Partial Synthetic PPARƳ Derivative Ameliorates Aorta Injury in Experimental Diabetic Rats Mediated by Activation of miR-126-5p Pi3k/AKT/PDK 1/mTOR Expression
Abstract
1. Introduction
2. Results
2.1. Effect of Streptozotocin on Serum Fasting Blood Glucose Adult Female Albino Rats with Experimentally-Induced Diabetes Mellitus
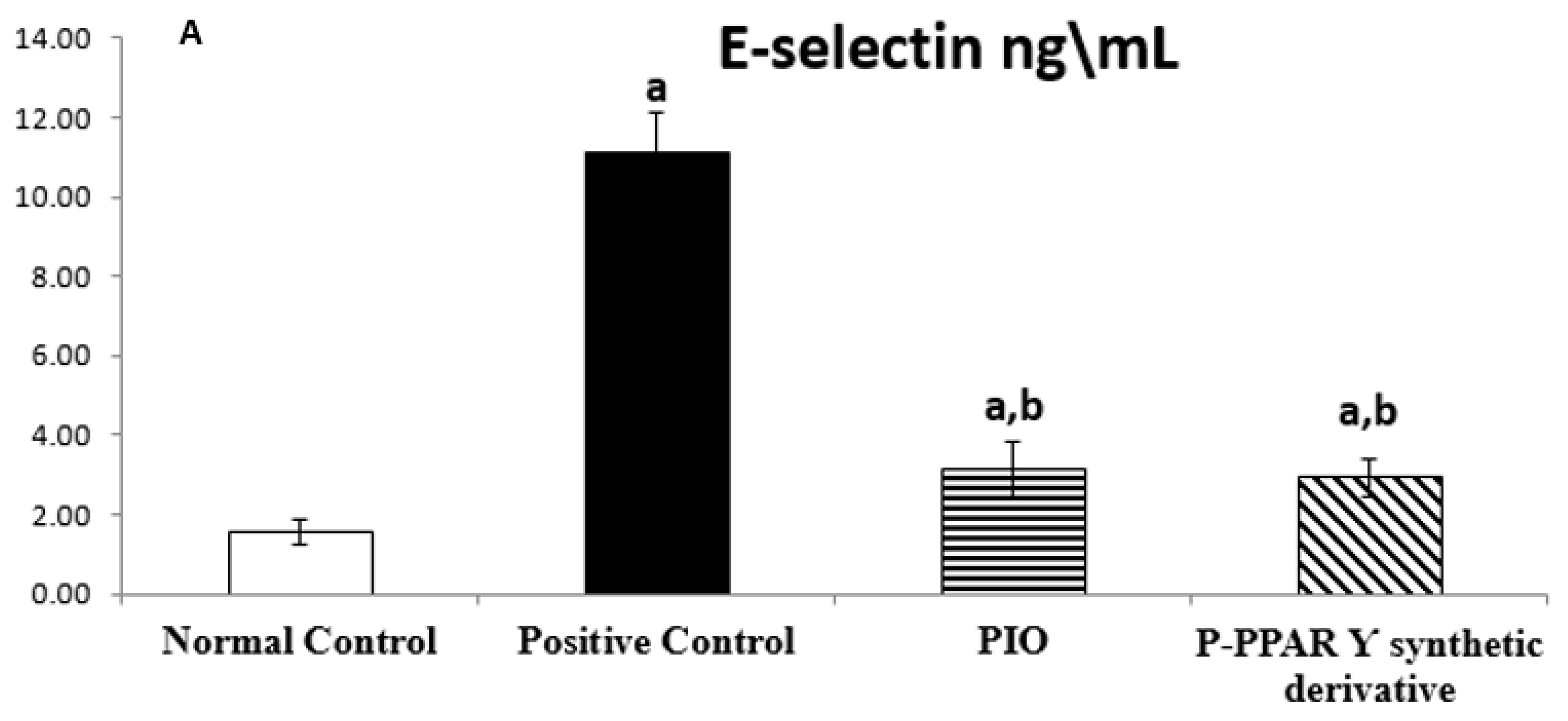
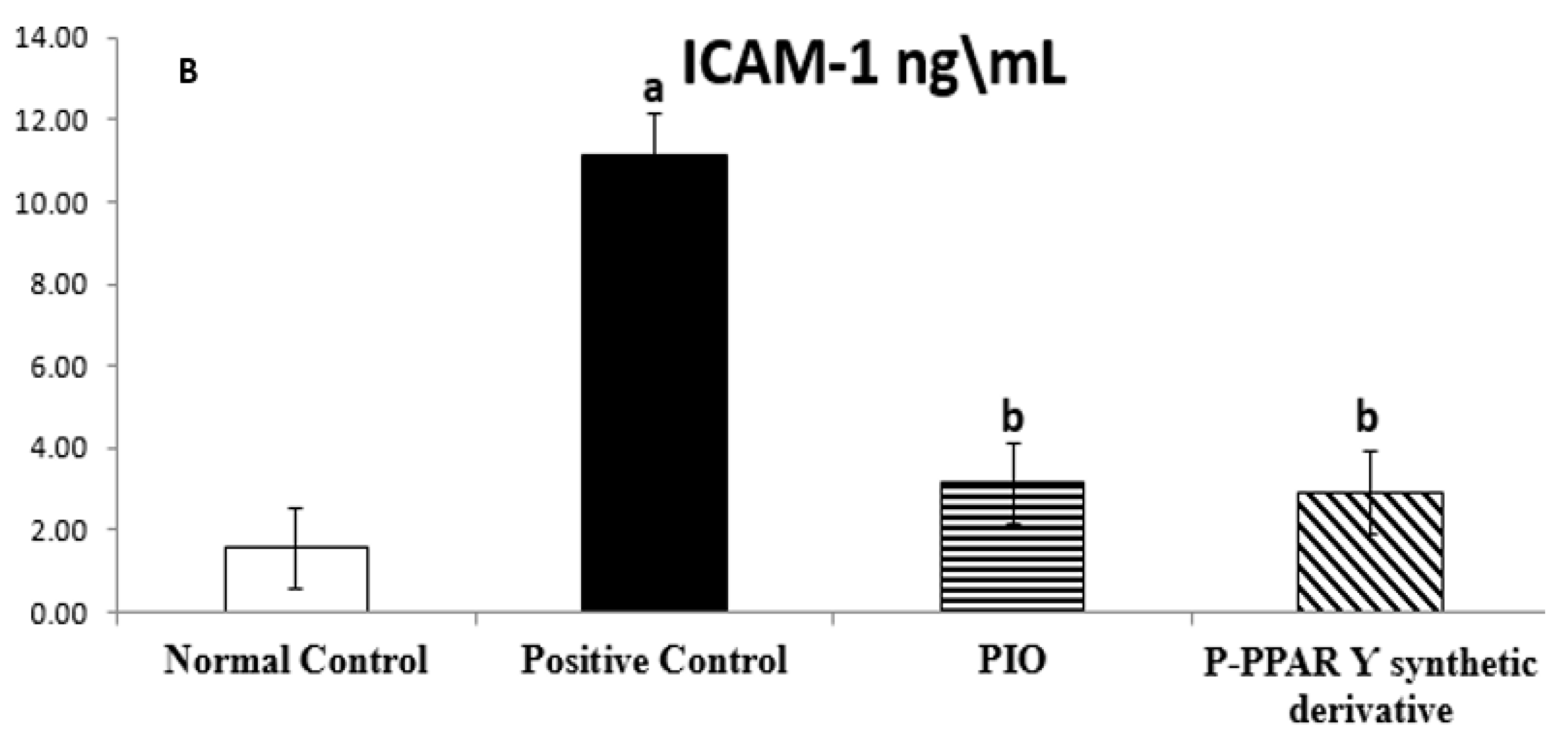
2.2. Effect of 2 Weeks of Treatment with P-PPARƳ Synthetic Derivative on Tissue E-Selectin and ICAM-1 Level in Adult Female Albino Rats with Experimentally Induced T2D Vascular Damage
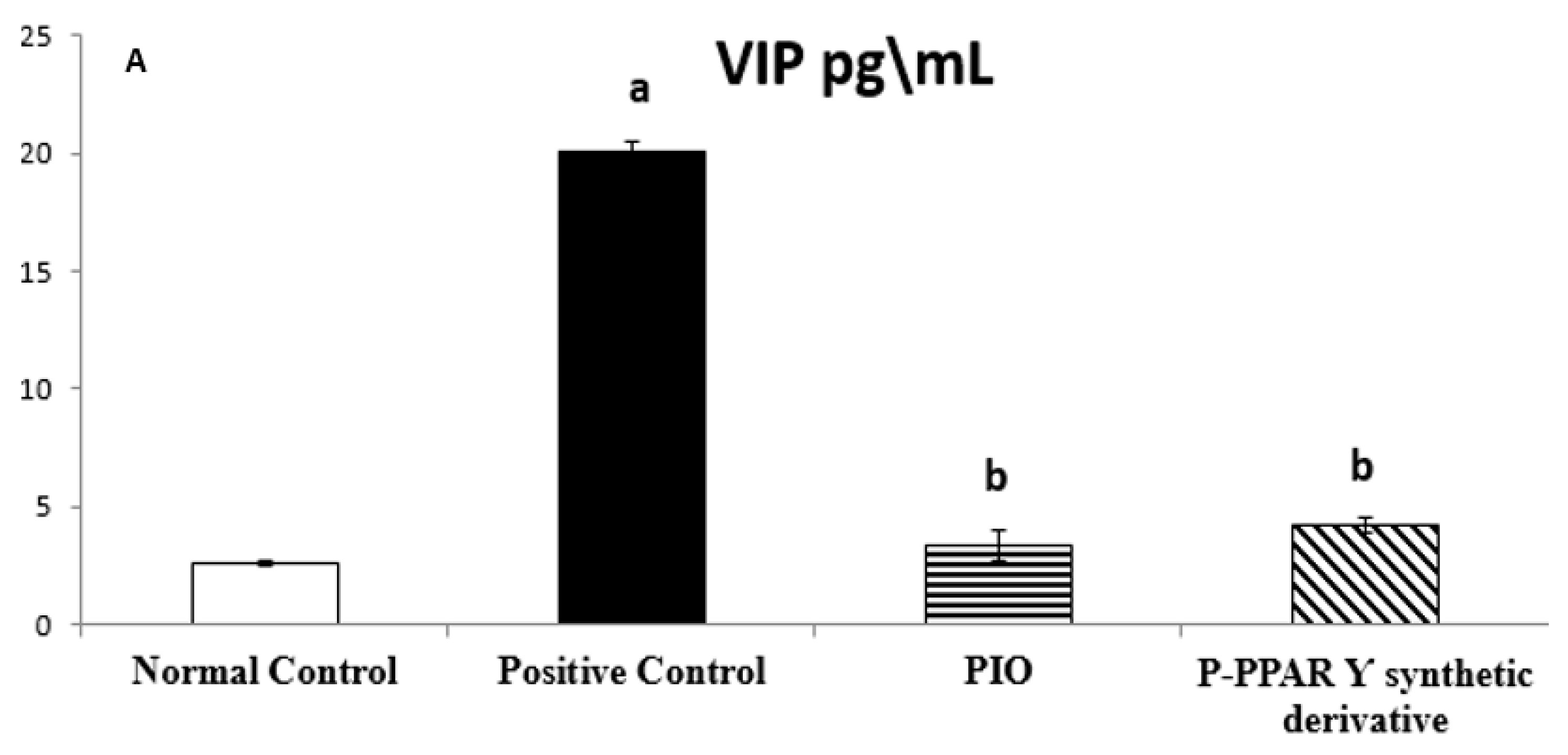
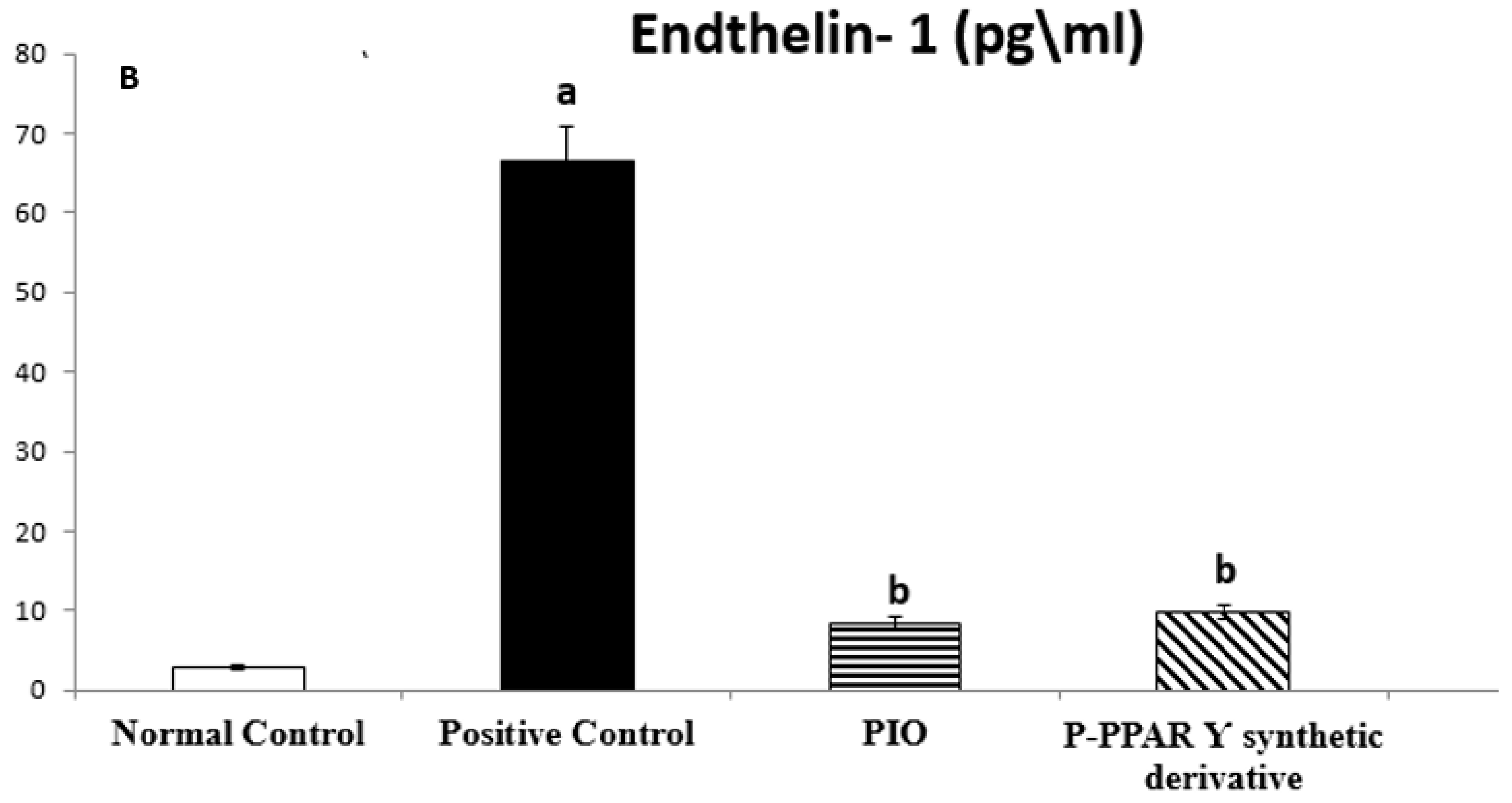
2.3. Effect of 2 Weeks of Treatment with P-PPA Ƴ Synthetic Derivative on Tissue VIP and ET-1 Level in Adult Female Albino Rats with Experimentally Induced T2D Vascular Damage
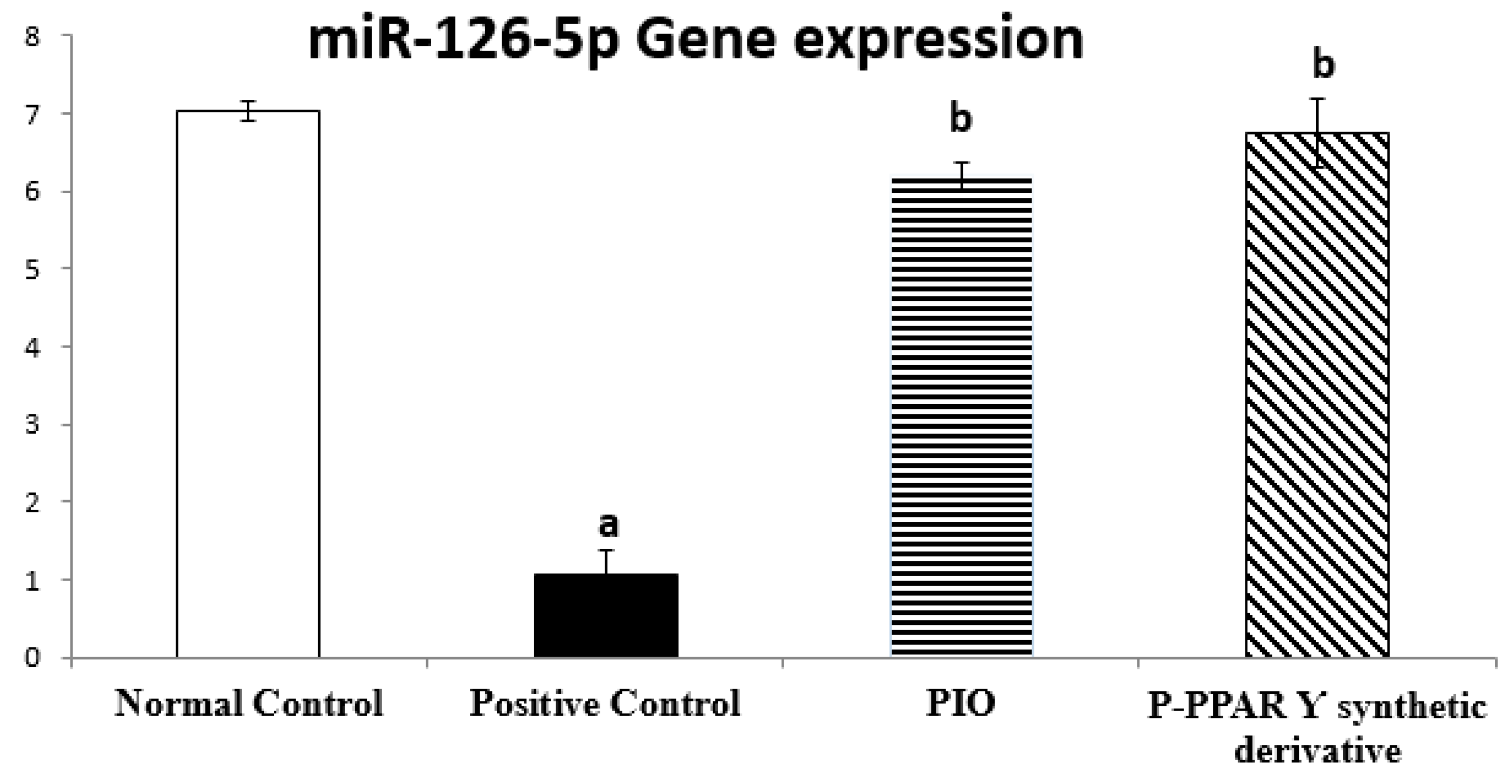
2.4. Effect of 2 Weeks of Treatment with P-PPARƳ Synthetic Derivative on Regulating miR-126-5p Gene Expression
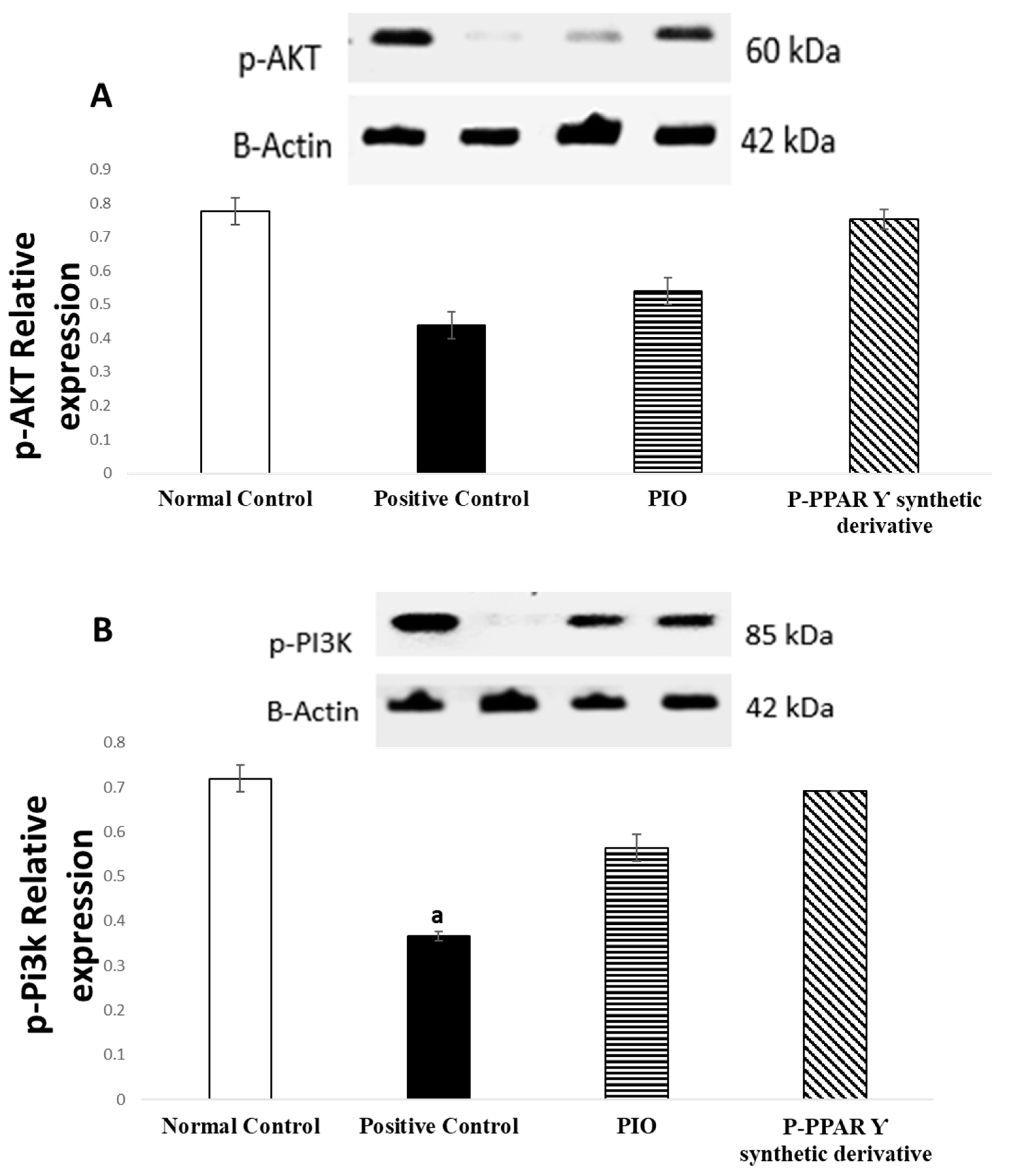
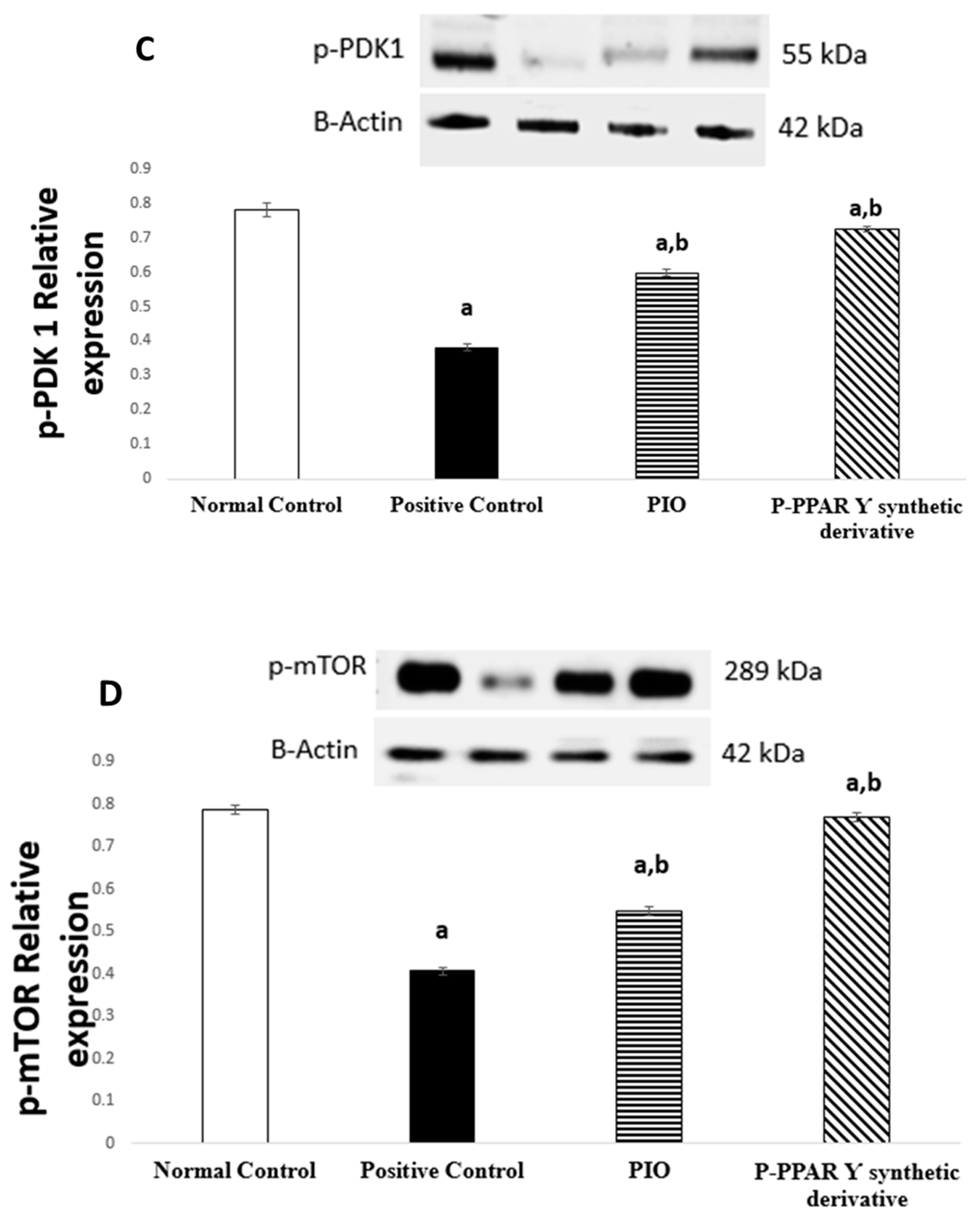
2.5. Effect of 2 Weeks of Treatment with P-PPARƳ Synthetic Derivative on Activation of p-AKT/p-Pi3k/p-PDK 1/p-mTOR Expression, Enhancing Vascular Endothelial Repair
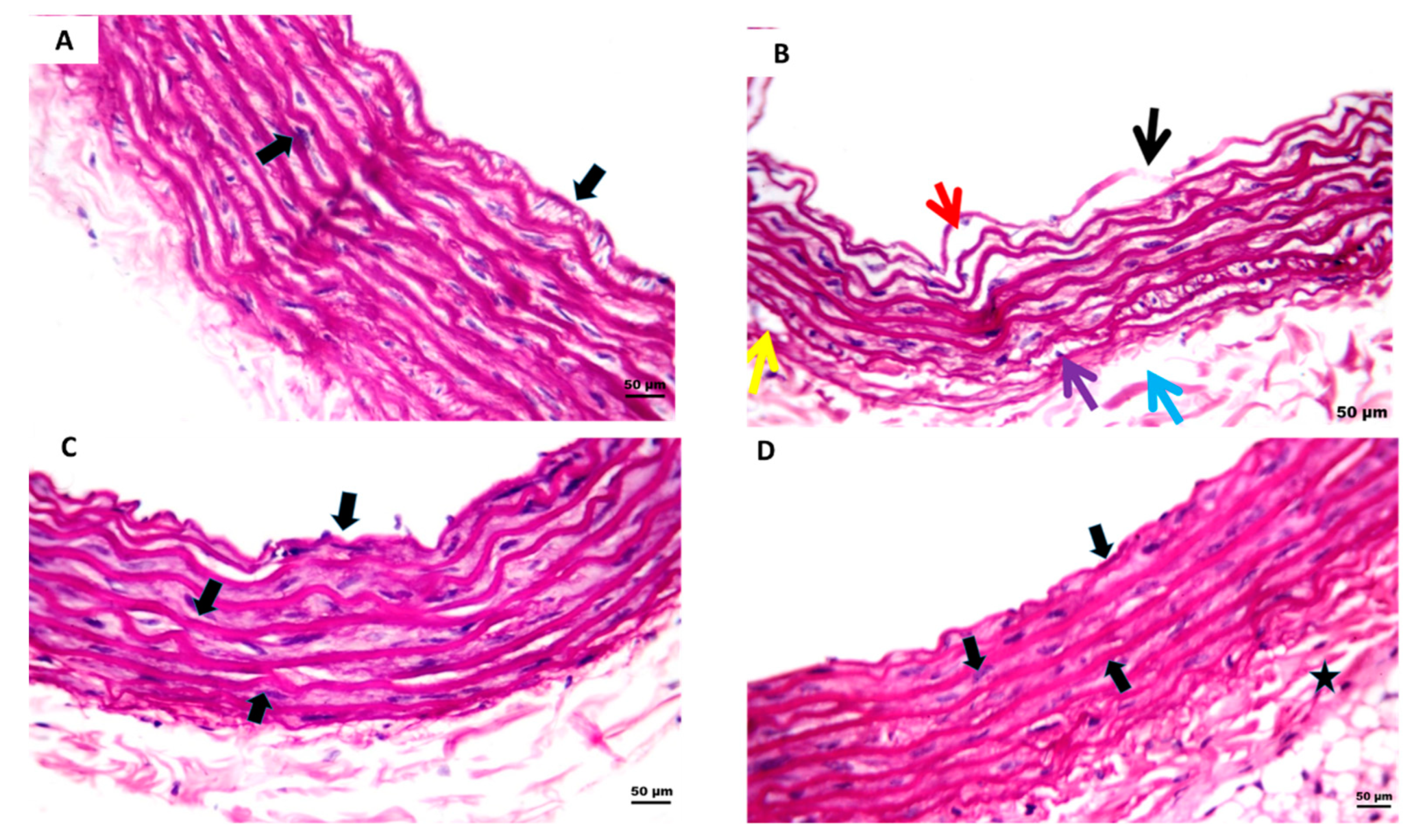
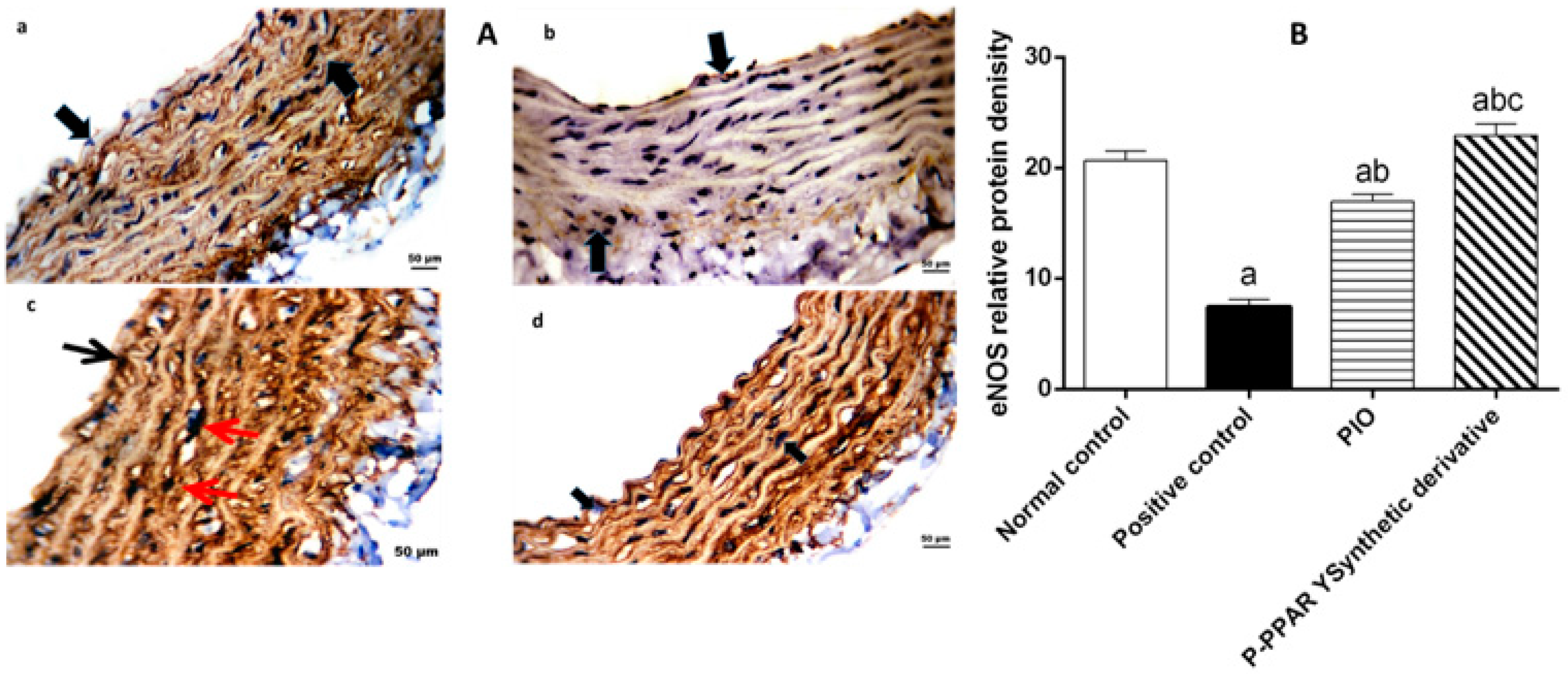
2.6. Effect of 2 Weeks of Treatment with P-PPARƳ Synthetic Derivative on Attenuating Histopathological Aortic Strip Endothelial Abrasions
2.7. Effect of 2 Weeks of Treatment with P-PPARƳ Synthetic Derivative on Mitigating ROS and Enhancing Tissue Antioxidant Defense Mechanism
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagent Kits, Antibodies, and Tested Agents
4.2. Animals
4.3. Animal Experimental Model
4.4. Isolation of Tissue and Preparation
4.5. ELISA Determination for Specific Tissue Endothelial Biomarkers
4.6. Western Blot Analysis for PI3k/AKT/mTOR Signaling Pathway
4.7. Histopathological Study
4.8. Immunohistochemical Assay
4.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) for Determination of miR-126-5p Expression Levels
