A Preclinical Study of an 125I-Labeled PSMA Ligand for Prostate-Cancer Puncture
Abstract
1. Introduction
2. Results
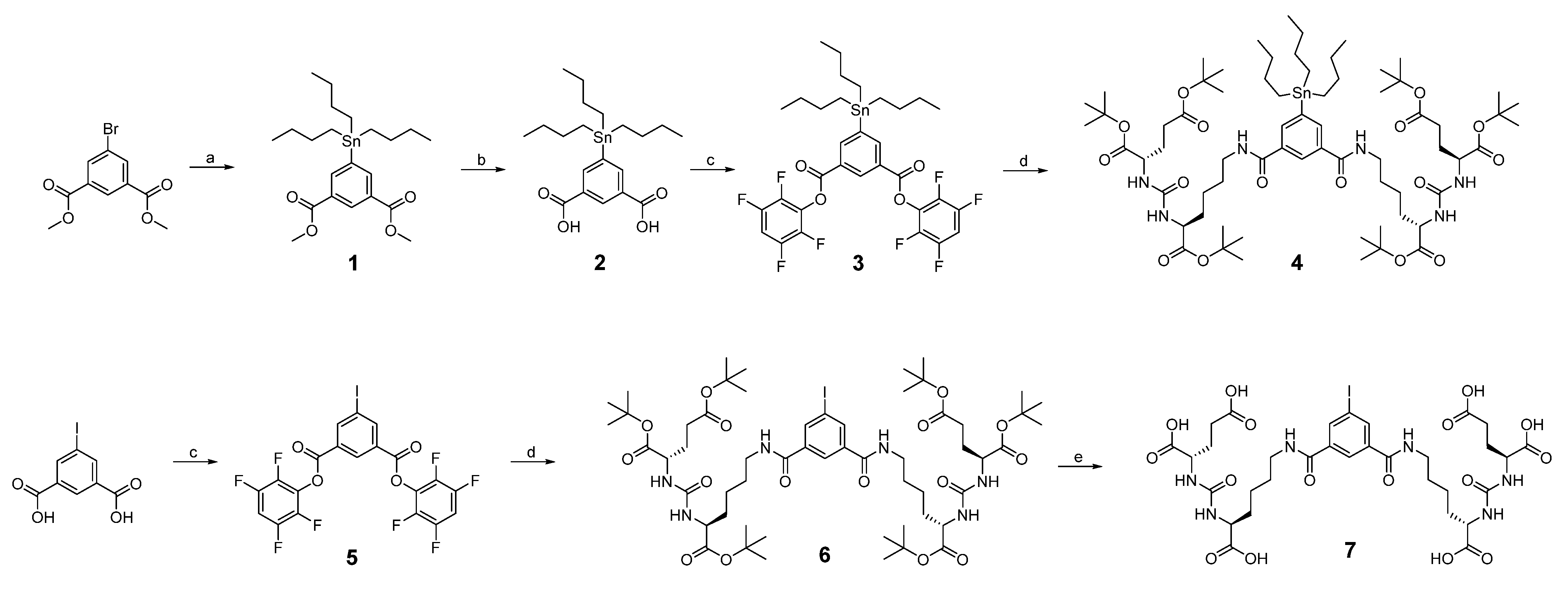
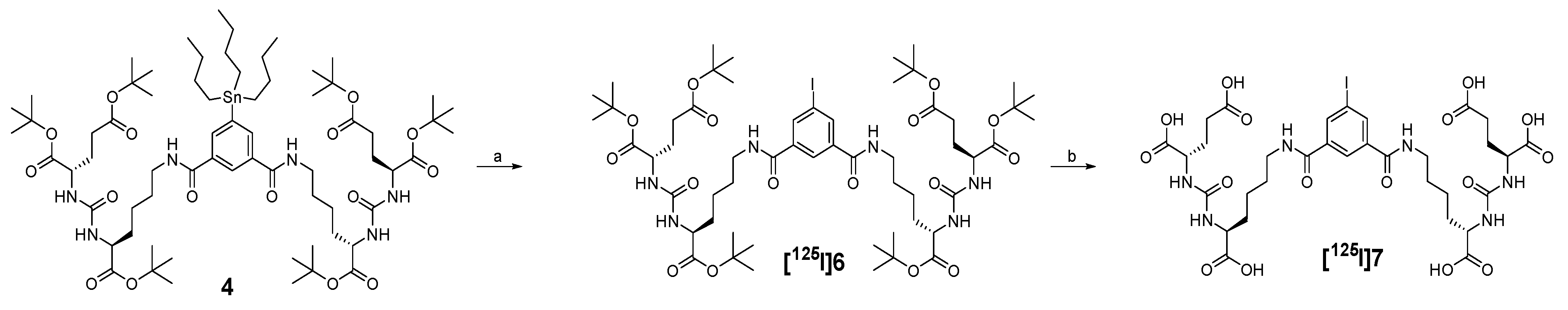
2.1. Synthesis and Radiochemistry
2.2. HPLC Purity Identification and MS Analysis
2.3. Determination of PSMA Inhibitory Activity
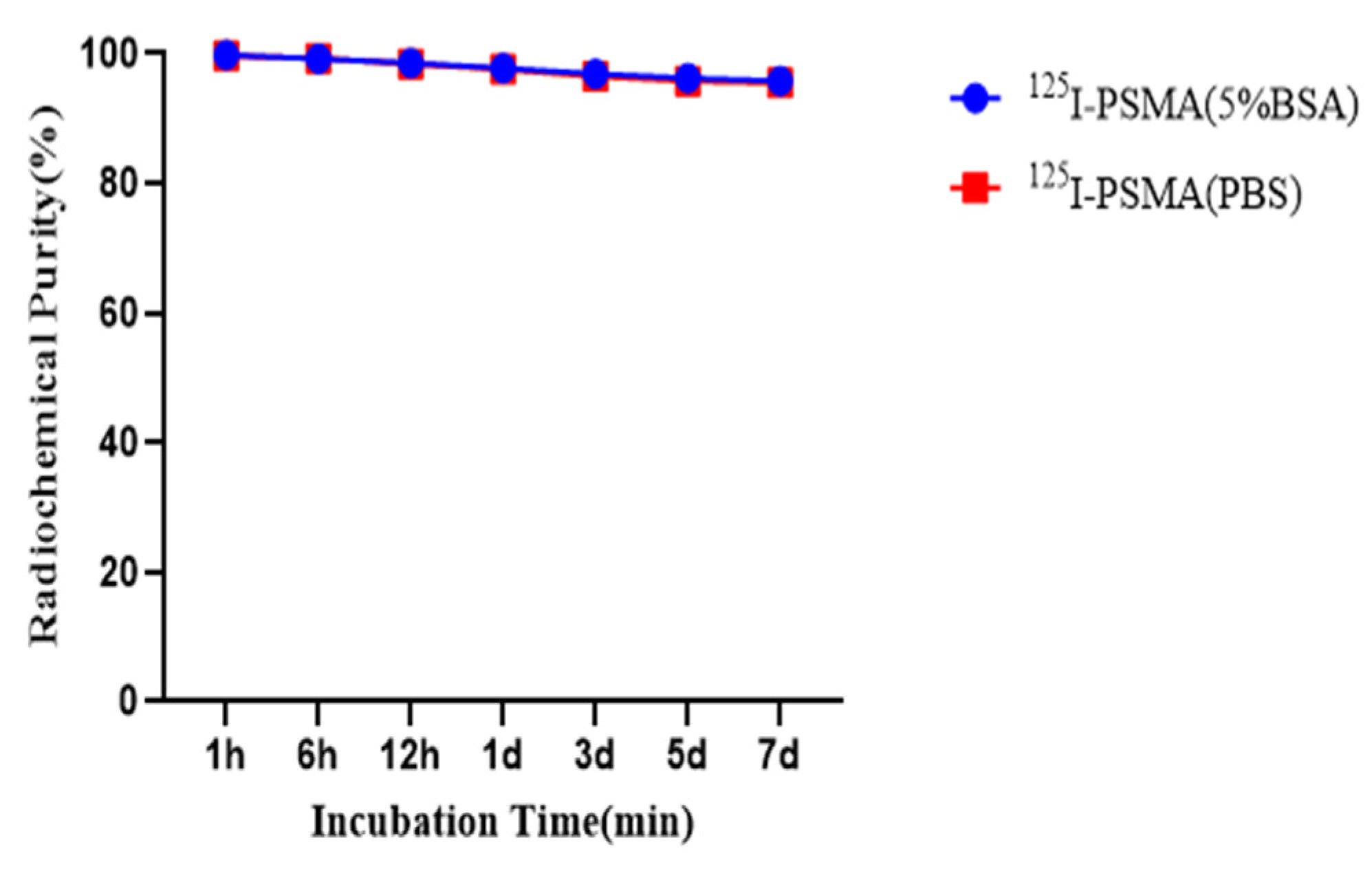
2.4. In Vitro Stability and Partition Coefficient
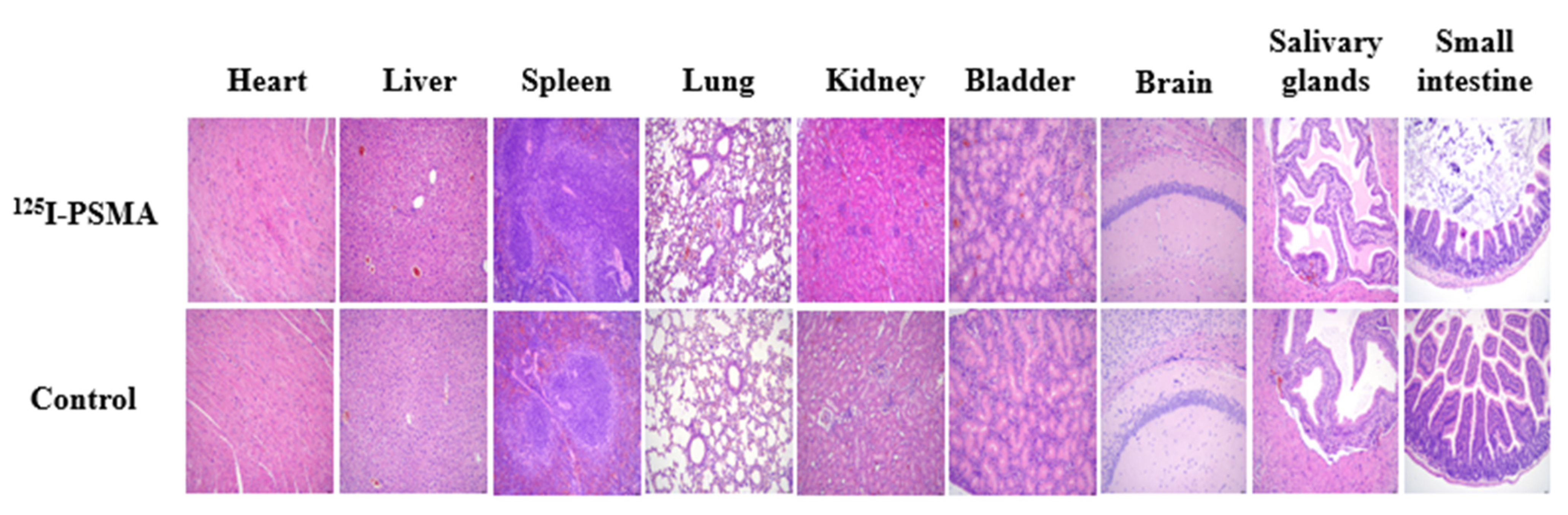
2.5. Acute Toxicity Test
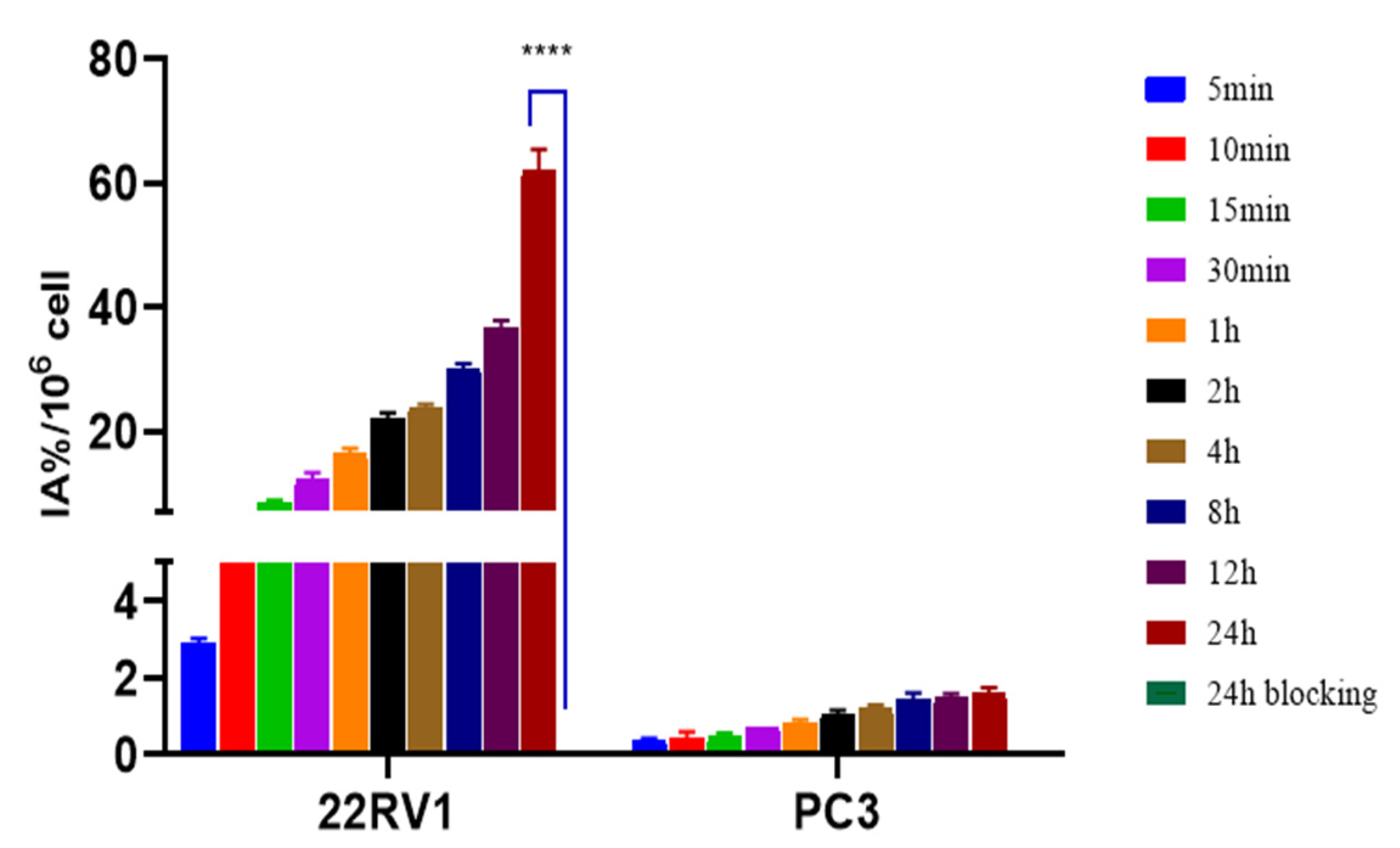
2.6. In Vitro Cellular Experiments
2.7. Pharmacokinetics
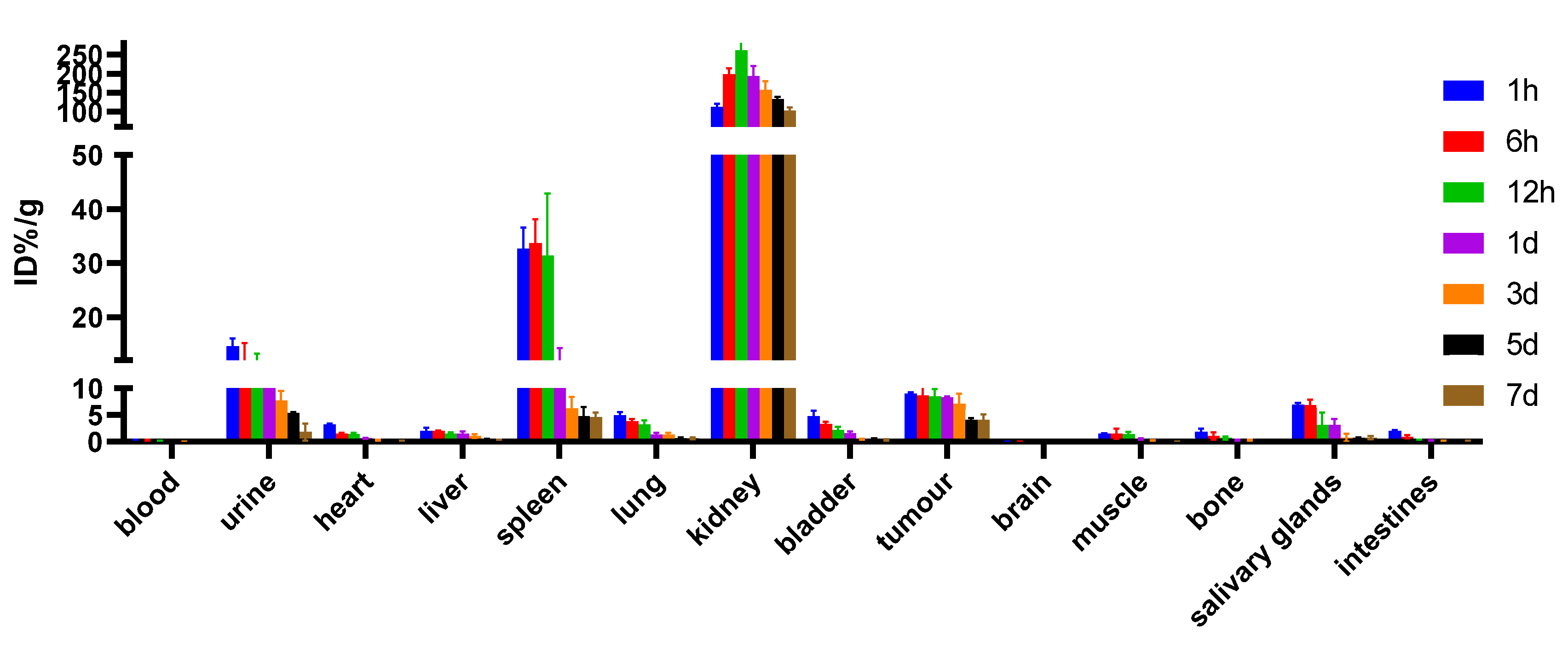
2.8. Biodistribution
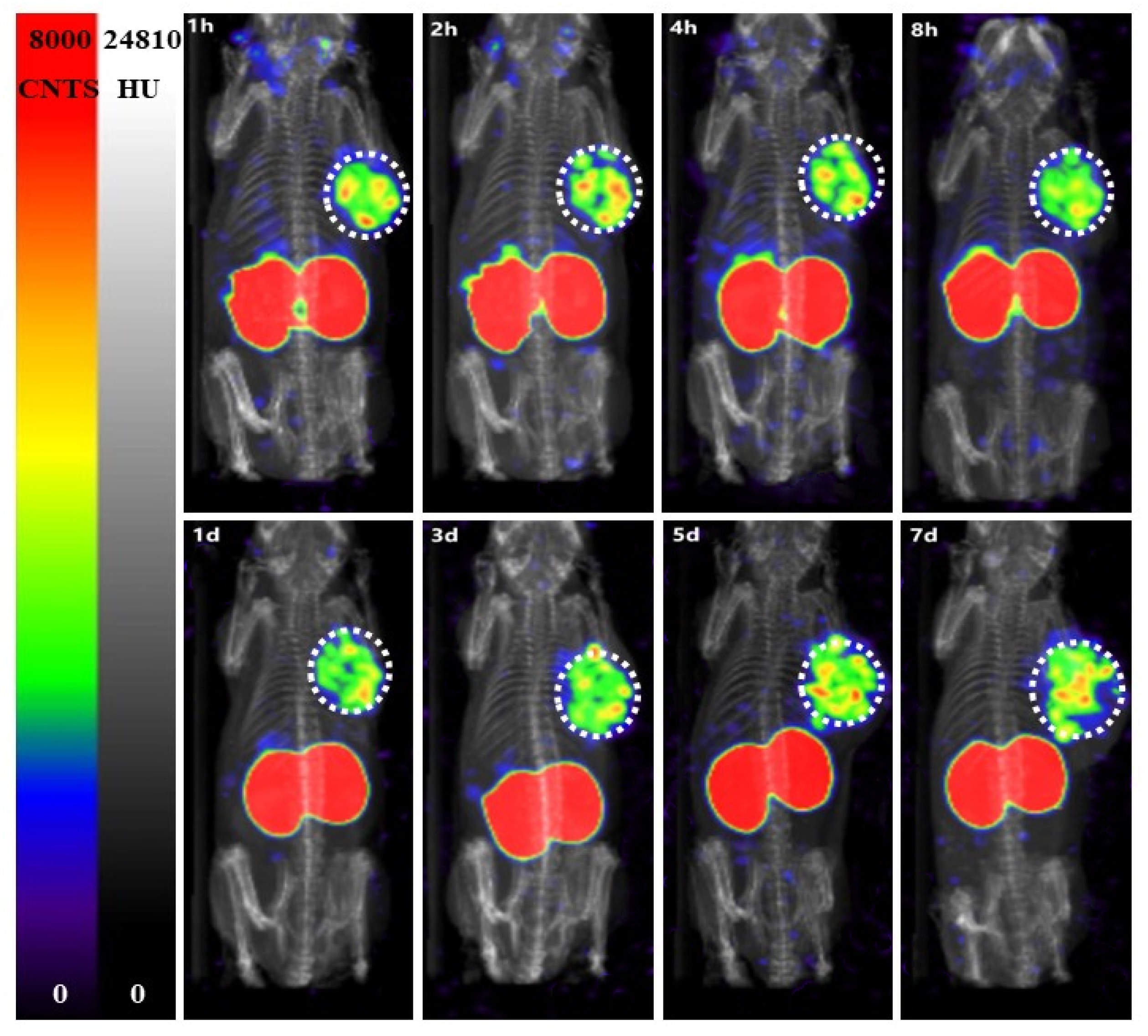
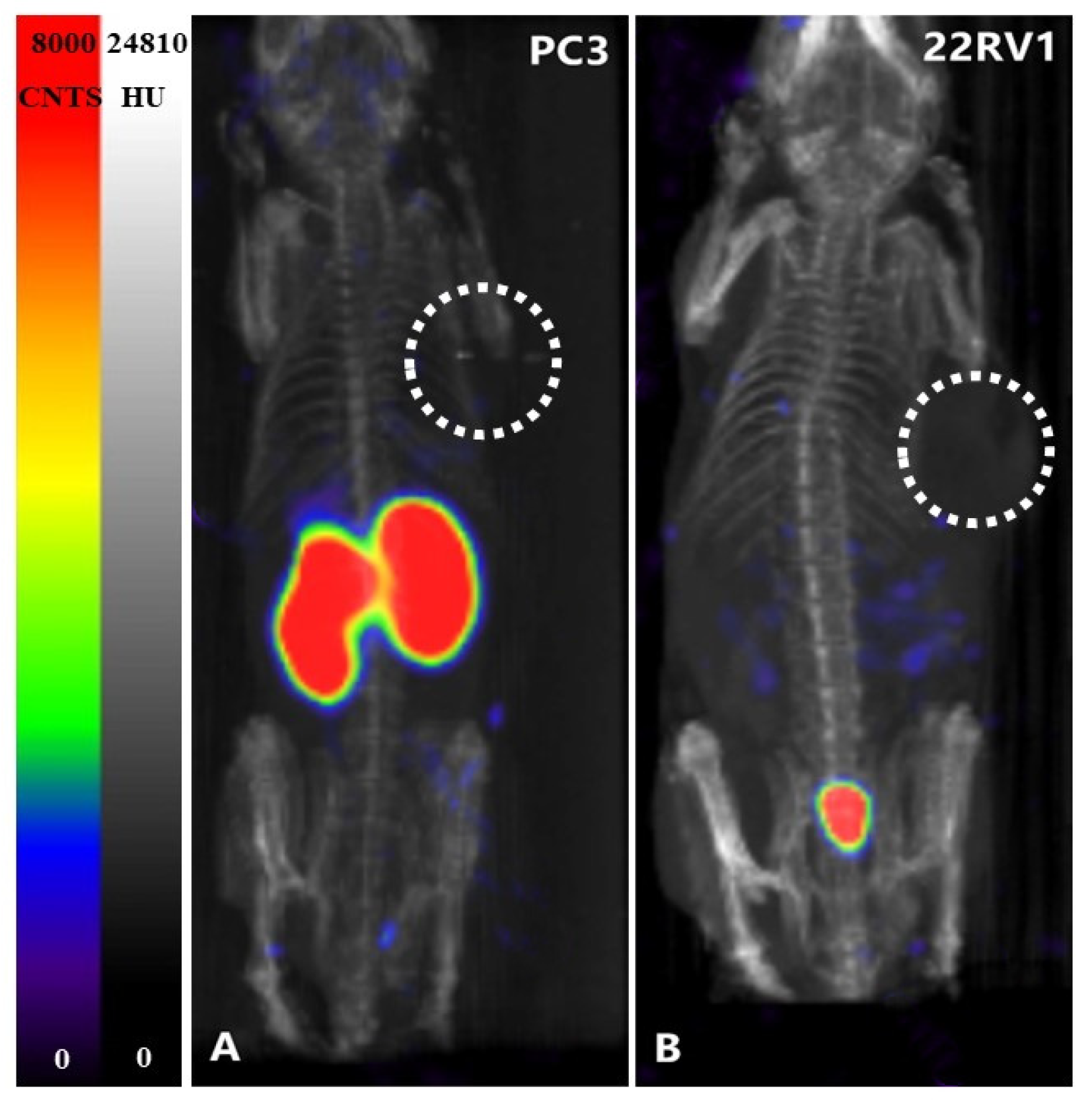
2.9. SPECT Imaging
3. Discussion
4. Materials and Methods
4.1. General Materials
4.2. Cell Lines and Mouse Models
4.3. Chemical and Radiochemical Syntheses
4.3.1. Synthesis of Compound 1
4.3.2. Synthesis of Compound 2
4.3.3. Synthesis of Compound 3
4.3.4. Synthesis of Compound 4
4.3.5. Synthesis of Compound 5
4.3.6. Synthesis of Compound 6
4.3.7. Synthesis of Compound 7
4.4. Radiochemistry
4.5. Determination of Radiochemical Purity by HPLC and MS Analysis
4.6. The Binding Affinity of 125I-PSMA-7 and PSMA
4.7. In Vitro Stability
4.8. Partition Coefficient
4.9. Acute Toxicity Test
4.10. In Vitro Cellular Experiments
4.11. Ex Vivo Biodistribution and Imaging
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, R.M.; Zong, Y.N.; Cao, S.M.; Xu, R.H. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Schoots, I.G. Omission of systematic transrectal ultrasound guided biopsy from the MRI targeted approach in men with previous negative prostate biopsy might still be premature. Ann. Transl. Med. 2016, 4, 205. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, G.; Simone, G.; Del Monte, M.; Ciardi, A.; Grompone, M.D.; Campa, R.; Indino, E.L.; Barchetti, F.; Sciarra, A.; et al. Negative Multiparametric Magnetic Resonance Imaging for Prostate Cancer: What’s Next? Eur. Urol. 2018, 74, 48–54. [Google Scholar] [CrossRef]
- Hübner, N.; Shariat, S.; Remzi, M. Prostate biopsy: Guidelines and evidence. Curr. Opin. Urol. 2018, 28, 354–359. [Google Scholar] [CrossRef]
- Fendler, W.P.; Schmidt, D.F.; Wenter, V.; Thierfelder, K.M.; Zach, C.; Stief, C.; Bartenstein, P.; Kirchner, T.; Gildehaus, F.J.; Gratzke, C.; et al. 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 1720–1725. [Google Scholar] [CrossRef]
- Zamboglou, C.; Drendel, V.; Jilg, C.A.; Rischke, H.C.; Beck, T.I.; Schultze-Seemann, W.; Krauss, T.; Mix, M.; Schiller, F.; Wetterauer, U.; et al. Comparison of (68)Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics 2017, 7, 228–237. [Google Scholar] [CrossRef]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet (Lond. Engl.) 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Eklund, M.; Jäderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Grönberg, H.; Nordström, T. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef]
- Van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naïve Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur. Urol. 2019, 75, 570–578. [Google Scholar]
- Wegelin, O.; van Melick, H.H.E.; Hooft, L.; Bosch, J.; Reitsma, H.B.; Barentsz, J.O.; Somford, D.M. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur. Urol. 2017, 71, 517–531. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, S.; Zhang, C.; Fu, Y.; Lv, X.; Zhang, Q.; Deng, Y.; Zhang, C.; Luo, R.; Zhao, X.; et al. Comparison of (68)Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J. Transl. Med. 2017, 15, 230. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, C.; Zhang, Q.; Fu, Y.; Zhao, X.; Chen, M.; Zhang, B.; Li, D.; Shi, J.; Wang, F.; et al. Diagnostic performance of (68)Ga-PSMA PET/CT for identification of aggressive cribriform morphology in prostate cancer with whole-mount sections. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1531–1541. [Google Scholar] [CrossRef]
- Bodar, Y.J.L.; Jansen, B.H.E.; van der Voorn, J.P.; Zwezerijnen, G.J.C.; Meijer, D.; Nieuwenhuijzen, J.A.; Boellaard, R.; Hendrikse, N.H.; Hoekstra, O.S.; van Moorselaar, R.J.A.; et al. Detection of prostate cancer with (18)F-DCFPyL PET/CT compared to final histopathology of radical prostatectomy specimens: Is PSMA-targeted biopsy feasible? The DeTeCT trial. World J. Urol. 2021, 39, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.; Morton, A.; Yaxley, J.; Ranasinghe, S.; Teloken, P.E.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. (68)Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is (68)Ga-PSMA PET/CT guided biopsy the future? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, H.; Liu, J.; Zhang, X.; Lin, M.; Schmidt, H.; Gao, J.; Xu, B. A Pilot Study of (18)F-DCFPyL PET/CT or PET/MRI and Ultrasound Fusion Targeted Prostate Biopsy for Intra-Prostatic PET-Positive Lesions. Front. Oncol. 2021, 11, 612157. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Zhang, Z.; Zhang, N.; Du, P.; Yang, Y.; Liu, Y.; Yu, W.; Li, N.; Gorin, M.A.; et al. (68)Ga-PSMA PET/CT Combined with PET/Ultrasound-Guided Prostate Biopsy Can Diagnose Clinically Significant Prostate Cancer in Men with Previous Negative Biopsy Results. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 1314–1319. [Google Scholar]
- Kumar, R.; Singh, S.K.; Mittal, B.R.; Vadi, S.K.; Kakkar, N.; Singh, H.; Krishnaraju, V.S.; Kumar, S.; Bhattacharya, A. Safety and Diagnostic Yield of (68)Ga Prostate-specific Membrane Antigen PET/CT-guided Robotic-assisted Transgluteal Prostatic Biopsy. Radiology 2022, 303, 392–398. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, W.C.; Xu, Z.; Jiang, N.; Zang, S.M.; Xu, L.W.; Huang, W.B.; Wang, F.; Sun, H.B. (68)Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: A prospective randomized single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Foss, C.A.; Byun, Y.; Nimmagadda, S.; Pullambhatla, M.; Fox, J.J.; Castanares, M.; Lupold, S.E.; Babich, J.W.; Mease, R.C.; et al. Radiohalogenated prostate-specific membrane antigen (PSMA)-based ureas as imaging agents for prostate cancer. J. Med. Chem. 2008, 51, 7933–7943. [Google Scholar] [CrossRef]
- Foss, C.A.; Mease, R.C.; Fan, H.; Wang, Y.; Ravert, H.T.; Dannals, R.F.; Olszewski, R.T.; Heston, W.D.; Kozikowski, A.P.; Pomper, M.G. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: In vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 4022–4028. [Google Scholar] [CrossRef]
- Harada, N.; Kimura, H.; Ono, M.; Saji, H. Preparation of asymmetric urea derivatives that target prostate-specific membrane antigen for SPECT imaging. J. Med. Chem. 2013, 56, 7890–7901. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Kang, C.M.; McDougald, D.; Minn, I.; Brummet, M.; Pomper, M.G.; Zalutsky, M.R. Brush border enzyme-cleavable linkers: Evaluation for reducing renal uptake of radiolabeled prostate-specific membrane antigen inhibitors. Nucl. Med. Biol. 2018, 62, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.A.; Laudicella, R.; Zeimpekis, K.; Mebert, I.; Müller, J.; Maurer, A.; Grünig, H.; Donati, O.; Sapienza, M.T.; Rueschoff, J.H.; et al. Hot needles can confirm accurate lesion sampling intraoperatively using [(18)F]PSMA-1007 PET/CT-guided biopsy in patients with suspected prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.F.; Slusher, B.S. Design of NAALADase inhibitors: A novel neuroprotective strategy. Curr. Med. Chem. 2001, 8, 949–957. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, Y.; Liu, Y.; Wu, Y.; Fang, Y.; Wang, B.; Xu, B. Targeted fluorescent imaging of a novel FITC-labeled PSMA ligand in prostate cancer. Amino Acids 2022, 54, 147–155. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Ren, Y.; Guo, X.; Jiang, J.; Xie, Q.; Xia, L.; Wang, F.; Zhu, H.; Yang, Z. Development of an Albumin-Based PSMA Probe with Prolonged Half-Life. Front. Mol. Biosci. 2020, 7, 585024. [Google Scholar] [CrossRef]
- Xiao, D.; Duan, X.; Gan, Q.; Zhang, X.; Zhang, J. Preparation and Biological Evaluation of [(99m)Tc]Tc-CNGU as a PSMA-Targeted Radiotracer for the Imaging of Prostate Cancer. Molecules 2020, 25, 5548. [Google Scholar] [CrossRef] [PubMed]





| . | Background | Tumor | Muscle |
|---|---|---|---|
| 4d | 42 | 1739 | 45 |
| 7d | 74 | 1404 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, X.; Zhou, H.; Chen, Y.; Zhang, X.; Cui, M.; Chen, K.; Xu, X.; Zhang, J.; Xu, B. A Preclinical Study of an 125I-Labeled PSMA Ligand for Prostate-Cancer Puncture. Pharmaceuticals 2022, 15, 1252. https://doi.org/10.3390/ph15101252
Luan X, Zhou H, Chen Y, Zhang X, Cui M, Chen K, Xu X, Zhang J, Xu B. A Preclinical Study of an 125I-Labeled PSMA Ligand for Prostate-Cancer Puncture. Pharmaceuticals. 2022; 15(10):1252. https://doi.org/10.3390/ph15101252
Chicago/Turabian StyleLuan, Xiaohui, Haoxi Zhou, Yimin Chen, Xiaojun Zhang, Mengchao Cui, Kuang Chen, Xiaodan Xu, Jinming Zhang, and Baixuan Xu. 2022. "A Preclinical Study of an 125I-Labeled PSMA Ligand for Prostate-Cancer Puncture" Pharmaceuticals 15, no. 10: 1252. https://doi.org/10.3390/ph15101252
APA StyleLuan, X., Zhou, H., Chen, Y., Zhang, X., Cui, M., Chen, K., Xu, X., Zhang, J., & Xu, B. (2022). A Preclinical Study of an 125I-Labeled PSMA Ligand for Prostate-Cancer Puncture. Pharmaceuticals, 15(10), 1252. https://doi.org/10.3390/ph15101252






