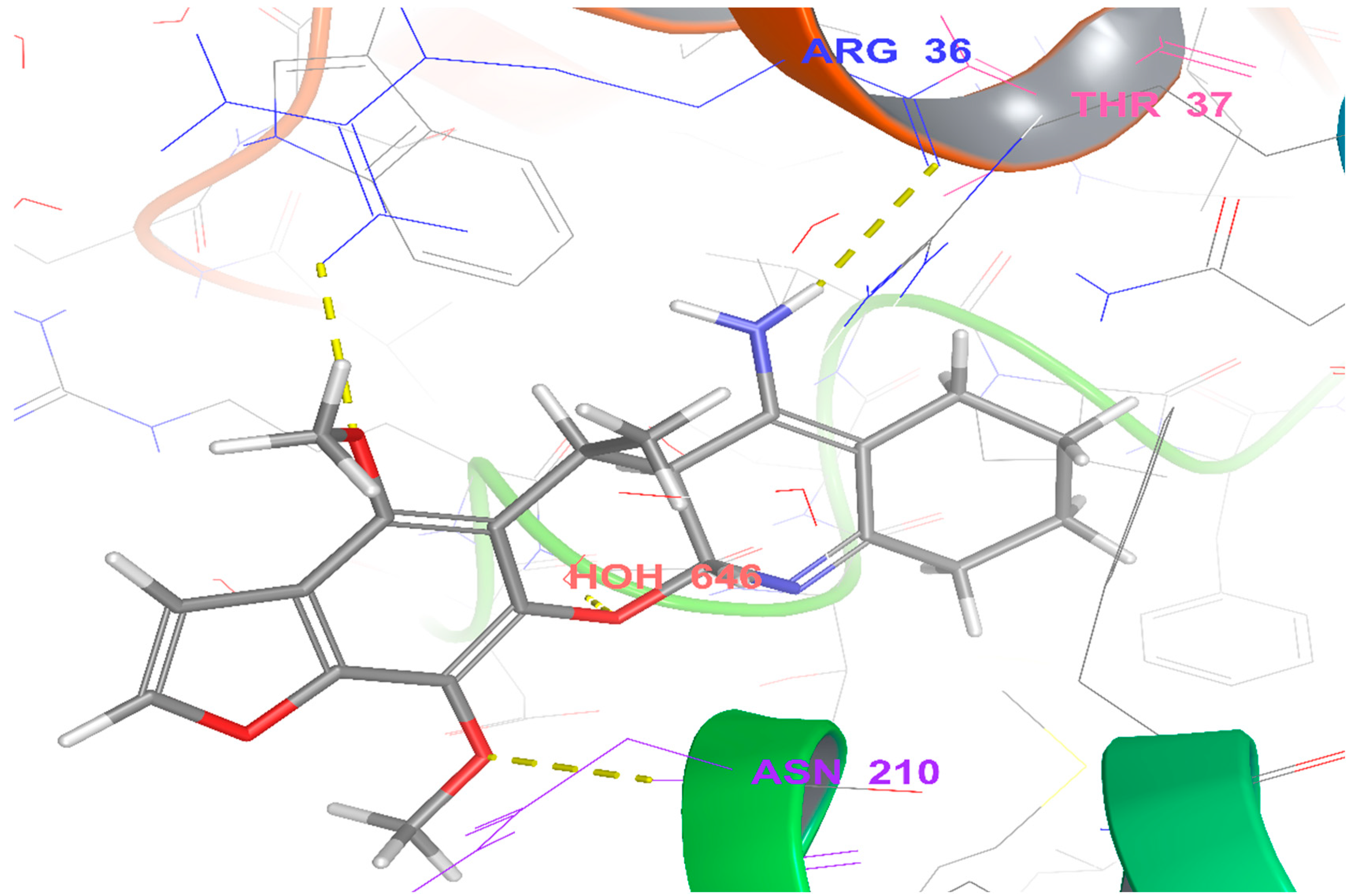
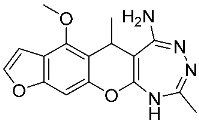
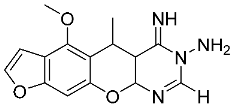
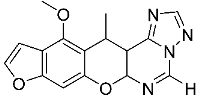
3. Materials and Methods
Through cooperation with other researchers, a research plan was made for the synthesis of new heterocyclic compounds. These new compounds were planned to study their antimicrobial activity, and the plan was successfully implemented.
3.1. General Information
All the melting points were assessed on an Electrothermal IA 9100 series digital melting point apparatus (Shimadzu, Tokyo, Japan). Elemental analyses were performed on Vario EL (Elementar, Langenselbold, Germany). Microanalytical data were processed at the microanalytical center of the Faculty of Science at Cairo University and National Research Centre. The IR spectra (KBr disc) were recorded using a Perkin-Elmer 1650 spectrometer (Waltham, MA, USA). NMR spectra were determined using JEOL 270 MHz and JEOL JMS-AX 500 MHz (JEOL, Tokyo, Japan) spectrometers with Me4Si as an internal standard. Mass spectra were recorded on an EI Ms-QP 1000 EX instrument (Shimadzu, Tokyo, Japan) at 70 eV. Biological evaluations were performed by the antimicrobial unit of Department of Chemistry of Natural and Microbial Products (National Research Centre, Giza 12622, Egypt). All starting materials and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
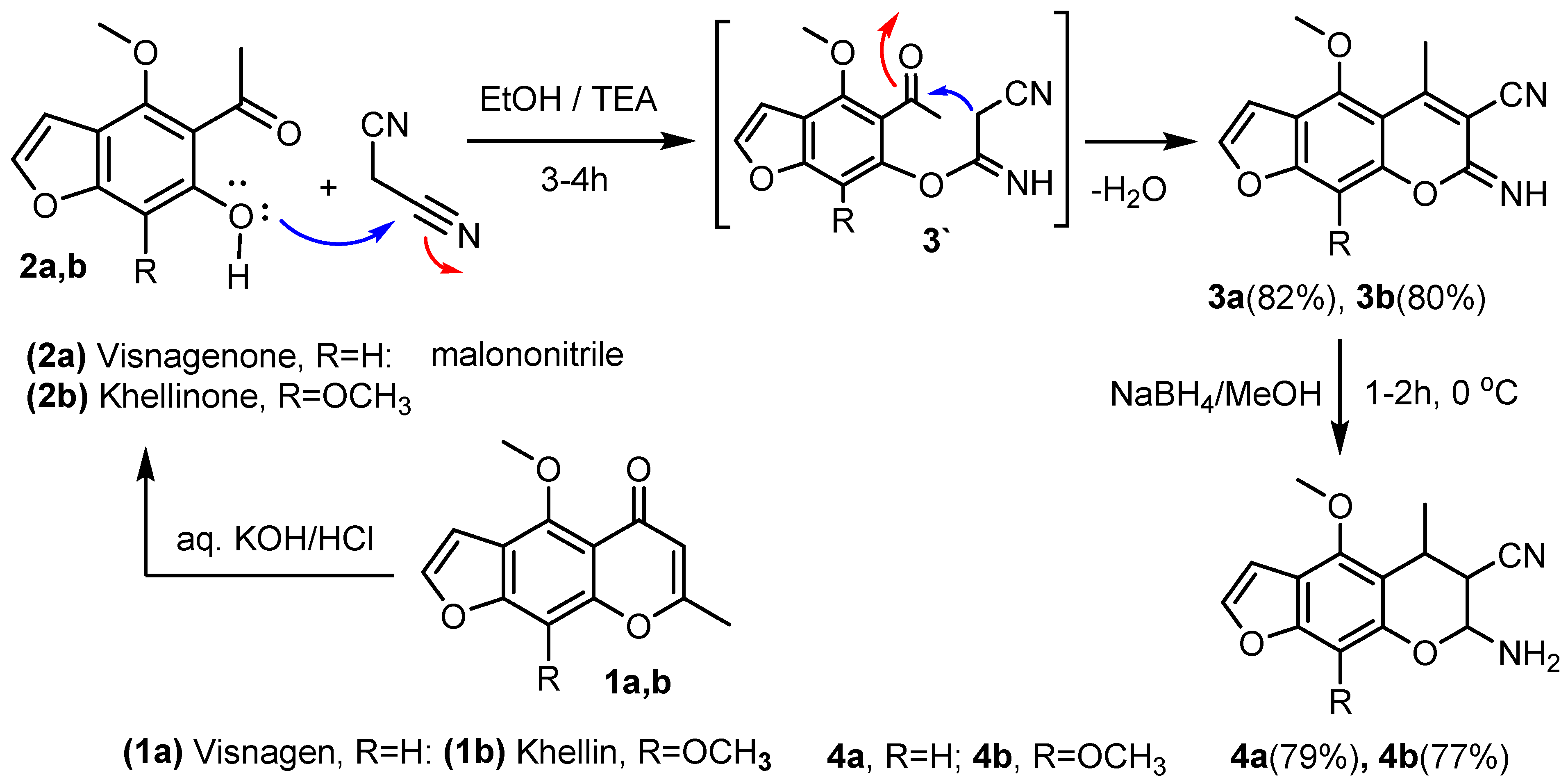
3.2. Synthesis of 7-imino-(4-methoxy or 4, 9-dimethoxy)-5-methyl-7H-furo[3,2-g] chromene-6-carbonitrile (3a,b)
Method A. General Procedure [
36]: To a stirred solution of visnaginone
2a (2.06 g, 0.01 mol) or khellinone (
2b) (2.36 g, 0.01 mol) and malononitrile (0.66 g, 0.01 mol) in absolute ethanol (50 mL) was added triethylamine (1 mL, 0.01 mol). The mixture was refluxed for 3–4 h (TLC) and then allowed to cool to room temperature. The final formed precipitate was isolated via filtration and washed with ethanol to get s pure product, then recrystallized from the proper solvent to give (
3a) and (
3b)
.Method B. To a stirred solution of visnaginone (2a) (2.06 g, 0.01 mol) or khellinone (2b) (2.36 g, 0.01 mol) in ethanolic sodium ethoxide solution (0.5 g, 0.02-atom of sodium 35 mL of ethanol), malononitrile (0.66 g, 0.01 mol) was added and the mixture was heated under reflux for 2–4 h and checked by TLC. After cooling, the final solid product was collected and recrystallized from the proper solvent to give (3a) and (3b), respectively.
3.3. Synthesis of 7-imino-4-methoxy-5-methyl-7H-furo[3,2-g]chromene-6-carbonitrile (3a)
The compound was obtained from the reaction of visnaginone (2a) (2.06 g, 0.01 mol) and malononitrile (0.66 g, 0.01 mol), as yellowish crystals, crystallized from dioxane (82%), melting point (M.p.): 208–210 °C. IR (ν, cm−1) KBr: 3300 (NH), 3055 (CH-aryl), 2965 (CH-aliph), 2240 (CN), 1630 (C=N), 1590 (C=C). 1H NMR (DMSO-d6, ppm) δ 2.35 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.80 (d, 1H, J = 2.35 Hz, furan), 7.10 (s, 1H, benzene), 7.30 (d, 1H, J = 2.38 Hz, furan), 9.50 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 18.5, 58.2 (2C, CH3, OCH3), 90.3 (1C, CH), 91.5, 116.7 (2C, C-CN), 103.1, 106.4, 110.2, 145.5, 150.7, 154.6, 155.2, 157.4, 161.2 (9C, Ar-C); MS (70 eV, %) m/z 254 (M+, 100%); Anal. Calc. (Found) for C14H10N2O3 (254.25): C, 66.14 (66.22); H, 3.96 (3.90); N, 11.02 (11.12).