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanner, C.; Lachin, J.M.; Inzucchi, S.E.; Fitchett, D.; Mattheus, M.; George, J.T.; Woerle, H.-J.; Broedl, U.C.; von Eynatten, M. Zinman, B. Empagliflozin and Clinical Outcomes in Patients with Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease. Circulation 2018, 137, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Aikaeli, F.; Njim, T.; Gissing, S.; Moyo, F.; Alam, U.; Mfinanga, S.G.; Okebe, J.; Ramaiya, K.; Webb, E.L.; Jaffar, S.; et al. Prevalence of microvascular and macrovascular complications of diabetes in newly diagnosed type 2 diabetes in low-and-middle-income countries: A systematic review and meta-analysis. PLoS Glob. Public Health 2022, 2, e0000599. [Google Scholar] [CrossRef]
- Sosale, A.; Kumar, K.P.; Sadikot, S.M.; Nigam, A.; Bajaj, S.; Zargar, A.H.; Singh, S.K. Chronic complications in newly diagnosed patients with Type 2 diabetes mellitus in India. Indian J. Endocrinol. Metab. 2014, 18, 355. [Google Scholar] [CrossRef] [PubMed]
- Polidori, N.; Mainieri, F.; Chiarelli, F.; Mohn, A.; Giannini, C. Early Insulin Resistance, Type 2 Diabetes, and Treatment Options in Childhood. Horm. Res. Paediatr. 2021, 95, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2021, 62, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Irudayaraj, S.S.; Jincy, J.; Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S.; Chandramohan, G.; Packiam, S.M. Antidiabetic with antilipidemic and antioxidant effects of flindersine by enhanced glucose uptake through GLUT4 translocation and PPARƳ agonism in type 2 diabetic rats. J. Ethnopharmacol. 2022, 285, 114883. [Google Scholar] [CrossRef]
- SantaCruz-Calvo, S.; Bharath, L.; Pugh, G.; SantaCruz-Calvo, L.; Lenin, R.R.; Lutshumba, J.; Liu, R.; Bachstetter, A.D.; Zhu, B.; Nikolajczyk, B.S. Adaptive immune cells shape obesity-associated type 2 diabetes mellitus and less prominent comorbidities. Nat. Rev. Endocrinol. 2021, 18, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.L.S.; Barbalho, S.M.; de Araujo, R.R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef]
