Immunostimulatory Effect of Postbiotics Prepared from Phellinus linteus Mycelial Submerged Culture via Activation of Spleen and Peyer’s Patch in C3H/HeN Mice
Abstract
1. Introduction
2. Results
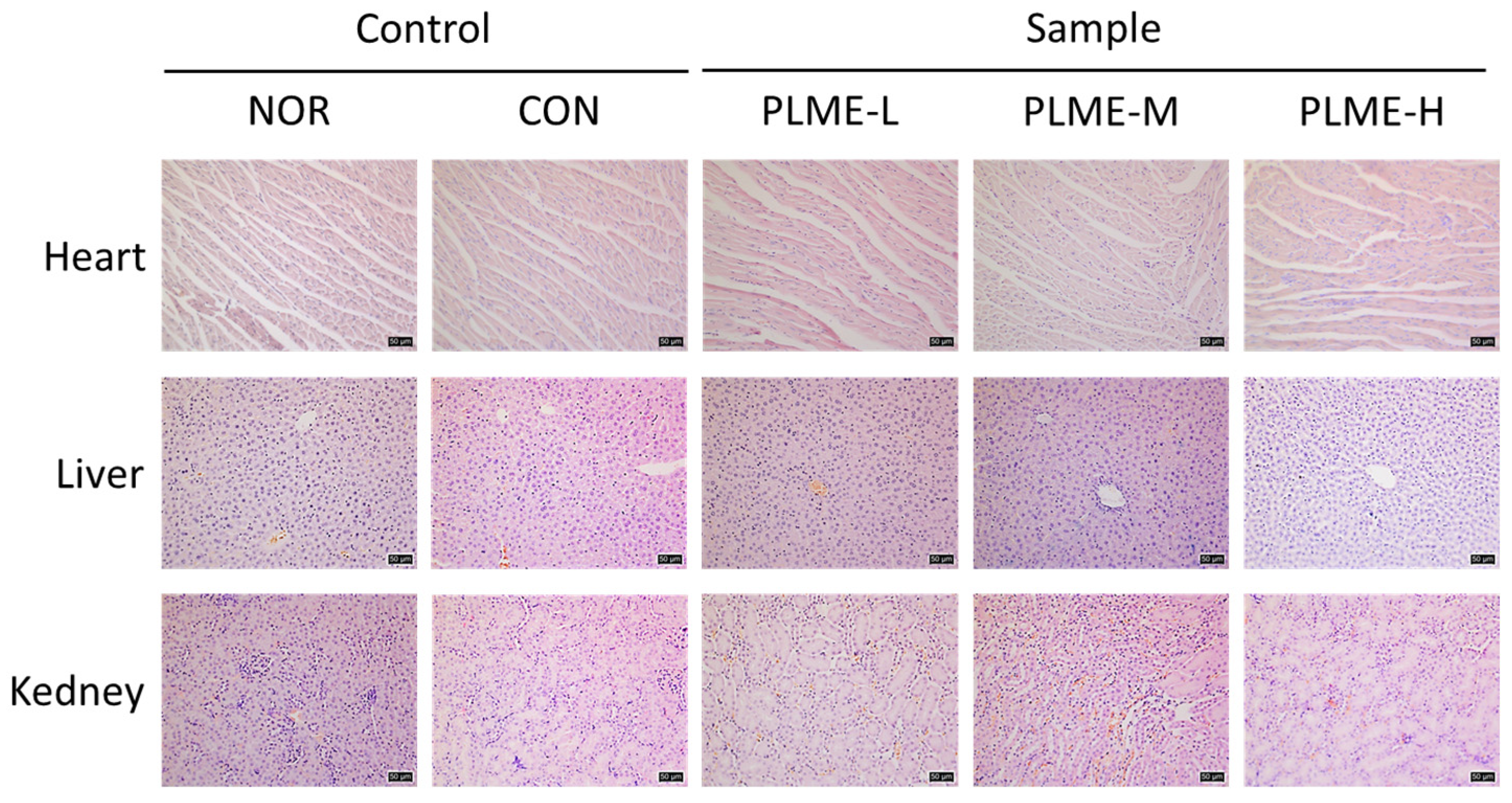
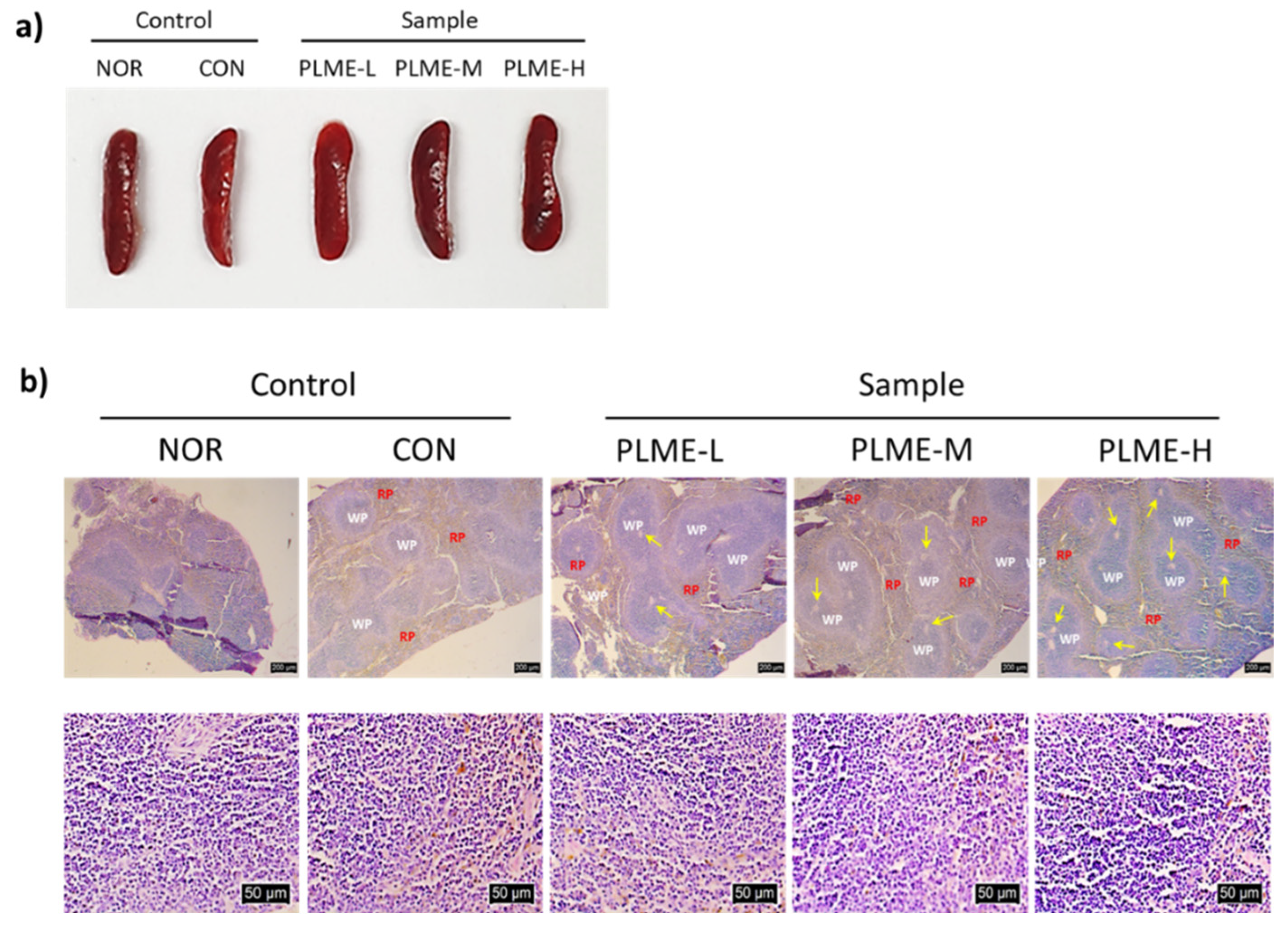
2.1. Effect of PLME Administration on the Major Organs
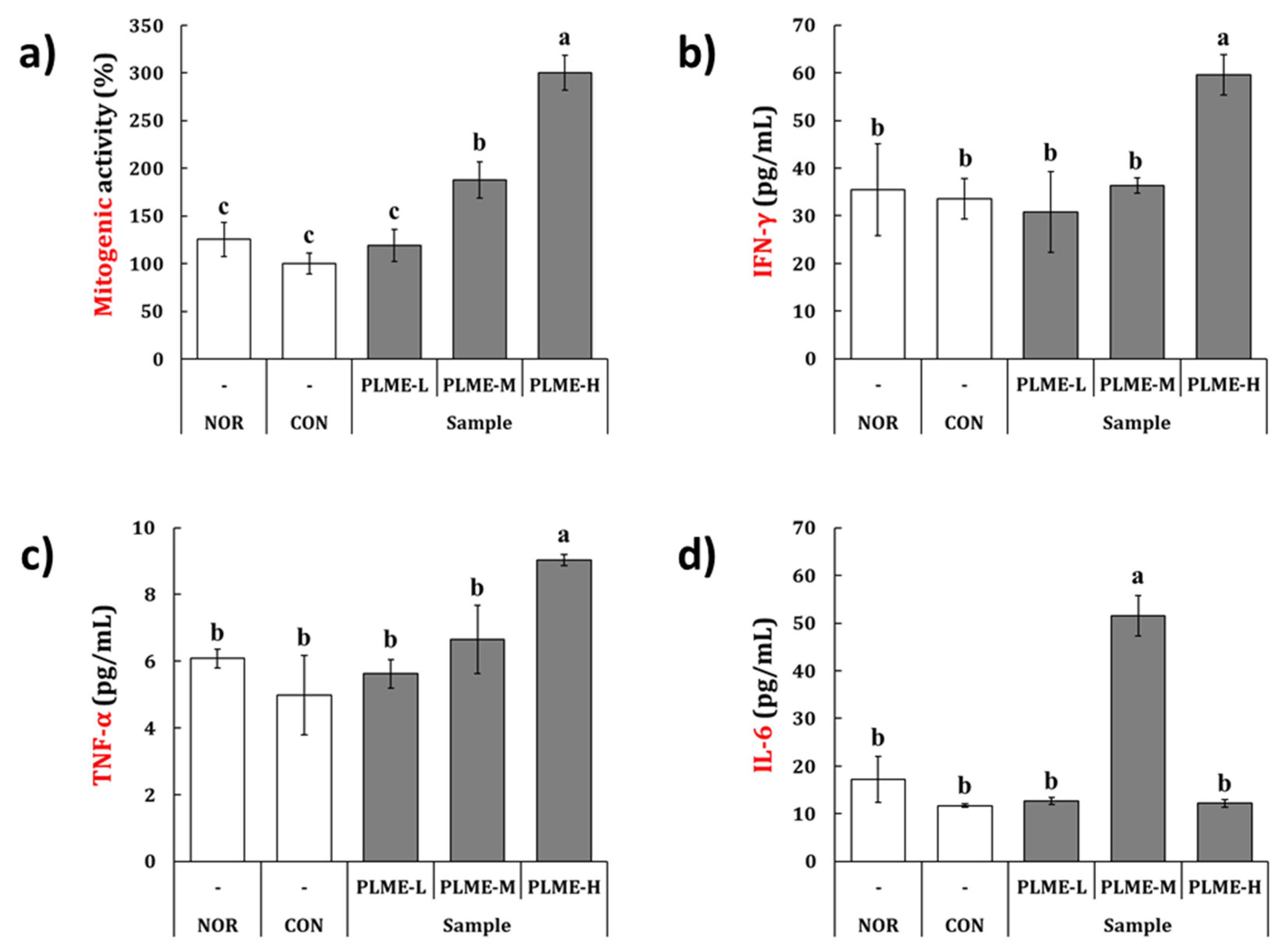
2.2. Effect of PLME Administration on the Spleen-Mediated Immunostimulation
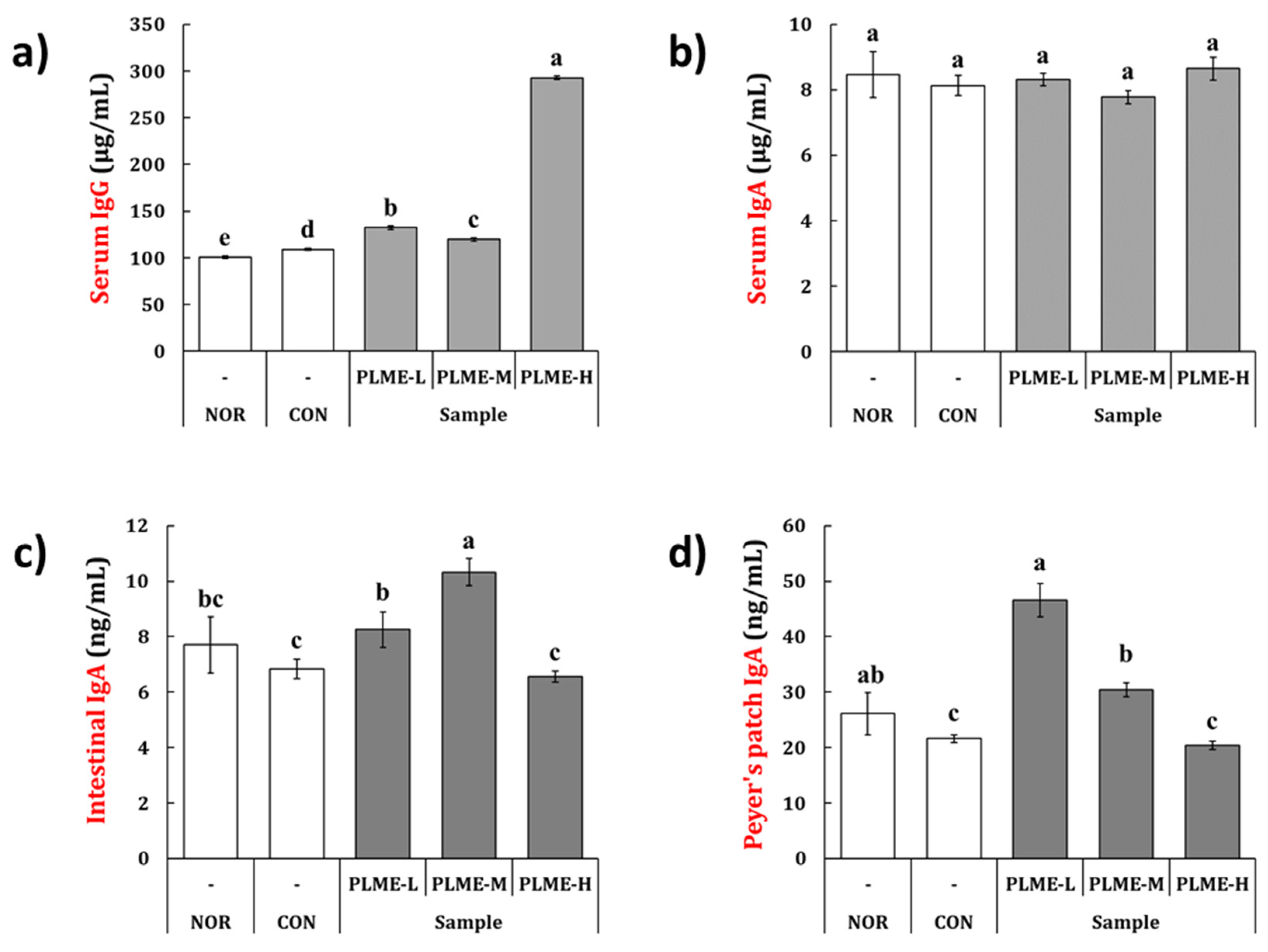
2.3. Effect of PLME Administration on the Immunoglobulin Production
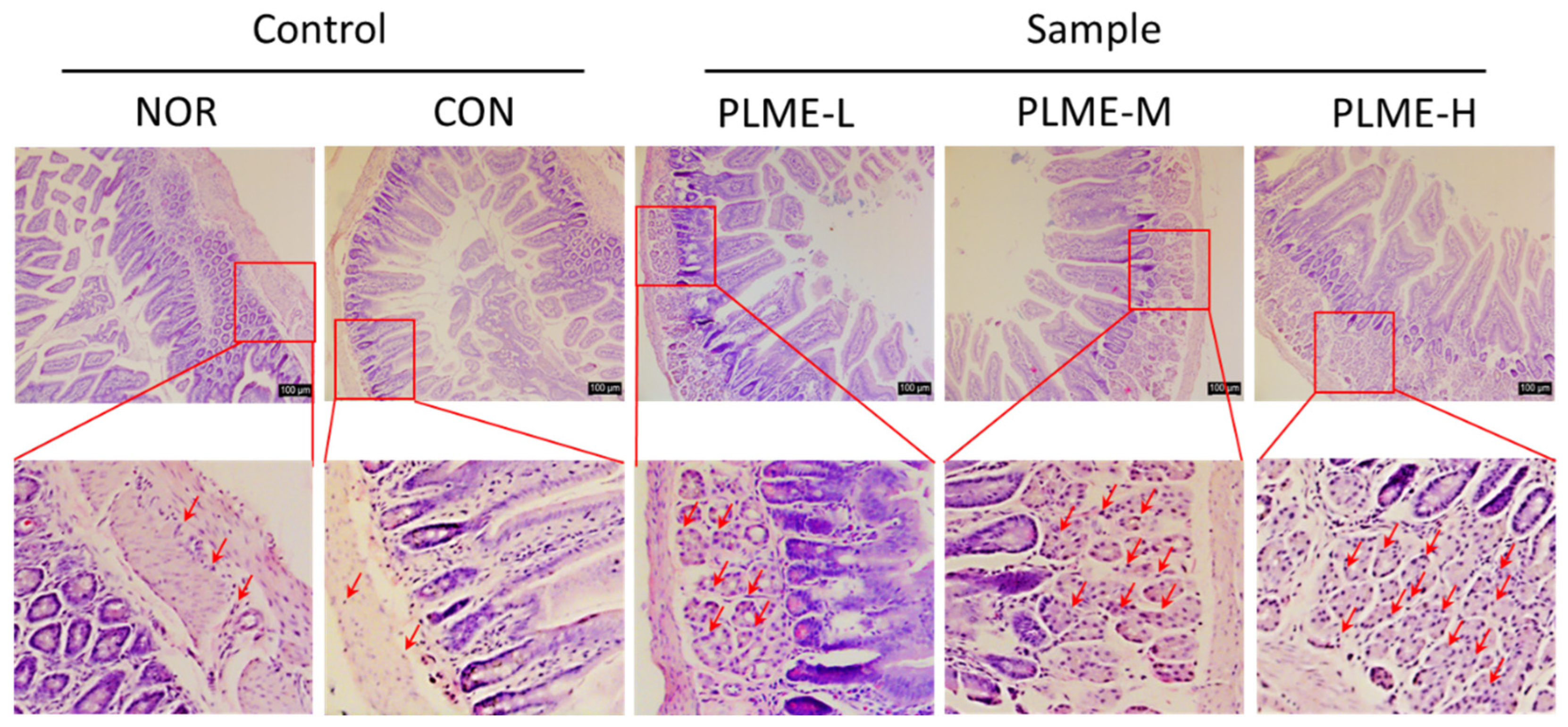
2.4. Effect of PLME Administration on the Intestinal Immune System-Modulating Activity through Peyer’s Patch
2.5. General Components and Sugar Composition of PLME
3. Discussion
4. Materials and Methods
4.1. Submerged Cultivation of P. linteus Mycelium
4.2. Preparation of Postbiotics Prepared from P. linteus Mycelial Submerged Culture
4.3. Animals and Administration Schedule
4.4. Histomorphometric Analysis
4.5. Mitogenic Activity and Cytokine Production by Splenocytes
4.6. Quantification of Immunoglobulin (Ig) in Serum, Small Intestinal Fluids, and Peyer’s Patch
4.7. Intestinal Immune System-Modulating Activity through Peyer’s Patch
4.8. Analyses of General Components and Sugar Composition
4.9. Quantification of α- and β-Glucan Contents in PLME
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. The role of edible mushrooms in health: Evaluation of the evidence. J. Funct. Foods 2012, 4, 687–709. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Chaturvedi, V.K.; Mishra, D.; Bajpeyee, A.; Tiwari, A.; Singh, M.P. Role of edible mushroom as a potent therapeutics for the diabetes and obesity. 3 Biotech 2019, 9, 450. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech 2018, 8, 334. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-based composite: The future sustainable biomaterial. Int. J. Biomater. 2022, 2022, 8401528. [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. fruiting bodies of edible fungi—A comparison of metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, J.-H.; Hwang, J.-H.; Jeong, H.-S.; Lee, H.-Y.; Yu, K.-W. Enhancement of immunostimulation and anti-metastasis in submerged culture of bearded tooth mushroom (Hericium erinaceum) mycelia by addition of ginseng extract. Food Sci. Biotechnol. 2010, 19, 1259–1266. [Google Scholar] [CrossRef]
- Kim, H.; Ra, K.S.; Hwang, J.H.; Yu, K.W. Immune enhancement of Hericium erinaceum mycelium cultured in submerged medium supplemented with ginseng extract. Korean J. Food Nutr. 2012, 25, 737–746. [Google Scholar] [CrossRef][Green Version]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Rad, A.H.; Abbasi, A.; Kafil, H.S.; Ganbarov, K. Potential pharmaceutical and food applications of postbiotics: A review. Curr. Pharm. Biotechnol. 2020, 21, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tan, H.; Liu, Q.; Zheng, X.; Zhang, H.; Liu, Y.; Xu, L. A review: The bioactivities and pharmacological applications of Phellinus linteus. Molecules 2019, 24, 1888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Kim, S.H.; Chen, C.Y. A medicinal mushroom: Phellinus linteus. Curr. Med. Chem. 2008, 15, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Wang, P.W.; Kuo, P.C.; Hung, H.Y.; Pan, T.L. Hepatoprotective principles and other chemical constituents from the mycelium of Phellinus linteus. Molecules 2018, 23, 1705. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Chang, C.C.; Lin, J.J.; Chen, C.C.; Chyau, C.C.; Peng, R.Y. Styrylpyrones from Phellinus linteus mycelia alleviate non-alcoholic fatty liver by modulating lipid and glucose metabolic homeostasis in high-fat and high-fructose diet-fed mice. Antioxidants 2022, 11, 898. [Google Scholar] [CrossRef]
