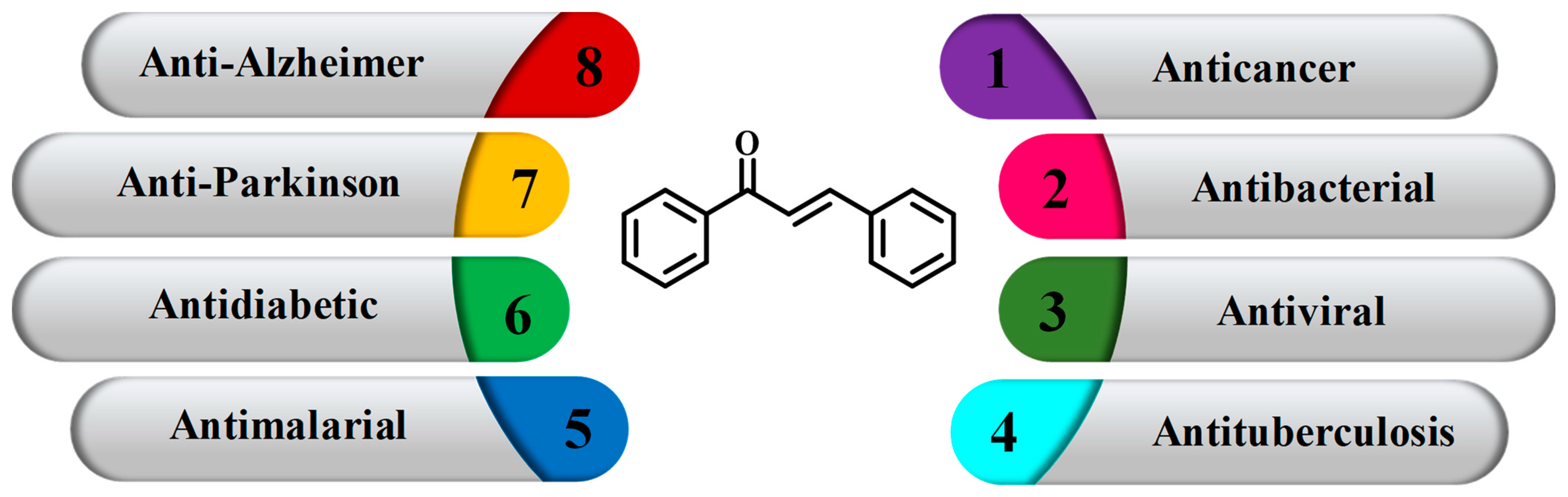
Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry
Abstract
1. Introduction
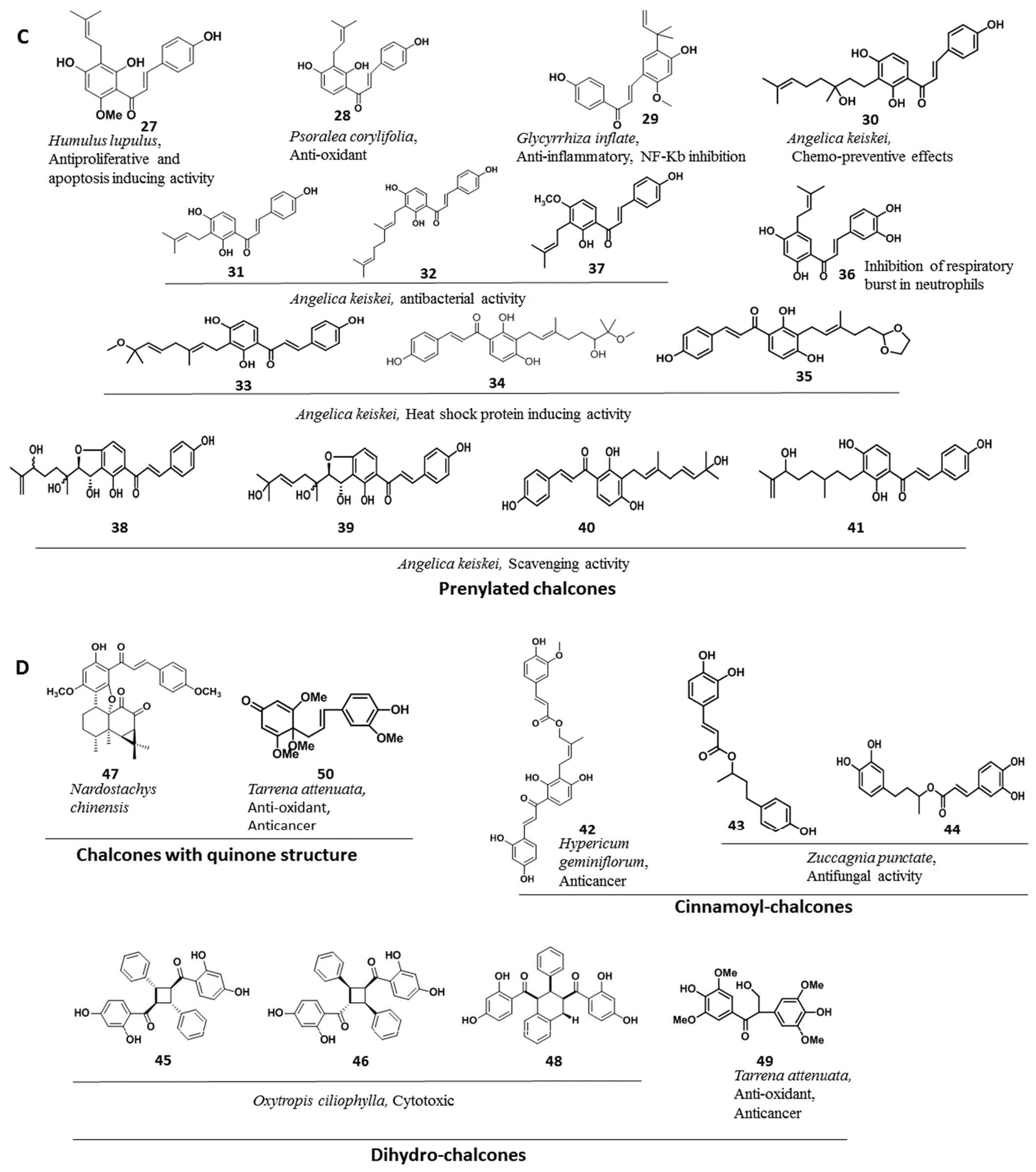
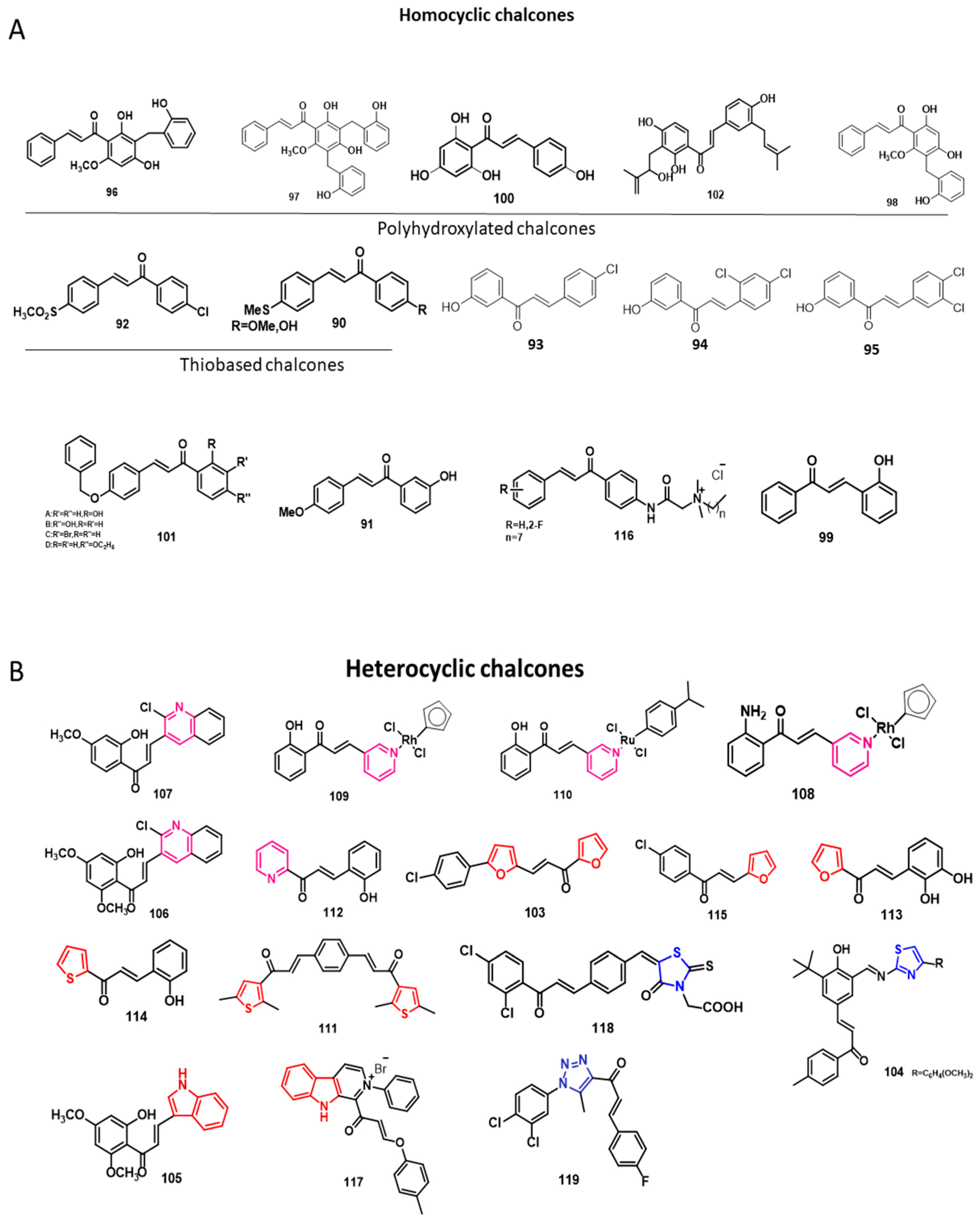
2. Chalcones from Natural Sources
3. Synthesis of Chalcones
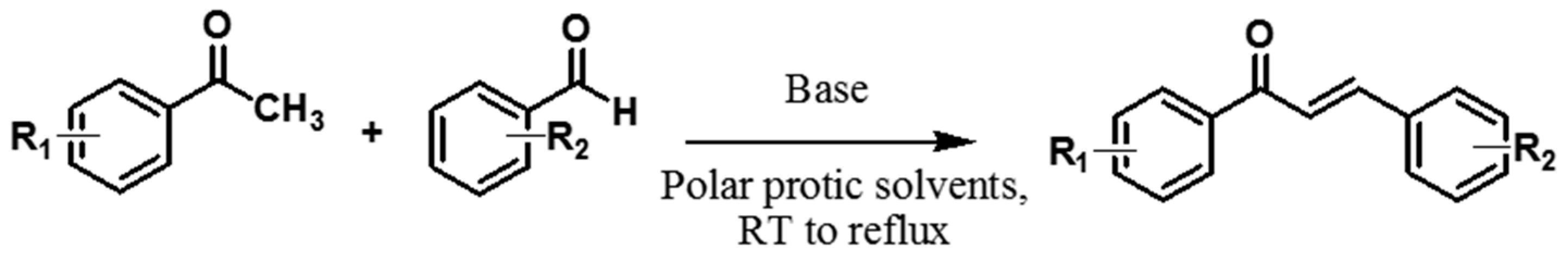
3.1. Conventional Synthesis of Chalcones
3.2. Greener Approaches for the Synthesis of Chalcones
3.2.1. Microwave-Assisted Method
3.2.2. Ultrasound-Irradiated Synthesis
3.2.3. Grinding Technique
3.3. Coupling Reactions
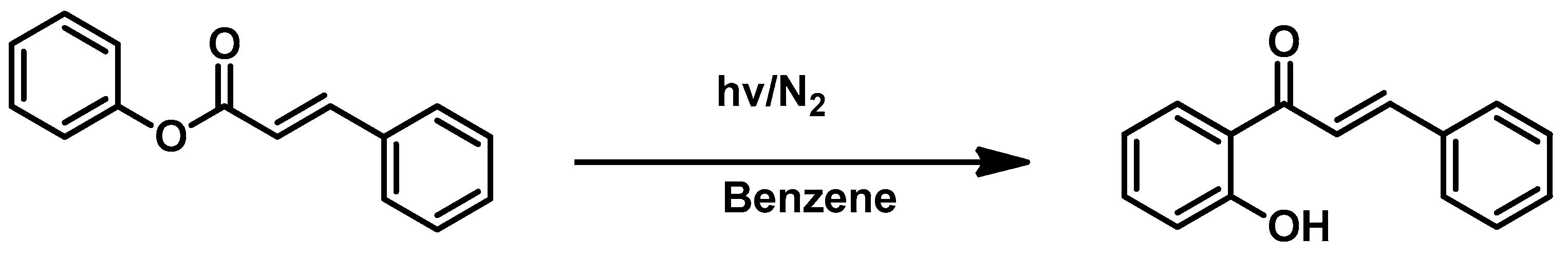
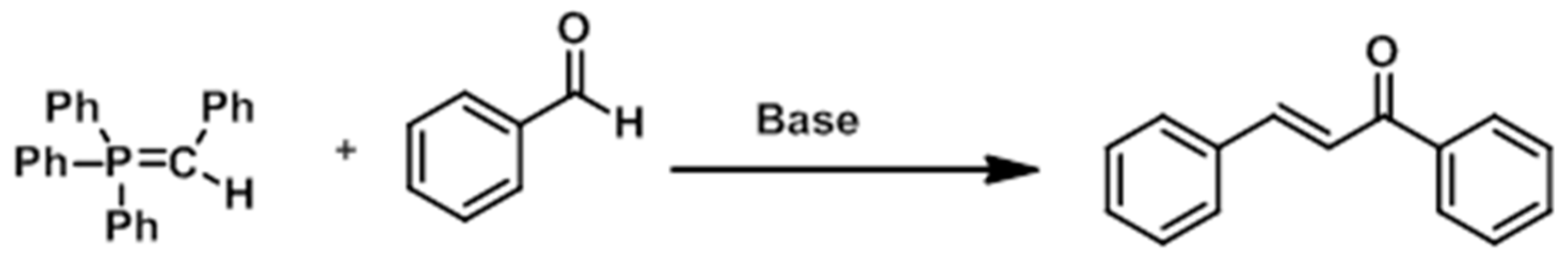
3.4. Miscellaneous Reactions
4. Chalcones against Infectious Diseases
4.1. Anti-Tubercular Activity
4.2. Antiviral Activity
4.3. Antimalarial Activity
4.4. Antibacterial Activity
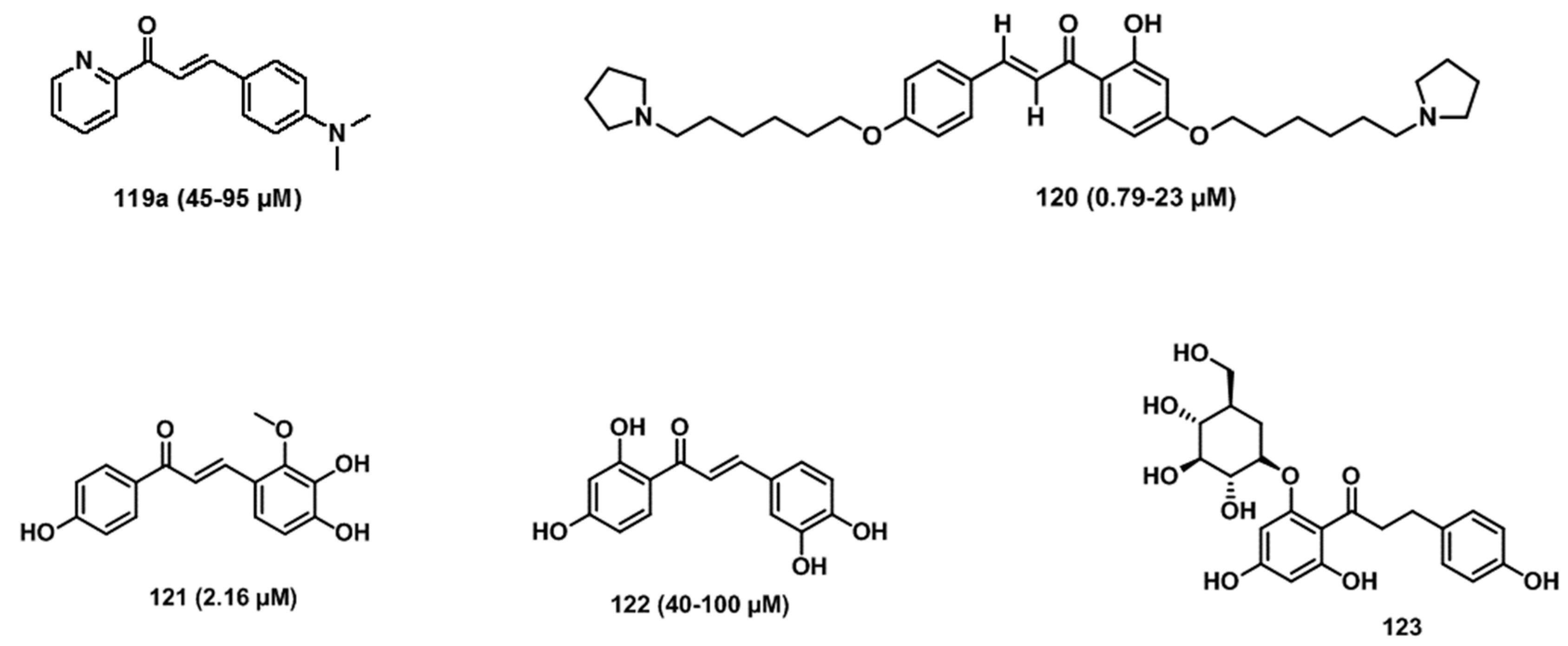
5. Chalcones for Non-Infectious Diseases
5.1. Anti-Alzheimer’s Activity
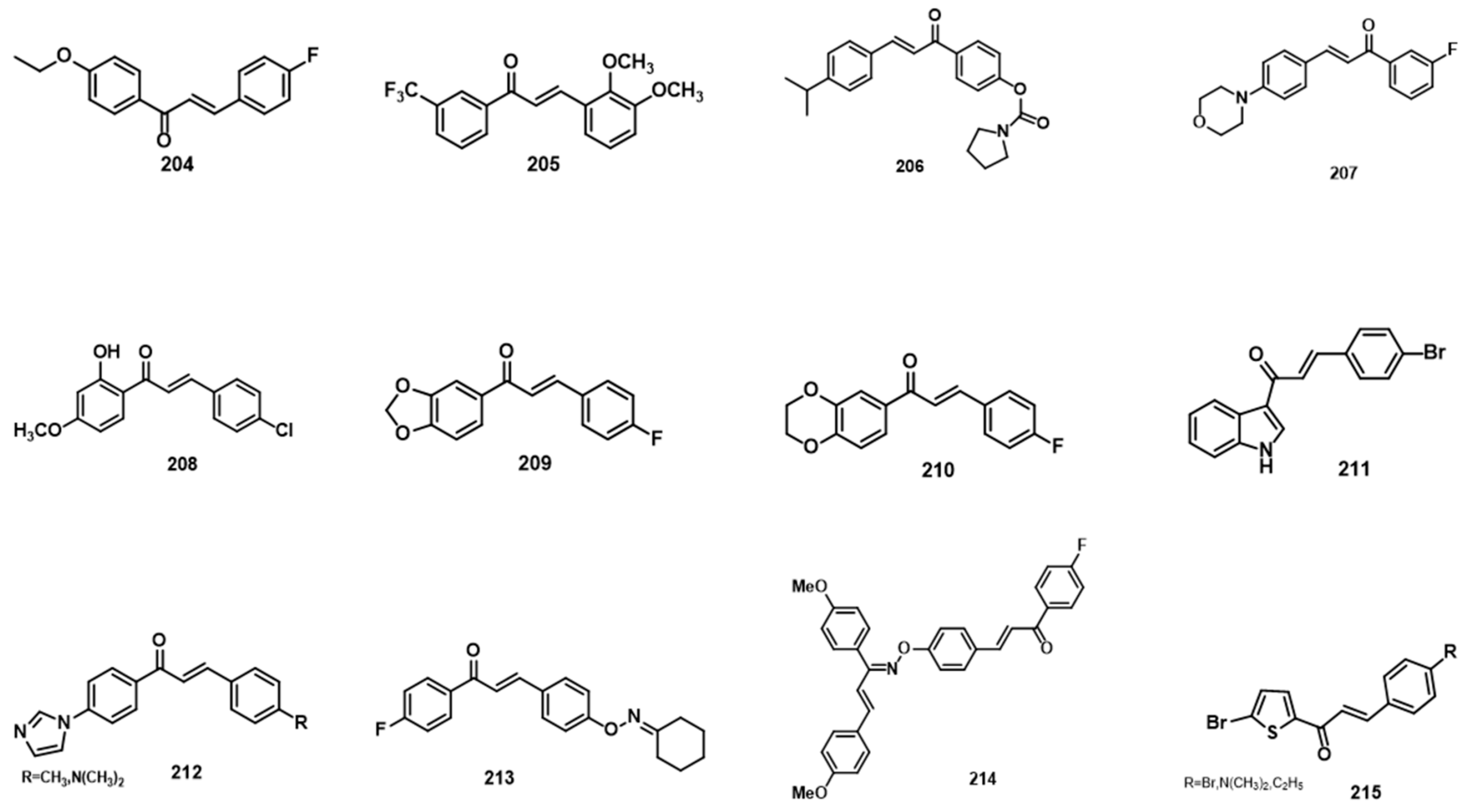
5.2. Anticancer Activity
5.2.1. Anti-Breast-Cancer Activity
5.2.2. Anti-Lung-Cancer Activity
5.2.3. Chalcones with Broad-Spectrum Anticancer Activities
5.3. Antidiabetic Activity
5.4. Anti-Parkinson’s Activity
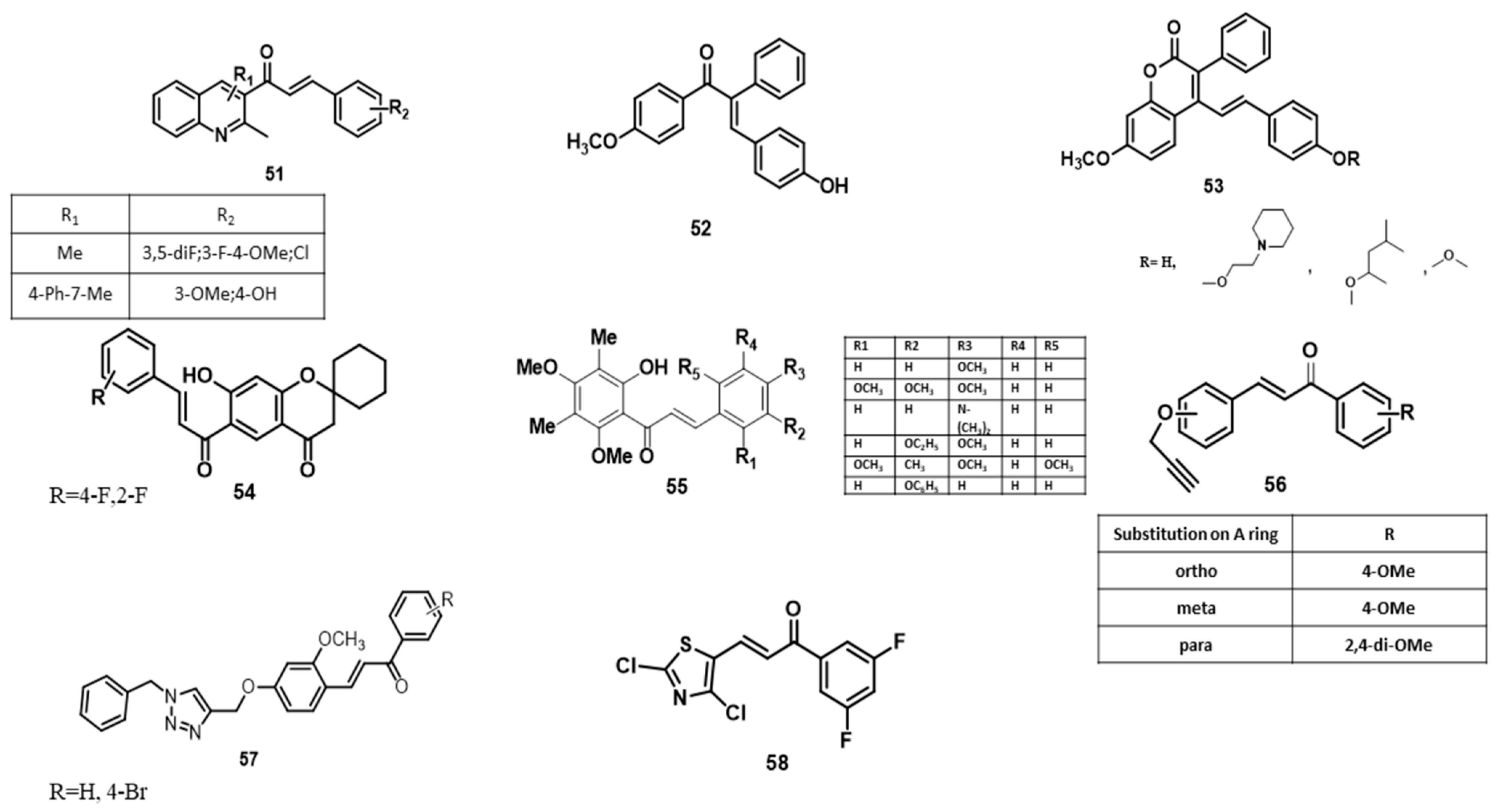
6. SAR Studies
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Narender, T.; Papi Reddy, K. A Simple and Highly Efficient Method for the Synthesis of Chalcones by Using Borontrifluoride-Etherate. Tetrahedron Lett. 2007, 48, 3177–3180. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N.; Jazvinš’cak, M.; Jembrek, J. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Mostafa, Y.A. A Facile Synthesis, Drug-Likeness, and in Silico Molecular Docking of Certain New Azidosulfonamide–Chalcones and Their in Vitro Antimicrobial Activity. Mon. Für Chem. Chem. Mon. 2020, 151, 417–427. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, D.; Zeng, H.; Ren, X.; Song, B.; Hu, D.; Gan, X. New chalcone derivatives: Synthesis, antiviral activity and mechanism of action. RSC Adv. 2020, 10, 24483–24490. [Google Scholar] [CrossRef]
- Osipova, V.P.; Polovinkina, M.A.; Telekova, L.R.; Velikorodov, A.V.; Stepkina, N.N.; Berberova, N.T. Synthesis and Antioxidant Activity of New Hydroxy Derivatives of Chalcones. Russ. Chem. Bull. 2020, 69, 504–509. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, W.; Huang, Y.; Zeng, X. Licochalcone B, a Chalcone Derivative from Glycyrrhiza Inflata, as a Multifunctional Agent for the Treatment of Alzheimer’s Disease. Nat. Prod. Res. 2020, 34, 736–739. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Peng, Y.; Jiang, Z.H.; Zhang, L.; Du, Z. Design, Synthesis and Anti-Tumor Activity of Novel Benzimidazole-Chalcone Hybrids as Non-Intercalative Topoisomerase II Catalytic Inhibitors. Molecules 2020, 25, 3180. [Google Scholar] [CrossRef]
- Rammohan, A.; Bhaskar, B.V.; Venkateswarlu, N.; Gu, W.; Zyryanov, G.V. Design, Synthesis, Docking and Biological Evaluation of Chalcones as Promising Antidiabetic Agents. Bioorg. Chem. 2020, 95, 103527. [Google Scholar] [CrossRef]
- Maliyakkal, N.; Saleem, U.; Anwar, F.; Shah, M.A.; Ahmad, B.; Umer, F.; Almoyad, M.A.A.; Parambi, D.G.T.; Beeran, A.A.; Nath, L.R.; et al. Ameliorative Effect of Ethoxylated Chalcone-Based MAO-B Inhibitor on Behavioural Predictors of Haloperidol-Induced Parkinsonism in Mice: Evidence of Its Antioxidative Role against Parkinson’s Diseases. Environ. Sci. Pollut. Res. 2022, 29, 7271–7282. [Google Scholar] [CrossRef]
- Hsieh, H.-K.; Tsao, L.-T.; Wang, J.-P.; Lin, C.-N. Synthesis and Anti-Inflammatory Effect of Chalcones. J. Pharm. Pharmacol. 2000, 52, 163–171. [Google Scholar] [CrossRef] [PubMed]
- de Campos-Buzzi, F.; de Campos, J.P.; Tonini, P.P.; Corrêa, R.; Yunes, R.A.; Boeck, P.; Cechinel-Filho, V. Antinociceptive Effects of Synthetic Chalcones Obtained from Xanthoxyline. Arch. der Pharm. 2006, 339, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.I.; Mahmood, A.; Madni, M.; Masood, S.; Kashif, M. Synthesis, Characterization, Theoretical, Anti-Bacterial and Molecular Docking Studies of Quinoline Based Chalcones as a DNA Gyrase Inhibitor. Bioorg. Chem. 2014, 54, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, A.; Altintop, M.D.; Sever, B.; Gençer, H.K.; Kapkaç, H.A.; Atli, Ö.; Baysal, M. A New Series of Pyrrole-Based Chalcones: Synthesis and Evaluation of Antimicrobial Activity, Cytotoxicity, and Genotoxicity. Molecules 2017, 22, 2112. [Google Scholar] [CrossRef]
- Mojarrab, M.; Soltani, R.; Aliabadi, A. Pyridine Based Chalcones: Synthesis and Evaluation of Antioxidant Activity of 1-Phenyl-3-(Pyridin-2-Yl) Prop-2-En-1-One Derivatives. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 125. [Google Scholar] [CrossRef]
- Özdemir, A.; Altintop, M.D.; Turan-Zitouni, G.; Çiftçi, G.A.; Ertorun, Ö.; Alataş, Ö.; Kaplancikli, Z.A. Synthesis and Evaluation of New Indole-Based Chalcones as Potential Antiinflammatory Agents. Eur. J. Med. Chem. 2015, 89, 304–309. [Google Scholar] [CrossRef]
- Chavan, H.V.; Adsul, L.K.; Kotmale, A.S.; Dhakane, V.D.; Thakare, V.N.; Bandgar, B.P. Design, Synthesis, Characterization and in Vitro and in Vivo Anti-Inflammatory Evaluation of Novel Pyrazole-Based Chalcones. J. Enzym. Inhib. Med. Chem. 2015, 30, 22–31. [Google Scholar] [CrossRef]
- Naik, N.; Kumar, V.H.; Dias, S.M.; Swami, R.J. Novel 4-methoxy-2-acetyl benzofuran based chalcones: A new perceptivity into their antioxidant potentials. Int. J. Pharm. Pharm. Sci. 2013, 5, 242–247. [Google Scholar]
- Rajeshirke, M.; Sreenath, M.C.; Chitrambalam, S.; Joe, I.H.; Sekar, N. Enhancement of NLO Properties in OBO Fluorophores Derived from Carbazole-Coumarin Chalcones Containing Carboxylic Acid at the N-Alykl Terminal End. J. Phys. Chem. C 2018, 122, 14313–14325. [Google Scholar] [CrossRef]
- Shaik, A.; Bhandare, R.R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Antimicrobial, Antioxidant, and Anticancer Activities of Some Novel Isoxazole Ring Containing Chalcone and Dihydropyrazole Derivatives. Molecules 2020, 25, 1047. [Google Scholar] [CrossRef]
- Parikh, K.; Joshi, D. Antibacterial and Antifungal Screening of Newly Synthesized Benzimidazole-Clubbed Chalcone Derivatives. Med. Chem. Res. 2013, 22, 3688–3697. [Google Scholar] [CrossRef]
- Bala, D.; Jinga, L.I.; Popa, M.; Hanganu, A.; Voicescu, M.; Bleotu, C.; Tarko, L.; Nica, S. Design, Synthesis, and Biological Evaluation of New Azulene-Containing Chalcones. Materials 2022, 15, 1629. [Google Scholar] [CrossRef]
- Singh, A.; Rani, A.; Gut, J.; Rosenthal, P.J.; Kumar, V. Piperazine-linked 4-aminoquinoline-chalcone/ferrocenyl-chalcone conjugates: Synthesis and antiplasmodial evaluation. Chem. Biol. Drug Des. 2017, 90, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, C.; Guo, S.; Fan, J.; Wang, L.; Shi, D. Chalcone-Analogue Fluorescent Probes for Detecting Thiophenols in Seawater Samples. Talanta 2019, 201, 301–308. [Google Scholar] [CrossRef]
- Malek, S.N.A.; Phang, C.W.; Ibrahim, H.; Wahab, N.A.; Sim, K.S. Phytochemical and Cytotoxic Investigations of Alpinia Mutica Rhizomes. Molecules 2011, 16, 583–589. [Google Scholar] [CrossRef]
- Yang, X.W.; Wang, J.S.; Wang, Y.H.; Xiao, H.T.; Hu, X.J.; Mu, S.Z.; Yan-Lin, M.; Lin, H.; He, H.P.; Li, N.; et al. Tarennane and Tarennone, Two Novel Chalcone Constituents from Tarenna Attenuata. Planta Med. 2007, 73, 496–498. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Kelsang, N.; Tu, G.; Kong, D.; Lu, J.; Zhang, Y.; Liang, H.; Tu, P.; Zhang, Q. Structurally Diverse Cytotoxic Dimeric Chalcones from Oxytropis Chiliophylla. J. Nat. Prod. 2018, 81, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Tanabe, S.; Tago, K.; Itoh, H.; Mashino, T.; Sonoda, Y.; Kasahara, T. Licochalcone A Potently Inhibits Tumor Necrosis Factor α-Induced Nuclear Factor-ΚB Activation through the Direct Inhibition of IκB Kinase Complex Activation. Mol Pharm. 2009, 76, 745–753. [Google Scholar] [CrossRef]
- Tomazela, D.M.; Pupo, M.T.; Passador, E.A.P.; da Silva, M.F.D.G.F.; Vieira, P.C.; Fernandes, J.B.; Rodrigues Fo, E.; Oliva, G.; Pirani, J.R. Pyrano Chalcones and a Flavone from Neoraputia Magnifica and Their Trypanosoma Cruzi Glycosomal Glyceraldehyde-3-Phosphate Dehydrogenase-Inhibitory Activities. Phytochemistry 2000, 55, 643–651. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Hasegawa, D.; Ukiya, M.; Kimura, Y.; Enjo, F.; Suzuki, T.; Nishino, H. Chalcones and Other Compounds from the Exudates of Angelica Keiskei and Their Cancer Chemopreventive Effects. J. Nat. Prod. 2006, 69, 38–42. [Google Scholar] [CrossRef]
- Cui, Y.; Ao, M.; Li, W.; Hu, J.; Yu, L. Anti-Inflammatory Activity of Licochalcone a Isolated from Glycyrrhiza Inflata. Z. Für Nat. Sect. C J. Biosci. 2008, 63, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.C.R.F.; Ehrenfried, C.A.; Lopez, B.G.C.; de Araujo, T.M.; Pascoal, V.D.B.; Gilioli, R.; Anheê, G.F.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Stefanello, M.A.; et al. Antiproliferative Activity and Induction of Apoptosis in PC-3 Cells by the Chalcone Cardamonin from Campomanesia Adamantium (Myrtaceae) in a Bioactivity-Guided Study. Molecules 2014, 19, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Tuntipaleepun, M.; Chakthong, S.; Ponglimanont, C.; Plodpai, P.; Voravuthikunchai, S.P. Antifungal and Cytotoxic Substances from the Stem Barks of Desmos Chinensis. Chin. Chem. Lett. 2012, 23, 587–590. [Google Scholar] [CrossRef]