- Sucato, V.; Coppola, G.; Manno, G.; Vadalà, G.; Novo, G.; Corrado, E.; Galassi, A.R. Coronary Artery Disease in South Asian Patients: Cardiovascular Risk Factors, Pathogenesis and Treatments. Curr. Probl. Cardiol. 2022, 101228. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Rotariu, M.; Turnea, M.-A.; Anghelescu, A.; Albadi, I.; Dogaru, G.; Silișteanu, S.C.; Ionescu, E.V.; Firan, F.C.; Ionescu, A.M.; et al. Topical Reappraisal of Molecular Pharmacological Approaches to Endothelial Dysfunction in Diabetes Mellitus Angiopathy. Curr. Issues Mol. Biol. 2022, 44, 3378–3397. [Google Scholar] [CrossRef]
- Arthi, P.S.; Annamalai, S. Diabetes Mellitus and Peripheral Vascular Disease. Int. J. Contemp. Med. Res. 2020, 7, G10–G13. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life 2021, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Vincent, R.; Kumarathasan, P.; Goegan, P.; Bjarnason, S.G.; Guénette, J.; Karthikeyan, S.; Thomson, E.M.; Adamson, I.Y.; Watkinson, W.P.; Battistini, B.; et al. Acute cardiovascular effects of inhaled ambient particulate matter: Chemical composition-related oxidative stress, endothelin-1, blood pressure, and ST-segment changes in Wistar rats. Chemosphere 2022, 296, 133933. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Couzens, E.; Hwang, A.; Russell, M.; Barlow, C.E.; Defina, L.F.; Hoofnagle, A.N.; Kim, F. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS ONE 2018, 13, e0192616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, J.; Xue, Z.; Wang, W.; Li, C.; Xu, F.; Du, Y.; Lyu, X. SIRT3 attenuates coronary atherosclerosis in diabetic patients by regulating endothelial cell function. J. Clin. Lab. Anal. 2022, 36, e24586. [Google Scholar] [CrossRef]
- Jia, W.; Liu, J.; Tian, X.; Jiang, P.; Cheng, Z.; Meng, C. MircoRNA-126–5p inhibits apoptosis of endothelial cell in vascular arterial walls via NF-κB/PI3K/AKT/mTOR signaling pathway in atherosclerosis. J. Mol. Histol. 2022, 53, 51–62. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, M.; Zuo, Z. Palmitate Induces Mitochondrial Energy Metabolism Disorder and Cellular Damage via the PPAR Signaling Pathway in Diabetic Cardiomyopathy. Diabetes Metab. Syndr. Obesity Targets Ther. 2022, 15, 2287. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; De la Fuente, S.; Palomeque, J.; Sheu, S. The role of mitochondria in metabolic disease: A special emphasis on heart dysfunction. J. Physiol. 2021, 599, 3477–3493. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Jian, L.; Cheung, C.W.; Zhang, L.; Xia, Z. Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovasc. Diabetol. 2021, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Oscai, L.B.; Caruso, R.A.; Wergeles, A.C. Lipoprotein lipase hydrolyzes endogenous triacylglycerols in muscle of exercised rats. J. Appl. Physiol. 1982, 52, 1059–1063. [Google Scholar] [CrossRef]
- Bouchareychas, L.; Raffai, R.L. Apolipoprotein E and Atherosclerosis: From Lipoprotein Metabolism to MicroRNA Control of Inflammation. J. Cardiovasc. Dev. Dis. 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Nazari-Jahantigh, M.; Neth, P.; Weber, C.; Schober, A. MicroRNA-126, -145, and -155: A therapeutic triad in atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2013, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.; Nazari-Jahantigh, M.; Wei, Y.; Bidzhekov, K.; Gremse, F.; Grommes, J.; Megens, R.T.; Heyll, K.; Noels, H.; Hristov, M.; et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat. Med. 2014, 20, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The Endothelial-Specific MicroRNA miR-126 Governs Vascular Integrity and Angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, W.; Zhang, L.; Wu, X.; Du, N.; Hu, Y.; Li, X.; Shen, N.; Xiao, D.; Zhang, H.; et al. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci. Rep. 2015, 5, 9401. [Google Scholar] [CrossRef]
- Tang, F.; Yang, T.-L. MicroRNA-126 alleviates endothelial cells injury in atherosclerosis by restoring autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 495, 1482–1489. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, H.; Li, W.-J.; Liu, Y.-H. LncRNA MALAT1 Promotes OGD-Induced Apoptosis of Brain Microvascular Endothelial Cells by Sponging miR-126 to Repress PI3K/Akt Signaling Pathway. Neurochem. Res. 2020, 45, 2091–2099. [Google Scholar] [CrossRef]
- Bonetti, J.; Corti, A.; Lerouge, L.; Pompella, A.; Gaucher, C. Phenotypic Modulation of Macrophages and Vascular Smooth Muscle Cells in Atherosclerosis—Nitro-Redox Interconnections. Antioxidants 2021, 10, 516. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, Y.; Sun, Z.; Shen, X.; Cai, Y.; Li, L.; Wang, Z. Role of PI3K in the Progression and Regression of Atherosclerosis. Front. Pharmacol. 2021, 12, 632378. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Xu, W.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; et al. Emodin alleviates acute hypoxia-induced apoptosis in gibel carp (Carassius gibelio) by upregulating autophagy through modulation of the AMPK/mTOR pathway. Aquaculture 2021, 548, 737689. [Google Scholar] [CrossRef]
- He, J.; Liu, J.; Huang, Y.; Tang, X.; Xiao, H.; Hu, Z. Oxidative Stress, Inflammation, and Autophagy: Potential Targets of Mesenchymal Stem Cells-Based Therapies in Ischemic Stroke. Front. Neurosci. 2021, 15, 641157. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, S.; Liu, J.; Dong, Z.; Guan, F.; Xu, A.; Zhao, J.; Ge, J. Ameliorating mechanism of nuciferine on high-fat diet-induced dyslipidemia and hepatic steatosis by regulating intestinal absorption and serum extracellular vesicles in rats. J. Funct. Foods 2022, 95, 105182. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y.; Tang, Z.; Zhang, H.; Qin, X.; Zhu, Y.; Guan, Y.; Wang, X.; Staels, B.; Chien, S.; et al. Suppression of pro-inflammatory adhesion molecules by PPAR-δ in human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, H. Structural Biology-Based Exploration of Subtype-Selective Agonists for Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2021, 22, 9223. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Liao, D.; He, W.; Ong, E.S.; Nelson, M.C.; Olefsky, J.M.; Boland, R.; Evans, R.M. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Effects of Selective Peroxisome Proliferator Activated Receptor Agonists on Corneal Epithelial Wound Healing. Pharmaceuticals 2021, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xie, Q.; Sun, C.; Wu, Y.; Zhang, W.; Li, W. Phospholipase A2 group IIA correlates with circulating high-density lipoprotein cholesterol and modulates cholesterol efflux possibly through regulation of PPAR-Ƴ/LXR-α/ABCA1 in macrophages. J. Transl. Med. 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Kucharska, A.Z.; Matuszewska, A.; Rapak, A.; Gomułkiewicz, A.; Dzimira, S.; Dzięgiel, P.; Nowak, B.; Trocha, M.; Magdalan, J.; et al. Cornelian Cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-Ƴ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model. Nutrients 2021, 13, 3621. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chuang, L.M.; Chang, Y.C.; Hsieh, M.L.; Tsou, L.; Chen, S.Y.; Ke, Y.Y.; Hung, M.S.; Hee, S.W.; Lee, H.L.; et al. Increasing endogenous PPARƳ ligands improves insulin sensitivity and protects against diet-induced obesity without side effects of thiazolidinediones. J. Res. Squar 2021, 1–34. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2021, 477, 15–38. [Google Scholar] [CrossRef]
- Vachher, M.; Bansal, S.; Kumar, B.; Yadav, S.; Arora, T.; Wali, N.M.; Burman, A. Contribution of organokines in the development of NAFLD/NASH associated hepatocellular carcinoma. J. Cell. Biochem. 2022; in press. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Natarajan, N.; Sadaf, S.; Florentin, J.; Dutta, P. Regulation of cardiovascular health and disease by visceral adipose tissue-derived metabolic hormones. J. Physiol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Y.; Ding, L.; He, X.; Takahashi, Y.; Gao, Y.; Shen, W.; Cheng, R.; Chen, Q.; Qi, X.; et al. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 15401–15406. [Google Scholar] [CrossRef] [PubMed]
- Guixé-Muntet, S.; Biquard, L.; Szabo, G.; Dufour, J.F.; Tacke, F.; Francque, S.; Rautou, P.E.; Gracia-Sancho, J. Vascular effects of PPARs in the context of NASH. Aliment. Pharmacol. Ther. 2022, 56, 209–233. [Google Scholar] [CrossRef]
- Wasim, R.; Ansari, T.M.; Ahsan, F.; Siddiqui, M.H.; Singh, A.; Shariq, M.; Parveen, S. Pleiotropic Benefits of Statins in Cardiovascular Diseases. Drug Res. 2022. [Google Scholar] [CrossRef]
- S Jain, K.; R Kulkarni, R.; Jain, D.P. Current drug targets for antihyperlipidemic therapy. Mini Rev. Med. Chem. 2010, 10, 232–262. [Google Scholar] [CrossRef] [PubMed]
- Das, E.K.; Lai, P.Y.; Robinson, A.T.; Pleuss, J.; Ali, M.M.; Haus, J.M.; Gutterman, D.D.; Phillips, S.A. Regular Aerobic, Resistance, and Cross-Training Exercise Prevents Reduced Vascular Function Following a High Sugar or High Fat Mixed Meal in Young Healthy Adults. Front. Physiol. 2018, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxidative Med. Cell. Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.A.M.; Budin, S.B.; Zainalabidin, S.; Jalil, J.; Sapian, S.; Jubaidi, F.F.; Anuar, N.N.M. The Role of Polyphenol in Modulating Associated Genes in Diabetes-Induced Vascular Disorders. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar]
- Koliaki, C.; Katsilambros, N. Repositioning the Role of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) on the TRAIL to the Development of Diabetes Mellitus: An Update of Experimental and Clinical Evidence. Int. J. Mol. Sci. 2022, 23, 3225. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.; Topol, E.J. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005, 294, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Katkar, G.D.; Sayed, I.M.; Anandachar, M.S.; Castillo, V.; Vidales, E.; Toobian, D.; Usmani, F.; Sawires, J.R.; Leriche, G.; Yang, J.; et al. Artificial intelligence-rationalized balanced PPARα/Ƴ dual agonism resets dysregulated macrophage processes in inflammatory bowel disease. Commun. Biol. 2022, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R. Angiocrine endothelium: From physiology to atherosclerosis and cardiac repair. Vasc. Pharmacol. 2022, 144, 106993. [Google Scholar] [CrossRef]
- le Noble, F.; Kupatt, C. Interdependence of Angiogenesis and Arteriogenesis in Development and Disease. Int. J. Mol. Sci. 2022, 23, 3879. [Google Scholar] [CrossRef]