- Shin, M.R.; Lee, J.H.; Lee, J.A.; Kim, M.J.; Park, H.J.; Park, B.W.; Seo, S.B.; Roh, S.S. Immunomodulatory and anti-inflammatory effects of Phellinus linteus mycelium. BMC Complementary Med. Ther. 2021, 21, 269. [Google Scholar] [CrossRef]
- Hwang, J.S.; Kwon, H.K.; Kim, J.E.; Rho, J.; Im, S.H. Immunomodulatory effect of water soluble extract separated from mycelium of Phellinus linteus on experimental atopic dermatitis. BMC Complementary Altern. Med. 2012, 12, 159. [Google Scholar] [CrossRef]
- Inagaki, N.; Shibata, T.; Itoh, T.; Suzuki, T.; Tanaka, H.; Nakamura, T.; Akiyama, Y.; Kawagishi, H.; Nagai, H. Inhibition of IgE-dependent mouse triphasic cutaneous reaction by a boiling water fraction separated from mycelium of Phellinus linteus. Evid. Based Complementary Altern. Med. 2005, 2, 369–374. [Google Scholar] [CrossRef]
- Shin, M.R.; Lee, J.A.; Kim, M.J.; Park, H.J.; Park, B.W.; Seo, S.B.; Roh, S.S. Protective effects of Phellinus linteus mycelium on the development of osteoarthritis after monosodium iodoacetate injection. Evid. Based Complementary Altern. Med. 2020, 2020, 7240858. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 2022, 54, 1–11. [Google Scholar] [CrossRef]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune modulation from five major mushrooms: Application to integrative oncology. Integr. Med. 2014, 13, 32–44. [Google Scholar]
- Suruga, K.; Tomita, T.; Kadokura, K. Medicinal mushroom mycelia: Characteristics, benefits, and utility in soybean fermentation. In Functional Food; Shiomi, N., Savitskaya, P.A., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.J.; Xu, X.Y.; Choi, H.S.; Kim, H.; Cho, I.H.; Yi, T.H.; Kim, Y.J. The immune-enhancing properties of Hwanglyeonhaedok-tang-mediated biosynthesized gold nanoparticles in macrophages and splenocytes. Int. J. Nanomed. 2022, 17, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Liu, L.; Gao, J.; Guo, B.; Zhu, B. Essential role of TNF-alpha in development of spleen fibroblastic reticular cells. Cell. Immunol. 2015, 293, 130–136. [Google Scholar] [CrossRef]
- Guimarães, E.S.; Martins, J.M.; Gomes, M.T.R.; Cerqueira, D.M.; Oliveira, S.C. Lack of interleukin-6 affects IFN-γ and TNF-α production and early in vivo control of Brucella abortus infection. Pathogens 2020, 9, 1040. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef]
- Kim, H.; Yu, K.-W.; Hong, H.-D.; Shin, K.-S. Effect of arabinoxylan- and rhamnogalacturonan I-rich polysaccharides isolated from young barley leaf on intestinal immunostimulatory activity. J. Funct. Foods 2017, 35, 384–390. [Google Scholar] [CrossRef]
- Cerutti, A.; Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 2008, 28, 740–750. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Draga, A. Measurement of β-glucan in mushrooms and mycelial products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.J.; Oyston, P.C.; Williamson, D.E. Potential of the beta-glucans to enhance innate resistance to biological agents. Expert Rev. Anti Infect. Ther. 2010, 8, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary yeast beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Bgatova, N.P.; Vetvicka, V. Glucan and mannan-Two peas in a pod. Int. J. Mol. Sci. 2019, 20, 3189. [Google Scholar] [CrossRef]
- Onitake, T.; Ueno, Y.; Tanaka, S.; Sagami, S.; Hayashi, R.; Nagai, K.; Hide, M.; Chayama, K. Pulverized konjac glucomannan ameliorates oxazolone-induced colitis in mice. Eur. J. Nutr. 2015, 54, 959–969. [Google Scholar] [CrossRef]
- Phipps, K.R.; Lee, H.Y.; Kim, H.; Jeon, B. Oral administration of a novel hydrolyzed chicken sternal cartilage extract (BioCell Collagen®) reduces UVB-induced photoaging in mice. J. Funct. Foods 2020, 68, 103870. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hwang, K.C.; Shin, H.Y.; Kim, W.J.; Seo, M.S.; Kim, H. Effects of a high-molecular-weight polysaccharides isolated from Korean persimmon on the antioxidant, anti-Inflammatory, and antiwrinkle activity. Molecules 2021, 26, 1600. [Google Scholar] [CrossRef] [PubMed]

| Group | Tissue Weight (g) | ||
|---|---|---|---|
| Heart | Kidney | Liver | |
| NOR | 0.095 ± 0.004 ns | 0.261 ± 0.011 ns | 0.901 ± 0.034 ns |
| CON | 0.105 ± 0.004 | 0.269 ± 0.005 | 0.912 ± 0.027 |
| PLME-L | 0.096 ± 0.005 | 0.271 ± 0.010 | 0.876 ± 0.037 |
| PLME-M | 0.096 ± 0.003 | 0.268 ± 0.004 | 0.881 ± 0.019 |
| PLME-H | 0.092 ± 0.002 | 0.264 ± 0.007 | 0.841 ± 0.013 |
| Chemical Composition | Mean ± S.D. (mg/g) |
|---|---|
| Neutral sugar | 969.1 ± 18.2 |
| Uronic acid | 37.1 ± 2.2 |
| Protein | 2.2 ± 0.6 |
| Component sugar | Mean ± S.D. (mole %) |
| Rhamnose | 0.3 ± 0.0 |
| Fucose | – |
| Arabinose | 1.0 ± 0.1 |
| Xylose | – |
| Mannose | 1.2 ± 0.0 |
| Galactose | 2.7 ± 0.3 |
| Glucose | 93.8 ± 0.6 |
| Galacturonic acid | 1.0 ± 0.3 |
| Glucuronic acid | – |
| Glucan composition | Mean ± S.D. (mg/g) |
| β-1,3:1,6-glucan | 88.5 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, M.G.; Shin, H.Y.; Jeong, E.-J.; Kim, G.; Jeong, S.B.; Ha, E.J.; Choi, S.-Y.; Moon, S.-K.; Yu, K.-W.; Suh, H.-J.; et al. Immunostimulatory Effect of Postbiotics Prepared from Phellinus linteus Mycelial Submerged Culture via Activation of Spleen and Peyer’s Patch in C3H/HeN Mice. Pharmaceuticals 2022, 15, 1215. https://doi.org/10.3390/ph15101215
Suh MG, Shin HY, Jeong E-J, Kim G, Jeong SB, Ha EJ, Choi S-Y, Moon S-K, Yu K-W, Suh H-J, et al. Immunostimulatory Effect of Postbiotics Prepared from Phellinus linteus Mycelial Submerged Culture via Activation of Spleen and Peyer’s Patch in C3H/HeN Mice. Pharmaceuticals. 2022; 15(10):1215. https://doi.org/10.3390/ph15101215
Chicago/Turabian StyleSuh, Min Geun, Hyun Young Shin, Eun-Jin Jeong, Gaeuleh Kim, Se Bin Jeong, Eun Ji Ha, Sang-Yong Choi, Sung-Kwon Moon, Kwang-Won Yu, Hyung-Joo Suh, and et al. 2022. "Immunostimulatory Effect of Postbiotics Prepared from Phellinus linteus Mycelial Submerged Culture via Activation of Spleen and Peyer’s Patch in C3H/HeN Mice" Pharmaceuticals 15, no. 10: 1215. https://doi.org/10.3390/ph15101215
APA StyleSuh, M. G., Shin, H. Y., Jeong, E.-J., Kim, G., Jeong, S. B., Ha, E. J., Choi, S.-Y., Moon, S.-K., Yu, K.-W., Suh, H.-J., & Kim, H. (2022). Immunostimulatory Effect of Postbiotics Prepared from Phellinus linteus Mycelial Submerged Culture via Activation of Spleen and Peyer’s Patch in C3H/HeN Mice. Pharmaceuticals, 15(10), 1215. https://doi.org/10.3390/ph15101215