3.4. Synthesis of 7-imino-4,9-dimethoxy-5-methyl-7H-furo[3,2-g]chromene-6-carbonitrile (3b)
The compound was obtained from the reaction of khellinone (2b) (2.36 g, 0.01mol) and malononitrile (0.66 g, 0.01 mol), as yellow crystals, crystallized from methanol (80%), M.p.: 220–222 °C. IR (ν, cm−1) KBr: 3310 (NH), 3060 (CH-aryl), 2970 (CH-aliph), 2235 (CN), 1634 (C=N), 1595 (C=C). 1H NMR (DMSO-d6, ppm) δ 2.30 (s, 3H, CH3), 3.85 (s, 6H, 2OCH3), 6.85 (d, 1H, J = 2.34 Hz, furan),7.05 (d, 1H, J = 2.37 Hz, furan), 9.60 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 18.6 (1C, CH3), 59.5 (2C, 2OCH3), 91.8, 116.9 (2C, C-CN), 103.5, 106.2, 113.1, 123.5, 145.1, 146.5, 148.4, 150.3, 157.2, 161.5 (10 C, Ar-C); MS (70 eV, %) m/z 284 (M+, 98%); Anal. Calc. (Found) for C15H12N2O4 (284.27): C, 63.38 (63.45); H, 4.26 (4.35); N, 9.85 (9.77).
3.5. Synthesis of 7-amino-(4-methoxy or 4,9-dimethoxy) -5-methyl-6,7-dihydro-5H-furo[3,2-g] chromene-6-carbonitrile (4a,b)
General procedure [
36]: To a solution of (
3a) (2.54 g, 0.01 mol) or (
3b) (2.84 g, 0.01 mol) in methanol (50 mL) was added sodium borohydride (0.38 g, 0.01 mol) at 0 °C. The reaction mixture was stirred for 1–2 h, under control (TLC). The reaction mixture was poured into water and the precipitated solid was filtered, washed with water, and dried and crystallized from the proper solvent to give (
4a) and (
4b), respectively.
3.6. Synthesis of 7-amino-4-methoxy-5-methyl-6, 7-dihydro-5H-furo[3,2-g]chromene-6- carbonitrile (4a)
The compound was obtained from the reaction of (3a) (2.54 g, 0.01mol) and sodium borohydride (0.38 g, 0.01 mol), as white crystals, crystallized from ethanol (79%), M.p.: 240–242 °C. IR (ν, cm−1) KBr: broad 3416 (NH2), 3052 (CH-aryl), 2970 (CH-aliph), 2244 (CN), 1585 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.45 (d, 3H, J = 6.80 Hz, CH3), 3.05 (m, 1H, CH, pyran ring), 3.15 (t, 1H, J = 6.85 Hz, CH, pyran ring), 3.81 (s, 3H, OCH3), 5.10 (d, 1H, J = 6.88 Hz, CH, pyran ring), 6.88 (d, 1H, J = 2.31 Hz, furan), 7.15 (s, 1H, benzene), 7.37 (d, 1H, J = 2.32 Hz, furan), 8.40 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.1 (1C, CH3), 21.3, 44.5, (2C, CH, pyran ring), 60.1 (1C, OCH3), 81.2 (1C, CH-NH2), 90.7 (1C, CH, benzene), 120.2 (1C, CN), 102.8, 105.1, 106.7, 145.9, 152.3, 154.8, 156.9 (7C, Ar-C); MS (70 eV, %) m/z 258 (M+, 100%); Anal. Calc. (Found) for C14H14N2O3 (258.28): C, 65.11 (65.20); H, 5.46 (5.52); N, 10.85 (10.77).
3.7. Synthesis of 7-amino-4,9-dimethoxy-5-methyl-6,7-dihydro-5H-furo[3,2-g]chromene-6- carbonitrile (4b)
The compound was obtained from the reaction of 3b (2.84 g, 0.01mol) and sodium borohydride (0.38 g, 0.01 mol), as yellowish crystals, crystallized from benzene (77%), M.p.: 260–262 °C. IR (ν, cm−1) KBr: broad 3420 (NH2), 3057 (CH-aryl), 2962 (CH-aliph), 2241 (CN), 1582 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.40 (d, 3H, J = 6.77 Hz, CH3), 3.08 (m, 1H, CH, pyran ring), 3.11 (t, 1H, J = 6.87 Hz, CH, pyran ring), 3.90 (s, 6H, 2OCH3), 5.15 (d, 1H, J = 6.81 Hz, CH, pyran ring), 6.84 (d, 1H, J = 2.37 Hz, furan), 7.40 (d, 1H, J = 2.39 Hz, furan), 8.45 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.5 (1C, CH3), 21.7, 44.8, (2C, CH, pyran ring), 60.6 (2C, 2OCH3), 82.4 (1C, CH-NH2), 122.1 (1C, CN), 105.1, 105.7, 110.2, 127.3, 145.1, 145.8, 146.5, 146.9 (8C, Ar-C); MS (70 eV, %) m/z 288 (M+, 95%); Anal. Calc. (Found) for C15H16N2O4 (288.30): C, 62.49 (62.55); H, 5.59 (5.66); N, 9.72 (9.65).
3.8. Synthesis of (6-methoxy or 6, 10-dimethoxy) -5-methyl-4a, 11a-dihydro-5H-furo[3′,2′: 6,7] chromeno[2,3-d] pyrimidin-4-amine (5a,b)
General procedure: Method A. A mixture of (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) and formamide (30 mL) was refluxed for 4–6 h under control (TLC). After cooling, the yellowish crystals were filtered off and washed with cold water and methanol, then recrystallized from the proper solvent to give (5a) and (5b), respectively.
Method B. A stream of NH3 gas was passed through (17a) (3.14 g, 0.01 mol) or (17b) (3.44 g, 0.01 mol) in a dioxane solution at room temperature for 2–4 h under control (TLC). The mixture was left in the refrigerator overnight, and the solid product that formed upon cooling was collected by filtration to give (5a) and (5b), respectively.
3.9. Synthesis of 6-methoxy-5-methyl-4a, 11a-dihydro-5H-furo[3′,2′: 6,7]chromeno[2,3-d] pyrimidin-4-amine (5a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and formamide (30 mL), as yellowish crystals, crystallized from methanol (75%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3425 (NH2), 3057 (CH-aryl), 2973 (CH-aliph), 1628 (C=N), 1588 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.38 (d, 3H, J = 6.75 Hz, CH3), 3.10 (m, 1H, CH, pyran ring), 3.20 (t, 1H, J = 6.80 Hz, CH, pyran ring), 3.85 (s, 3H, OCH3), 5.05 (d, 1H, J = 6.79 Hz, CH, pyran ring), 6.45 (s, 2H, NH2, D2O exchangeable), 6.75 (d, 1H, J = 2.35 Hz, furan), 7.18 (s, 1H, benzene), 7.40 (d, 1H, J = 2.34 Hz, furan), 8.08 (s, 1H, CH, pyrimidine ring); 13C NMR (DMSO-d6) δ 19.5 (1C, CH3), 23.5, 46.2, (2C, CH, pyran ring), 60.4 (1C, OCH3), 85.3 (1C, CH, pyran ring), 90.5 (1C, CH, benzene), 104.2, 105.5, 106.4, 147.2, 152.3, 154.1, 155.9, 157.4, 158.2 (9C, Ar-C); MS (70 eV, %) m/z 285 (M+, 100%); Anal. Calc. (Found) for C15H15N3O3 (285.30): C, 63.15 (63.22); H, 5.30 (5.39); N, 14.73 (14.66).
3.10. Synthesis of 6, 10-dimethoxy-5-methyl-4a, 11a-dihydro-5H-furo[3′,2′: 6,7]chromeno[2,3-d] pyrimidin-4-amine (5b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and formamide (30 mL), as yellow crystals, crystallized from ethanol (73%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3422 (NH2), 3059 (CH-aryl), 2977 (CH-aliph), 1629 (C=N), 1584 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.35 (d, 3H, J = 6.73 Hz, CH3), 3.20 (m, 1H, CH, pyran ring), 3.30 (t, 1H, J = 6.77 Hz, CH, pyran ring), 3.91 (s, 6H, 2OCH3), 5.10 (d, 1H, J = 6.81 Hz, CH, pyran ring), 6.50 (s, 2H, NH2, D2O exchangeable), 6.80 (d, 1H, J = 2.37 Hz, furan), 7.45 (d, 1H, J = 2.38 Hz, furan), 8.11 (s, 1H, CH, pyrimidine ring); 13C NMR (DMSO-d6) δ 19.8 (1C, CH3), 24.2, 47.6, (2C, CH, pyran ring), 60.9 (2C, 2OCH3), 86.1 (1C, CH, pyran ring), 105.1, 106.3, 110.2, 127.4, 144.7, 145.5, 146.5, 147.1, 157.7, 158.5 (10 C, Ar-C); MS (70 eV, %) m/z 315 (M+, 100%); Anal. Calc. (Found) for C16H17N3O4 (315.33): C, 60.94 (60.88); H, 5.43 (5.50); N, 13.33 (13.40).
3.11. Synthesis of (6-methoxy or 6, 10-dimethoxy)-5-methyl-3, 5-dihydro-4H-furo[3′,2′: 6,7] chromeno[2,3-d]pyrimidin-4-one (6a,b)
General procedure: Method A. A solution of (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) and formic acid (25 mL) was heated under reflux for 7–10 h under control (TLC). The reaction solution was allowed to cool to room temperature and poured into water. The formed solid precipitate was collected by filtration, washed with ethanol, dried, and crystallized from the proper solvent to give (6a) and (6b).
Method B. A mix of (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) and formic acid (10 mL) in formamide (35 mL) was refluxed for 5–8 h. After cooling, the solution was poured into cold water. The solid precipitate that formed was collected by filtration, washed with cold water/ethanol, and recrystallized from the proper solvent to give (6a) and (6b), respectively.
3.12. Synthesis of 6-methoxy-5-methyl-3, 5-dihydro-4H-furo[3′,2′: 6, 7]chromeno[2,3-d] pyrimidin-4-one (6a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and formic acid (25 mL), as brownish crystals, crystallized from dioxane (81%), M.p. > 350 °C. IR (ν, cm−1) KBr: 3295 (br. NH), 3063 (CH-aryl), 2962 (CH-aliph), 1680 (CO), 1630 (C=N), 1582 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.48 (d, 3H, J = 6.79 Hz, CH3), 3.65 (q, 1H, J = 6.76 Hz, CH, pyran ring), 3.92 (s, 3H, OCH3), 6.81 (d, 1H, J = 2.32 Hz, furan), 7.07 (s, 1H, benzene), 7.45 (d, 1H, J = 2.37 Hz, furan), 8.12 (s, 1H, CH, pyrimidine ring), 10.70 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.4, (1C, CH, pyran ring), 22.1 (1C, CH3), 60.7 (1C, OCH3), 90.8 (1C, CH, benzene), 105.8, 106.3, 107.5, 109.6, 146.4, 150.7, 151.2, 153.8, 156.1, 160.5 (10C, Ar-C), 163.4 (1C, C=O); MS (70 eV, %) m/z 284 (M+, 100%); Anal. Calc. (Found) for C15H12N2O4 (284.27): C, 63.38 (63.45); H, 4.26 (4.35); N, 9.85 (9.77).