- Liu, M.L.; Duan, Y.H.; Hou, Y.L.; Li, C.; Gao, H.; Dai, Y.; Yao, X.S. Nardoaristolones A and B, Two Terpenoids with Unusual Skeletons from Nardostachys Chinensis Batal. Org. Lett. 2013, 15, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.B.; Zhang, K.; Cheng, L.Y.; Mack, P. Butein, a Specific Protein Tyrosine Kinase Inhibitor. Biochem. Biophys. Res. Commun. 1998, 245, 435–438. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, C.; Qian, Y.; Chai, H.B.; Chen, X.; Goetz, M.; Kinghorn, A.D. Constituents of an Extract of Cryptocarya Rubra Housed in a Repository with Cytotoxic and Glucose Transport Inhibitory Effects. J. Nat. Prod. 2014, 77, 550–556. [Google Scholar] [CrossRef]
- Rees, K.A.; Bermudez, C.; Edwards, D.J.; Elliott, A.G.; Ripen, J.E.; Seta, C.; Huang, J.X.; Cooper, M.A.; Fraser, J.A.; Yeo, T.C.; et al. Flemingin-Type Prenylated Chalcones from the Sarawak Rainforest Plant Desmodium Congestum. J. Nat. Prod. 2015, 78, 2141–2144. [Google Scholar] [CrossRef]
- Inamori, Y.; Baba, K.; Tsujibo, H.; Taniguch, M.; Nakata, K.; Kozawa, M. Antibacterial Activity of Two Chalcones, Xanthoangelol and 4-Hydroxyderricin, Isolated from the Root of Angelica Keiskei KOIDZUMI. Chem. Pharm. Bull. 1991, 39, 1604–1605. [Google Scholar] [CrossRef]
- Costa, G.M.; Endo, E.H.; Cortez, D.A.G.; Nakamura, T.U.; Nakamura, C.V.; Dias Filho, B.P. Antimicrobial Effects of Piper Hispidum Extract, Fractions and Chalcones against Candida Albicans and Staphylococcus Aureus. J. Mycol. Médicale 2016, 26, 217–226. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Han, S.; Wang, D.; Mo, S.; Yu, L.; Huang, H.; Tsui, K.; Shen, J.; Chen, J. Dietary Compound Isoliquiritigenin Inhibits Breast Cancer Neoangiogenesis via VEGF/VEGFR-2 Signaling Pathway. PLoS ONE 2013, 8, e68566. [Google Scholar] [CrossRef] [PubMed]
- Agarkar, S.A.; Kulkarni, R.R.; Dhas, V.V.; Chinchansure, A.A.; Hazra, P.; Joshi, S.P.; Ogale, S.B. Isobutrin from Butea Monosperma (Flame of the Forest): A Promising New Natural Sensitizer Belonging to Chalcone Class. ACS Appl. Mater. Interfaces 2011, 3, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural Chalcones in Chinese Materia Medica: Licorice. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.S.; Bilal, A.; Ullah, R.; Iqbal, M.; Khan, S.; Ahmed, I.; Krohn, K.; Saleem, R.S.Z.; Hussain, H.; Faisal, A. Natural and Semisynthetic Chalcones as Dual FLT3 and Microtubule Polymerization Inhibitors. J. Nat. Prod. 2020, 83, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Muko, M.; Ohta, E.; Ohta, S. C-Geranylated Chalcones from the Stems of Angelica Keiskei with Superoxide-Scavenging Activity. J. Nat. Prod. 2008, 71, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.S.; Choi, S.K.; Lee, Y.S.; Jafari, M.; Seo, E.K. Chalcones from Angelica Keiskei: Evaluation of Their Heat Shock Protein Inducing Activities. J. Nat. Prod. 2015, 78, 2481–2487. [Google Scholar] [CrossRef]
- Daikonya, A.; Katsuki, S.; Kitanaka, S. Antiallergic Agents from Natural Sources 9. Inhibition of Nitric Oxide Production by Novel Chalcone Derivatives from Mallotus Philippinensis (Euphorbiaceae). Chem. Pharm. Bull. 2004, 52, 1326–1329. [Google Scholar] [CrossRef]
- Fu, L.C.; Huang, X.A.; Lai, Z.Y.; Hu, Y.J.; Liu, H.J.; Cai, X.L. A New 3-Benzylchroman Derivative from Sappan Lignum (Caesalpinia Sappan). Molecules 2008, 13, 1923–1930. [Google Scholar] [CrossRef]
- Phrutivorapongkul, A.; Lipipun, V.; Ruangrungsi, N.; Kirtikara, K.; Nishikawa, K.; Maruyama, S.; Watanabe, T.; Ishikawa, T. Studies on the Chemical Constituents of Stem Bark of Millettia Leucantha: Isolation of New Chalcones with Cytotoxic, Anti-Herpes Simplex Virus and Anti-Inflammatory Activities. Chem. Pharm. Bull. 2003, 51, 187–190. [Google Scholar] [CrossRef]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.E.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal Chalcones and New Caffeic Acids Esters from Zuccagnia Punctata Acting against Soybean Infecting Fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar] [CrossRef]
- Wang, J.P.; Tsao, L.T.; Raung, S.L.; Lin, C.N. Investigation of the Inhibitory Effect of Broussochalcone A on Respiratory Burst in Neutrophils. Eur. J. Pharmacol. 1997, 320, 201–208. [Google Scholar] [CrossRef]
- Ohnogi, H.; Kudo, Y.; Tahara, K.; Sugiyama, K.; Enoki, T.; Hayami, S.; Sagawa, H.; Tanimura, Y.; Aoi, W.; Naito, Y.; et al. Six New Chalcones from Angelica Keiskei Inducing Adiponectin Production in 3T3-L1 Adipocytes. Biosci. Biotechnol. Biochem. 2012, 76, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Inoue, J.; Tamura, Y.; Mizutani, K. Antioxidative components of Psoralea corylifolia (Leguminosae). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Tupe, S.G.; Gample, S.P.; Chandgude, M.G.; Sarkar, D.; Deshpande, M.V.; Joshi, S.P. Antifungal Dimeric Chalcone Derivative Kamalachalcone e from Mallotus Philippinensis. Nat. Prod. Res. 2014, 28, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, J.; Gerhauser, C. Anti-Proliferative and Apoptosis-Inducing Properties of Xanthohumol, a Prenylated Chalcone from Hops (Humulus Lupulus L.). In Natural Compounds as Inducers of Cell Death; Springer: Dordrecht, The Netherlands, 2012; Volume 1, pp. 69–93. [Google Scholar] [CrossRef]
- McRae, J.M.; Yang, Q.; Crawford, R.J.; Palombo, E.A. Acylated Flavonoid Tetraglycoside from Planchonia Careya Leaves. Phytochem. Lett. 2008, 1, 99–102. [Google Scholar] [CrossRef]
- Chung, M.-I.; Weng, J.-R.; Lai, M.-H.; Yen, M.-H.; Lin, C.-N. A New Chalcone, Xanthones, and a Xanthonolignoid from Hypericum geminiflorum. J. Nat. Prod. 1999, 62, 1033–1035. [Google Scholar] [CrossRef]
- Maxwell, C.A.; Hartwig, U.A.; Joseph, C.M.; Phillips, D.A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce Nod Genes of Rhizobium Meliloti. Plant Physiol 1989, 91, 842–847. [Google Scholar] [CrossRef]
- Nair, A.D.; Athira, C.K.; Manikandan, P.; Ramani, P. One-Pot Synthesis of Modified 4-Aryl-4H-Chromenes and Their Preliminary Anti-Cancer Studies. J. Indian Chem. Soc. 2019, 96, 19–22. [Google Scholar]
- Pandurangan, N.; Bose, C.; Banerji, A. Synthesis and Antioxygenic Activities of Seabuckthorn Flavone-3-Ols and Analogs. Bioorg. Med. Chem. Lett. 2011, 21, 5328–5330. [Google Scholar] [CrossRef]
- Hernawan; Purwono, B.; Triyono; Hanafi, M. The Use of Chitosan as a Solid Base Catalyst for the Chalcones Synthesis. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 2055. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Velty, A. Activated Hydrotalcites as Catalysts for the Synthesis of Chalcones of Pharmaceutical Interest. J. Catal. 2004, 221, 474–482. [Google Scholar] [CrossRef]
- Mohammad, S.A.G.; Khoerunnisa, F.; Rigolet, S.; Jean Daou, T.; Ling, T.C.; Ng, E.P. Hierarchical Cs–Pollucite Nanozeolite Modified with Novel Organosilane as an Excellent Solid Base Catalyst for Claisen–Schmidt Condensation of Benzaldehyde and Acetophenone. Processes 2020, 8, 96. [Google Scholar] [CrossRef]
- Winter, C.; Caetano, J.N.; Araújo, A.B.C.; Chaves, A.R.; Ostroski, I.C.; Vaz, B.G.; Pérez, C.N.; Alonso, C.G. Activated Carbons for Chalcone Production: Claisen-Schmidt Condensation Reaction. Chem. Eng. J. 2016, 303, 604–610. [Google Scholar] [CrossRef]
- Elamathi, P.; Chandrasekar, G.; Balamurali, M.M. Nanoporous AlSBA-15 Catalysed Claisen–Schmidt Condensation for the Synthesis of Novel and Biologically Active Chalcones. J. Porous Mater. 2020, 27, 817–829. [Google Scholar] [CrossRef]
- Rafiee, E.; Rahimi, F. A Green Approach to the Synthesis of Chalcones via Claisen-Schmidt Condensation Reaction Using Cesium Salts of 12-Tungstophosphoric Acid as a Reusable Nanocatalyst. Mon. Für Chem. Chem. Mon. 2013, 144, 361–367. [Google Scholar] [CrossRef]
- Das, S.; Porashar, B.; Saikia, S.; Borah, R. Brönsted Acidic Ionic Liquids Catalysed Sequential Michael-Like Addition of Indole with Chalcones via Claisen-Schmidt Condensation. ChemistrySelect 2020, 5, 3041–3047. [Google Scholar] [CrossRef]
- Sazegar, M.R.; Mahmoudian, S.; Mahmoudi, A.; Triwahyono, S.; Jalil, A.A.; Mukti, R.R.; Nazirah Kamarudin, N.H.; Ghoreishi, M.K. Catalyzed Claisen–Schmidt Reaction by Protonated Aluminate Mesoporous Silica Nanomaterial Focused on the (E)-Chalcone Synthesis as a Biologically Active Compound. RSC Adv. 2016, 6, 11023–11031. [Google Scholar] [CrossRef]
- Jioui, I.; Dânoun, K.; Solhy, A.; Jouiad, M.; Zahouily, M.; Essaid, B.; Len, C.; Fihri, A. Modified Fluorapatite as Highly Efficient Catalyst for the Synthesis of Chalcones via Claisen–Schmidt Condensation Reaction. J. Ind. Eng. Chem. 2016, 39, 218–225. [Google Scholar] [CrossRef]