- Melly, L.; Banfi, A. Fibrin-based factor delivery for therapeutic angiogenesis: Friend or foe? Cell Tissue Res. 2022, 387, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Gaetani, E.; Flex, A.; Aprahamian, T.; Hopkins, T.; Straface, G.; Pecorini, G.; Stigliano, E.; Smith, R.C.; Angelini, F.; et al. Selective activation of peroxisome proliferator–activated receptor (PPAR) α and PPARƳ induces neoangiogenesis through a vascular endothelial growth factor–dependent mechanism. Diabetes 2008, 57, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Marei, I.; Chidiac, O.; Thomas, B.; Pasquier, J.; Dargham, S.; Robay, A.; Vakayil, M.; Jameesh, M.; Triggle, C.; Rafii, A.; et al. Angiogenic content of microparticles in patients with diabetes and coronary artery disease predicts networks of endothelial dysfunction. Cardiovasc. Diabetol. 2022, 21, 17. [Google Scholar] [CrossRef]
- Sahiba, N.; Sethiya, A.; Soni, J.; Agarwal, D.K.; Agarwal, S. Saturated five-membered thiazolidines and their derivatives: From synthesis to biological applications. Top. Curr. Chem. 2020, 378, 34. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, H.A.; Mansour, S.M.; Elyamany, M.F. Vitamin D Combined with Pioglitazone Mitigates Type-2 Diabetes-Induced Hepatic Injury Through Targeting Inflammation, Apoptosis, and Oxidative Stress. Inflammation 2022, 45, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Kim, J.E.; Park, J.W.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.Y. Fermented mulberry (Morus alba) leaves suppress high fat diet-induced hepatic steatosis through amelioration of the inflammatory response and autophagy pathway. BMC Complementary Med. Ther. 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Bachetti, T.; D’Anna, S.E.; Galloway, B.; Bianco, A.; D’Agnano, V.; Papa, A.; Motta, A.; Perrotta, F.; Maniscalco, M. Mechanisms and Clinical Implications of Endothelial Dysfunction in Arterial Hypertension. J. Cardiovasc. Dev. Dis. 2022, 9, 136. [Google Scholar] [CrossRef]
- Shih, M.H.; Xu, Y.Y.; Yang, Y.S.; Lin, G.L. A facile synthesis and antimicrobial activity evaluation of sydnonyl-substituted thiazolidine derivatives. Molecules 2015, 20, 6520–6532. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, K.R.; Oruganti, L.; Ganjayi, M.S.; