Calcitonin Gene-Related Peptide (CGRP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Migraine Pathogenesis
Abstract
1. Introduction
2. Human Models of Migraine
3. Calcitonin Gene-Related Peptide
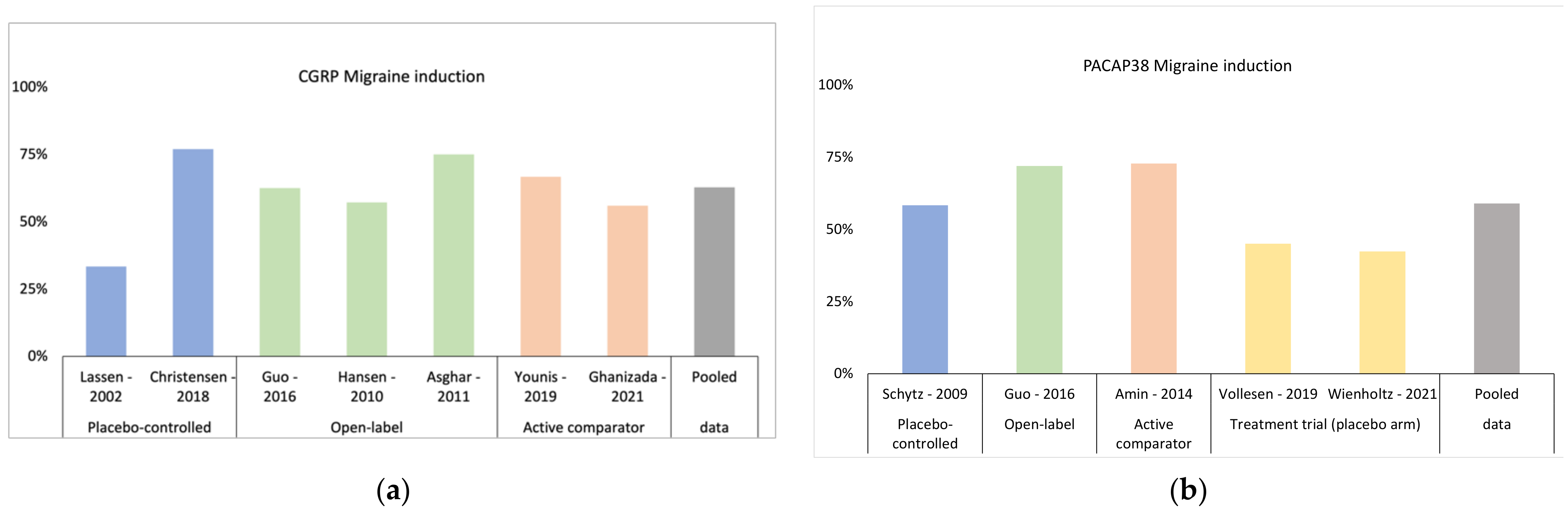
3.1. Triggering Headache and Migraine Attacks with CGRP
3.2. CGRP’s Mode of Action
3.3. CGRP’s Site of Action
3.4. CGRP-Targeted Treatment
3.4.1. Erenumab
3.4.2. Fremanezumab
3.4.3. Galcanezumab
3.4.4. Eptinezumab
3.5. Clinical Relevance of Anti-CGRP Antibody Therapy
4. Pituitary Adenylate Cyclase-Activating Polypeptide
4.1. Triggering Headache and Migraine Attacks with PACAP
4.2. PACAP’s Mode of Action
4.3. PACAP’s Site of Action
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and Systems of Care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Giffin, N.J.; Ruggiero, L.; Lipton, R.B.; Silberstein, S.D.; Tvedskov, J.F.; Olesen, J.; Altman, J.; Goadsby, P.J.; Macrae, A. Premonitory Symptoms in Migraine: An Electronic Diary Study. Neurology 2003, 60, 935–940. [Google Scholar] [CrossRef]
- Bose, P.; Goadsby, P.J. The Migraine Postdrome. Curr. Opin. Neurol. 2016, 29, 299–301. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the Trigeminovascular System—40 Years and Counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Ashina, M.; Terwindt, G.M.; Al-Karagholi, M.A.M.; de Boer, I.; Lee, M.J.; Hay, D.L.; Schulte, L.H.; Hadjikhani, N.; Sinclair, A.J.; Ashina, H.; et al. Migraine: Disease Characterisation, Biomarkers, and Precision Medicine. Lancet 2021, 397, 1496–1504. [Google Scholar] [CrossRef]
- Pellegrino, A.B.W.; Davis-Martin, R.E.; Houle, T.T.; Turner, D.P.; Smitherman, T.A. Perceived Triggers of Primary Headache Disorders: A Meta-Analysis. Cephalalgia 2017, 38, 1188–1198. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; á Dunga, B.O.; Olesen, J. Human Models of Migraine—Short-Term Pain for Long-Term Gain. Nat. Rev. Neurol. 2017, 13, 713–724. [Google Scholar] [CrossRef]
- Thomsen, L.L.; Kruuse, C.; Iversen, H.K.; Olesen, J. A Nitric Oxide Donor (Nitroglycerin) Triggers Genuine Migraine Attacks. Eur. J. Neurol. 1994, 1, 73–80. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive Peptide Release in the Extracerebral Circulation of Humans during Migraine Headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Tvedskov, J.F.; Lipka, K.; Ashina, M.; Iversen, H.K.; Schifter, S.; Olesen, J. No Increase of Calcitonin Gene-Related Peptide in Jugular Blood during Migraine. Ann. Neurol. 2005, 58, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Lassen, L.H.; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP May Play a Causative Role in Migraine. Cephalalgia 2002, 22, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.S.; Hansen, A.E.; Amin, F.M.; van der Geest, R.J.; van der Koning, P.; Larsson, H.B.W.; Olesen, J.; Ashina, M. Evidence for a Vascular Factor in Migraine. Ann. Neurol. 2011, 69, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vollesen, A.L.H.; Olesen, J.; Ashina, M. Premonitory and Nonheadache Symptoms Induced by CGRP and PACAP38 in Patients with Migraine. Pain 2016, 157, 2773–2781. [Google Scholar] [CrossRef]
- Hansen, J.M.; Hauge, A.W.; Olesen, J.; Ashina, M. Calcitonin Gene-Related Peptide Triggers Migraine-like Attacks in Patients with Migraine with Aura. Cephalalgia 2010, 30, 1179–1186. [Google Scholar] [CrossRef]
- Asghar, M.S.; Hansen, A.E.; Kapijimpanga, T.; van der Geest, R.J.; van der Koning, P.; Larsson, H.B.W.; Olesen, J.; Ashina, M. Dilation by CGRP of Middle Meningeal Artery and Reversal by Sumatriptan in Normal Volunteers. Neurology 2010, 75, 1520–1526. [Google Scholar] [CrossRef]
- Christensen, C.E.; Amin, F.M.; Younis, S.; Lindberg, U.; de Koning, P.; Petersen, E.T.; Paulson, O.B.; Larsson, H.B.W.; Ashina, M. Sildenafil and Calcitonin Gene-Related Peptide Dilate Intradural Arteries: A 3T MR Angiography Study in Healthy Volunteers. Cephalalgia 2019, 39, 264–273. [Google Scholar] [CrossRef]
- Christensen, C.E.; Younis, S.; Lindberg, U.; Boer, V.O.; de Koning, P.; Petersen, E.T.; Paulson, O.B.; Larsson, H.B.W.; Amin, F.M.; Ashina, M. Ultra-High Field MR Angiography in Human Migraine Models: A 3.0 T/7.0 T Comparison Study. J. Headache Pain 2019, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Schytz, H.W.; Ashina, M. CGRP in Human Models of Primary Headaches. Cephalalgia 2016, 38, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed]
- Kruuse, C.; Thomsen, L.L.; Birk, S.; Olesen, J. Migraine Can Be Induced by Sildenafil without Changes in Middle Cerebral Artery Diameter. Brain 2003, 126, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.; Christensen, C.E.; Toft, N.M.; Søborg, T.; Amin, F.M.; Hougaard, A.; Ashina, M. Investigation of Distinct Molecular Pathways in Migraine Induction Using Calcitonin Gene-Related Peptide and Sildenafil. Cephalalgia 2019, 39, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, H.; Iljazi, A.; Ashina, H.; Do, T.P.; Al-Karagholi, M.A.M.; Amin, F.M.; Ashina, M. Nocebo Response in Human Models of Migraine: A Systematic Review and Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Trials in Migraine without Aura and Healthy Volunteers. Cephalalgia 2021, 41, 99–111. [Google Scholar] [CrossRef]
- Hansen, J.M.; Thomsen, L.L.; Olesen, J.; Ashina, M. Calcitonin Gene-Related Peptide Does Not Cause the Familial Hemiplegic Migraine Phenotype. Neurology 2008, 71, 841–847. [Google Scholar] [CrossRef]
- Brain, S.D.; Grant, A.D. Vascular Actions of Calcitonin Gene-Related Peptide and Adrenomedullin. Physiol. Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin Gene-Related Peptide and Pain: A Systematic Review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.A.; Lassen, L.H.; Birk, S.; Lesko, L.; Olesen, J. BIBN4096BS Antagonizes Human Alpha-Calcitonin Gene Related Peptide-Induced Headache and Extracerebral Artery Dilatation. Clin. Pharm. Ther. 2005, 77, 202–213. [Google Scholar] [CrossRef]
- Amin, F.M.; Hougaard, A.; Schytz, H.W.; Asghar, M.S.; Lundholm, E.; Parvaiz, A.I.; de Koning, P.J.H.; Andersen, M.R.; Larsson, H.B.W.; Fahrenkrug, J.; et al. Investigation of the Pathophysiological Mechanisms of Migraine Attacks Induced by Pituitary Adenylate Cyclase-Activating Polypeptide-38. Brain 2014, 137, 779–794. [Google Scholar] [CrossRef]
- Amin, F.M.; Asghar, M.S.; Hougaard, A.; Hansen, A.E.; Larsen, V.A.; de Koning, P.J.; Larsson, H.B.; Olesen, J.; Ashina, M. Magnetic Resonance Angiography of Intracranial and Extracranial Arteries in Patients with Spontaneous Migraine without Aura: A Cross-Sectional Study. Lancet Neurol. 2013, 12, 454–461. [Google Scholar] [CrossRef]
- Christensen, C.E.; Younis, S.; Lindberg, U.; de Koning, P.; Tolnai, D.; Paulson, O.B.; Larsson, H.B.W.; Amin, F.M.; Ashina, M. Intradural Artery Dilation during Experimentally Induced Migraine Attacks. Pain 2021, 162, 176–183. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPS Regulate the Transport and Ligand Specificity of the Calcitonin- Receptor-like Receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Olesen, I.; Jørgensen, L.; Engel, U.; Edvinsson, L. In-Depth Characterization of CGRP Receptors in Human Intracranial Arteries. Eur. J. Pharm. 2003, 481, 207–216. [Google Scholar] [CrossRef]
- Grände, G.; Nilsson, E.; Edvinsson, L. Comparison of Responses to Vasoactive Drugs in Human and Rat Cerebral Arteries Using Myography and Pressurized Cerebral Artery Method. Cephalalgia 2013, 33, 152–159. [Google Scholar] [CrossRef]
- Christensen, S.L.; Ernstsen, C.; Olesen, J.; Kristensen, D.M. No Central Action of CGRP Antagonising Drugs in the GTN Mouse Model of Migraine. Cephalalgia 2020, 40, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, R.; Olesen, J. Calcitonin Gene-Related Peptide Modulators–The History and Renaissance of a N. Migraine Drug Class. Headache 2019, 59, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, E.D.; Joshi, A.D.; Sanabria-Bohórquez, S.; Fan, H.; Zeng, Z.; Purcell, M.; Gantert, L.; Riffel, K.; Williams, M.; O’Malley, S.; et al. In Vivo Quantification of Calcitonin Gene-Related Peptide Receptor Occupancy by Telcagepant in Rhesus Monkey and Human Brain Using the Positron Emission Tomography Tracer [11C]MK-4232. J. Pharmacol. Exp. Ther. 2013, 347, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Yamaguchi, A.; Yamatani, T.; Nakamura, A.; Morishita, T.; Inui, T.; Fukase, M.; Noda, T.; Fujita, T. Calcitonin Gene-Related Peptide Receptor Antagonist Human CGRP-(8-37). Am. J. Physiol. Endocrinol. Metab. 1989, 256, E331–E335. [Google Scholar] [CrossRef]
- Doods, H.; Hallermayer, G.; Wu, D.; Entzeroth, M.; Rudolf, K.; Engel, W.; Eberlein, W. Pharmacological Profile of BIBN4096BS, the First Selective Small Molecule CGRP Antagonist. Br. J. Pharm. 2000, 129, 420–423. [Google Scholar] [CrossRef]
- Olesen, J.; Diener, H.-C.; Husstedt, I.W.; Goadsby, P.J.; Hall, D.; Meier, U.; Pollentier, S.; Lesko, L.M. BIBN 4096 BS Clinical Proof of Concept Study Group Calcitonin Gene–Related Peptide Receptor Antagonist BIBN 4096 BS for the Acute Treatment of Migraine. N. Engl. J. Med. 2004, 350, 1104–1110. [Google Scholar] [CrossRef]
- Ho, T.W.; Connor, K.M.; Zhang, Y.; Pearlman, E.; Koppenhaver, J.; Fan, X.; Lines, C.; Edvinsson, L.; Goadsby, P.J.; Michelson, D. Randomized Controlled Trial of the CGRP Receptor Antagonist Telcagepant for Migraine Prevention. Neurology 2014, 83, 958–966. [Google Scholar] [CrossRef]
- Ho, T.W.; Ho, A.P.; Ge, Y.J.; Assaid, C.; Gottwald, R.; MacGregor, E.A.; Mannix, L.K.; van Oosterhout, W.P.J.; Koppenhaver, J.; Lines, C.; et al. Randomized Controlled Trial of the CGRP Receptor Antagonist Telcagepant for Prevention of Headache in Women with Perimenstrual Migraine. Cephalalgia 2016, 36, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Ailani, J.; Lipton, R.B.; Goadsby, P.J.; Guo, H.; Miceli, R.; Severt, L.; Finnegan, M.; Trugman, J.M. Atogepant for the Preventive Treatment of Migraine. N. Engl. J. Med. 2021, 385, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Lipton, R.B.; Ailani, J.; Lu, K.; Finnegan, M.; Trugman, J.M.; Szegedi, A. Ubrogepant for the Treatment of Migraine. N. Engl. J. Med. 2019, 381, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Croop, R.; Lipton, R.B.; Kudrow, D.; Stock, D.A.; Kamen, L.; Conway, C.M.; Stock, E.G.; Coric, V.; Goadsby, P.J. Oral Rimegepant for Preventive Treatment of Migraine: A Phase 2/3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2021, 397, 51–60. [Google Scholar] [CrossRef]
- Croop, R.; Goadsby, P.J.; Stock, D.A.; Conway, C.M.; Forshaw, M.; Stock, E.G.; Coric, V.; Lipton, R.B. Efficacy, Safety, and Tolerability of Rimegepant Orally Disintegrating Tablet for the Acute Treatment of Migraine: A Randomised, Phase 3, Double-Blind, Placebo-Controlled Trial. Lancet 2019, 394, 737–745. [Google Scholar] [CrossRef]
- Charles, A.; Pozo-Rosich, P. Targeting Calcitonin Gene-Related Peptide: A New Era in Migraine Therapy. Lancet 2019, 394, 1765–1774. [Google Scholar] [CrossRef]
- Dodick, D.W. CGRP Ligand and Receptor Monoclonal Antibodies for Migraine Prevention: Evidence Review and Clinical Implications. Cephalalgia 2019, 39, 445–458. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Approves Novel Preventive Treatment for Migraine. Available online: www.fda.gov/newsevents/newsroom/pressannouncements/ucm608120.htm (accessed on 10 September 2022).
- Sun, H.; Dodick, D.W.; Silberstein, S.; Goadsby, P.J.; Reuter, U.; Ashina, M.; Saper, J.; Cady, R.; Chon, Y.; Dietrich, J.; et al. Safety and Efficacy of AMG 334 for Prevention of Episodic Migraine: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2016, 15, 382–390. [Google Scholar] [CrossRef]
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. ARISE: A Phase 3 Randomized Trial of Erenumab for Episodic Migraine. Cephalalgia 2018, 38, 1026–1037. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Silberstein, S.D.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Effect of Fremanezumab Compared with Placebo for Prevention of Episodic Migraine a Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2018, 319, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, V.L.; Dodick, D.W.; Zhang, Q.; Carter, J.N.; Ailani, J.; Conley, R.R. Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Skljarevski, V.; Matharu, M.; Millen, B.A.; Ossipov, M.H.; Kim, B.K.; Yang, J.Y. Efficacy and Safety of Galcanezumab for the Prevention of Episodic Migraine: Results of the EVOLVE-2 Phase 3 Randomized Controlled Clinical Trial. Cephalalgia 2018, 38, 1442–1454. [Google Scholar] [CrossRef]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in Chronic Migraine: The Randomized, Double-Blind, Placebo-Controlled REGAIN Study. Neurology 2018, 91, E2211–E2221. [Google Scholar] [CrossRef]
- Ashina, M.; Saper, J.; Cady, R.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Smith, J. Eptinezumab in Episodic Migraine: A Randomized, Double-Blind, Placebo-Controlled Study (PROMISE-1). Cephalalgia 2020, 40, 241–254. [Google Scholar] [CrossRef]
- Lipton, R.B.; Goadsby, P.J.; Smith, J.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Cady, R. Efficacy and Safety of Eptinezumab in Patients with Chronic Migraine: PROMISE-2. Neurology 2020, 94, E1365–E1377. [Google Scholar] [CrossRef]
- Sacco, S.; Bendtsen, L.; Ashina, M.; Reuter, U.; Terwindt, G.; Mitsikostas, D.D.; Martelletti, P. European Headache Federation Guideline on the Use of Monoclonal Antibodies Acting on the Calcitonin Gene Related Peptide or Its Receptor for Migraine Prevention. J. Headache Pain 2019, 20, 1–19. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharm. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Schytz, H.W.; Birk, S.; Wienecke, T.; Kruuse, C.; Olesen, J.; Ashina, M. PACAP38 Induces Migraine-like Attacks in Patients with Migraine without Aura. Brain 2009, 132, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.M.; Asghar, M.S.; Guo, S.; Hougaard, A.; Hansen, A.E.; Schytz, H.W.; van der Geest, R.; de Koning, P.J.; Larsson, H.B.; Olesen, J.; et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2011, 32, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, H.; Al-Karagholi, M.A.-M.; Arngrim, N.; Ghanizada, M.; Larsson, H.B.W.; Amin, F.M.; Ashina, M. Effect of pituitary adenylate cyclase-activating polypeptide-27 on cerebral hemodynamics in healthy volunteers: A 3T MRI study. Peptides 2019, 121, 170134. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, H.; Al-Karagholi, M.A.M.; Arngrim, N.; Olesen, J.; Ashina, M. PACAP27 Induces Migraine-like Attacks in Migraine Patients. Cephalalgia 2020, 40, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vollesen, A.L.H.; Hansen, R.D.; Esserlind, A.L.; Amin, F.M.; Christensen, A.F.; Olesen, J.; Ashina, M. Part I: Pituitary Adenylate Cyclase-Activating Polypeptide-38 Induced Migraine-like Attacks in Patients with and without Familial Aggregation of Migraine. Cephalalgia 2017, 37, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wienholtz, N.K.F.; Christensen, C.E.; Zhang, D.G.; Coskun, H.; Ghanizada, H.; Al-Karagholi, M.A.M.; Hannibal, J.; Egeberg, A.; Thyssen, J.P.; Ashina, M. Early Treatment with Sumatriptan Prevents PACAP38-Induced Migraine: A Randomised Clinical Trial. Cephalalgia 2021, 41, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Knutsson, M.; Ca, L.E. Distribution of MRNA for VIP and PACAP Receptors in Human Cerebral Arteries and Cranial Ganglia. Neuroreport 2002, 13, 507–509. [Google Scholar] [CrossRef]
- Pellesi, L.; Al-Karagholi, M.A.-M.; de Icco, R.; Coskun, H.; Elbahi, F.A.; Lopez-Lopez, C.; Snellman, J.; Hannibal, J.; Amin, F.M.; Ashina, M. Effect of Vasoactive Intestinal Polypeptide on Development of Migraine Headaches: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2118543. [Google Scholar] [CrossRef]
- Amin, F.M.; Asghar, M.S.; Ravneberg, J.W.; de Koning, P.J.H.; Larsson, H.B.W.; Olesen, J.; Ashina, M. The Effect of Sumatriptan on Cephalic Arteries: A 3T MR-Angiography Study in Healthy Volunteers. Cephalalgia 2013, 33, 1009–1016. [Google Scholar] [CrossRef]
- Vollesen, L.H.; Guo, S.; Andersen, M.R.; Ashina, M. Effect of the H 1 -Antihistamine Clemastine on PACAP38 Induced Migraine. Cephalalgia 2019, 39, 597–607. [Google Scholar] [CrossRef]
- Ghanizada, H.; Al-Karagholi, M.A.M.; Arngrim, N.; Mørch-Rasmussen, M.; Metcalf-Clausen, M.; Larsson, H.B.W.; Amin, F.M.; Ashina, M. Investigation of Sumatriptan and Ketorolac Trometamol in the Human Experimental Model of Headache. J. Headache Pain 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J.; Hannibal, J.; Tams, J.; Georg, B. Immunohistochemical Localization of the VIP 1 Receptor (VPAC 1 R) in Rat Cerebral Blood Vessels: Relation to PACAP and VIP Containing Nerves. J. Cereb. Blood Flow Metab. 2000, 20, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Boni, L.; Ploug, K.; Olesen, J.; Jansen-Olesen, I.; Gupta, S. The in Vivo Effect of VIP, PACAP-38 and PACAP-27 and MRNA Expression of Their Receptors in Rat Middle Meningeal Artery. Cephalalgia 2009, 29, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.H.; la Cour, S.H.; Calloe, K.; Hauser, F.; Olesen, J.; Klaerke, D.A.; Jansen-Olesen, I. PACAP-38 and PACAP(638) Degranulate Rat Meningeal Mast Cells via the Orphan MrgB3-Receptor. Front. Cell. Neurosci. 2019, 13, 1–11. [Google Scholar] [CrossRef]
- Ashina, M.; Doležil, D.; Bonner, J.H.; Zhou, L.; Klatt, J.; Picard, H.; Mikol, D.D. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial of AMG 301, a Pituitary Adenylate Cyclase-Activating Polypeptide PAC1 Receptor Monoclonal Antibody for Migraine Prevention. Cephalalgia 2021, 41, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Baun, M.; de Vries, R.; van den Bogaerdt, A.J.; Dirven, C.M.F.; Danser, A.H.J.; Jansen-Olesen, I.; Olesen, J.; Villalón, C.M.; Maassenvandenbrink, A.; et al. Pharmacological Characterization of VIP and PACAP Receptors in the Human Meningeal and Coronary Artery. Cephalalgia 2011, 31, 181–189. [Google Scholar] [CrossRef]
- FM, A.; HW, S. Transport of the Pituitary Adenylate Cyclase-Activating Polypeptide across the Blood-Brain Barrier: Implications for Migraine. J. Headache Pain 2018, 19, 1–6. [Google Scholar] [CrossRef]
- Amin, F.M.; Hougaard, A.; Cramer, S.P.; Christensen, C.E.; Wolfram, F.; Larsson, H.B.W.; Ashina, M. Intact Blood−brain Barrier during Spontaneous Attacks of Migraine without Aura: A 3T DCE-MRI Study. Eur. J. Neurol. 2017, 24, 1116–1124. [Google Scholar] [CrossRef]
- Hougaard, A.; Amin, F.M.; Christensen, C.E.; Younis, S.; Wolfram, F.; Cramer, S.P.; Larsson, H.B.W.; Ashina, M. Increased Brainstem Perfusion, but No Blood- Brain Barrier Disruption, during Attacks of Migraine with Aura. Brain 2017, 140, 1633–1642. [Google Scholar] [CrossRef]
- Khan, S.; Amin, F.M.; Fliedner, F.P.; Christensen, C.E.; Tolnai, D.; Younis, S.; Olinger, A.C.R.; Birgens, H.; Daldrup-Link, H.; Kjær, A.; et al. Investigating Macrophage-Mediated Inflammation in Migraine Using Ultrasmall Superparamagnetic Iron Oxide-Enhanced 3T Magnetic Resonance Imaging. Cephalalgia 2019, 39, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.M.; Lundholm, E.; Hougaard, A.; Arngrim, N.; Wiinberg, L.; de Koning, P.J.; Larsson, H.B.; Ashina, M. Measurement Precision and Biological Variation of Cranial Arteries Using Automated Analysis of 3 T Magnetic Resonance Angiography. J Headache Pain 2014, 15, 25. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, C.E.; Ashina, M.; Amin, F.M. Calcitonin Gene-Related Peptide (CGRP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Migraine Pathogenesis. Pharmaceuticals 2022, 15, 1189. https://doi.org/10.3390/ph15101189
Christensen CE, Ashina M, Amin FM. Calcitonin Gene-Related Peptide (CGRP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Migraine Pathogenesis. Pharmaceuticals. 2022; 15(10):1189. https://doi.org/10.3390/ph15101189
Chicago/Turabian StyleChristensen, Casper Emil, Messoud Ashina, and Faisal Mohammad Amin. 2022. "Calcitonin Gene-Related Peptide (CGRP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Migraine Pathogenesis" Pharmaceuticals 15, no. 10: 1189. https://doi.org/10.3390/ph15101189
APA StyleChristensen, C. E., Ashina, M., & Amin, F. M. (2022). Calcitonin Gene-Related Peptide (CGRP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Migraine Pathogenesis. Pharmaceuticals, 15(10), 1189. https://doi.org/10.3390/ph15101189