- Heidarzadeh, T.; Nami, N.; Zareyee, D. Preparation of (MWCNTs)-COOH/CeO2Hybrid as an Efficient Catalyst for Claisen-Schmidt Condensation. J. Appl. Chem. Res. 2021, 15, 44–57. [Google Scholar]
- Ke, F.; Qiu, L.G.; Zhu, J. Fe3O4@MOF Core-Shell Magnetic Microspheres as Excellent Catalysts for the Claisen-Schmidt Condensation Reaction. Nanoscale 2014, 6, 1596–1601. [Google Scholar] [CrossRef]
- Paul, A.; Devi, M.; Dhar, S.S. Incorporation of Nanosized ZnWO4 and Fe3O4 on Graphitic Carbon Nitride to Fabricate a Novel, Highly Active Magnetically Recoverable Catalyst in Claisen–Schmidt Condensation. J. Phys. Chem. Solids 2020, 136, 109117. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Han, H.; Liu, Y.; Song, J.; Guo, W.; Chu, W.; Sun, Z. Graphene-Supported ZnO Nanoparticles: An Efficient Heterogeneous Catalyst for the Claisen-Schmidt Condensation Reaction without Additional Base. Tetrahedron Lett. 2017, 58, 3984–3988. [Google Scholar] [CrossRef]
- Srivastava, Y.K. Ecofriendly Microwave Assisted Synthesis of Some Chalcones. Rasayan J. Chem. 2008, 1, 884–886. [Google Scholar]
- Farooq, S.; Ngaini, Z.; Mortadza, N.A. Microwave-Assisted Synthesis and Molecular Docking Study of Heteroaromatic Chalcone Derivatives as Potential Antibacterial Agents. Bull. Korean Chem. Soc. 2020, 41, 918–924. [Google Scholar] [CrossRef]
- Calvino, V.; Picallo, M.; López-Peinado, A.J.; Martín-Aranda, R.M.; Durán-Valle, C.J. Ultrasound Accelerated Claisen–Schmidt Condensation: A Green Route to Chalcones. Appl. Surf. Sci. 2006, 252, 6071–6074. [Google Scholar] [CrossRef]
- Perozo-Rondón, E.; Martín-Aranda, R.M.; Casal, B.; Durán-Valle, C.J.; Lau, W.N.; Zhang, X.F.; Yeung, K.L. Sonocatalysis in Solvent Free Conditions: An Efficient Eco-Friendly Methodology to Prepare Chalcones Using a New Type of Amino Grafted Zeolites. Catal. Today 2006, 114, 183–187. [Google Scholar] [CrossRef]
- Homerin, G.; Nica, A.S.; Farce, A.; Dubois, J.; Ghinet, A. Ultrasounds-Mediated 10-Seconds Synthesis of Chalcones as Potential Farnesyltransferase Inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127149. [Google Scholar] [CrossRef]
- Kumar, S.; Lamba, M.S.; Makrandi, J.K. An Efficient Green Procedure for the Synthesis of Chalcones Using C-200 as Solid Support under Grinding Conditions. Green Chem. Lett. Rev. 2008, 1, 123–125. [Google Scholar] [CrossRef]
- Yeshwant, S.M.; Nanded, M.; Nalwar, Y.; Zangade, S.; Mokle, S.; Vibhute, A.; Vibhute, Y. An Efficient and Operationally Simple Synthesis of Some New Chalcones by Using Grinding Technique. Chem. Sci. J. 2009, 2011, 13. [Google Scholar]
- Al-Masum, M.; Ng, E.; Wai, M.C. Palladium-Catalyzed Direct Cross-Coupling of Potassium Styryltrifluoroborates and Benzoyl Chlorides—A One Step Method for Chalcone Synthesis. Tetrahedron Lett. 2011, 52, 1008–1010. [Google Scholar] [CrossRef]
- Diana, E.J.; Kanchana, U.S.; Mathew, T.V.; Anilkumar, G. Recent Developments in the Metal Catalysed Cross-Coupling Reactions for the Synthesis of the Enone System of Chalcones. Appl. Organomet. Chem. 2020, 34, e5987. [Google Scholar] [CrossRef]
- Braun, R.U.; Ansorge, M.; Müller, T.J.J. Coupling-Isomerization Synthesis of Chalcones. Chemistry 2006, 12, 9081–9094. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Ötvös, S.B.; Wu, Y.C.; Mándity, I.M.; Chang, F.R.; Fülöp, F. Highly Selective Continuous-Flow Synthesis of Potentially Bioactive Deuterated Chalcone Derivatives. ChemPlusChem 2015, 80, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.L.N.; Venkatesh, V.; Jadhav, D.N. A Palladium Catalyzed Atom-Efficient Cross-Coupling Reactivity of Triarylbismuths with α,β-Unsaturated Acyl Chlorides. J. Organomet. Chem. 2008, 693, 2494–2498. [Google Scholar] [CrossRef]
- Yamakawa, T.; Kinoshita, H.; Miura, K. Synthetic Utility of Tribenzyltin Hydride and Its Derivatives as Easily Accessible, Removable, and Decomposable Organotin Reagents. J. Organomet. Chem. 2013, 724, 129–134. [Google Scholar] [CrossRef]
- Kim, S.; Bae, S.W.; Lee, J.S.; Park, J. Recyclable Gold Nanoparticle Catalyst for the Aerobic Alcohol Oxidation and C–C Bond Forming Reaction between Primary Alcohols and Ketones under Ambient Conditions. Tetrahedron 2009, 65, 1461–1466. [Google Scholar] [CrossRef]
- Li, C.J. Cross-Dehydrogenative Coupling (CDC): Exploring C-C Bond Formations beyond Functional Group Transformations. Acc. Chem. Res. 2009, 42, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, S.; Tripathi, V.D.; Srivastava, S. Synthesis of Chalcones and Flavanones Using Julia–Kocienski Olefination. Tetrahedron 2010, 66, 9445–9449. [Google Scholar] [CrossRef]
- Tan, P.; Wang, S.R. Reductive (3 + 2) Annulation of Benzils with Pyrylium Salts: Stereoselective Access to Furyl Analogues of Cis-Chalcones. Org. Lett. 2019, 21, 6029–6033. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-Cancer Chalcones: Structural and Molecular Target Perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.T.; Kagan, J. The Photochemical Synthesis of 2′-Hydroxychalcones from Phenyl Cinnamates. J. Org. Chem. 1970, 35, 2901–2904. [Google Scholar] [CrossRef]
- Wang, C.Q. Solvent-Free Stereoselective Synthesis of Chalcones via Wittig Reaction of Arsonium Ylide by Grinding. In Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2014; Volume 864, pp. 2132–2135. [Google Scholar]
- Huang, Z.; Wang, L.; Huang, X. Stereoselective Synthesis of α-Bromo-α,β-Unsaturated Ketones via Wittig Reaction. Synth. Commun. 2006, 33, 757–762. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2021, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Banik, B.K.; Mahato, A.K.; Shanthi, C.N.; Mohantad, B.C. Microwave-assisted synthesis of antitubercular agents: A novel approach. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design; Elsevier: Amsterdam, The Netherlands, 2020; pp. 779–818. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, R.; Kant, R. Synthesis, Molecular Docking, and Evaluation of Triazole and Chalcone Conjugate as Antitubercular Agent. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Bhoot, D.; Khunt, R.C.; Parekh, H.H. Synthesis and Biological Evaluation of Chalcones and Acetyl Pyrazoline Derivatives Comprising Furan Nucleus as an Antitubercular Agents. Med. Chem. Res. 2012, 21, 3233–3239. [Google Scholar] [CrossRef]
- Muradás, T.C.; Abbadi, B.L.; Villela, A.D.; Macchi, F.S.; Bergo, P.F.; de Freitas, T.F.; Sperotto, N.D.M.; Timmers, L.F.S.M.; de Souza, O.N.; Picada, J.N.; et al. Pre-Clinical Evaluation of Quinoxaline-Derived Chalcones in Tuberculosis. PLoS ONE 2018, 13, e0202568. [Google Scholar] [CrossRef]
- Rao, N.S.; Shaik, A.B.; Routhu, S.R.; Hussaini, S.M.A.; Sunkari, S.; Rao, A.V.S.; Reddy, A.M.; Alarifi, A.; Kamal, A. New Quinoline Linked Chalcone and Pyrazoline Conjugates: Molecular Properties Prediction, Antimicrobial and Antitubercular Activities. ChemistrySelect 2017, 2, 2989–2996. [Google Scholar] [CrossRef]
- Yadav, D.K.; Ahmad, I.; Shukla, A.; Khan, F.; Negi, A.S.; Gupta, A. QSAR and Docking Studies on Chalcone Derivatives for Antitubercular Activity against M.Tuberculosis H37Rv. J. Chemom. 2014, 28, 499–507. [Google Scholar] [CrossRef]
- Mujahid, M.; Yogeeswari, P.; Sriram, D.; Basavanag, U.M.V.; Díaz-Cervantes, E.; Córdoba-Bahena, L.; Robles, J.; Gonnade, R.G.; Karthikeyan, M.; Vyas, R.; et al. Spirochromone-chalcone conjugates as antitubercular agents: Synthesis, bio evaluation and molecular modeling studies. RSC Adv. 2015, 5, 106448–106460. [Google Scholar] [CrossRef]
- Anandam, R.; Jadav, S.S.; Ala, V.B.; Ahsan, M.J.; Bollikolla, H.B. Synthesis of New C-Dimethylated Chalcones as Potent Antitubercular Agents. Med. Chem. Res. 2018, 27, 1690–1704. [Google Scholar] [CrossRef]
- Hans, R.H.; Guantai, E.M.; Lategan, C.; Smith, P.J.; Wan, B.; Franzblau, S.G.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis, Antimalarial and Antitubercular Activity of Acetylenic Chalcones. Bioorg. Med. Chem. Lett. 2010, 20, 942–944. [Google Scholar] [CrossRef]
- Kasetti, A.B.; Singhvi, I.; Nagasuri, R.; Bhandare, R.R.; Shaik, A.B. Thiazole–Chalcone Hybrids as Prospective Antitubercular and Antiproliferative Agents: Design, Synthesis, Biological, Molecular Docking Studies and In Silico ADME Evaluation. Molecules 2021, 26, 2847. [Google Scholar] [CrossRef]
- Trivedi, J.C.; Bariwal, J.B.; Upadhyay, K.D.; Naliapara, Y.T.; Joshi, S.K.; Pannecouque, C.C.; de Clercq, E.; Shah, A.K. Improved and Rapid Synthesis of New Coumarinyl Chalcone Derivatives and Their Antiviral Activity. Tetrahedron Lett. 2007, 48, 8472–8474. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Al-Hashimi, I.; Al Moustafa, A.E.; Khalil, A. A comprehensive review on the antiviral activities of chalcones. J. Drug Target. 2021, 29, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Onyilagha, J.C.; Malhotra, B.; Elder, M.; French, C.J.; Towers, G.N. Comparative studies of inhibitory activities of chalcones on tomato ringspot virus (ToRSV). Can. J. Plant Pathol. 1997, 19, 133–137. [Google Scholar] [CrossRef]
- Cole, A.L.; Hossain, S.; Cole, A.M.; Phanstiel, O. Synthesis and Bioevaluation of Substituted Chalcones, Coumaranones and Other Flavonoids as Anti-HIV Agents. Bioorg. Med. Chem. 2016, 24, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xie, D.; He, F.; Song, B.; Hu, D. Antiviral Properties and Interaction of Novel Chalcone Derivatives Containing a Purine and Benzenesulfonamide Moiety. Bioorg. Med. Chem. Lett. 2018, 28, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Wang, Y.; Hu, D.; Song, B. Design, Synthesis, and Antiviral Activity of Novel Chalcone Derivatives Containing a Purine Moiety. Chin. J. Chem. 2017, 35, 665–672. [Google Scholar] [CrossRef]
- Mateeva, N.; Eyunni, S.V.K.; Redda, K.K.; Ononuju, U.; Hansberry, T.D.; Aikens, C.; Nag, A. Functional Evaluation of Synthetic Flavonoids and Chalcones for Potential Antiviral and Anticancer Properties. Bioorg. Med. Chem. Lett. 2017, 27, 2350–2356. [Google Scholar] [CrossRef]
- Duran, N.; Polat, M.F.; Aktas, D.A.; Alagoz, M.A.; Ay, E.; Cimen, F.; Tek, E.; Anil, B.; Burmaoglu, S.; Algul, O. New Chalcone Derivatives as Effective against SARS-CoV-2 Agent. Int. J. Clin. Pract. 2021, 75, e14846. [Google Scholar] [CrossRef]
- Tang, X.; Su, S.; Chen, M.; He, J.; Xia, R.; Guo, T.; Chen, Y.; Zhang, C.; Wang, J.; Xue, W. Novel chalcone derivatives containing a 1, 2, 4-triazine moiety: Design, synthesis, antibacterial and antiviral activities. RSC Adv. 2019, 9, 6011–6020. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhou, D.G.; He, F.C.; Chen, J.X.; Chen, Y.Z.; Gan, X.H.; Hu, D.Y.; Song, B.A. Synthesis and Antiviral Bioactivity of Novel Chalcone Derivatives Containing Purine Moiety. Chin. Chem. Lett. 2018, 29, 127–130. [Google Scholar] [CrossRef]
- Gan, X.; Hu, D.; Chen, Z.; Wang, Y.; Song, B. Synthesis and Antiviral Evaluation of Novel 1,3,4-Oxadiazole/Thiadiazole-Chalcone Conjugates. Bioorg. Med. Chem. Lett. 2017, 27, 4298–4301. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, B.M.; Fanelli, A.; Piccinino, D.; De Angelis, M.; Dolfa, C.; Palamara, A.T.; Nencioni, L.; Zippilli, C.; Crucianelli, M.; Saladino, R.; et al. Synthesis of stilbene and chalcone inhibitors of influenza A virus by SBA-15 supported Hoveyda-Grubbs metathesis. Catalysts 2019, 9, 983. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Hu, D.; Dong, L.; Pan, J.; Luo, L.; Zhang, W.; Xue, W.; Jin, L.; Song, B. Synthesis, Antiviral Activity, and 3D-QSAR Study of Novel Chalcone Derivatives Containing Malonate and Pyridine Moieties. Arab. J. Chem. 2019, 12, 2685–2696. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, D.; Li, P.; Xie, D.; Gan, X. Synthesis, antiviral bioactivity of novel 4-thioquinazoline derivatives containing chalcone moiety. Molecules 2015, 20, 11861–11874. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Mishra, N.; Kumar, B.; Sharma, M.; Bhattacharya, A.; Mishra, L.C.; Bhasin, V.K. Potent Antimalarial Activity of Newly Synthesized Substituted Chalcone Analogs in Vitro. Med. Chem. Res. 2009, 18, 407–420. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhang, Z.W.; Lekkala, R.; Alsulami, H.; Rakesh, K.P. Chalcone Hybrids as Privileged Scaffolds in Antimalarial Drug Discovery: A Key Review. Eur. J. Med. Chem. 2020, 193, 112215. [Google Scholar] [CrossRef]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Vélez, I.D.; Upegui, Y.; Muñoz, J.A.; et al. An Efficient Synthesis of New Caffeine-Based Chalcones, Pyrazolines and Pyrazolo[3,4-b][1,4]Diazepines as Potential Antimalarial, Antitrypanosomal and Antileishmanial Agents. Eur. J. Med. Chem. 2015, 93, 401–413. [Google Scholar] [CrossRef]
- De Oliveira, M.E.; Cenzi, G.; Nunes, R.R.; Andrighetti, C.R.; Valadão, D.M.D.S.; Dos Reis, C.; Simões, C.M.O.; Nunes, R.J.; Junior, M.C.; Taranto, A.G.; et al. Antimalarial activity of 4-metoxychalcones: Docking studies as falcipain/plasmepsin inhibitors, ADMET and lipophilic efficiency analysis to identify a putative oral lead candidate. Molecules 2013, 18, 15276–15287. [Google Scholar] [CrossRef]
- Yadav, N.; Dixit, S.K.; Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Antimalarial Activity of Newly Synthesized Chalcone Derivatives In Vitro. Chem. Biol. Drug Des. 2012, 80, 340–347. [Google Scholar] [CrossRef]
- Hameed, A.; Masood, S.; Hameed, A.; Ahmed, E.; Sharif, A.; Abdullah, M.I. Anti-Malarial, Cytotoxicity and Molecular Docking Studies of Quinolinyl Chalcones as Potential Anti-Malarial Agent. J. Comput. Mol. Des. 2019, 33, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Gayam, V.; Ravi, S. Cinnamoylated Chloroquine Analogues: A New Structural Class of Antimalarial Agents. Eur. J. Med. Chem. 2017, 135, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, R.; Biot, C.; Delaney, G.; Roussel, P.; Pascual, A.; Pradines, B.; Klahn, A.H. Cyrhetrenyl Chalcones: Synthesis, Characterization and Antimalarial Evaluation. J. Organomet. Chem. 2013, 723, 143–148. [Google Scholar] [CrossRef]
- Syahri, J.; Nasution, H.; Nurohmah, B.A.; Purwono, B.; Yuanita, E.; Zakaria, N.H.; Hassan, N.I. Design, synthesis and biological evaluation of aminoalkylated chalcones as antimalarial agent. Sains Malays. 2020, 49, 2667–2677. [Google Scholar] [CrossRef]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Smith, P.J.; Chibale, K. Design, Synthesis and in Vitro Antimalarial Evaluation of Triazole-Linked Chalcone and Dienone Hybrid Compounds. Bioorg. Med. Chem. 2010, 18, 8243–8256. [Google Scholar] [CrossRef]
- Gutteridge, C.E.; Thota, D.S.; Curtis, S.M.; Kozar, M.P.; Li, Q.; Xie, L.; Zhang, J.; Melendez, V.; Asher, C.O.; Luong, T.T.; et al. In Vitro Biotransformation, in Vivo Efficacy and Pharmacokinetics of Antimalarial Chalcones. Pharmacology 2011, 87, 96–104. [Google Scholar] [CrossRef]
- Kumar, R.; Mohanakrishnan, D.; Sharma, A.; Kaushik, N.K.; Kalia, K.; Sinha, A.K.; Sahal, D. Reinvestigation of Structure–Activity Relationship of Methoxylated Chalcones as Antimalarials: Synthesis and Evaluation of 2,4,5-Trimethoxy Substituted Patterns as Lead Candidates Derived from Abundantly Available Natural β-Asarone. Eur. J. Med. Chem. 2010, 45, 5292–5301. [Google Scholar] [CrossRef]
- Tadigoppula, N.; Korthikunta, V.; Gupta, S.; Kancharla, P.; Khaliq, T.; Soni, A.; Srivastava, R.K.; Srivastava, K.; Puri, S.K.; Raju, K.S.R.; et al. Synthesis and Insight into the Structure-Activity Relationships of Chalcones as Antimalarial Agents. J. Med. Chem. 2013, 56, 31–45. [Google Scholar] [CrossRef]
- Sarveswari, S.; Vijayakumar, V.; Siva, R.; Priya, R. Synthesis of 4-Hydroxy-2(1h)-Quinolone Derived Chalcones, Pyrazolines and Their Antimicrobial, in Silico Antimalarial Evaluations. Appl. Biochem. Biotechnol. 2015, 175, 43–64. [Google Scholar] [CrossRef]
- Tomar, V.; Bhattacharjee, G.; Kamaluddin; Rajakumar, S.; Srivastava, K.; Puri, S. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J. Med. Chem. 2010, 45, 745–751. [Google Scholar] [CrossRef]
- Syahri, J.; Rullah, K.; Armunanto, R.; Yuanita, E.; Nurohmah, B.A.; Aluwi, M.F.F.M.; Kok, L.; Wai, B.P. Synthesis, biological evaluation, QSAR analysis, and molecular docking of chalcone derivatives for antimalarial activity. Parasite 2016, 4, 8. [Google Scholar] [CrossRef]
- Smit, F.J.; N’Da, D.D. Synthesis, in vitro antimalarial activity and cytotoxicity of novel 4-aminoquinolinyl-chalcone amides. Bioorg. Med. Chem. 2014, 22, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Jyoti; Gaur, R.; Kumar, Y.; Cheema, H.S.; Kapkoti, D.S.; Darokar, M.P.; Khan, F.; Bhakuni, R.S. Synthesis, Molecular Modelling Studies of Indolyl Chalcone Derivatives and Their Antimalarial Activity Evaluation. Nat. Prod. Res. 2021, 35, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Mouzié, C.M.; Guefack, M.-G.F.; Kianfé, B.Y.; Serondo, H.U.; Ponou, B.K.; Siwe-Noundou, X.; Teponno, R.B.; Krause, R.W.M.; Kuete, V.; Tapondjou, L.A. A New Chalcone and Antimicrobial Chemical Constituents of Dracaena Stedneuri. Pharmaceuticals 2022, 15, 725. [Google Scholar] [CrossRef]
- Lagu, S.B.; Yejella, R.P.; Bhandare, R.R.; Shaik, A.B. Design, Synthesis, and Antibacterial and Antifungal Activities of Novel Trifluoromethyl and Trifluoromethoxy Substituted Chalcone Derivatives. Pharmaceuticals 2020, 13, 375. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Prabhawathi, V.; Doble, M. Antibacterial Activity and QSAR of Chalcones against Biofilm-Producing Bacteria Isolated from Marine Waters. SAR QSAR Environ. Res. 2010, 21, 247–263. [Google Scholar] [CrossRef]
- Satokata, A.A.C.; Souza, J.H.; Silva, L.L.O.; Santiago, M.B.; Ramos, S.B.; de Assis, L.R.; Theodoro, R.D.S.; e Oliveira, L.R.; Regasini, L.O.; Martins, C.H.G. Chalcones with potential antibacterial and antibiofilm activities against periodontopathogenic bacteria. Anaerobe 2022, 76, 102588. [Google Scholar] [CrossRef]
- Moreira Osório, T.; Delle Monache, F.; Domeneghini Chiaradia, L.; Mascarello, A.; Regina Stumpf, T.; Roberto Zanetti, C.; Bardini Silveira, D.; Regina Monte Barardi, C.; de Fatima Albino Smânia, E.; Viancelli, A.; et al. Antibacterial Activity of Chalcones, Hydrazones and Oxadiazoles against Methicillin-Resistant Staphylococcus Aureus. Bioorg. Med. Chem. Lett. 2012, 22, 225–230. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Ganesan, S.; Veluchamy, P.; Doble, M. Novel Chalcones and 1,3,5-Triphenyl-2-Pyrazoline Derivatives as Antibacterial Agents. Chem. Biol. Drug Des. 2010, 76, 407–411. [Google Scholar] [CrossRef]
- Li, Y.; Sun, B.; Zhai, J.; Fu, L.; Zhang, S.; Zhang, J.; Liu, H.; Xie, W.; Deng, H.; Chen, Z.; et al. Synthesis and Antibacterial Activity of Four Natural Chalcones and Their Derivatives. Tetrahedron Lett. 2019, 60, 151165. [Google Scholar] [CrossRef]
- Abdula, A.; Abdula, A.M. Synthesis, Characterization and Antibacterial Activity of (E)-Chalcone Derivatives. Eur. J. Chem. 2013, 4, 207–210. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Rao, K.B.; Kushwaha, P.; Modukuri, R.K.; Singh, P.; Soni, I.; Shukla, P.K.; Chopra, S.; Pasupuleti, M. Novel Chalcone-Thiazole Hybrids as Potent Inhibitors of Drug Resistant Staphylococcus Aureus. ACS Med. Chem. Lett. 2015, 6, 809–813. [Google Scholar] [CrossRef]
- Venkatesan, P.; Sumathi, S. Piperidine Mediated Synthesis of N-heterocyclic Chalcones and Their Antibacterial Activity. J. Heterocycl. Chem. 2009, 47, 81–84. [Google Scholar] [CrossRef]
- Dkhar, L.; Banothu, V.; Pinder, E.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Ru, Rh and Ir Metal Complexes of Pyridyl Chalcone Derivatives: Their Potent Antibacterial Activity, Comparable Cytotoxicity Potency and Selectivity to Cisplatin. Polyhedron 2020, 185, 114606. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A. Synthesis and anti-bacterial activities of a bis-chalcone derived from thiophene and its bis-cyclized products. Molecules 2011, 16, 523–531. [Google Scholar] [CrossRef]
- Tran, T.-D.; Nguyen, T.-T.; Do, T.-H.; Huynh, T.-N.; Tran, C.-D.; Thai, K.-M. Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Jiang, S.M.; Chen, Z.H.; Ye, B.J.; Piao, H.R. Synthesis and Anti-Bacterial Activity of Some Heterocyclic Chalcone Derivatives Bearing Thiofuran, Furan, and Quinoline Moieties. Arch. der Pharm. 2011, 344, 689–695. [Google Scholar] [CrossRef]
- Chu, W.C.; Bai, P.Y.; Yang, Z.Q.; Cui, D.Y.; Hua, Y.G.; Yang, Y.; Yang, Q.Q.; Zhang, E.; Qin, S. Synthesis and Antibacterial Evaluation of Novel Cationic Chalcone Derivatives Possessing Broad Spectrum Antibacterial Activity. Eur. J. Med. Chem. 2018, 143, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana Reddy, P.O.; Hridhay, M.; Nikhil, K.; Khan, S.; Jha, P.N.; Shah, K.; Kumar, D. Synthesis and Investigations into the Anticancer and Antibacterial Activity Studies of β-Carboline Chalcones and Their Bromide Salts. Bioorg. Med. Chem. Lett. 2018, 28, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Zheng, C.J.; Sun, L.P.; Piao, H.R. Synthesis of New Chalcone Derivatives Containing a Rhodanine-3-Acetic Acid Moiety with Potential Anti-Bacterial Activity. Eur. J. Med. Chem. 2010, 45, 5739–5743. [Google Scholar] [CrossRef] [PubMed]
- Santosh, R.; Selvam, M.K.; Kanekar, S.U.; Nagaraja, G.K. Synthesis, Characterization, Antibacterial and Antioxidant Studies of Some Heterocyclic Compounds from Triazole-Linked Chalcone Derivatives. ChemistrySelect 2018, 3, 6338–6343. [Google Scholar] [CrossRef]
- Rani, A.; Singh, A.; Kaur, J.; Singh, G.; Bhatti, R.; Gumede, N.; Kisten, P.; Singh, P.; Kumar, V. 1H-1, 2, 3-triazole grafted tacrine-chalcone conjugates as potential cholinesterase inhibitors with the evaluation of their behavioral tests and oxidative stress in mice brain cells. Bioorg. Chem. 2021, 114, 105053. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Haridas, A.; Uçar, G.; Baysal, I.; Joy, M.; Mathew, G.E.; Lakshmanan, B.; Jayaprakash, V. Synthesis, Biochemistry, and Computational Studies of Brominated Thienyl Chalcones: A New Class of Reversible MAO-B Inhibitors. ChemMedChem 2016, 11, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Rampa, A.; Montanari, S.; Pruccoli, L.; Bartolini, M.; Falchi, F.; Feoli, A.; Cavalli, A.; Belluti, F.; Gobbi, S.; Tarozzi, A.; et al. Chalcone-Based Carbamates for Alzheimer’s Disease Treatment. Futur. Med. Chem. 2017, 9, 749–764. [Google Scholar] [CrossRef]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and Its Analogs: Therapeutic and Diagnostic Applications in Alzheimer’s Disease. Bioorg. Chem. 2021, 108, 104681. [Google Scholar] [CrossRef]
- Zhang, X.; Rakesh, K.P.; Bukhari, S.N.A.; Balakrishna, M.; Manukumar, H.M.; Qin, H.L. Multi-Targetable Chalcone Analogs to Treat Deadly Alzheimer’s Disease: Current View and Upcoming Advice. Bioorg. Chem. 2018, 80, 86–93. [Google Scholar] [CrossRef]
- Cong, L.; Dong, X.; Wang, Y.; Deng, Y.; Li, B.; Dai, R. On the Role of Synthesized Hydroxylated Chalcones as Dual Functional Amyloid-β Aggregation and Ferroptosis Inhibitors for Potential Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 166, 11–21. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhou, L.Y.; Tan, R.X.; Liang, G.P.; Fang, S.X.; Li, W.; Xie, M.; Wen, Y.H.; Wu, J.Q.; Chen, Y.P. Design, Synthesis, and Evaluation of Chalcone Derivatives as Multifunctional Agents against Alzheimer’s Disease. Chem. Biodivers. 2021, 18, e2100341. [Google Scholar] [CrossRef]
- Bai, P.; Wang, K.; Zhang, P.; Shi, J.; Cheng, X.; Zhang, Q.; Zheng, C.; Cheng, Y.; Yang, J.; Lu, X.; et al. Development of Chalcone-O-Alkylamine Derivatives as Multifunctional Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 183, 111737. [Google Scholar] [CrossRef]
- Lee, D.S.; Jeong, G.S. Butein Provides Neuroprotective and Anti-neuroinflammatory Effects through Nrf2/ARE-dependent Haem Oxygenase 1 Expression by Activating the PI3K/Akt Pathway. Br. J. Pharmacol. 2016, 173, 2894. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An Important Scaffold for Medicinal Chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchio, F.; Baudry, A.; Lepiniec, L.; Grotewold, E. The regulation of flavonoid biosynthesis. In The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 97–122. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.L.S.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Zachariah, S.M.; Ramani, P. 4-Aryl-4H-Chromene-3-Carbonitrile Derivates: Synthesis and Preliminary Anti-Breast Cancer Studies. J. Heterocycl. Chem. 2016, 53, 1778–1782. [Google Scholar] [CrossRef]
- Doroghazi, J.R.; Albright, J.C.; Goering, A.W.; Ju, K.S.; Haines, R.R.; Tchalukov, K.A.; Labeda, D.P.; Kelleher, N.L.; Metcalf, W.W. A Roadmap for Natural Product Discovery Based on Large-Scale Genomics and Metabolomics. Nat. Chem. Biol. 2014, 10, 963–968. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, E.; Baek, K.H.; Kwon, H.B.; Woo, H.; Lee, E.S.; Kwon, Y.; Na, Y. Chalcones, Inhibitors for Topoisomerase I and Cathepsin B and L, as Potential Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2013, 23, 3320–3324. [Google Scholar] [CrossRef]
- Wang, G.; Liu, W.; Gong, Z.; Huang, Y.; Li, Y.; Peng, Z. Design, Synthesis, Biological Evaluation and Molecular Docking Studies of New Chalcone Derivatives Containing Diaryl Ether Moiety as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Bioorg. Chem. 2020, 95, 103565. [Google Scholar] [CrossRef]
- Wang, G.; Liu, W.; Gong, Z.; Huang, Y.; Li, Y.; Peng, Z. Synthesis, Biological Evaluation, and Molecular Modelling of New Naphthalene-Chalcone Derivatives as Potential Anticancer Agents on MCF-7 Breast Cancer Cells by Targeting Tubulin Colchicine Binding Site. J. Enzym. Inhib. Med. Chem. 2020, 35, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mokale, S.N.; Dube, P.N.; Bhavale, S.A.; Sayed, I.; Begum, A.; Nevase, M.C.; Shelke, V.R.; Mujaheed, A. Synthesis, in-Vitro Screening, and Docking Analysis of Novel Pyrrolidine and Piperidine-Substituted Ethoxy Chalcone as Anticancer Agents. Med. Chem. Res. 2015, 24, 1842–1856. [Google Scholar] [CrossRef]
- Khanapure, S.; Jagadale, M.; Bansode, P.; Choudhari, P.; Rashinkar, G. Anticancer Activity of Ruthenocenyl Chalcones and Their Molecular Docking Studies. J. Mol. Struct. 2018, 1173, 142–147. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.J.; Sun, J.; Yang, X.H.; Zhao, T.T.; Lu, X.; Gong, H.B.; Zhu, H.L. Design, Synthesis and Biological Evaluation of Novel Chalcone Derivatives as Antitubulin Agents. Bioorg. Med. Chem. 2012, 20, 3212–3218. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.B.; Reddy, M.B.M.; Reddy, R.; Chhajed, S.; Gupta, P.P. Molecular Docking, PKPD, and Assessment of Toxicity of Few Chalcone Analogues as EGFR Inhibitor in Search of Anticancer Agents. Struct. Chem. 2020, 31, 2249–2255. [Google Scholar] [CrossRef]
- Sharma, A.; Chakravarti, B.; Gupt, M.P.; Siddiqui, J.A.; Konwar, R.; Tripathi, R.P. Synthesis and Anti Breast Cancer Activity of Biphenyl Based Chalcones. Bioorg. Med. Chem. 2010, 18, 4711–4720. [Google Scholar] [CrossRef]
- Lu, C.-F.; Wang, S.-H.; Pang, X.-J.; Zhu, T.; Li, H.-L.; Li, Q.-R.; Li, Q.-Y.; Gu, Y.-F.; Mu, Z.-Y.; Jin, M.-J.; et al. Synthesis and biological evaluation of amino chalcone derivatives as antiproliferative agents. Molecules 2020, 25, 5530. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Hager, E.; Pettit, C.; Gurulingappa, H.; Davidson, N.E.; Khan, S.R. Design, synthesis, and evaluation of novel boronic-chalcone derivatives as antitumor agents. J. Med.Chem. 2003, 46, 2813–2815. [Google Scholar] [CrossRef] [PubMed]
- Ngameni, B.; Cedric, K.; Mbaveng, A.T.; Erdoğan, M.; Simo, I.; Kuete, V.; Daştan, A. Design, Synthesis, Characterization, and Anticancer Activity of a Novel Series of O-Substituted Chalcone Derivatives. Bioorg. Med. Chem. Lett. 2021, 35, 127827. [Google Scholar] [CrossRef]
- Kumar, D.; Raj, K.K.; Malhotra, S.V.; Rawat, D.S. Synthesis and anticancer activity evaluation of resveratrol–chalcone conjugates. MedChemComm 2014, 5, 528–535. [Google Scholar] [CrossRef]
- Saxena, H.O.; Faridi, U.; Kumar, J.K.; Luqman, S.; Darokar, M.P.; Shanker, K.; Chanotiya, C.S.; Gupta, M.M.; Negi, A.S. Synthesis of Chalcone Derivatives on Steroidal Framework and Their Anticancer Activities. Steroids 2007, 72, 892–900. [Google Scholar] [CrossRef]
- Shenvi, S.; Kumar, K.; Hatti, K.S.; Rijesh, K.; Diwakar, L.; Reddy, G.C. Synthesis, Anticancer and Antioxidant Activities of 2,4,5-Trimethoxy Chalcones and Analogues from Asaronaldehyde: Structure–Activity Relationship. Eur. J. Med. Chem. 2013, 62, 435–442. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Ko, P.W.; Chang, Y.J.; Kapoor, M.; Liang, Y.C.; Chu, H.L.; Lin, H.H.; Horng, J.C.; Hsu, M.H. Design and synthesis of benzimidazole-chalcone derivatives as potential anticancer agents. Molecules 2019, 24, 3259. [Google Scholar] [CrossRef]
- Kamal, A.; Reddy, J.S.; Ramaiah, M.J.; Dastagiri, D.; Bharathi, E.V.; Sagar, M.V.P.; Pushpavalli, S.N.C.V.L.; Ray, P.; Pal-Bhadra, M. Design, synthesis and biological evaluation of imidazopyridine/pyrimidine-chalcone derivatives as potential anticancer agents. MedChemComm 2010, 1, 355–360. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Masaret, G.S.; Muhammad, Z.A.; Harras, M.F. Discovery of Thiazole-Based-Chalcones and 4-Hetarylthiazoles as Potent Anticancer Agents: Synthesis, Docking Study and Anticancer Activity. Bioorg. Chem. 2020, 98, 103761. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Shang, Z.P.; Jantan, I.; Tan, O.U.; Hussain, M.A.; Sher, M.; Bukhari, S.N.A. Molecular Docking Studies and Biological Evaluation of Chalcone Based Pyrazolines as Tyrosinase Inhibitors and Potential Anticancer Agents. RSC Adv. 2015, 5, 46330–46338. [Google Scholar] [CrossRef]
- Sankappa Rai, U.; Isloor, A.M.; Shetty, P.; Pai, K.S.R.; Fun, H.K. Synthesis and in Vitro Biological Evaluation of New Pyrazole Chalcones and Heterocyclic Diamides as Potential Anticancer Agents. Arab. J. Chem. 2015, 8, 317–321. [Google Scholar] [CrossRef]
- Madhavi, S.; Sreenivasulu, R.; Yazala, J.P.; Raju, R.R. Synthesis of Chalcone Incorporated Quinazoline Derivatives as Anticancer Agents. Saudi Pharm. J. 2017, 25, 275–279. [Google Scholar] [CrossRef]
- Mirzaei, S.; Hadizadeh, F.; Eisvand, F.; Mosaffa, F.; Ghodsi, R. Synthesis, Structure-Activity Relationship and Molecular Docking Studies of Novel Quinoline-Chalcone Hybrids as Potential Anticancer Agents and Tubulin Inhibitors. J. Mol. Struct. 2020, 1202, 127310. [Google Scholar] [CrossRef]
- Kamal, A.; Srinivasulu, V.; Nayak, V.L.; Sathish, M.; Shankaraiah, N.; Bagul, C.; Reddy, N.V.S.; Rangaraj, N.; Nagesh, N. Design and Synthesis of C3-Pyrazole/Chalcone-Linked Beta-Carboline Hybrids: Antitopoisomerase I, DNA-Interactive, and Apoptosis-Inducing Anticancer Agents. ChemMedChem 2014, 9, 2084–2098. [Google Scholar] [CrossRef]
- Alswah, M.; Bayoumi, A.H.; Elgamal, K.; Elmorsy, A.; Ihmaid, S.; Ahmed, H.E. Design, synthesis and cytotoxic evaluation of novel chalcone derivatives bearing triazolo [4,3-a]-quinoxaline moieties as potent anticancer agents with dual EGFR kinase and tubulin polymerization inhibitory effects. Molecules 2017, 23, 48. [Google Scholar] [CrossRef]
- Suma, V.R.; Sreenivasulu, R.; Rao, M.V.B.; Subramanyam, M.; Ahsan, M.J.; Alluri, R.; Rao, K.R.M. Design, Synthesis, and Biological Evaluation of Chalcone-Linked Thiazole-Imidazopyridine Derivatives as Anticancer Agents. Med. Chem. Res. 2020, 29, 1643–1654. [Google Scholar] [CrossRef]
- Ammar, Y.A.; Fayed, E.A.; Bayoumi, A.H.; Ezz, R.R.; Alsaid, M.S.; Soliman, A.M.; Ghorab, M.M. New Chalcones Bearing Isatin Scaffold: Synthesis, Molecular Modeling and Biological Evaluation as Anticancer Agents. Res. Chem. Intermed. 2017, 43, 6765–6786. [Google Scholar] [CrossRef]
- Bagul, C.; Rao, G.K.; Makani, V.K.K.; Tamboli, J.R.; Pal-Bhadra, M.; Kamal, A. Synthesis and biological evaluation of chalcone-linked pyrazolo [1,5-a] pyrimidines as potential anticancer agents. MedChemComm 2017, 8, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Alam, M.A.; Atkinson, T.; Gurrapu, S.; Sravan Kumar, J.; Bicknese, C.; Johnson, J.L.; Williams, M. Synthesis and Evaluation of P-N,N-Dialkyl Substituted Chalcones as Anti-Cancer Agents. Med. Chem. Res. 2013, 22, 4610–4614. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Elaasser, M.M.; Asiri, A.M. Synthesis of novel chalcone-based phenothiazine derivatives as antioxidant and anticancer agents. Molecules 2020, 25, 4566. [Google Scholar] [CrossRef] [PubMed]
- Peerzada, M.N.; Khan, P.; Ahmad, K.; Hassan, M.I.; Azam, A. Synthesis, Characterization and Biological Evaluation of Tertiary Sulfonamide Derivatives of Pyridyl-Indole Based Heteroaryl Chalcone as Potential Carbonic Anhydrase IX Inhibitors and Anticancer Agents. Eur. J. Med. Chem. 2018, 155, 13–23. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Mohamed, M.S.; Shouman, S.A.; Fathi, M.M.; Abdelhamid, I.A. Synthesis and Biological Evaluation of a Novel Series of Chalcones Incorporated Pyrazole Moiety as Anticancer and Antimicrobial Agents. Appl. Biochem. Biotechnol. 2012, 168, 1153–1162. [Google Scholar] [CrossRef]
- Pragathi, Y.J.; Veronica, D.; Anitha, K.; Rao, M.V.B.; Raju, R.R. Synthesis and Biological Evaluation of Chalcone Derivatives of 1,2,4-Thiadiazol-Benzo[d]Imidazol-2-Yl)Quinolin-2(1H)-One as Anticancer Agents. Chem. Data Collect. 2020, 30, 100556. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Singh, A.K.; Meena, S.; Lohani, M.; Singh, A.; Arya, R.K.; Cheruvu, S.H.; Sarkar, J.; Gayen, J.R.; Datta, D.; et al. Synthesis of Novel β-Carboline Based Chalcones with High Cytotoxic Activity against Breast Cancer Cells. Bioorg. Med. Chem. Lett. 2014, 24, 2820–2824. [Google Scholar] [CrossRef]
- Gurrapu, N.; Praveen Kumar, E.; Kolluri, P.K.; Putta, S.; Sivan, S.K.; Subhashini, N.J.P. Synthesis, Biological Evaluation and Molecular Docking Studies of Novel 1,2,3-Triazole Tethered Chalcone Hybrids as Potential Anticancer Agents. J. Mol. Struct. 2020, 1217, 128356. [Google Scholar] [CrossRef]
- Coşkun, D.; Tekin, S.; Sandal, S.; Coşkun, M.F. Synthesis, Characterization, and Anticancer Activity of New Benzofuran Substituted Chalcones. J. Chem. 2016, 2016, 7678486. [Google Scholar] [CrossRef]
- Djemoui, A.; Naouri, A.; Ouahrani, M.R.; Djemoui, D.; Lahcene, S.; Lahrech, M.B.; Boukenna, L.; Albuquerque, H.M.T.; Saher, L.; Rocha, D.H.A.; et al. A Step-by-Step Synthesis of Triazole-Benzimidazole-Chalcone Hybrids: Anticancer Activity in Human Cells+. J. Mol. Struct. 2020, 1204, 127487. [Google Scholar] [CrossRef]
- Dong, J.; Huang, G.; Zhang, Q.; Wang, Z.; Cui, J.; Wu, Y.; Meng, Q.; Li, S. Development of benzochalcone derivatives as selective CYP1B1 inhibitors and anticancer agents. MedChemComm 2019, 10, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.S.; Patel, H.H.; Mathew, N.S.; Nayak, Y. Synthesis of Newer Piperidinyl Chalcones and Their Anticancer Activity in Human Cancer Cell Lines. Res. Chem. Intermed. 2016, 42, 3673–3688. [Google Scholar] [CrossRef]
- Khan, N.S.; Khan, P.; Ansari, M.F.; Srivastava, S.; Hasan, G.M.; Husain, M.; Hassan, M.I. Thienopyrimidine-Chalcone Hybrid Molecules Inhibit Fas-Activated Serine/Threonine Kinase: An Approach to Ameliorate Antiproliferation in Human Breast Cancer Cells. Mol. Pharm. 2018, 15, 4173–4189. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.J.; Maluleka, M.M.; Parbhoo, N.; Malindisa, S.T. Synthesis, Evaluation for Cytotoxicity and Molecular Docking Studies of Benzo[c]Furan-Chalcones for Potential to Inhibit Tubulin Polymerization and/or EGFR-Tyrosine Kinase Phosphorylation. Int. J. Mol. Sci. 2018, 19, 2552. [Google Scholar] [CrossRef]
- Tatsuzaki, J.; Bastow, K.F.; Nakagawa-Goto, K.; Nakamura, S.; Itokawa, H.; Lee, K.H. Dehydrozingerone, Chalcone, and Isoeugenol Analogues as in Vitro Anticancer Agents. J. Nat. Prod. 2006, 69, 1445–1449. [Google Scholar] [CrossRef]
- Wan, M.; Xu, L.; Hua, L.; Li, A.; Li, S.; Lu, W.; Pang, Y.; Cao, C.; Liu, X.; Jiao, P. Synthesis and Evaluation of Novel Isoxazolyl Chalcones as Potential Anticancer Agents. Bioorg. Chem. 2014, 54, 38–43. [Google Scholar] [CrossRef]
- Kumar, D.; Maruthi Kumar, N.; Tantak, M.P.; Ogura, M.; Kusaka, E.; Ito, T. Synthesis and Identification of α-Cyano Bis(Indolyl)Chalcones as Novel Anticancer Agents. Bioorg. Med. Chem. Lett. 2014, 24, 5170–5174. [Google Scholar] [CrossRef]
- Rahimzadeh Oskuei, S.; Mirzaei, S.; Reza Jafari-Nik, M.; Hadizadeh, F.; Eisvand, F.; Mosaffa, F.; Ghodsi, R. Design, Synthesis and Biological Evaluation of Novel Imidazole-Chalcone Derivatives as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Bioorg. Chem. 2021, 112, 104904. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S. Synthesis and Biological Screening of a Combinatorial Library of β-Chlorovinyl Chalcones as Anticancer, Anti-Inflammatory and Antimicrobial Agents. Bioorg. Med. Chem. 2010, 18, 2060–2065. [Google Scholar] [CrossRef]
- Kamal, A.; Mallareddy, A.; Suresh, P.; Shaik, T.B.; Lakshma Nayak, V.; Kishor, C.; Shetti, R.V.C.R.N.C.; Sankara Rao, N.; Tamboli, J.R.; Ramakrishna, S.; et al. Synthesis of Chalcone-Amidobenzothiazole Conjugates as Antimitotic and Apoptotic Inducing Agents. Bioorg. Med. Chem. 2012, 20, 3480–3492. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, L.; Hong, G.; Yang, X.; Liu, T. Design, Synthesis and Anticancer Activity of Matrine–1H-1,2,3-Triazole–Chalcone Conjugates. Bioorg. Med. Chem. Lett. 2015, 25, 2540–2544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hedblom, A.; Koerner, S.K.; Li, M.; Jernigan, F.E.; Wegiel, B.; Sun, L. Novel Synthetic Chalcones Induce Apoptosis in the A549 Non-Small Cell Lung Cancer Cells Harboring a KRAS Mutation. Bioorg. Med. Chem. Lett. 2016, 26, 5703–5706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, R.; Zheng, W.; Chen, D.; Yue, X.; Cao, Y.; Qin, W.; Sun, H.; Wang, Y.; Liu, Z.; et al. Synthesis and Evaluation of Anticancer Activity of BOC26P, an Ortho-Aryl Chalcone Sodium Phosphate as Water-Soluble Prodrugs in Vitro and in Vivo. Biomed. Pharmacother. 2017, 96, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, E.H.; Kim, J.; Kim, S.H.; Kim, I. Biological Evaluation of Indolizine-Chalcone Hybrids as New Anticancer Agents. Eur. J. Med. Chem. 2018, 144, 435–443. [Google Scholar] [CrossRef]
- Jain, U.K.; Bhatia, R.K.; Rao, A.R.; Singh, R.; Saxena, A.K.; Sehar, I. Design and Development of Halogenated Chalcone Derivatives as Potential Anticancer Agents. Trop. J. Pharm. Res. 2014, 13, 73–80. [Google Scholar] [CrossRef]
- de Vasconcelos, A.; Campos, V.F.; Nedel, F.; Seixas, F.K.; Dellagostin, O.A.; Smith, K.R.; de Pereira, C.M.P.; Stefanello, F.M.; Collares, T.; Barschak, A.G. Cytotoxic and Apoptotic Effects of Chalcone Derivatives of 2-Acetyl Thiophene on Human Colon Adenocarcinoma Cells. Cell Biochem. Funct. 2013, 31, 289–297. [Google Scholar] [CrossRef]
- Gupta, S.; Maurya, P.; Upadhyay, A.; Kushwaha, P.; Krishna, S.; Siddiqi, M.I.; Sashidhara, K.V.; Banerjee, D. Synthesis and Bio-Evaluation of Indole-Chalcone Based Benzopyrans as Promising Antiligase and Antiproliferative Agents. Eur. J. Med. Chem. 2018, 143, 1981–1996. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Cheng, C.-C.; Shen, L.-L.; Wang, Z.-K.; Wu, S.-B.; Li, W.-L.; Chen, S.-H.; Zhou, R.-P.; Qiu, P.-H. Synthetic chalcones with potent antioxidant ability on H2O2-induced apoptosis in PC12 cells. Int. J. Mol.Sci. 2014, 15, 18525–18539. [Google Scholar] [CrossRef]
- Singh, A.; Fong, G.; Liu, J.; Wu, Y.-H.; Chang, K.; Park, W.; Kim, J.; Tam, C.; Cheng, L.W.; Land, K.M.; et al. Synthesis and preliminary antimicrobial analysis of isatin–ferrocene and isatin–ferrocenyl chalcone conjugates. ACS Omega 2018, 3, 5808–5813. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Ozten Kandas, N.; Dag, A.; Sonmez, F.; Kucukislamoglu, M. Synthesis and Biological Evaluation of Novel Coumarin-Chalcone Derivatives Containing Urea Moiety as Potential Anticancer Agents. Arab. J. Chem. 2020, 13, 1120–1129. [Google Scholar] [CrossRef]
- Xu, F.; Li, W.; Shuai, W.; Yang, L.; Bi, Y.; Ma, C.; Yao, H.; Xu, S.; Zhu, Z.; Xu, J. Design, Synthesis and Biological Evaluation of Pyridine-Chalcone Derivatives as Novel Microtubule-Destabilizing Agents. Eur. J. Med. Chem. 2019, 173, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stanojković, T.; Marković, V.; Matić, I.Z.; Mladenović, M.P.; Petrović, N.; Krivokuća, A.; Petković, M.; Joksović, M.D. Highly Selective Anthraquinone-Chalcone Hybrids as Potential Antileukemia Agents. Bioorg. Med. Chem. Lett. 2018, 28, 2593–2598. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.R.; Pallela, V.R.; Cosenza, S.C.; Mallireddigari, M.R.; Patti, R.; Bonagura, M.; Truongcao, M.; Akula, B.; Jatiani, S.S.; Reddy, E.P. Design, Synthesis and Evaluation of (E)-α-Benzylthio Chalcones as Novel Inhibitors of BCR-ABL Kinase. Bioorg. Med. Chem. 2010, 18, 2317–2326. [Google Scholar] [CrossRef]
- Ferrer, R.; Lobo, G.; Gamboa, N.; Rodrigues, J.; Abramjuk, C.; Jung, K.; Lein, M.; Charris, J.E. Synthesis of [(7-Chloroquinolin-4-Yl)Amino]Chalcones: Potential Antimalarial and Anticancer Agents. Sci. Pharm. 2009, 77, 725–742. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.M.; Akamatsu, K.; Kusaka, E.; Harada, H.; Ito, T. Synthesis and Biological Evaluation of Indolyl Chalcones as Antitumor Agents. Bioorg. Med. Chem. Lett. 2010, 20, 3916–3919. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2009, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Reddy, V.S.; Santosh, K.; Bharath Kumar, G.; Shaik, A.B.; Mahesh, R.; Chourasiya, S.S.; Sayeed, I.B.; Kotamraju, S. Synthesis of Imidazo[2,1-b][1,3,4]Thiadiazole–Chalcones as Apoptosis Inducing Anticancer Agents. Medchemcomm 2014, 5, 1718–1723. [Google Scholar] [CrossRef]
- Wu, C.M.; Lin, K.W.; Teng, C.H.; Huang, A.M.; Chen, Y.C.; Yen, M.H.; Wu, W.B.; Pu, Y.S.; Lin, C.N. Chalcone derivatives inhibit human platelet aggregation and inhibit growth in human bladder cancer cells. Biol. Pharm. Bull. 2014, 37, 1191–1198. [Google Scholar] [CrossRef]
- Gargantilla, M.; López-Fernández, J.; Camarasa, M.J.; Persoons, L.; Daelemans, D.; Priego, E.M.; Pérez-Pérez, M.J. Inhibition of XPO-1 Mediated Nuclear Export through the Michael-Acceptor Character of Chalcones. Pharmaceuticals 2021, 14, 1131. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Syahidah, H.N.; Subarnas, A.; Yusuf, M.; Bryant, S.D.; Langer, T. Molecular Docking and 3D-Pharmacophore Modeling to Study the Interactions of Chalcone Derivatives with Estrogen Receptor Alpha. Pharmaceuticals 2017, 10, 81. [Google Scholar] [CrossRef]
- Alam, M.J.; Alam, O.; Perwez, A.; Rizvi, M.A.; Naim, M.J.; Naidu, V.G.M.; Imran, M.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Design, Synthesis, Molecular Docking, and Biological Evaluation of Pyrazole Hybrid Chalcone Conjugates as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Pharmaceuticals 2022, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, J.; Hu, J.; Pang, Y.; Huang, L.; Li, X. Synthesis, Biological Evaluation and Mechanism Study of Chalcone Analogues as Novel Anti-Cancer Agents. RSC Adv. 2015, 5, 68128–68135. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and Biological Evaluation of Simple Methoxylated Chalcones as Anticancer, Anti-Inflammatory and Antioxidant Agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef]
- Venkatarao, V.; Kumar, L.; Jha, A.; Sridhar, G. Synthesis and Biological Evaluation of Chalcone Fused Quinoline Derivatives as Anticancer Agents. Chem. Data Collect. 2019, 22, 100236. [Google Scholar] [CrossRef]
- Konidala, S.K.; Kotra, V.; Danduga, R.C.S.R.; Kola, P.K. Coumarin-Chalcone Hybrids Targeting Insulin Receptor: Design, Synthesis, Anti-Diabetic Activity, and Molecular Docking. Bioorg. Chem. 2020, 104, 104207. [Google Scholar] [CrossRef]
- Adelusi, T.I.; Du, L.; Chowdhury, A.; Xiaoke, G.; Lu, Q.; Yin, X. Signaling Pathways and Proteins Targeted by Antidiabetic Chalcones. Life Sci. 2021, 284, 118982. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Kaur, M. Bio-Medical Potential of Chalcone Derivatives and Their Metal Complexes as Antidiabetic Agents: A Review. J. Coord. Chem. 2021, 74, 725–742. [Google Scholar] [CrossRef]
- Cai, C.Y.; Rao, L.; Rao, Y.; Guo, J.X.; Xiao, Z.Z.; Cao, J.Y.; Huang, Z.S.; Wang, B. Analogues of Xanthones—Chalcones and Bis-Chalcones as α-Glucosidase Inhibitors and Anti-Diabetes Candidates. Eur. J. Med. Chem. 2017, 130, 51–59. [Google Scholar] [CrossRef]
- Shin, J.; Jang, M.G.; Park, J.C.; Koo, Y.D.; Lee, J.Y.; Park, K.S.; Chung, S.S.; Park, K. Antidiabetic Effects of Trihydroxychalcone Derivatives via Activation of AMP-Activated Protein Kinase. J. Ind. Eng. Chem. 2018, 60, 177–184. [Google Scholar] [CrossRef]
- Shukla, P.; Satyanarayana, M.; Verma, P.C.; Tiwari, J.; Dwivedi, A.P.; Srivastava, R.; Rehuja, N.; Srivastava, S.P.; Gautam, S.; Tamrakar, A.K.; et al. Chalcone-based aryloxypropanolamine as a potential antidiabetic and antidyslipidaemic agent. Curr. Sci. 2017, 112, 1675–1689. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. Chalcones and Their Therapeutic Targets for the Management of Diabetes: Structural and Pharmacological Perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.S.; Salunke, S.D. Facile synthesis of some triazine based chalcones as potential antioxidant and anti-diabetic agents. J. Chem. Pharm. 2015, 7, 114–120. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Hsieh, T.J.; El-Shazly, M.; Chuang, D.W.; Tsai, Y.H.; Yen, C.T.; Wu, S.F.; Wu, Y.C.; Chang, F.R. Synthesis of Chalcone Derivatives as Potential Anti-Diabetic Agents. Bioorg. Med. Chem. Lett. 2012, 22, 3912–3915. [Google Scholar] [CrossRef] [PubMed]
- Chinthala, Y.; Thakur, S.; Tirunagari, S.; Chinde, S.; Domatti, A.K.; Arigari, N.K.; Srinivas, K.V.N.S.; Alam, S.; Jonnala, K.K.; Khan, F.; et al. Synthesis, Docking and ADMET Studies of Novel Chalcone Triazoles for Anti-Cancer and Anti-Diabetic Activity. Eur. J. Med. Chem. 2015, 93, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Królicka, E.; Kie, K.; Ła, D. Chalcones as Potential Ligands for the Treatment of Parkinson’s Disease. Pharmaceuticals 2022, 15, 847. [Google Scholar] [CrossRef]
- Hammuda, A.; Shalaby, R.; Rovida, S.; Edmondson, D.E.; Binda, C.; Khalil, A. Design and Synthesis of Novel Chalcones as Potent Selective Monoamine Oxidase-B Inhibitors. Eur. J. Med. Chem. 2016, 114, 162–169. [Google Scholar] [CrossRef]
- Mathew, B.; Parambi, D.G.T.; Mathew, G.E.; Uddin, M.S.; Inasu, S.T.; Kim, H.; Marathakam, A.; Unnikrishnan, M.K.; Carradori, S. Emerging Therapeutic Potentials of Dual-Acting MAO and AChE Inhibitors in Alzheimer’s and Parkinson’s Diseases. Arch. Pharm. Chem. Life Sci. 2019, 352, 1900177. [Google Scholar] [CrossRef]
- Hitge, R.; Smit, S.; Petzer, A.; Petzer, J.P. Evaluation of Nitrocatechol Chalcone and Pyrazoline Derivatives as Inhibitors of Catechol-O-Methyltransferase and Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2020, 30, 127188. [Google Scholar] [CrossRef]
- Parambi, D.G.T.; Saleem, U.; Shah, M.A.; Anwar, F.; Ahmad, B.; Manzar, A.; Itzaz, A.; Harilal, S.; Uddin, M.S.; Kim, H.; et al. Exploring the Therapeutic Potentials of Highly Selective Oxygenated Chalcone Based MAO-B Inhibitors in a Haloperidol-Induced Murine Model of Parkinson’s Disease. Neurochem. Res. 2020, 45, 2786–2799. [Google Scholar] [CrossRef]
- Sasidharan, R.; Manju, S.L.; Uçar, G.; Baysal, I.; Mathew, B. Identification of Indole-Based Chalcones: Discovery of a Potent, Selective, and Reversible Class of MAO-B Inhibitors. Arch. Pharm. 2016, 349, 627–637. [Google Scholar] [CrossRef]
- Sasidharan, R.; Baek, S.C.; Sreedharannair Leelabaiamma, M.; Kim, H.; Bijo, M. Imidazole Bearing Chalcones as a New Class of Monoamine Oxidase Inhibitors. Biomed. Pharmacother. 2018, 106, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Rangarajan, T.M.; Chaudhary, R.; Singh, R.P.; Singh, M.; Singh, R.P.; Tondo, A.R.; Gambacorta, N.; Nicolotti, O.; Mathew, B.; et al. Novel class of chalcone oxime ethers as potent monoamine oxidase-B and acetylcholinesterase inhibitors. Molecules 2020, 25, 2356. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Ahmad, A.; Oswal, N.; Dandawate, P.; Rub, R.A.; Deshpande, J.; Swamy, K.V.; Sarkar, F.H. Fluorinated 2′-Hydroxychalcones as Garcinol Analogs with Enhanced Antioxidant and Anticancer Activities. Bioorg. Med. Chem. Lett. 2010, 20, 5818–5821. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, I.S.; Moon, A. 2-Hydroxychalcone and Xanthohumol Inhibit Invasion of Triple Negative Breast Cancer Cells. Chem. Interact. 2013, 203, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.I.; Yamali, C.; Gunesacar, G.; Sakagami, H.; Okudaira, N.; Uesawa, Y.; Kagaya, H. Cytotoxicity, apoptosis, and QSAR studies of phenothiazine derived methoxylated chalcones as anticancer drug candidates. Med. Chem. Res. 2018, 27, 2366–2378. [Google Scholar] [CrossRef]
- Prabhakar, V.; Balasubramanian, R.; Sathe, P.; Krishna, C.M.; Juvekar, A. In Vitro Anticancer Activity of Monosubstituted Chalcone Derivatives. Int. J. Tumor Ther. 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Schmitt, F.; Draut, H.; Biersack, B.; Schobert, R. Halogenated naphthochalcones and structurally related naphthopyra-zolines with antitumor activity. Bioorg. Med. Chem. Lett. 2016, 26, 5168–5171. [Google Scholar] [CrossRef]
- Mai, C.W.; Yaeghoobi, M.; Abd-Rahman, N.; Kang, Y.B.; Pichika, M.R. Chalcones with Electron-Withdrawing and Electron-Donating Substituents: Anticancer Activity against TRAIL Resistant Cancer Cells, Structure–Activity Relationship Analysis and Regulation of Apoptotic Proteins. Eur. J. Med. Chem. 2014, 77, 378–387. [Google Scholar] [CrossRef]
- Echeverria, C.; Santibañez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Ramirez-Tagle, R. Structural Antitumoral Activity Relationships of Synthetic Chalcones. Int. J. Mol. Sci. 2009, 10, 221–231. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone Synthesis, Properties and Medicinal Applications: A Review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]



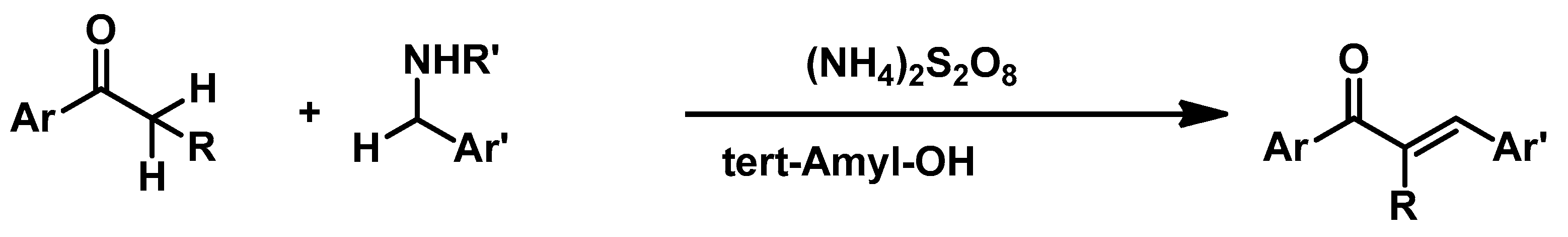
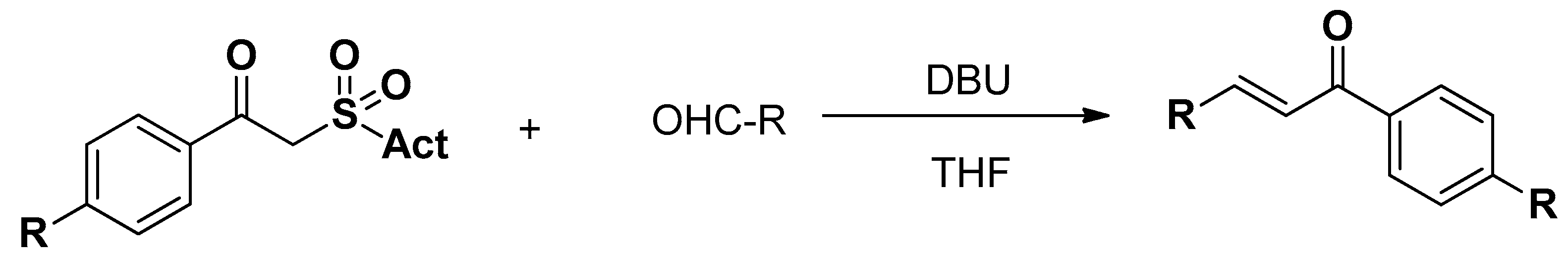




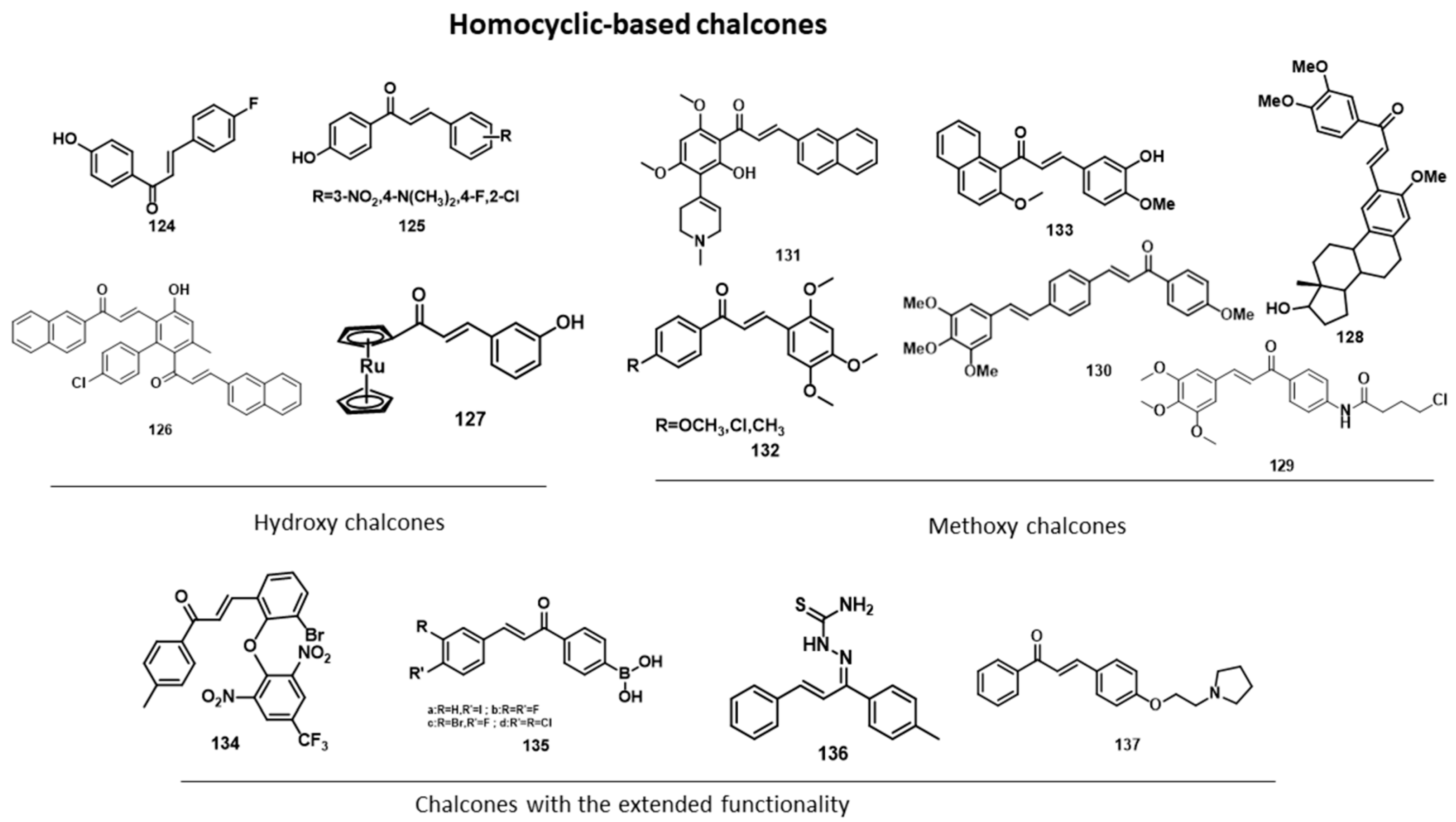






| Homocyclic chalcone derivatives (breast cancer cell lines; IC50 values in µM) | |||||
| Hydroxylated chalcones | Methoxylated chalcones | Chalcones with extended functionality | |||
| 124 | 1.37 | 128 | 7.3 | 134 | 0.03 |
| 125 | - | 129 | 2.54 | 135 | 3.5 |
| 126 | 4.4 | 130 | 30.55 | 136 | - |
| 127 | 91.4 | 131 | 23.45 | 137 | <10 |
| - | - | 132 | 2.2 | - | - |
| - | - | 133 | 1.2 | - | - |
| Heterocyclic chalcone derivatives (breast cancer cell lines; IC50 values in µM) | |||||
| Furano-based chalcones | Imidazole-based chalcones | Thiazole-based chalcones | |||
| 138 | 2.2 | 142 | 8.91 | 145 | 1.97 |
| 139 | 0.00035–0.59 | 143 | 0.56 | 146 | 0.18 |
| 140 | 1.45 | 144 | 5.89 | 147 | 12 |
| 141 | 1.8 | - | - | - | - |
| Pyrimidine-based chalcones | Indole-based chalcones | Oxazoline/pyrazole/quinoline/pyridine-based chalcones | |||
| 148 | 7.4 | 151 | 31.66 | 154 | 0.35 |
| 149 | 6.52 | 152 | 2.25 | 155 | 3.9 |
| 150 | 0.14 | 153 | 12 | 156 | 2.32 |
| - | - | - | - | 157 | 1.8 |
| Compound Number | Structure of Chalcones | Types of Cancer |
|---|---|---|
| 173 | 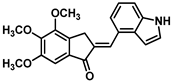 | Cervical cancer (0.027 ± 0.01 µM), prostate cancer (0.031 ± 0.05 µM), leukemia (0.031 ± 0.12 µM), lung cancer (0.026 ± 0.03 µM) [236] |
| 174 | 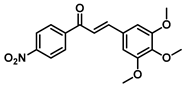 | Lung cancer, colon cancer, renal adenocarcinoma, pancreatic carcinoma [237] |
| 175 | 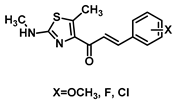 | Lung cancer (1.39–3.17 µM), breast cancer (1.97–4.14 µM), hepatocarcinoma (1.56–3.79 µM) [186] |
| 176 | 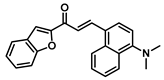 | Breast cancer (2.2 ± 0.3 µM), prostate cancer(0.9 ± 0.5 µM), lung cancer (1.10 ± 0.5 µM), pancreatic carcinoma (1.2 ± 0.2 µM) [187] |
| 177 | 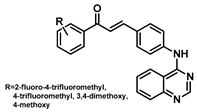 | Lung cancer (0.10–2.90 µM), breast cancer (0.14–0.17 µM), colon adenocarcinomas (0.13–2.89 µM) [189] |
| 178 |  | Lung cancer (0.66 ± 0.071 µM), breast cancer (0.18 ± 0.094 µM), prostate carcinoma (1.03 ± 0.45 µM) [193] |
| 179 | 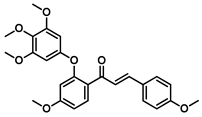 | Breast cancer (3.44 ± 0.19 µM), liver carcinoma (4.64 ± 0.23 µM), adenocarcinoma (6.31 ± 0.27 μM) [171] |
| 180 |  | Colon cancer (11.78 µM), breast cancer (31.66 µM), liver cancer (13.95 µM) [194] |
| 181 | 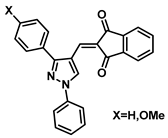 | Breast cancer (3.9–4.1 µM), liver cancer (3.8–5.0 µM), colorectal cancer (3.3 µM) [199] |
| 182 | 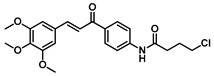 | Breast cancer (2.54 µM), colorectal cancer (1.83 µM), gastric carcinoma (1.52 µM) [178] |
| 183 | 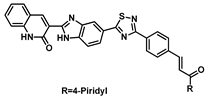 | Breast cancer (0.012 ± 0.007 µM), lung cancer (0.074 ± 0.004 µM), colon cancer (0.074 ± 0.004 μM), ovarian cancer (0.083 ± 0.002 μM) [200] |
| 184 | 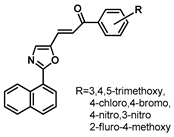 | Breast cancer (0.33–0.89 µM), melanoma (0.11–1.28 µM), lung cancer (0.34–7.56 µM) [238] |
| 185 | 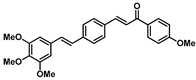 | Ovarian carcinoma (6.66 µM), breast cancer (30.55 µM), lung cancer (36.35 µM) [181] |
| 186 |  | Colon carcinoma (33.31 µM), cervical carcinoma (21.80 µM), breast cancer (23.45 µM), lung cancer (4.28 µM) [206] |
| 187 | 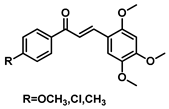 | Breast cancer, synovial carcinoma, cervical carcinoma (2.2–4.5 µM) [183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals 2022, 15, 1250. https://doi.org/10.3390/ph15101250
Rajendran G, Bhanu D, Aruchamy B, Ramani P, Pandurangan N, Bobba KN, Oh EJ, Chung HY, Gangadaran P, Ahn B-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals. 2022; 15(10):1250. https://doi.org/10.3390/ph15101250
Chicago/Turabian StyleRajendran, Gayathri, Deepu Bhanu, Baladhandapani Aruchamy, Prasanna Ramani, Nanjan Pandurangan, Kondapa Naidu Bobba, Eun Jung Oh, Ho Yun Chung, Prakash Gangadaran, and Byeong-Cheol Ahn. 2022. "Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry" Pharmaceuticals 15, no. 10: 1250. https://doi.org/10.3390/ph15101250
APA StyleRajendran, G., Bhanu, D., Aruchamy, B., Ramani, P., Pandurangan, N., Bobba, K. N., Oh, E. J., Chung, H. Y., Gangadaran, P., & Ahn, B.-C. (2022). Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals, 15(10), 1250. https://doi.org/10.3390/ph15101250