3.13. Synthesis of 6,10-dimethoxy-5-methyl-3, 5-dihydro-4H-furo[3′,2′: 6,7]chromeno[2,3-d] pyrimidin-4-one (6b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and formic acid (25 mL), as yellowish crystals, crystallized from methanol (80%), M.p. > 350 °C. IR (ν, cm−1) KBr: 3290 (broad NH), 3060 (CH-aryl), 2959 (CH-aliph), 1682 (CO), 1633 (C=N), 1588 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.50 (d, 3H, J = 6.80 Hz, CH3), 3.70 (q, 1H, J = 6.78 Hz, CH, pyran ring), 3.95 (s, 6H, 2OCH3), 6.84 (d, 1H, J = 2.35 Hz, furan), 7.50 (d, 1H, J = 2.34 Hz, furan), 8.14 (s, 1H, CH, pyrimidine ring), 10.75 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.7, (1C, CH, pyran ring), 22.5 (1C, CH3), 60.9 (2C, 2OCH3), 106.1, 107.4, 110.6, 113.1, 124.3, 139.1, 145.8, 146.5, 146.9, 150.5, 161.8 (11C, Ar-C), 163.9 (1C, C=O); MS (70 eV, %) m/z 314 (M+, 100%); Anal. Calc. (Found) for C16H14N2O5 (314.30): C, 61.14 (61.22); H, 4.49 (4.55); N, 8.91 (8.84).
3.14. Synthesis of N-(6-cyano-(4-methoxy or 4, 9-dimethoxy)-5-methyl-5H-furo[3,2-g] chromen-7-yl) acetamide (7a,b)
General procedure: A mix of (4a) (2.58 g, 0.01 mol) and (4b) (2.88 g, 0.01 mol) was refluxed in acetic anhydride (30 mL) for 3–5 h, and then allowed to cool to room temperature and poured into cold water (50 mL). The solid product that formed was collected by filtration and washed with cold water. The final products were recrystallized from the proper solvent to give (7a) and (7b).
3.15. Synthesis of N-(6-cyano-4-methoxy-5-methyl-5H-furo[3,2-g]chromen-7-yl)acetamide (7a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01mol) and acetic anhydride (30 mL) as yellowish crystals, crystallized from ethanol (90%), M.p.: 318–320 °C. IR (ν, cm−1) KBr: 3300 (br. NH), 3057 (CH-aryl), 2962 (CH-aliph), 2225 (CN), 1691 (C=O), 1583 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.30 (d, 3H, J = 6.83 Hz, CH3), 1.90 (s, 3H, CH3), 3.63 (q, 1H, J = 6.84 Hz, CH, pyran ring), 3.87 (s, 3H, OCH3), 6.79 (d, 1H, J = 2.37 Hz, furan), 7.07 (s, 1H, benzene), 7.42 (d, 1H, J = 2.38 Hz, furan), 9.35 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.6 (1C, CH, pyran ring), 22.7, 24.4 (2C, 2CH3), 60.2 (1C, OCH3), 68.1 (1C, C-CN, pyran ring), 118.6 (1C, CN), 90.1 (1C, CH, benzene), 104.5, 105.4, 108.8, 146.1, 150.5, 154.2, 155.1, 162.4 (8C, Ar-C), 168.5 (1C,C=O); MS (70 eV, %) m/z 298 (M+, 100%); Anal. Calc. (Found) for C16H14N2O4 (298.30): C, 64.42 (64.50); H, 4.73 (4.65); N, 9.39 (9.32).
3.16. Synthesis of N-(6-cyano-4,9-dimethoxy-5-methyl-5H-furo[3,2-g]chromen-7-yl)acetamide (7b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01mol) and acetic anhydride (30 mL) as yellow crystals, crystallized from methanol (85%), M.p.: 332–334 °C. IR (ν, cm−1) KBr: 3310 (br. NH), 3058 (CH-aryl), 2966 (CH-aliph), 2223 (CN), 1688 (C=O), 1580 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.28 (d, 3H, J = 6.87 Hz, CH3), 1.84 (s, 3H, CH3), 3.59 (q, 1H, J = 6.78 Hz, CH, pyran ring), 3.90 (s, 6H, 2OCH3), 6.81 (d, 1H, J = 2.38 Hz, furan), 7.47(d, 1H, J = 2.40 Hz, furan), 9.30 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.3 (1C, CH, pyran ring), 22.1, 24.8 (2C, 2CH3), 61.3 (2C, 2OCH3), 68.5 (1C, C-CN, pyran ring), 118.8 (1C, CN), 106.2, 110.5, 112.7, 124.2, 139.6, 145.9, 146.4, 147.1, 163.7 (9C, Ar-C), 168.1 (1C,C=O); MS (70 eV, %) m/z 328 (M+, 100%); Anal. Calc. (Found) for C17H16N2O5 (328.32): C, 62.19 (62.27); H, 4.91 (4.84); N, 8.53 (8.60).
3.17. Synthesis of (6-methoxy or 6,10-dimethoxy)-2, 5-dimethyl-5H-furo [3′,2′: 6,7]chromeno [2,3-d]pyrimidin-4-ol (8a,b)
General procedure: Method A. A mixture of (4a) (2.58 g, 0.01 mol) and (4b) (2.88 g, 0.01 mol) in an acetic anhydride/pyridine mixture (30 mL:15 mL) was heated in a water bath for 10–12 h under control (TLC), allowed to cool, and poured into 30 mL of acidified cold water. The solid precipitate that formed was collected via filtration and washed with cold water. The precipitate products were recrystallized from the proper solvent to give (8a) and (8b).
Method B. A solution of (7a) (2.98 g, 0.01 mol) or (7b) (3.28 g, 0.01 mol) in absolute ethanol (25 mL) with pyridine (5 mL) was heated and refluxed on water bath for 6–9 h under control (TLC), after cooling the solid precipitate was collected via filtration, washed with water, dried and recrystallized from appropriate solvent to give (8a) and (8b).
3.18. Synthesis of 6-methoxy-2,5-dimethyl-5H-furo[3′,2′:6,7]chromeno[2,3-d]pyrimidin-4-ol (8a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and acetic anhydride/pyridine as yellow crystals, crystallized from toluene (84%), M.p. > 350 °C. IR (ν, cm−1) KBr: 3412 (br. OH), 3071 (CH-aryl), 2960 (CH-aliph), 1632 (C=N), 1586 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.50 (d, 3H, J = 6.78 Hz, CH3), 2.08 (s, 3H, CH3), 4.10 (q, 1H, J = 6.81 Hz, CH, pyran ring), 3.82 (s, 3H, OCH3), 6.80 (d, 1H, J = 2.39 Hz, furan), 7.01 (s, 1H, benzene), 7.55 (d, 1H, J = 2.35 Hz, furan), 12.10 (s, 1H, OH, D2O exchangeable); 13C NMR (DMSO-d6) δ 22.1, 24.5 (2C, 2CH3), 24.9 (1C, CH, pyran ring), 60.4 (1C, OCH3), 93.2 (1C, CH, benzene), 105.6, 107.1, 112.7, 119.3, 146.4, 150.2, 153.8, 154.7, 156.1, 164.5, 169.8 (11C, Ar-C); MS (70 eV, %) m/z 298 (M+, 100%); Anal. Calc. (Found) for C16H14N2O4 (298.30): C, 64.42 (64.35); H, 4.73 (4.80); N, 9.39 (9.46).
3.19. Synthesis of 6, 10-dimethoxy-2, 5-dimethyl-5H-furo[3′,2′: 6,7]chromeno[2,3-d] pyrimidin-4-ol (8b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and acetic anhydride/pyridine as yellowish crystals, crystallized from benzene (82%), M.p. > 350 °C. IR (ν, cm−1) KBr: 3408 (br. OH), 3073 (CH-aryl), 2962 (CH-aliph), 1636 (C=N), 1588 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.58 (d, 3H, J = 6.74 Hz, CH3), 2.13 (s, 3H, CH3), 4.18 (q, 1H, J = 6.82 Hz, CH, pyran ring), 3.94 (s, 6H, 2OCH3), 6.82 (d, 1H, J = 2.41 Hz, furan), 7.57 (d, 1H, J = 2.40 Hz, furan), 12.15 (s, 1H, OH, D2O exchangeable); 13C NMR (DMSO-d6) δ 22.6, 23.8 (2C, 2CH3), 24.7 (1C, CH, pyran ring), 62.3 (2C, 2OCH3), 106.5, 115.2, 115.8, 119.5, 128.5, 136.7, 144.9, 146.3, 146.8, 154.5, 165.6, 107.1 (12C, Ar-C); MS (70 eV, %) m/z 328 (M+, 100%); Anal. Calc. (Found) for C17H16N2O5 (328.32): C, 62.19 (62.27); H, 4.91 (4.85); N, 8.53 (8.60).
3.20. Synthesis of (7-methoxy or 7,11-dimethoxy)-2,6-dimethyl-1,6-dihydrofuro[3′,2′:6,7] chromeno[2,3-e][1,2,4]triazepin-5-amine (9a,b)
General procedure: A mix of (7a) (2.98 g, 0.01 mol) and (7b) (3.28 g, 0.01 mmol) and hydrazine hydrate (5 mL) in ethanol (40 mL) containing (0.1 mL) of piperidine was refluxed for 4–7 h under control (TLC). The reaction solution was concentrated under reduced pressure and the residue was triturated through methanol. The formed solid product was filtered, washed with methanol, and recrystallized from the appropriate solvent to give (9a) and (9b), respectively.
3.21. Synthesis of 7-methoxy-2,6-dimethyl-1,6-dihydrofuro[3′,2′:6,7]chromeno[2,3-e][1,2,4] triazepin-5-amine (9a)
The compound was obtained from the reaction of (7a) (2.98 g, 0.01 mol) and hydrazine hydrate as yellow crystals, crystallized from DMF (78%), M.p. >350 °C. IR (ν, cm−1) KBr: broad 3420–3395 (NH2), 3305 (br. NH), 3050 (CH-aryl), 2960 (CH-aliph), 1630 (C=N), 1585 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.28 (d, 3H, J = 6.90 Hz, CH3), 1.70 (s, 3H, CH3), 3.68 (q, 1H, J = 6.80 Hz, CH, pyran ring), 3.82 (s, 3H, OCH3), 6.77 (d, 1H, J = 2.30 Hz, furan), 6.85 (s, 2H, NH2, D2O exchangeable), 7.01 (s, 1H, benzene), 7.50 (d, 1H, J = 2.31 Hz, furan), 10.10 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 18.1 (1C, CH, pyran ring), 22.3, 24.1 (2C, 2CH3), 60.5 (1C, OCH3), 80.2 (1C, triazepin ring), 90.6 (1C, CH, benzene), 105.8, 106.7, 109.5, 146.4, 148.5, 148.6, 151.1, 153.8, 155.7, 160.2 (10 C, Ar-C); MS (70 eV, %) m/z 312 (M+, 90%); Anal. Calc. (Found) for C16H16N4O3 (312.33): C, 61.53 (61.60); H, 5.16 (5.21); N, 17.94 (17.88).
3.22. Synthesis of 7,11-dimethoxy-2,6-dimethyl-1,6-dihydrofuro[3′,2′:6,7]chromeno [2,3-e][1,2,4]triazepin-5-amine (9b)
The compound was obtained from the reaction of (7b) (3.28 g, 0.01 mmol) and hydrazine hydrate as yellowish crystals, crystallized from DMF (74%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3415–3390 (NH2), 3301 (br. NH), 3052 (CH-aryl), 2963 (CH-aliph), 1634 (C=N), 1587 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.29 (d, 3H, J = 6.92 Hz, CH3), 1.72 (s, 3H, CH3), 3.70 (q, 1H, J = 6.83 Hz, CH, pyran ring), 3.88 (s, 6H, 2OCH3), 6.80 (d, 1H, J = 2.31 Hz, furan), 6.90 (s, 2H, NH2, D2O exchangeable), 7.55 (d, 1H, J = 2.36 Hz, furan), 10.20 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 18.7 (1C, CH, pyran ring), 22.9, 24.4 (2C, 2CH3), 61.8 (2C, 2OCH3), 80.6 (1C, triazepin ring), 106.1, 110.4, 112.5, 124.3, 139.2, 145.6, 146.2, 146.9, 148.5, 148.7, 160.4 (11 C, Ar-C); MS (70 eV, %) m/z 342 (M+, 85%); Anal. Calc. (Found) for C17H18N4O4 (342.35): C, 59.64 (59.71); H, 5.30 (5.37); N, 16.37 (16.30).
3.23. Synthesis of 7-amino-(4-methoxy or 4, 9-dimethoxy)-5-methyl-5H-furo[3,2-g]chromene- 6-carboxamide (10a,b)
General procedure: Method A. Compound (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) was stirred in concentrated H2SO4 (30 mL) for 20–24 h at room temperature. The reaction mixture was poured dropwise over crushed ice. The solid product was filtered, washed with water, left to dry and recrystallized from the appropriate solvent to give (10a) or (10b), respectively.
Method B. Compound (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) was added dropwise with stirring to concentrated cold sulfuric acid at 20 °C (15 mL); so long as the temperature did not exceed 40 °C, the solution was stirred for a further 2 h at room temperature and poured into ice-cold water (20 mL). The reaction solution was left overnight in the refrigerator. The final solid precipitate was filtered off and recrystallized from the proper solvent to give (10a) or (10b).
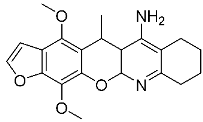
3.24. Synthesis of 7-amino-4-methoxy-5-methyl-5H-furo[3,2-g]chromene-6-carboxamide (10a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and concentrated cold sulfuric acid as brownish crystals, crystallized from methanol (73%), M.p.: 345–347 °C. IR (ν, cm−1) KBr: broad 3415, 3405 (2NH2), 3080 (CH-aryl), 2930 (CH-aliph), 1660 (C=O), 1581 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.38 (d, 3H, J = 6.72 Hz, CH3), 3.65 (q, 1H, J = 6.75 Hz, CH, pyran ring), 3.87 (s, 3H, OCH3), 6.74 (d, 1H, J = 2.35 Hz, furan), 6.86 (s, 2H, NH2, D2O exchangeable), 7.09 (s, 1H, benzene), 7.25 (s, 2H, 2NH2, D2O exchangeable), 7.74 (d, 1H, J = 2.42 Hz, furan); 13C NMR (DMSO-d6) δ 19.2 (1C, CH, pyran ring), 21.5 (1C, CH3), 60.2 (1C, OCH3), 84.5 (1C, pyran ring), 90.7 (1C, CH, benzene), 105.2, 106.6, 110.3, 146.7, 150.9, 153.5, 155.8, 159.2 (8C, Ar-C), 170.1(1C,C=O); MS (70 eV, %) m/z 274 (M+, 100%); Anal. Calc. (Found) for C14H14N2O4 (274.28): C, 61.31 (61.38); H, 5.15 (5.22); N, 10.21 (10.27).
3.25. Synthesis of 7-amino-4,9-dimethoxy-5-methyl-5H-furo[3,2-g]chromene-6-carboxamide (10b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and conc. cold sulfuric acid as yellowish crystals, crystallized from ethanol (70%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3410, 3402 (2NH2), 3085 (CH-aryl), 2935 (CH-aliph), 1665 (C=O), 1583 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.40 (d, 3H, J = 6.71 Hz, CH3), 3.70 (q, 1H, J = 6.79 Hz, CH, pyran ring), 3.92 (s, 6H, 2OCH3), 6.80 (d, 1H, J = 2.40 Hz, furan), 6.91 (s, 2H, NH2, D2O exchangeable), 7.30 (s, 2H, 2NH2, D2O exchangeable), 7.70 (d, 1H, J = 2.44 Hz, furan); 13C NMR (DMSO-d6) δ 18.9 (1C, CH, pyran ring), 21.7 (1C, CH3), 61.8 (2C, 2OCH3), 85.1 (1C, pyran ring), 106.4, 111.5, 113.7, 124.3, 138.5, 144.9, 146.4, 147.1, 158.7 (9C, Ar-C), 173.5 (1C,C=O); MS (70 eV, %) m/z 304 (M+, 92%); Anal. Calc. (Found) for C15H16N2O5 (304.30): C, 59.21 (59.30); H, 5.30 (5.39); N, 9.21 (9.14).
3.26. Synthesis of (6-methoxy or 6,10-dimethoxy)-5-methyl-2-phenyl-3,5-dihydro-4H-furo[3′,2′: 6,7]chromeno[2,3-d]pyrimidin-4-one (11a,b)
General procedure: A mix of compounds (10a) (2.74 g, 0.01 mol) and (10b) (3.04 g, 0.01 mol) and the suitable acid chloride (0.01 mol), namely benzoyl chloride (1.2 mL, 0.01 mol), was refluxed in acetic acid (25 mL) for 9–12 h under control (TLC). The reaction solution was allowed to cool, then poured onto ice-cold water. The solid precipitate was filtered, washed with water, and recrystallized from the proper solvent to give (11a) and (11b).
3.27. Synthesis of 6-methoxy-5-methyl-2-phenyl-3,5-dihydro-4H-furo[3′,2′: 6,7]chromeno[2,3-d] pyrimidin-4-one (11a)
The compound was obtained from the reaction of (10a) (2.74 g, 0.01 mol) and benzoyl chloride as yellowish crystals, crystallized from dioxane (71%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3270 (NH), 3084 (CH-aryl), 2935 (CH-aliph), 1686 (C=O), 1585 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.40 (d, 3H, J = 6.80 Hz, CH3), 3.70 (q, 1H, J = 6.81 Hz, CH, pyran ring), 3.90 (s, 3H, OCH3), 6.78 (d, 1H, J = 2.39 Hz, furan), 7.07 (s, 1H, benzene),7.35–7.72 (m, 5H, phenyl), 7.78 (d, 1H, J = 2.41 Hz, furan), 11.10 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.5 (1C, CH, pyran ring), 21.8 (1C, CH3), 60.7 (1C, OCH3), 90.8 (1C, CH, benzene), 105.4, 106.1, 108.1, 109.3, 128.1, 128.6, 130.4, 131.5, 146.2, 150.5, 153.1, 155.6, 158.7, 160.5 (16C, Ar-C), 165.2(1C,C=O); MS (70 eV, %) m/z 360 (M+, 100%); Anal. Calc. (Found) for C21H16N2O4 (360.37): C, 69.99 (69.90); H, 4.48 (4.41); N, 7.77 (7.84).
3.28. Synthesis of 6, 10-dimethoxy-5-methyl-2-phenyl-3, 5-dihydro-4H-furo[3′,2′: 6,7]chromeno [2,3-d]pyrimidin-4-one (11b)
The compound was obtained from the reaction of (10b) (3.04 g, 0.01 mol) and benzoyl chloride as brownish crystals, crystallized from THF (68%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3275 (NH), 3080 (CH-aryl), 2932 (CH-aliph), 1682 (C=O), 1580 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.41 (d, 3H, J = 6.82 Hz, CH3), 3.68 (q, 1H, J = 6.84 Hz, CH, pyran ring), 3.94 (s, 6H, 2OCH3), 6.80 (d, 1H, J = 2.37 Hz, furan), 7.40–7.79 (m, 5H, phenyl), 7.85 (d, 1H, J = 2.34 Hz, furan), 11.22 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.2 (1C, CH, pyran ring), 22.1 (1C, CH3), 60.9 (2C, 2OCH3), 106.5, 107.8, 110.5,112.6, 123.5, 128.4, 128.9, 130.5, 131.9, 139.2, 145.4, 146.6, 146.9, 157.1, 160.8 (17C, Ar-C), 166.1 (1C,C=O); MS (70 eV, %) m/z 390 (M+, 94%); Anal. Calc. (Found) for C22H18N2O5 (390.39): C, 67.69 (67.75); H, 4.65 (4.71); N, 7.18 (7.10).
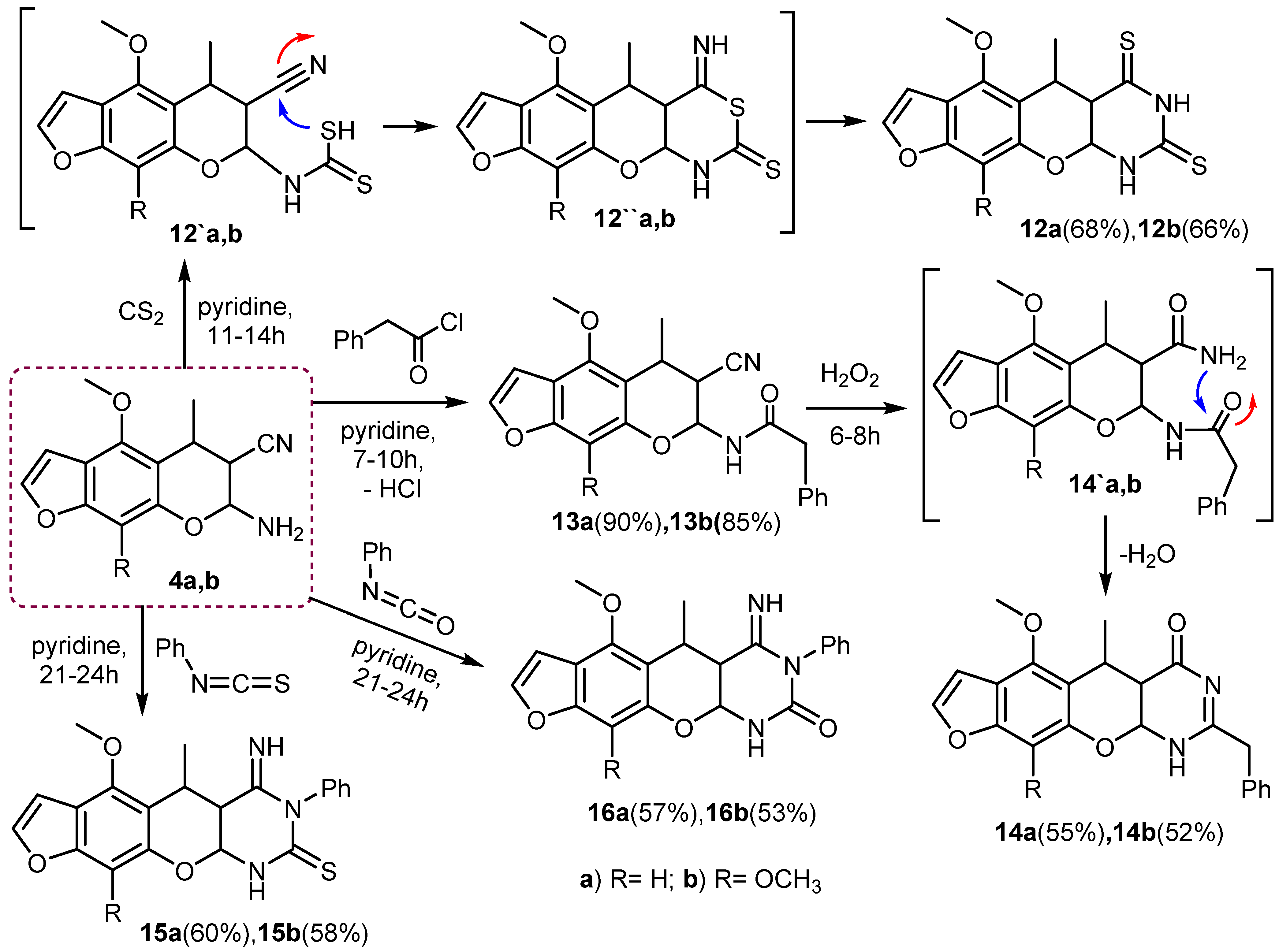
3.29. Synthesis of (6-methoxy or 6,10-dimethoxy)-5-methyl-1,4a,5,11a-tetrahydro-2H-furo[3′,2′: 6,7]chromeno [2,3-d]pyrimidine-2,4(3H)-dithione (12a,b)
General procedure: A solution of compound (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) and carbon disulfide (4 mL) in 40 mL of pyridine was heated and stirred under reflux on a water bath for 11–14 h with TLC. The solid product precipitated so formed was filtered off while hot and washed several times with ethanol. The final products were recrystallized from the suitable solvent to give (12a) or (12b).
3.30. Synthesis of 6-methoxy-5-methyl-1,4a,5,11a-tetrahydro-2H-furo[3′,2′:6,7]chromeno[2,3-d] pyrimidine-2,4(3H)-dithione (12a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and carbon disulfide, as pale yellow crystals, crystallized from DMF (68%), M.p. > 350 °C. IR (ν, cm−1) KBr: IR (ν, cm−1) KBr: broad 3235-3210 (2NH), 3059 (CH-aryl), 2977 (CH-aliph), 1581 (C=C), 1300–1295 (2C=S), 1H NMR (DMSO-d6, ppm) δ 1.33 (d, 3H, J = 6.74 Hz, CH3), 3.08 (m, 1H, CH, pyran ring), 3.12 (t, 1H, J = 6.75 Hz, CH, pyran ring), 3.90 (s, 3H, OCH3), 5.20 (d, 1H, J = 6.77 Hz, CH, pyran ring), 6.80 (d, 1H, J = 2.34 Hz, furan), 7.10 (s, 1H, benzene), 7.42 (d, 1H, J = 2.37 Hz, furan), 9.10, 12.02 (s, 2H, 2NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.3 (1C, CH3), 32.5 (1C, CH, pyran ring), 60.8 (1C, OCH3), 70.2, 90.5 (2C, 2CH, pyran ring), 92.1 (1C, CH, benzene), 103.6, 105.4, 106.9, 146.5, 151.6, 154.1, 155.7 (7C, Ar-C), 180.1, 195.8 (2C, C=S); MS (70 eV, %) m/z 334 (M+, 92%); Anal. Calc. (Found) for C15H14N2O3S2 (334.41): C, 53.88 (53.80); H, 4.22 (4.30); N, 8.38 (8.45).
3.31. Synthesis of 6,10-dimethoxy-5-methyl-1,4a,5,11a-tetrahydro-2H-furo[3′,2′:6,7]chromeno [2, 3-d]pyrimidine-2,4(3H)-dithione (12b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and carbon disulfide, as yellowish crystals, crystallized from dioxane (66%), M.p. > 350 °C. IR (ν, cm−1) KBr: IR (ν, cm−1) KBr: broad 3231–3207 (2NH), 3060 (CH-aryl), 2980 (CH-aliph), 1580 (C=C), 1302–1297 (2C=S), 1H NMR (DMSO-d6, ppm) δ 1.28 (d, 3H, J = 6.71 Hz, CH3), 3.12 (m, 1H, CH, pyran ring), 3.20 (t, 1H, J = 6.76 Hz, CH, pyran ring), 3.95 (s, 6H, 2OCH3), 5.30 (d, 1H, J = 6.78 Hz, CH, pyran ring), 6.82 (d, 1H, J = 2.30 Hz, furan), 7.48 (d, 1H, J = 2.32 Hz, furan), 9.20, 12.15 (s, 2H, 2NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.7 (1C, CH3), 33.1 (1C, CH, pyran ring), 61.8 (2C, 2OCH3), 70.5, 90.9 (2C, 2CH, pyran ring), 105.5, 106.3, 110.4, 127.1, 144.7, 145.6, 146.4, 146.8 (8C, Ar-C), 180.5, 196.1 (2C, C=S); MS (70 eV, %) m/z 364 (M+, 85%); Anal. Calc. (Found) for C16H16N2O4S2 (364.43): C, 52.73 (52.80); H, 4.43 (4.35); N, 7.69 (7.78).
3.32. Synthesis of N-(6-cyano-(4-methoxy or 4,9-dimethoxy)-5-methyl-6,7-dihydro-5H-furo[3, 2-g]chromen-7-yl)-2-phenylacetamide (13a,b)
General procedure: A mixture of compound (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) and 2-phenylacetyl chloride (1.55 g, 0.01 mol) in 35 mL of pyridine was heated and refluxed for 7–10 h with TLC. The reaction solution was allowed to cool at room temperature and then poured into acidified cold water. The final precipitate was filtered, washed with cold water, dried, and crystallized with the appropriate solvent to give (13a) or (13b).
3.33. Synthesis of N-(6-cyano-4-methoxy-5-methyl-6,7-dihydro-5H-furo[3,2-g]chromen-7-yl)- 2-phenyl- acetamide (13a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01mol) and 2-phenylacetyl chloride (1.55 g, 0.01mol), as brownish crystals, crystallized from n-hexane (90%), M.p.: 292–294 °C. IR (ν, cm−1) KBr: broad 3215 (NH), 3045 (CH-aryl), 2962 (CH-aliph), 2240 (CN), 1690 (C=O), 1582 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.41 (d, 3H, J = 6.73 Hz, CH3), 3.21 (m, 1H, CH, pyran ring), 3.28 (t, 1H, J = 6.79 Hz, CH, pyran ring), 3.35 (s, 2H, CH2), 3.88 (s, 3H, OCH3), 5.35 (d, 1H, J = 6.83 Hz, CH, pyran ring), 6.74 (d, 1H, J = 2.39 Hz, furan), 7.02–7.26 (s, 5H, benzene), 7.30 (s, 1H, benzene), 7.50 (d, 1H, J = 2.38 Hz, furan), 9.22 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.4 (1C, CH3), 20.5, 38.7, (2C, CH, pyran ring), 39.5 (1C, CH2), 60.6 (1C, OCH3), 82.3 (1C, CH-pyran ring), 91.1 (1C, CH, benzene), 120.5 (1C, CN), 103.4, 105.3, 106.5, 127.4, 128.8, 129.1, 136.1, 146.7, 152.1, 153.9, 156.5 (13C, Ar-C), 170.8 (1C, C=O); MS (70 eV, %) m/z 376 (M+, 100%); Anal. Calc. (Found) for C22H20N2O4 (376.41): C, 70.20 (70.28); H, 5.36 (5.45); N, 7.44 (7.52).
3.34. Synthesis of N-(6-cyano-4, 9-dimethoxy-5-methyl-6,7-dihydro-5H-furo[3,2-g]chromen- 7-yl)-2-phenylacetamide (13b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01mol) and 2-phenylacetyl chloride (1.55 g, 0.01 mol), as yellowish crystals, crystallized from benzene (85%), M.p.: 305–307 °C. IR (ν, cm−1) KBr: broad 3220 (NH), 3050 (CH-aryl), 2966 (CH-aliph), 2244 (CN), 1692 (C=O), 1585 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.38 (d, 3H, J = 6.72 Hz, CH3), 3.19 (m, 1H, CH, pyran ring), 3.25 (t, 1H, J = 6.75 Hz, CH, pyran ring), 3.33 (s, 2H, CH2), 3.95 (s, 6H, 2OCH3), 5.45 (d, 1H, J = 6.84 Hz, CH, pyran ring), 6.71 (d, 1H, J = 2.45 Hz, furan), 7.05–7.30 (s, 5H, benzene), 7.55 (d, 1H, J = 2.44 Hz, furan), 9.30 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.6 (1C, CH3), 20.8, 40.2, (2C, CH, pyran ring), 39.7(1C, CH2), 61.2 (2C, 2OCH3), 82.6 (1C, CH-pyran ring),120.7 (1C, CN), 106.1, 107.4, 110.2, 126.7, 127.5, 129.4, 129.8, 136.9, 145.1, 146.2, 146.5, 146.9 (14C, Ar-C), 171.3 (1C, C=O); MS (70 eV, %) m/z 406 (M+, 88%); Anal. Calc. (Found) for C23H22N2O5 (406.44): C, 67.97 (67.90); H, 5.46 (5.55); N, 6.89 (6.80).
3.35. Synthesis of 2-benzyl-(6-methoxy or 6,10-dimethoxy)-5-methyl-1,4a,5,11a-tetrahydro- 4H-furo[3′,2′: 6,7]chromeno [2,3-d]pyrimidin-4-one (14a,b)
General procedure: To a well-stirred cold mixture of compound (13a) (3.76 g, 0.01 mol) or (13b) (4.06 g, 0.01 mol) in 20 mL of (HCl: AcOH/1:1), a cold solution of H2O2 (20 mL) was added dropwise in an ice bath (0–5 °C), and the reaction solution was stirred for 6–8 h at room temperature. The solid that precipitated was collected by filtration, then redissolved in NaOH (30 mL 10%), heated under reflux for 30–60 min, and cooled. The result was acidified with HCl (25 mL) and the final solid product was collected and crystallized from the proper solvent to give (14a) or (14b).
3.36. Synthesis of 2-benzyl-6-methoxy-5-methyl-1,4a,5,11a-tetrahydro-4H-furo[3′,2′:6,7] chromeno[2,3-d]pyrimidin-4-one (14a)
The compound was obtained from the reaction of (13a) (3.76 g, 0.01 mol) and H2O2, as yellowish crystals, crystallized from DMF (55%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3290 (NH), 3075 (CH-aryl), 2970 (CH-aliph), 1677 (C=O), 1626 (C=N), 1585 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.37 (d, 3H, J = 6.71 Hz, CH3), 3.15 (m, 1H, CH, pyran ring), 3.31 (t, 1H, J = 6.74 Hz, CH, pyran ring), 3.62 (s, 2H, CH2), 3.80 (s, 3H, OCH3), 5.22 (d, 1H, J = 6.79 Hz, CH, pyran ring), 6.71 (d, 1H, J = 2.41 Hz, furan), 7.10 (s, 1H, benzene), 7.20–7.40 (s, 5H, benzene), 7.60 (d, 1H, J = 2.38 Hz, furan), 9.50 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.8 (1C, CH3), 30.5 (1C, CH, pyran ring), 38.9 (1C, CH2), 60.2 (1C, OCH3), 63.5, 84.7 (1C, CH-pyran ring), 91.8 (1C, CH, benzene), 103.6, 105.5, 106.8, 126.1, 128.4, 129.3, 135.8, 146.4, 152.5, 154.2, 155.8, 157.4 (14C, Ar-C), 172.1 (1C, C=O); MS (70 eV, %) m/z 376 (M+, 77%); Anal. Calc. (Found) for C22H20N2O4 (376.41): C, 70.20 (70.12); H, 5.36 (5.28); N, 7.44 (7.57).
3.37. Synthesis of 2-benzyl-6,10-dimethoxy-5-methyl-1,4a,5,11a-tetrahydro-4H-furo[3′,2′:6, 7]chromeno[2,3-d]pyrimidin-4-one (14b)
The compound was obtained from the reaction of (13b) (4.06 g, 0.01 mol) and H2O2, as yellow crystals, crystallized from dioxane (52%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3285 (NH), 3070 (CH-aryl), 2972 (CH-aliph), 1675 (C=O), 1628 (C=N), 1582 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.33 (d, 3H, J = 6.73 Hz, CH3), 3.20 (m, 1H, CH, pyran ring), 3.35 (t, 1H, J = 6.71 Hz, CH, pyran ring), 3.66 (s, 2H, CH2), 3.94 (s, 6H, 2OCH3), 5.30 (d, 1H, J = 6.83 Hz, CH, pyran ring), 6.85 (d, 1H, J = 2.45 Hz, furan), 7.22–7.42 (s, 5H, benzene), 7.68 (d, 1H, J = 2.43 Hz, furan), 9.60 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 18.9 (1C, CH3), 30.8 (1C, CH, pyran ring), 39.4 (1C, CH2), 61.1 (2C, 2OCH3), 63.8, 85.1 (1C, CH-pyran ring), 106.1, 106.7, 110.4, 125.9, 126.6, 128.8, 129.5, 135.9, 145.1, 146.5, 146.8, 147.2, 157.1 (15C, Ar-C), 172.5 (1C, C=O); MS (70 eV, %) m/z 406 (M+, 70%); Anal. Calc. (Found) for C23H22N2O5 (406.44): C, 67.97 (67.91); H, 5.46 (5.40); N, 6.89 (6.79).
3.38. Synthesis of 4-imino-(6-methoxy or 6,10-dimethoxy)-5-methyl-3-phenyl-1,3,4,4a,5,11a- hexahydro-2H-furo [3′,2′: 6,7]chromeno [2,3-d]pyrimidine-2-thione (15a,b) and 4-imino-(6- methoxy or 6,10-dimethoxy)-5-methyl-3-phenyl-1,3,4,4a,5,11a-hexahydro-2H-furo[3′,2′:6,7] chromeno[2,3-d]pyrimidin-2-one (16a,b)
General procedure: A solution of compound (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) and phenylisothiocyanate (1.60 mL, 0.01 mol) or phenylisocyanate (1.10 mL, 0.01 mol) in 40 mL of pyridine was heated and refluxed for 21–24 h under control (TLC). The mixture was cooled and poured into cold water, filtrated, washed several times with ethanol, and dried. The final product was recrystallized from the proper solvent to give (15a), (15b), (16a), or (16b), respectively.
3.39. Synthesis of 4-imino-6-methoxy-5-methyl-3-phenyl-1,3,4,4a,5,11a-hexahydro-2H-furo [3′,2′:6,7]chromeno[2,3-d]pyrimidine-2-thione (15a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and phenylisothiocyanate as brownish crystals, crystallized from methanol (60%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3300-3225 (2NH), 3071(CH-aryl), 2966 (CH-aliph), 1633 (C=N), 1585 (C=C), 1335 (C=S), 1H NMR (DMSO-d6, ppm) δ 1.32 (d, 3H, J = 6.74 Hz, CH3), 3.10 (m, 1H, CH, pyran ring), 3.27 (t, 1H, J = 6.72 Hz, CH, pyran ring), 3.78 (s, 3H, OCH3), 5.15 (d, 1H, J = 6.73 Hz, CH, pyran ring), 6.74 (d, 1H, J = 2.38 Hz, furan), 7.14 (s, 1H, benzene), 7.35–7.58 (s, 5H, benzene ring), 7.70 (d, 1H, J = 2.36 Hz, furan), 9.10 (s, 1H, NH, D2O exchangeable), 9.60 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.5 (1C, CH3), 30.1, 53.8 (2C, 2CH, pyran ring), 60.6 (1C, OCH3), 88.8 (1C, CH-pyran ring), 91.9 (1C, CH, benzene), 103.3, 105.2, 106.4, 128.1, 129.2, 131.4, 133.2, 146.1, 152.4, 154.1, 155.6, 157.1 (14C, Ar-C), 175.5 (1C, C=S); MS (70 eV, %) m/z 393 (M+, 80%); Anal. Calc. (Found) for C21H19N3O3S (393.46): C, 64.11 (64.20); H, 4.87 (4.80); N, 10.68 (10.61).
3.40. Synthesis of 4-imino-6,10-dimethoxy-5-methyl-3-phenyl-1,3,4,4a,5,11a-hexahydro-2H-furo [3′,2′:6,7]chromeno[2,3-d]pyrimidine-2-thione (15b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and phenylisothiocyanate as yellowish crystals, crystallized from n-hexane (58%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3305–3230 (2NH), 3074 (CH-aryl), 2968 (CH-aliph), 1631 (C=N), 1583 (C=C), 1332 (C=S), 1H NMR (DMSO-d6, ppm) δ 1.28 (d, 3H, J = 6.70 Hz, CH3), 3.08 (m, 1H, CH, pyran ring), 3.24 (t, 1H, J = 6.77 Hz, CH, pyran ring), 3.88 (s, 6H, 2OCH3), 5.23 (d, 1H, J = 6.78 Hz, CH, pyran ring), 6.80 (d, 1H, J = 2.40 Hz, furan),7.38–7.61(s, 5H, benzene ring), 7.82 (d, 1H, J = 2.42 Hz, furan), 9.20 (s, 1H, NH, D2O exchangeable), 9.70 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.1 (1C, CH3), 30.7, 54.2 (2C, 2CH, pyran ring), 60.9 (2C, 2OCH3), 89.5 (1C, CH-pyran ring), 105.9, 106.4, 110.2, 126.8, 128.6, 129.5, 131.7, 134.3, 145.1, 146.2, 146.7, 147.1, 157.3 (15C, Ar-C), 175.8 (1C, C=S); MS (70 eV, %) m/z 423 (M+, 75%); Anal. Calc. (Found) for C22H21N3O4S (423.49): C, 62.40 (62.50); H, 5.00 (5.10); N, 9.92 (9.83).
3.41. Synthesis of 4-imino-6-methoxy-5-methyl-3-phenyl-1,3,4,4a,5,11a-hexahydro-2H-furo [3′,2′:6,7]chromeno[2,3-d]pyrimidin-2-one (16a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and phenylisocyanate as yellow crystals, crystallized from toluene (57%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3290–3240 (2NH), 3080 (CH-aryl), 2971 (CH-aliph), 1688 (C=O), 1635 (C=N), 1590 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.36 (d, 3H, J = 6.77 Hz, CH3), 3.08 (m, 1H, CH, pyran ring), 3.30 (t, 1H, J = 6.79 Hz, CH, pyran ring), 3.91 (s, 3H, OCH3), 5.25 (d, 1H, J = 6.78 Hz, CH, pyran ring), 6.79 (d, 1H, J = 2.41 Hz, furan), 7.27 (s, 1H, benzene), 7.39–7.61 (s, 5H, phenyl ring), 7.68 (d, 1H, J = 2.43 Hz, furan), 9.05 (s, 1H, NH, D2O exchangeable), 9.52 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.2 (1C, CH3), 31.4, 54.1 (2C, 2CH, pyran ring), 60.8 (1C, OCH3), 89.2 (1C, CH-pyran ring), 92.1 (1C, CH, benzene), 102.8, 104.5, 106.2, 127.7, 128.4, 129.6, 132.9, 146.3, 152.5, 153.9, 155.7, 156.8 (14C, Ar-C), 159.1 (1C, C=O); MS (70 eV, %) m/z 377 (M+, 72%); Anal. Calc. (Found) for C21H19N3O4 (377.40): C, 66.83 (66.75); H, 5.07 (5.15); N, 11.13 (11.05).
3.42. Synthesis of 4-imino-6,10-dimethoxy-5-methyl-3-phenyl-1,3,4,4a,5,11a-hexahydro-2H-furo [3′,2′:6,7]chromeno[2,3-d]pyrimidin-2-one (16b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and phenylisocyanate as pale yellow crystals, crystallized from benzene (53%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3282–3233 (2NH), 3073 (CH-aryl), 2960 (CH-aliph), 1682 (C=O), 1633 (C=N), 1581 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.32 (d, 3H, J = 6.73 Hz, CH3), 3.14 (m, 1H, CH, pyran ring), 3.35 (t, 1H, J = 6.70 Hz, CH, pyran ring), 3.95 (s, 6H, 2OCH3), 5.30 (d, 1H, J = 6.72 Hz, CH, pyran ring), 6.82 (d, 1H, J = 2.40 Hz, furan), 7.45–7.65 (s, 5H, phenyl ring), 7.72 (d, 1H, J = 2.44 Hz, furan), 9.17 (s, 1H, NH, D2O exchangeable), 9.50 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.5 (1C, CH3), 31.7, 54.5 (2C, 2CH, pyran ring), 61.1 (2C, 2OCH3), 90.3 (1C, CH-pyran ring), 105.5, 106.2, 110.4, 126.8, 127.9, 128.5, 129.2, 133.1, 144.8, 145.7, 146.3, 146.8, 156.7 (15C, Ar-C), 160.5 (1C, C=O); MS (70 eV, %) m/z 407 (M+, 69%); Anal. Calc. (Found) for C22H21N3O5 (407.43): C, 64.86 (64.80); H, 5.20 (5.12); N, 10.31 (10.38).
3.43. Synthesis of Ethyl -N-(6-cyano-(4-methoxy or 4, 9-dimethoxy)-5-methyl-6,7-dihydro-5H- furo[3,2-g]chromen-7-yl)formimidate (17a,b)
General procedure: A mix of compound (4a) (2.58 g, 0.01 mol) or (4b) (2.88 g, 0.01 mol) and triethyl-orthoformate (1.50 mL, 0.01 mol) and acetic anhydride (40 mL) was refluxed for 5–8 h under control (TLC). The solvent was removed under reduced pressure and the separated solid was recrystallized from the proper solvent to give (17a) or (17b).
