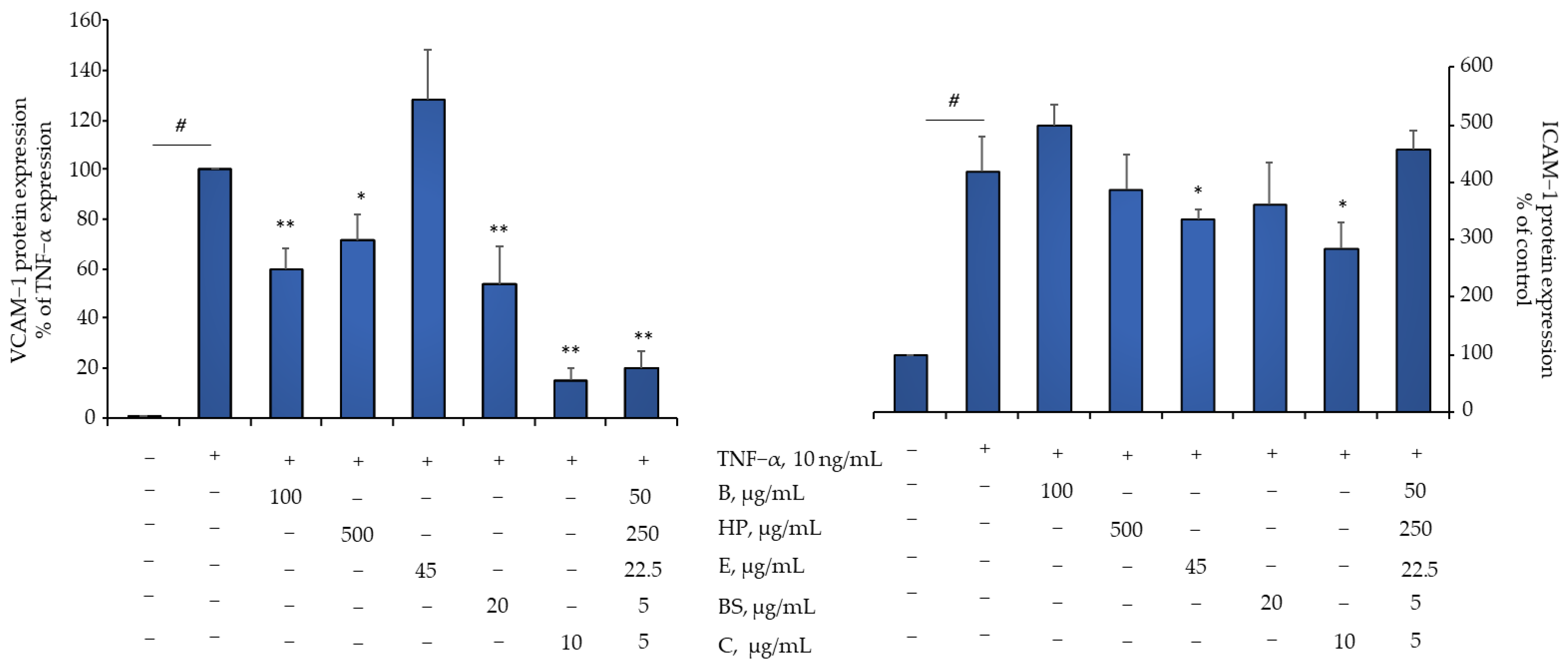
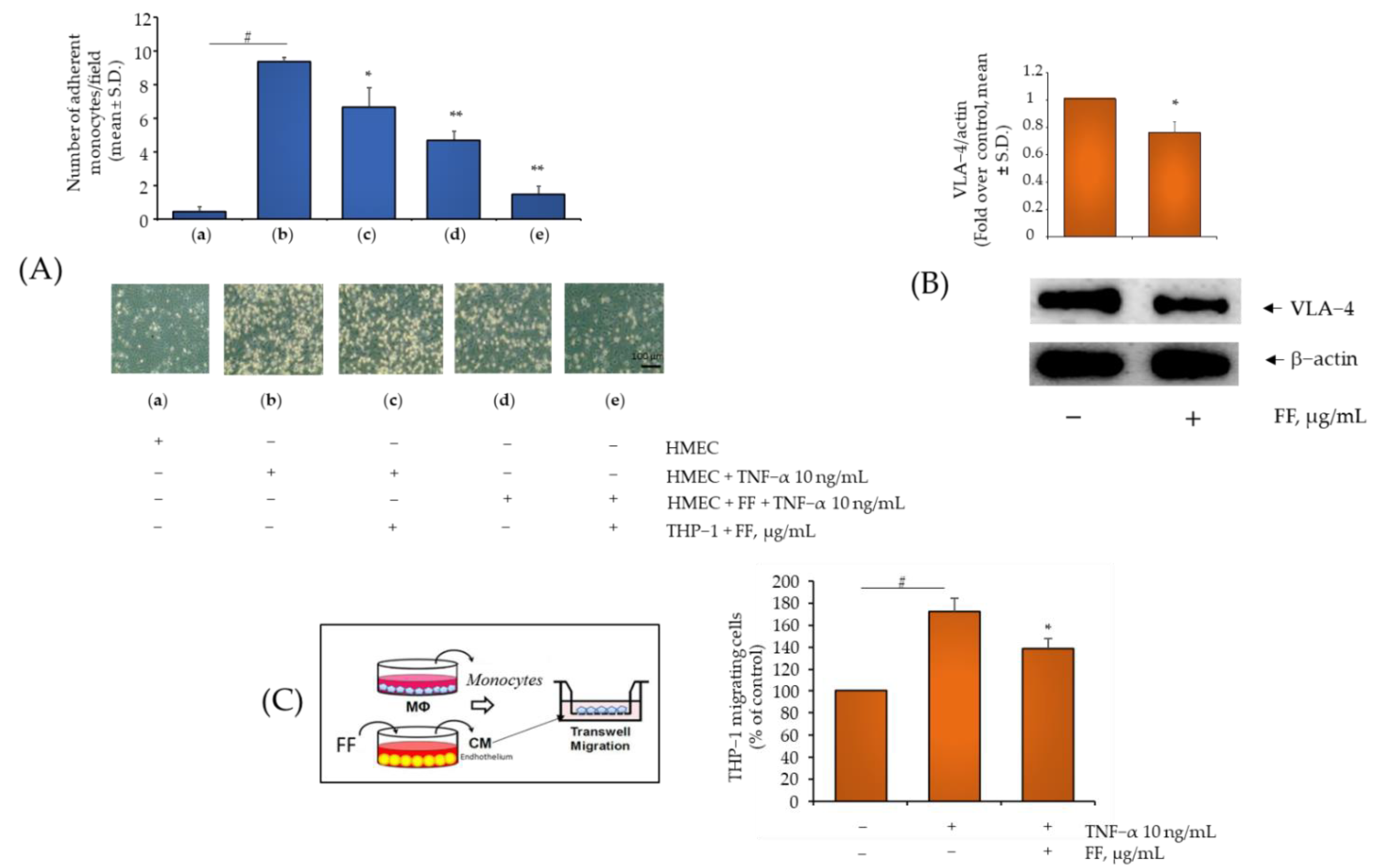
2.4. Comparison of B, HP, BS, C, and E Activity in Relation to Endothelial Expression of Genes and Proteins Involved in Inflammation, Leukocyte Adhesion, and Migration
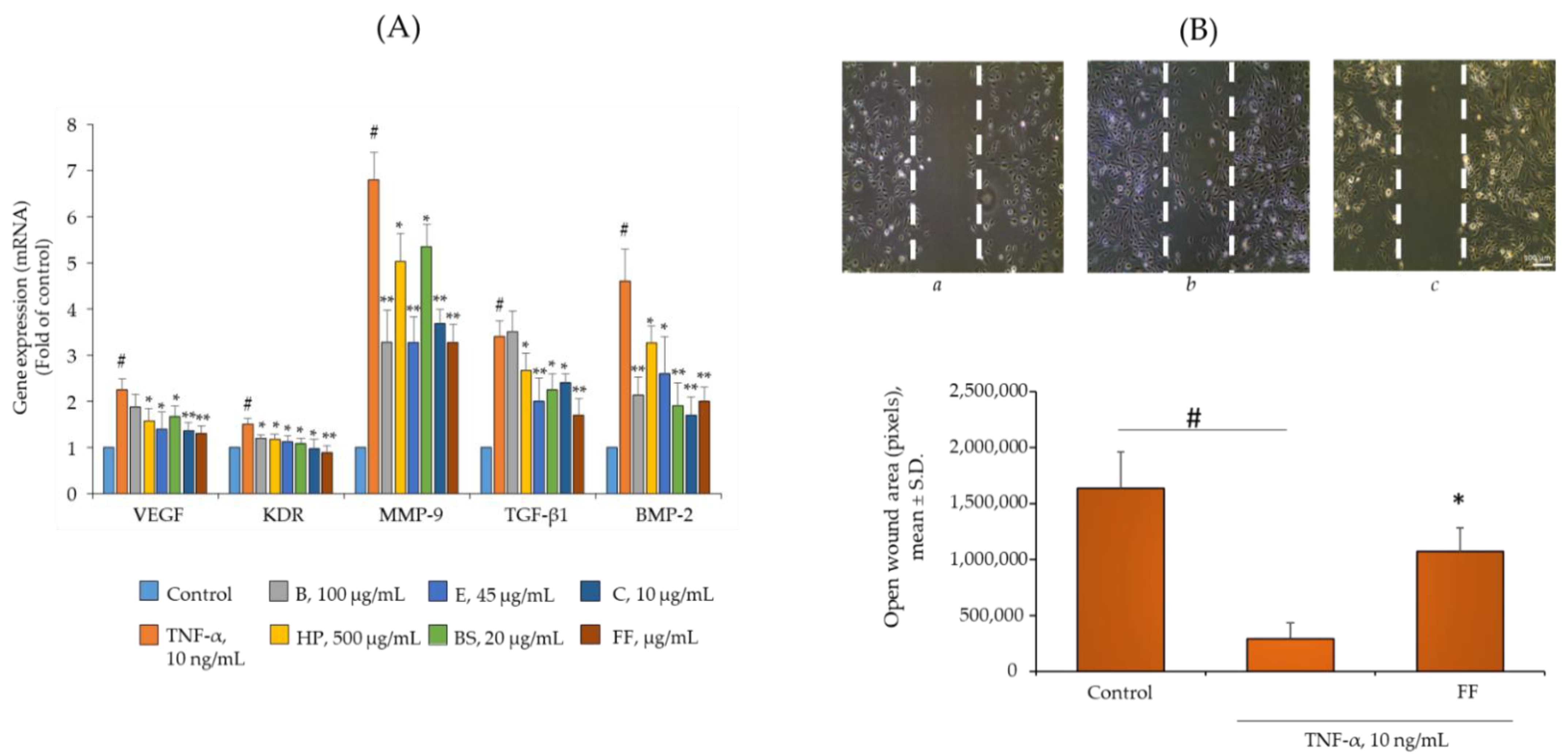
2.5. Comparison of B, HP, BS, C, and E Activity on the Endothelial Expression of Gene and Protein Involved in Angiogenesis and Osteophyte Formation
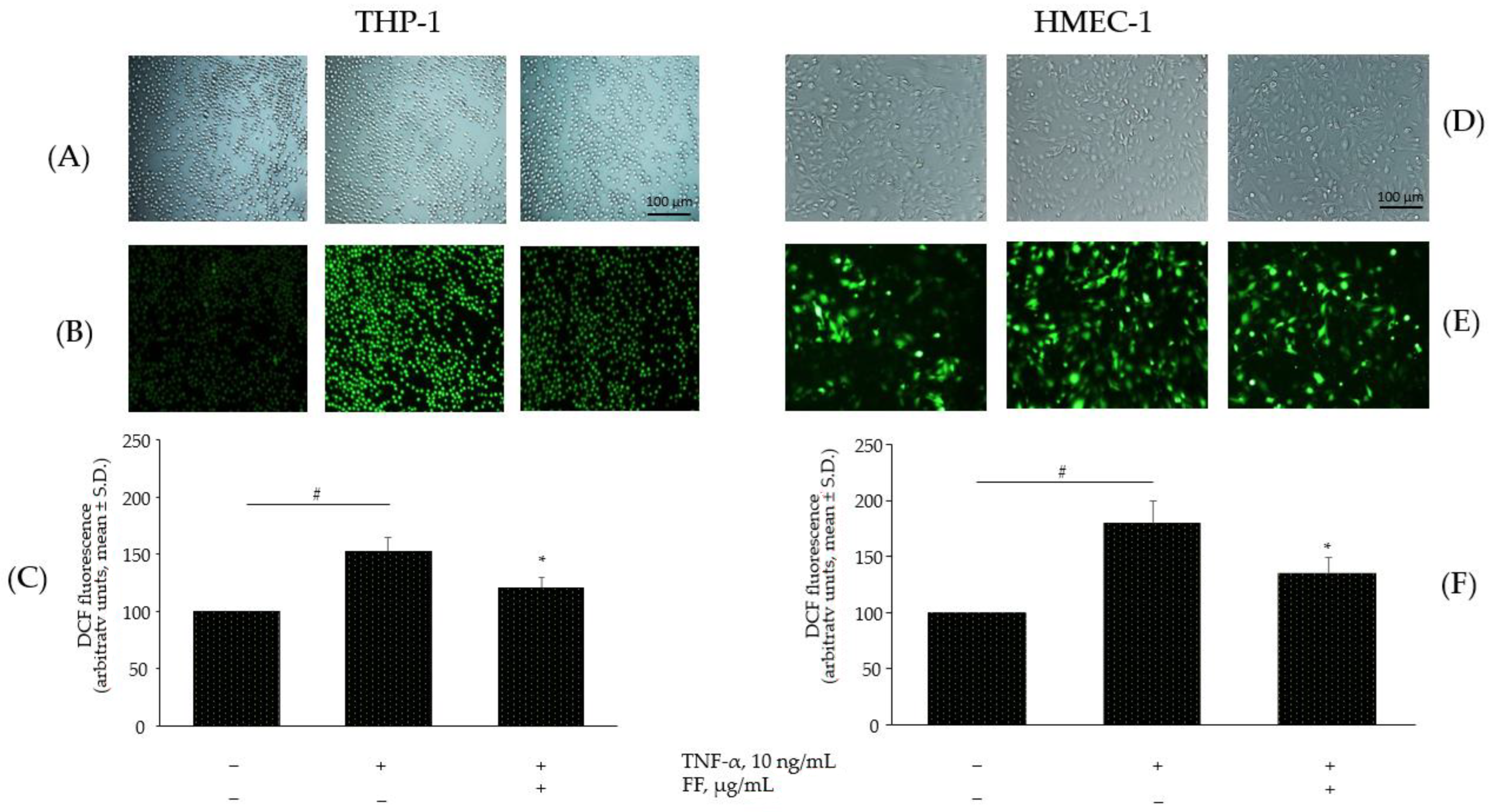
2.6. Effect of the Combination of Flonat Fast® Components on Monocytes and Endothelial Dysregulation in ROS Production
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Determination of the Total Phenolic Content
4.3. Antioxidant Activity Assays
4.4. Cell Culture and Treatment
4.5. Cell Viability
4.6. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
4.7. Cell Lysis and Immunoblotting
4.8. Cell Surface Immunoassay
4.9. Leukocyte-Endothelial Adhesion Assay
4.10. In Vitro THP-1 Chemotaxis Assay
4.11. Cell Migration Assay
4.12. Measurement of Intracellular ROS Production
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Griffin, T.M.; Scanzello, C.R. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 57–63. [Google Scholar]
- Hügle, T.; Geurts, J. What drives osteoarthritis?—Synovial versus subchondral bone pathology. Rheumatology 2016, 56, 1461–1471. [Google Scholar] [CrossRef]
- de Lange-Brokaar, B.J.E.; Ioan-Facsinay, A.; van Osch, G.J.V.M.; Zuurmond, A.M.; Schoones, J.; Toes, R.E.M.; Huizinga, T.W.J.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Seeling, M.; Hillenhoff, U.; David, J.P.; Schett, G.; Tuckermann, J.; Lux, A.; Nimmerjahn, F. Inflammatory monocytes and Fcγ receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10729–10734. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; Bonnet, C.S.; Turner, E.L.; Wilson, D.; Situ, M.; McWilliams, D.F. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthr. Cartil. 2007, 15, 743–751. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Nakamura, H.; Obata, K.; Yamada, H.; Hayakawa, T.; Fujikawa, K.; Okada, Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann. Rheum. Dis. 2000, 59, 455–461. [Google Scholar] [CrossRef]
- Dreier, R.; Grassel, S.; Fuchs, S.; Schaumburger, J.; Bruckner, P. Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Exp. Cell Res. 2004, 297, 303–312. [Google Scholar] [CrossRef]
- Dean, D.D.; Martel-Pelletier, J.; Pelletier, J.P.; Howell, D.S.; Woessner, J.F., Jr. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J. Clin. Investig. 1989, 84, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Hampson, V.; Tilman, R.; Goupille, P.; Taiwo, Y.; Hoyland, J.A. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann. Rheum. Dis. 1997, 56, 542–549. [Google Scholar] [CrossRef]
- Masuhara, K.; Nakai, T.; Yamaguchi, K.; Yamasaki, S.; Sasaguri, Y. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthritis Rheum. 2002, 46, 2625–2631. [Google Scholar] [CrossRef]
- Italiano, G.; Raimondo, M.; Giannetti, G.; Gargiulo, A. Benefits of a Food Supplement Containing Boswellia serrata and Bromelain for Improving the Quality of Life in Patients with Osteoarthritis: A Pilot Study. J. Altern. Complement. Med. 2019, 26, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Farpour, H.R.; Rajabi, N.; Ebrahimi, B. The Efficacy of Harpagophytum procumbens (Teltonal) in Patients with Knee Osteoarthritis: A Randomized Active-Controlled Clinical Trial. Evid. Based Complement. Alternat. Med. 2021, 2021, 5596892. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Priem, F.; Mobasheri, A. Curcumin: A new paradigm and therapeutic opportunity for the treatment of osteoarthritis: Curcumin for osteoarthritis management. SpringerPlus 2013, 2, 56. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Hallajzadeh, J.; Rezaeipour, M. Evaluation of the effect of polyphenol of escin compared with ibuprofen and dexamethasone in synoviocyte model for osteoarthritis: An in vitro study. Clin. Rheumatol. 2018, 37, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Zhang, M.L.; Cui, X.Y.; Gao, F.; He, Q.; Li, X.J.; Zhang, J.W.; Fawcett, J.P.; Gu, J.K. Comparative pharmacokinetics and bioavailability of escin Ia and isoescin Ia after administration of escin and of pure escin Ia and isoescin Ia in rat. J. Ethnopharmacol. 2012, 139, 201–206. [Google Scholar] [CrossRef] [PubMed]
- White, R.R.; Crawley, F.E.; Vellini, M.; Rovati, L.A. Bioavailability of 125I bromelain after oral administration to rats. Biopharm. Drug Dispos. 1988, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Peruru, R.; Usha Rani, R.; Thatiparthi, J.; Sampathi, S.; Dodoala, S.; Prasad, K.V.S.R.G. Devil’s claw (Harpagophytum procumbens) ameliorates the neurobehavioral changes and neurotoxicity in female rats exposed to arsenic. Heliyon 2020, 6, e03921. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Thawani, V.; Hingorani, L.; Shrivastava, M.; Bhate, V.R.; Khiyani, R. Pharmacokinetic study of 11-Keto β-Boswellic Acid. Phytomedicine 2004, 11, 255–260. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Hilkens, C.M.U. Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Front. Immunol. 2021, 12, 678757. [Google Scholar] [CrossRef] [PubMed]
- Walther, M.; Harms, H.; Krenn, V.; Radke, S.; Faehndrich, T.P.; Gohlke, F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001, 44, 331–338. [Google Scholar] [CrossRef]
- Lin, K.-C.; Castro, A.C. Very late antigen 4 (VLA4) antagonists as anti-inflammatory agents. Curr. Opin. Chem. Biol. 1998, 2, 453–457. [Google Scholar] [CrossRef]
- Szade, A.; Grochot-Przeczek, A.; Florczyk, U.; Jozkowicz, A.; Dulak, J. Cellular and molecular mechanisms of inflammation-induced angiogenesis. IUBMB Life 2015, 67, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Sainson, R.C.A.; Johnston, D.A.; Chu, H.C.; Holderfield, M.T.; Nakatsu, M.N.; Crampton, S.P.; Davis, J.; Conn, E.; Hughes, C.C.W. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008, 111, 4997–5007. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, H. Mathematical rules for synergistic, additive, and antagonistic effects of multi-drug combinations and their application in research and development of combinatorial drugs and special medical food combinations. Food Sci. Hum. Wellness 2019, 8, 136–141. [Google Scholar] [CrossRef]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef]
- Pothacharoen, P.; Chaiwongsa, R.; Chanmee, T.; Insuan, O.; Wongwichai, T.; Janchai, P.; Vaithanomsat, P. Bromelain Extract Exerts Antiarthritic Effects via Chondroprotection and the Suppression of TNF-α–Induced NF-κB and MAPK Signaling. Plants 2021, 10, 2273. [Google Scholar] [CrossRef]
- Huang, J.R.; Wu, C.C.; Hou, R.C.; Jeng, K.C. Bromelain inhibits lipopolysaccharide-induced cytokine production in human THP-1 monocytes via the removal of CD14. Immunol. Investig. 2008, 37, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.F.; Bundy, R.; Hicks, S.M.; Middleton, R.W. Bromelain reduces mild acute knee pain and improves well-being in a dose-dependent fashion in an open study of otherwise healthy adults. Phytomedicine 2002, 9, 681–686. [Google Scholar] [CrossRef]
- Kasemsuk, T.; Saengpetch, N.; Sibmooh, N.; Unchern, S. Improved WOMAC score following 16-week treatment with bromelain for knee osteoarthritis. Clin. Rheumatol. 2016, 35, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.; Prescott, P.; Lewith, G. Meta-analysis of the related nutritional supplements dimethyl sulfoxide and methylsulfonylmethane in the treatment of osteoarthritis of the knee. Evid. Based Complement. Alternat. Med. 2011, 2011, 528403. [Google Scholar] [CrossRef]
- Nathan, M.; Scholten, R. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Ann. Intern. Med. 1999, 130, 459. [Google Scholar] [CrossRef]
- Inaba, K.; Murata, K.; Naruto, S.; Matsuda, H. Inhibitory effects of devil’s claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. J. Nat. Med. 2010, 64, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef]
- Wegener, T.; Lüpke, N.P. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil’s claw (Harpagophytum procumbens DC.). Phytother. Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Börner, F.; Werner, M.; Ertelt, J.; Meins, J.; Abdel-Tawab, M.; Werz, O. Analysis of Boswellic Acid Contents and Related Pharmacological Activities of Frankincense-Based Remedies That Modulate Inflammation. Pharmaceuticals 2021, 14, 660. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R.; Bahadoram, M.; Alghasi, A. COVID-19: A Case for Inhibiting NLRP3 Inflammasome, Suppression of Inflammation with Curcumin? Basic Clin. Pharmacol. Toxicol. 2021, 128, 37–45. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Karimian, M.S.; Pirro, M.; Majeed, M.; Sahebkar, A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017, 33, 55–63. [Google Scholar] [CrossRef]
- Singh, H.; Sidhu, S.; Chopra, K.; Khan, M.U. The novel role of β-aescin in attenuating CCl(4)-induced hepatotoxicity in rats. Pharm. Biol. 2017, 55, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.M.; Wei, J. Effects of beta-Aescin on the expression of nuclear factor-kappaB and tumor necrosis factor-alpha after traumatic brain injury in rats. J. Zhejiang Univ. Sci. B 2005, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Smiljanovic, B.; Grün, J.R.; Steinbrich-Zöllner, M.; Stuhlmüller, B.; Häupl, T.; Burmester, G.R.; Radbruch, A.; Grützkau, A.; Baumgrass, R. Defining TNF-α- and LPS-induced gene signatures in monocytes to unravel the complexity of peripheral blood transcriptomes in health and disease. J. Mol. Med. 2010, 88, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Stavchansky, S.A.; Bowden, R.A.; Bowman, P.D. Effect of interleukin-1β and tumor necrosis factor-α on gene expression in human endothelial cells. Am. J. Physiol.-Cell Physiol. 2003, 284, C1577–C1583. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Alaaeddine, N.; Pelletier, J.P. Cytokines and their role in the pathophysiology of osteoarthritis. Front. Biosci. 1999, 4, D694-703. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Pelletier, J.-P.; Fahmi, H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin. Arthritis Rheum. 2003, 33, 155–167. [Google Scholar] [CrossRef]
- Nakata, K.; Hanai, T.; Take, Y.; Osada, T.; Tsuchiya, T.; Shima, D.; Fujimoto, Y. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: A systematic review. Osteoarthr. Cartil. 2018, 26, 1263–1273. [Google Scholar] [CrossRef]
- Vasanthkumar, T.; Hanumanthappa, M.; Lakshminarayana, R. Curcumin and capsaicin modulates LPS induced expression of COX-2, IL-6 and TGF-β in human peripheral blood mononuclear cells. Cytotechnology 2019, 71, 963–976. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Muñoz, E.; Rose, T.; Weiss, G.; McGregor, G.P. Molecular targets of the antiinflammatory Harpagophytum procumbens (devil’s claw): Inhibition of TNFα and COX-2 gene expression by preventing activation of AP-1. Phytother. Res. 2012, 26, 806–811. [Google Scholar] [CrossRef]
- Gayathri, B.; Manjula, N.; Vinaykumar, K.S.; Lakshmi, B.S.; Balakrishnan, A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFalpha, IL-1beta, NO and MAP kinases. Int. Immunopharmacol. 2007, 7, 473–482. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Montopoli, M.; Froldi, G.; Comelli, M.C.; Prosdocimi, M.; Caparrotta, L. Aescin protection of human vascular endothelial cells exposed to cobalt chloride mimicked hypoxia and inflammatory stimuli. Planta Med. 2007, 73, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Binion, D.G.; Otterson, M.F.; Rafiee, P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 2008, 57, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, S.; Du, B.; Sakamoto, K.; Khan, K.M.F.; Natarajan, C.; Breyer, R.M.; Dannenberg, A.J.; Falcone, D.J. Targeting Prostaglandin E2 Receptors as an Alternative Strategy to Block Cyclooxygenase-2-dependent Extracellular Matrix-induced Matrix Metalloproteinase-9 Expression by Macrophages. J. Biol. Chem. 2006, 281, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef]
- Massaro, M.; Zampolli, A.; Scoditti, E.; Carluccio, M.A.; Storelli, C.; Distante, A.; De Caterina, R. Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: Anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc. Res. 2010, 86, 311–320. [Google Scholar] [CrossRef]
- Galasso, O.; Familiari, F.; De Gori, M.; Gasparini, G. Recent Findings on the Role of Gelatinases (Matrix Metalloproteinase-2 and -9) in Osteoarthritis. Adv. Orthop. 2012, 2012, 834208. [Google Scholar] [CrossRef]
- Mixon, A.; Bahar-Moni, A.S.; Faisal, T.R. Mechanical characterization of articular cartilage degraded combinedly with MMP-1 and MMP-9. J. Mech. Behav. Biomed. Mater. 2022, 129, 105131. [Google Scholar] [CrossRef]
- Kennedy, A.; Ng, C.T.; Biniecka, M.; Saber, T.; Taylor, C.; O’Sullivan, J.; Veale, D.J.; Fearon, U. Angiogenesis and blood vessel stability in inflammatory arthritis. Arthritis Rheum. 2010, 62, 711–721. [Google Scholar] [CrossRef]
- Lainer-Carr, D.; Brahn, E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat. Clin. Pract. Rheumatol. 2007, 3, 434–442. [Google Scholar] [CrossRef]
- Bougelet, C.; Roland, I.H.; Ninane, N.; Arnould, T.; Remacle, J.; Michiels, C. Effect of aescine on hypoxia-induced neutrophil adherence to umbilical vein endothelium. Eur. J. Pharmacol. 1998, 345, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Shah, H.; Rink, C.; Phillips, C.; Preuss, H.; Subbaraju, G.V.; Trimurtulu, G.; Krishnaraju, A.V.; Bagchi, M.; et al. Human genome screen to identify the genetic basis of the anti-inflammatory effects of Boswellia in microvascular endothelial cells. DNA Cell Biol. 2005, 24, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Yu, Y.-M.; Cheng, A.-C. Curcumin suppresses pro-inflammatory adhesion response in Human Umbilical Vein Endothelial Cells. J. Food Biochem. 2018, 42, e12623. [Google Scholar] [CrossRef]
- Weber, C.; Springer, T.A. Interaction of very late antigen-4 with VCAM-1 supports transendothelial chemotaxis of monocytes by facilitating lateral migration. J. Immunol. 1998, 161, 6825–6834. [Google Scholar] [PubMed]
- Attur, M.; Dave, M.; Abramson, S.B.; Amin, A. Activation of diverse eicosanoid pathways in osteoarthritic cartilage: A lipidomic and genomic analysis. Bull. NYU Hosp. Jt. Dis. 2012, 70, 99–108. [Google Scholar]
- Shen, J.; Li, S.; Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014, 2, 14002. [Google Scholar] [CrossRef]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef]
- van Beuningen, H.M.; Glansbeek, H.L.; van der Kraan, P.M.; van den Berg, W.B. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthr. Cartil. 1998, 6, 306–317. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Glansbeek, H.L.; van Beuningen, H.M.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J. Immunol. 2002, 169, 507–514. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Wang, X.; Fan, D.; Cao, X.; Ye, Q.; Wang, Q.; Zhang, M.; Xiao, C. The Role of Reactive Oxygen Species in the Rheumatoid Arthritis-Associated Synovial Microenvironment. Antioxidants 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an Immortalized Human Microvascular Endothelial Cell Line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Basta, G.; Lazzerini, G.; Carluccio, M.A.; Bosetti, F.; Solaini, G.; Visioli, F.; Paolicchi, A.; De Caterina, R. Quenching of intracellular ROS generation as a mechanism for oleate-induced reduction of endothelial activation and early atherogenesis. Thromb. Haemost. 2002, 88, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Rifkin, D.B. Autocrine activities of basic fibroblast growth factor: Regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J. Cell Biol. 1988, 107, 1199–1205. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
| Extract | Total Polyphenols Content (µg GAE/mg Dry Extract) Mean ± S.D. | Radical Scavenging Activity (µmol TE/mg Dry Extract) Mean ± S.D. |
|---|---|---|
| B | 6.29 ± 2.20 | 1.72 ± 0.50 |
| HP | 7.42 ± 0.47 | 0.66 ± 0.05 |
| E | 136.65 ± 19.10 | 15.28 ± 1.49 |
| BS | 43.79 ± 6.81 | 7.72 ± 1.61 |
| C | 694.51 ± 132.58 | 11.59 ± 1.82 |
| Cell type | Bioactives | B | HP | BS | E | C | FF | ||
|---|---|---|---|---|---|---|---|---|---|
| TPC | |||||||||
| RSA | |||||||||
| Gene | |||||||||
| Monocyte | IL-6 | ||||||||
| IL-8 | |||||||||
| MMP-9 | |||||||||
| COX-2 | |||||||||
| Endothelial cell | VCAM-1 | ||||||||
| ICAM-1 | |||||||||
| MCP-1 | |||||||||
| COX-2 | |||||||||
| VEGF | |||||||||
| KDR | |||||||||
| MMP-9 | |||||||||
| TGF-β1 | |||||||||
| BMP-2 | |||||||||
| Monocyte adhesion | |||||||||
| No effect on gene induction | |||||||||
| Effect on gene induction | |||||||||
| Blue gradients indicate increase in total polyphenol content (TPC) or radical scavenging activity (RSA) | |||||||||
| Full Name | Forward Primer (5′-3′) | Revers Primer (3′-5′) | Accession Number | |
|---|---|---|---|---|
| MCP-1/CCL-2 | Monocyte chemoattractant protein-1/C-C Motif chemokine ligand 2 | CCCCAGTCACCTGCTGTTAT | TCCTGAACCCACTTCTGCTT | NM_002982.3 |
| PTGS2/COX-2 | Prostaglandin G/H synthase 2/cyclooxygenase-2 | TGCTGTGGAGCTGTATCCTG | GAAACCCACTTCTCCACCA | NM_000963.2 |
| IL-6 | Interleukin-6 | AGGAGACTTGCCTGGTGAAA | CAGGGGTGGTTATTGCATCT | NM_000600.5 |
| IL-8 | Interleukin-8 | GTGCAGTTTTGCCAAGGAGT | CTCTGCACCCAGTTTTCCTT | NM_001354840.3 |
| BMP-2 | Bone Morphogenetic Protein-2 | AGACCTGTATCGCAGGCACT | CCTCCGTGGGGATAGAACTT | NM_001200.4 |
| TGF-β1 | Transforming Growth Factor β-1 | CACGTGGAGCTGTACCAGAA | GAACCCGTTGATGTCCACTT | NM_000660.7 |
| KDR/VEGFR-2 | Kinase Insert Domain Receptor/Vascular Endothelial Growth Factor Receptor 2 | AGCGATGGCCTCTTCTGTAA | ACACGACTCCATGTTGGTCA | NM_002253.2 |
| VEGF | Vascular Endothelial Growth Factor | GACACACCCACCCACATACA | TCTCCTCCTCTTCCCTGTCA | NM_001171626.1 |
| MMP-9 | Matrix metalloproteinase-9 | TTGACAGCGACAAGAAGTGG | GCCATTCACGTCGTCCTTAT | NM_004994.2 |
| ICAM-1 | Intercellular adhesion molecule-1 | AGACATAGCCCCACCATGAG | CAAGGGTTGGGGTCAGTAGA | NM_000201.2 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 | CATGGAATTCGAACCCAAAC | CCTGGCTCAAGCATGTCATA | NM_001078.3 |
| 18S | 18 ribosomal RNA | AAACGGCTACCACATCCAAG | CCTCCAATGGATCCTCGTTA | NR_003286.2 |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | ATGGCCTTCCGTGTCCCCAC | ACGCCTGCTTCACCACCTTC | NM_002046.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarta, S.; Santarpino, G.; Carluccio, M.A.; Calabriso, N.; Scoditti, E.; Siculella, L.; Damiano, F.; Maffia, M.; Verri, T.; De Caterina, R.; et al. Analysis of the Anti-Inflammatory and Anti-Osteoarthritic Potential of Flonat Fast®, a Combination of Harpagophytum Procumbens DC. ex Meisn., Boswellia Serrata Roxb., Curcuma longa L., Bromelain and Escin (Aesculus hippocastanum), Evaluated in In Vitro Models of Inflammation Relevant to Osteoarthritis. Pharmaceuticals 2022, 15, 1263. https://doi.org/10.3390/ph15101263
Quarta S, Santarpino G, Carluccio MA, Calabriso N, Scoditti E, Siculella L, Damiano F, Maffia M, Verri T, De Caterina R, et al. Analysis of the Anti-Inflammatory and Anti-Osteoarthritic Potential of Flonat Fast®, a Combination of Harpagophytum Procumbens DC. ex Meisn., Boswellia Serrata Roxb., Curcuma longa L., Bromelain and Escin (Aesculus hippocastanum), Evaluated in In Vitro Models of Inflammation Relevant to Osteoarthritis. Pharmaceuticals. 2022; 15(10):1263. https://doi.org/10.3390/ph15101263
Chicago/Turabian StyleQuarta, Stefano, Giuseppe Santarpino, Maria Annunziata Carluccio, Nadia Calabriso, Egeria Scoditti, Luisa Siculella, Fabrizio Damiano, Michele Maffia, Tiziano Verri, Raffaele De Caterina, and et al. 2022. "Analysis of the Anti-Inflammatory and Anti-Osteoarthritic Potential of Flonat Fast®, a Combination of Harpagophytum Procumbens DC. ex Meisn., Boswellia Serrata Roxb., Curcuma longa L., Bromelain and Escin (Aesculus hippocastanum), Evaluated in In Vitro Models of Inflammation Relevant to Osteoarthritis" Pharmaceuticals 15, no. 10: 1263. https://doi.org/10.3390/ph15101263
APA StyleQuarta, S., Santarpino, G., Carluccio, M. A., Calabriso, N., Scoditti, E., Siculella, L., Damiano, F., Maffia, M., Verri, T., De Caterina, R., & Massaro, M. (2022). Analysis of the Anti-Inflammatory and Anti-Osteoarthritic Potential of Flonat Fast®, a Combination of Harpagophytum Procumbens DC. ex Meisn., Boswellia Serrata Roxb., Curcuma longa L., Bromelain and Escin (Aesculus hippocastanum), Evaluated in In Vitro Models of Inflammation Relevant to Osteoarthritis. Pharmaceuticals, 15(10), 1263. https://doi.org/10.3390/ph15101263