3.44. Synthesis of Ethyl-N-(6-cyano-4-methoxy-5-methyl-6,7-dihydro-5H-furo[3,2-g]chromen -7-yl) formimidate (17a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01mol) and triethyl-orthoformate as brownish crystals, crystallized from methanol (73%), M.p.: 270–272 °C. IR (ν, cm−1) KBr: 3049 (CH-aryl), 2955 (CH-aliph), 2220 (CN), 1635(C=N), 1580 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.25 (t, 3H, J = 6.72 Hz, CH3), 1.35 (d, 3H, J = 6.75 Hz, CH3), 3.27 (m, 1H, J = 6.80 Hz, CH, pyran ring), 3.35 (t, 1H, J = 6.85 Hz, CH, pyran ring), 3.39 (d, 1H, J = 6.68 Hz, CH, pyran ring), 3.66 (q, 2H, J = 6.65 Hz, CH2), 3.80 (s, 3H, OCH3), 6.82 (d, 1H, J = 2.33 Hz, furan), 7.17 (s, 1H, benzene), 7.50 (d, 1H, J = 2.46 Hz, furan), 8.02 (s, 1H,CH, methine proton); 13C NMR (DMSO-d6) δ 18.5, 20.7 (2C, 2CH3), 21.5, 45.2 (2C, 2CH, pyran ring), 60.8 (1C, OCH3), 64.2 (1C, CH2), 90.6 (1C, benzene ring), 92.3 (1C, pyran ring), 119.5 (1C, CN), 103.6, 105.1, 106.3, 146.5, 152.1, 154.7, 155.6 (7C, Ar-C), 159.1 (1C,C=N); MS (70 eV, %) m/z 314 (M+, 100%); Anal. Calc. (Found) for C17H18N2O4 (314.34): C, 64.96 (64.88); H, 5.77 (5.70); N, 8.91 (8.98).
3.45. Synthesis of Ethyl-N-(6-cyano-4,9-dimethoxy-5-methyl-6,7-dihydro-5H-furo[3,2-g] chromen-7-yl)formimidate (17b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01mol) and triethyl-orthoformate as yellowish crystals, crystallized from acetone (67%), M.p.: 280–282 °C. IR (ν, cm−1) KBr: 3045 (CH-aryl), 2951 (CH-aliph), 2218 (CN), 1633 (C=N), 1583 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.20 (t, 3H, J = 6.68 Hz, CH3), 1.31 (d, 3H, J = 6.69 Hz, CH3), 3.23 (m, 1H, J = 6.73 Hz, CH, pyran ring), 3.29 (t, 1H, J = 6.76 Hz, CH, pyran ring), 3.37 (d, 1H, J = 6.74 Hz, CH, pyran ring), 3.61 (q, 2H, J = 6.67 Hz, CH2), 3.91 (s, 6H, 2OCH3), 6.85 (d, 1H, J = 2.35 Hz, furan), 7.55 (d, 1H, J = 2.44 Hz, furan), 8.06 (s, 1H,CH, methine proton); 13C NMR (DMSO-d6) δ 19.2, 20.9 (2C, 2CH3), 21.8, 45.7 (2C, 2CH, pyran ring), 62.5 (2C, 2OCH3), 64.6 (1C, CH2), 91.9 (1C, pyran ring), 119.7 (1C, CN), 106.1, 106.7, 110.5, 127.1, 145.1, 145.8, 146.3, 146.9 (8C, Ar-C), 159.7 (1C,C=N); MS (70 eV, %) m/z 344 (M+, 97%); Anal. Calc. (Found) for C18H20N2O5 (344.37): C, 62.78 (62.85); H, 5.85 (5.77); N, 8.13 (8.21).
3.46. Synthesis of (6-methoxy or 6,10-dimethoxy)-3,5-dimethyl-3,4a,5,11a-tetrahydro-4H-furo [3′,2′:6,7]chromeno[2,3-d]pyrimidin-4-imine (18a,b)
General procedure: A mix of compound (17a) (3.14 g, 0.01 mol) or (17b) (3.44 g, 0.01 mol) and methylamine (0.04 mL, 0.01 mol) in absolute ethanol (40 mL) was heated and stirred at room temperature for 2–4 h with TLC. The resulting product was collected via filtration and recrystallized from the appropriate solvent to give (18a) or (18b).
3.47. Synthesis of 6-methoxy-3,5-dimethyl-3,4a,5,11a-tetrahydro-4H-furo[3′,2′:6,7]chromeno[2, 3-d]pyrimidin -4-imine (18a)
The compound was obtained from the reaction of (17a) (3.14 g, 0.01 mol) and methylamine as white crystals, crystallized from dioxane (82%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3250 (NH), 3045 (CH-aryl), 2960 (CH-aliph), 1636 (C=N), 1589 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.25 (d, 3H, J = 6.85 Hz, CH3), 3.23 (m, 1H, CH, pyran ring), 3.30 (t, 1H, J = 6.88 Hz, CH, pyran ring), 3.38 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 5.50 (d, 1H, J = 6.87 Hz, CH, pyran ring), 6.82 (d, 1H, J = 2.44 Hz, furan), 7.30 (s, 1H, benzene), 7.70 (s, 1H, pyrimidine ring), 7.80 (d, 1H, J = 2.43 Hz, furan), 9.65 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.4, 27.8 (2C, 2CH3), 29.5, 52.4 (2C, 2CH, pyran ring), 60.9 (1C, OCH3), 90.1 (1C, CH-pyran ring), 91.7 (1C, CH, benzene), 103.6, 105.5, 106.7, 146.4, 152.1, 152.7, 154.3, 155.8, 157.6 (9C, Ar-C); MS (70 eV, %) m/z 299 (M+, 98%); Anal. Calc. (Found) for C16H17N3O3 (299.33): C, 64.20 (64.29); H, 5.72 (5.65); N, 14.04 (14.10).
3.48. Synthesis of 6,10-dimethoxy-3,5-dimethyl-3,4a,5,11a-tetrahydro-4H-furo[3′,2′:6,7] chromeno [2,3-d]pyrimidin-4-imine (18b)
The compound was obtained from the reaction of (17b) (3.44 g, 0.01 mol) and methylamine as brownish crystals, crystallized from DMF (78%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3245 (NH), 3048 (CH-aryl), 2967 (CH-aliph), 1632 (C=N), 1581 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.28 (d, 3H, J = 6.87 Hz, CH3), 3.26 (m, 1H, CH, pyran ring), 3.35 (t, 1H, J = 6.86 Hz, CH, pyran ring), 3.40 (s, 3H, CH3), 3.93 (s, 6H, 2OCH3), 5.58 (d, 1H, J = 6.82 Hz, CH, pyran ring), 6.85 (d, 1H, J = 2.41 Hz, furan), 7.66 (s, 1H, pyrimidine ring), 7.85 (d, 1H, J = 2.40 Hz, furan), 9.62 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.6, 28.1 (2C, 2CH3), 30.2, 52.7 (2C, 2CH, pyran ring), 61.8 (2C, 2OCH3), 90.5 (1C, CH-pyran ring), 105.4, 106.1, 110.6, 127.2, 145.1, 145.7, 146.2, 147.1, 152.5, 156.9 (10 C, Ar-C); MS (70 eV, %) m/z 329 (M+, 95%); Anal. Calc. (Found) for C17H19N3O4 (329.36): C, 62.00 (62.10); H, 5.81 (5.75); N, 12.76 (12.70).
3.49. Synthesis of 4-imino-(6-methoxy or 6,10-dimethoxy)-5-methyl-4a, 11a-dihydro-4H-furo [3′, 2′:6,7]chromeno [2,3-d]pyrimidin-3(5H)-amine (19a,b)
General procedure: A mixture of compound (17a) (3.14 g, 0.01 mol) or (17b) (3.44 g, 0.01 mol) and hydrazine hydrate (1 mL, excess) was created in absolute ethanol (45 mL). The reaction solution was refluxed for 3–5 h with TLC, the reaction mixture was concentrated, and the precipitate product that separated out was filtered off and recrystallized from the suitable solvent to give (19a) or (19b).
3.50. Synthesis of 4-imino-6-methoxy-5-methyl-4a, 11a-dihydro-4H-furo [3′,2′: 6,7]chromeno [2, 3-d]pyrimidin-3(5H)-amine (19a)
The compound was obtained from the reaction of (17a) (3.14 g, 0.01 mol) and hydrazine hydrate as yellowish crystals, crystallized from methanol (76%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3425 (NH2), 3260 (NH), 3052 (CH-aryl), 2964 (CH-aliph), 1637 (C=N), 1590 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.27 (d, 3H, J = 6.78 Hz, CH3), 3.31 (m, 1H, CH, pyran ring), 3.39 (t, 1H, J = 6.90 Hz, CH, pyran ring), 3.84 (s, 3H, OCH3), 5.60 (d, 1H, J = 6.91 Hz, CH, pyran ring), 6.40 (s, 2H, NH2, D2O exchangeable), 6.73 (d, 1H, J = 2.41 Hz, furan), 7.26 (s, 1H, benzene), 7.65 (s, 1H, pyrimidine ring), 7.77 (d, 1H, J = 2.40 Hz, furan), 9.70 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 19.4 (1C, CH3), 26.9, 51.2 (2C, 2CH, pyran ring), 60.2 (1C, OCH3), 85.5 (1C, CH-pyran ring), 91.1 (1C, CH, benzene), 103.8, 105.4, 106.5, 146.2, 147.3, 152.6, 154.1, 155.9, 156.8 (9C, Ar-C); MS (70 eV, %) m/z 300 (M+, 100%); Anal. Calc. (Found) for C15H16N4O3 (300.32): C, 59.99 (59.90); H, 5.37 (5.45); N, 18.66 (18.58).
3.51. Synthesis of 4-imino-6,10-dimethoxy-5-methyl-4a,11a-dihydro-4H-furo [3′,2′:6,7] chromeno [2,3-d]pyrimidin-3(5H)-amine (19b)
The compound was obtained from the reaction of (17b) (3.44 g, 0.01 mol) and hydrazine hydrate as yellow crystals, crystallized from ethyl-acetate (71%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3422 (NH2), 3258 (NH), 3051 (CH-aryl), 2962 (CH-aliph), 1633 (C=N), 1581 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.25 (d, 3H, J = 6.76 Hz, CH3), 3.29 (m, 1H, CH, pyran ring), 3.41 (t, 1H, J = 6.87 Hz, CH, pyran ring), 3.94 (s, 6H, 2OCH3), 5.68 (d, 1H, J = 6.85 Hz, CH, pyran ring), 6.35 (s, 2H, NH2, D2O exchangeable), 6.70 (d, 1H, J = 2.42 Hz, furan), 7.61 (s, 1H, pyrimidine ring), 7.85 (d, 1H, J = 2.39 Hz, furan), 9.75 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 20.2 (1C, CH3), 28.3, 52.4 (2C, 2CH, pyran ring), 61.9 (2C, 2OCH3), 86.1 (1C, CH-pyran ring), 106.1, 106.8, 110.3, 126.8, 145.5, 146.1, 146.7, 147.4, 147.7, 157.2 (10C, Ar-C); MS (70 eV, %) m/z 330 (M+, 90%); Anal. Calc. (Found) for C16H18N4O4 (330.34): C, 58.17 (58.25); H, 5.49 (5.41); N, 16.96 (16.88).
3.52. Synthesis of (12-methoxy or 8,12-dimethoxy)-13-methyl-6a,13a-dihydro-13H-furo [3′,2′: 6, 7]chromeno [3,2-e][1,2,4]triazolo [1,5-c]pyrimidine (20a,b)
General procedure: A mixture of compound (19a) (3.00 g, 0.01 mol) or (19b) (3.30 g, 0.01 mol), excess of triethyl-orthoformate (6 mL), and acetic anhydride (30 mL) was refluxed for 4–6 h under control (TLC). The solvent was removed under reduced pressure; the separated solid was filtered off and recrystallized from the proper solvent to give triazolopyrimidines (20a) and (20b).
3.53. Synthesis of 12-methoxy-13-methyl-6a,13a-dihydro-13H-furo [3′,2′:6,7]chromeno [3,2-e][1,2,4]triazolo [1,5-c]pyrimidine (20a)
The compound was obtained from the reaction of (19a) (3.00 g, 0.01 mol) and triethylorthoformate as brownish crystals, crystallized from dioxane (68%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3045 (CH-aryl), 2937 (CH-aliph), 1633 (C=N), 1582 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.23 (d, 3H, J = 6.80 Hz, CH3), 3.22 (m, 1H, CH, pyran ring), 3.31 (t, 1H, J = 6.87 Hz, CH, pyran ring), 3.82 (s, 3H, OCH3), 5.40 (d, 1H, J = 6.83 Hz, CH, pyran ring), 6.10 (s, 1H,CH, triazole ring), 6.71 (d, 1H, J = 2.35 Hz, furan), 7.15 (s, 1H, benzene), 7.72 (s, 1H, pyrimidine ring), 7.83 (d, 1H, J = 2.33 Hz, furan); 13C NMR (DMSO-d6) δ 21.8 (1C, CH3), 27.3, 52.7 (2C, 2CH, pyran ring), 60.6 (1C, OCH3), 90.8 (1C, CH, benzene), 95.2 (1C, CH-pyran ring), 102.9, 105.1, 106.3, 139.7, 146.5, 151.4, 152.2, 152.6, 154.1, 156.2 (10C, Ar-C); MS (70 eV, %) m/z 310 (M+, 85%); Anal. Calc. (Found) for C16H14N4O3 (310.31): C, 61.93 (61.84); H, 4.55 (4.63); N, 18.06 (18.14).
3.54. Synthesis of 8,12-dimethoxy-13-methyl-6a,13a-dihydro-13H-furo [3′,2′:6,7]chromeno [3,2-e] [1,2,4]triazolo [1, 5-c]pyrimidine (20b)
The compound was obtained from the reaction of (19b) (3.30 g, 0.01 mol) and triethylorthoformate as pale brown crystals, crystallized from DMF (64%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3050 (CH-aryl), 2940 (CH-aliph), 1630 (C=N), 1580 (C=C), 1H NMR (DMSO-d6, ppm) δ 1.28 (d, 3H, J = 6.84 Hz, CH3), 3.26 (m, 1H, CH, pyran ring), 3.37 (t, 1H, J = 6.85 Hz, CH, pyran ring), 3.90 (s, 6H, 2OCH3), 5.45 (d, 1H, J = 6.81 Hz, CH, pyran ring), 6.05 (s, 1H,CH, triazole ring), 6.88 (d, 1H, J = 2.31 Hz, furan), 7.76 (s, 1H, pyrimidine ring), 7.88 (d, 1H, J = 2.37 Hz, furan); 13C NMR (DMSO-d6) δ 21.2 (1C, CH3), 27.8, 53.1 (2C, 2CH, pyran ring), 62.3 (2C, 2OCH3), 99.1 (1C, CH-pyran ring), 105.5, 106.4, 110.6, 126.8, 139.9, 145.2, 146.1, 146.6, 147.3, 151.5, 152.7 (11C, Ar-C); MS (70 eV, %) m/z 340 (M+, 80%); Anal. Calc. (Found) for C17H16N4O4 (340.34): C, 60.00 (60.10); H, 4.74 (4.66); N, 16.46 (16.55).
3.55. Synthesis of (4-methoxy or 4,13-dimethoxy)-5-methyl-5a,7,8,9,10,11a-hexahydro-5H-furo [3′,2′:6,7]chromeno [2,3-b]quinolin-6-amine (21a,b)
General procedure for the preparation [
37]: aluminum chloride (1.33 g, 0.01 mol) was suspended in dry 1, 2-dichloroethane (20 mL) at room temperature under an argon atmosphere. After stirring the suspension for a few minutes, the corresponding compound, (
4a) (2.58 g, 0.01 mol) or (
4b) (2.88 g, 0.01 mol), and cyclohexanone (1.03 mL, 0.01 mol) were added to the mixture and the reaction mixture was heated under reflux for 23–27 h. The reaction was monitored via TLC. After accomplishment of the reaction, an aqueous solution of sodium hydroxide (10%) was added dropwise to the mixture until the aqueous solution became basic. After stirring for 60 min, the final precipitate was filtered, washed with water, and recrystallized from the suitable solvent to give (
21a) or (
21b).
3.56. Synthesis of 4-methoxy-5-methyl-5a,7,8,9,10,11a-hexahydro-5H-furo [3′,2′:6,7]chromeno [2, 3-b]quinolin-6-amine (21a)
The compound was obtained from the reaction of (4a) (2.58 g, 0.01 mol) and cyclohexanone as pale yellow crystals, crystallized from DMF (75%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3415 (NH2), 3057 (CH-aryl), 2963 (CH-aliph), 1638 (C=N), 1589 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.32 (d, 3H, J = 6.83 Hz, CH3), 1.45–2.25 (m, 8H, cyclohexane ring), 3.30 (m, 1H, J = 6.85 Hz, CH, pyran ring), 3.40 (t, 1H, J = 6.88 Hz, CH, pyran ring), 3.87 (s, 3H, OCH3), 5.38 (d, 1H, J = 6.68 Hz, CH, pyran ring), 6.65 (s, 2H, NH2, D2O exchangeable), 6.88 (d, 1H, J = 2.37 Hz, furan), 7.29 (s, 1H, benzene), 7.70 (d, 1H, J = 2.39 Hz, furan); 13C NMR (DMSO-d6) δ 19.1 (1C, CH3), 22.3, 25.5, 26.8, 30.9 (4C, 4CH2, cyclohexane ring), 31.6, 55.5 (2C, 2CH, pyran ring), 60.7 (1C, OCH3), 90.8 (1C, benzene ring), 92.1, 98.4 (2C, pyridine ring), 103.4, 105.2, 106.1, 146.7, 152.5, 153.3, 154.1, 156.5, 165.2 (9C, Ar-C); MS (70 eV, %) m/z 338 (M+, 93%); Anal. Calc. (Found) for C20H22N2O3 (338.41): C, 70.99 (70.90); H, 6.55 (6.63); N, 8.28 (8.21).
3.57. Synthesis of 4,13-dimethoxy-5-methyl-5a,7,8,9,10,11a-hexahydro-5H-furo [3′,2′:6,7] chromeno [2,3-b]quinolin-6-amine (21b)
The compound was obtained from the reaction of (4b) (2.88 g, 0.01 mol) and cyclohexanone as yellowish crystals, crystallized from dioxane (73%), M.p. > 350 °C. IR (ν, cm−1) KBr: broad 3412 (NH2), 3059 (CH-aryl), 2966 (CH-aliph), 1631 (C=N), 1582 (C=C). 1H NMR (DMSO-d6, ppm) δ 1.26 (d, 3H, J = 6.79 Hz, CH3), 1.47–2.27 (m, 8H, cyclohexane ring), 3.35 (m, 1H, J = 6.75 Hz, CH, pyran ring), 3.44 (t, 1H, J = 6.77 Hz, CH, pyran ring), 3.91 (s, 6H, 2OCH3), 5.41 (d, 1H, J = 6.74 Hz, CH, pyran ring), 6.71 (s, 2H, NH2, D2O exchangeable), 6.90 (d, 1H, J = 2.35 Hz, furan), 7.75 (d, 1H, J = 2.31 Hz, furan); 13C NMR (DMSO-d6) δ 19.4 (1C, CH3), 22.5, 25.8, 27.1, 31.3 (4C, 4CH2, cyclohexane ring), 32.2, 55.8 (2C, 2CH, pyran ring), 61.9 (2C, 2OCH3), 92.6, 98.7 (2C, pyridine ring), 105.3, 105.7, 110.5, 127.5, 145.2, 145.5, 146.4, 146.8, 153.5, 165.7 (10C, Ar-C); MS (70 eV, %) m/z 368 (M+, 90%); Anal. Calc. (Found) for C21H24N2O4 (368.43): C, 68.46 (68.52); H, 6.57 (6.50); N, 7.60 (7.68).
3.58. Biological Screening (Materials and Methods, In Vitro)
The antimicrobial activity of the newly prepared compounds was tested in vitro against Gram-negative bacteria
Klebsiella pneumoniae (ATCC
® 10031™) and
Escherichia coli (ATCC
® 25922™); Gram-positive bacteria
Streptococcus pyogenes (ATCC
® 19615™) and
Staphylococcus aureus (ATCC
® 6538™); and the fungi
Candida albicans (ATCC
® 10231™),
Curvularia lunata, Alternaria alternate, and
Aspergillus niger (ATCC
® 16888™). The newly synthesized compounds were dissolved in dimethyl sulfoxide (DMSO) and tested for their antimicrobial activity by the agar disk diffusion technique. Cefotaxime sodium and nystatin [
9,
10,
33,
34,
35,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54] were used as standard drugs for the antibacterial and antifungal assays, respectively. A solution of 100 μg mL
−1 of the tested compound and microplate wells 1 cm in diameter were used. Zones of inhibition were measured with calipers or automated scanners and paralleled with those of the standards. Cefotaxime sodium (0.15 μmol mL
−1) and nystatin (0.037 μmol mL
−1) were used as the standard drugs for antibacterial and antifungal activity, respectively. Compound-impregnated disks were placed on an agar plate containing a standard suspension of microorganisms. The plate was incubated for 24 h at 37 °C. For the assessment of the minimum inhibitory concentration (MIC) by serial plate dilution [
9,
10,
33,
34,
35,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54], 5 mg of each tested compound was dissolved in 1 mL of DMSO separately to prepare stock solutions. Serial dilutions were prepared from each stock solution. The plates were incubated at 37 °C for 24 h. MIC is defined as the lowest concentration (μmol mL
−1) of the tested compound that results in no visible growth on the plates. DMSO was used as the solvent control to ensure that the solvent had no effect on bacterial growth. The results are shown in
Table 1 and
Table 2.
3.58.1. Ethical Approval and Consent to Participate
No humans or animals were used in this study; nevertheless, all the procedures were carried out under the approval of the Medical Research Ethics Committee of the National Research Centre, Department of Chemistry of Natural and Microbial Products, Giza 12622, Egypt.
3.58.2. Human and Animal Rights
No human or animal subjects were used in the study. The research was conducted according to ethical standards in vitro.
3.58.3. Chemicals and Drugs
Types of Gram-positive bacteria Staphylococcus aureus and Streptococcus pyogenes, Gram-negative bacteria Escherichia coli and Klebsiella pneumoniae, and fungi Aspergillus niger, Alternaria alternate, Curvularia lunata, and Candida albicans were from the National Research Centre, Department of Chemistry of Natural and Microbial Products, Giza, Egypt, and cefotaxime sodium, nystatin, and DMSO were purchased from Sigma-Aldrich.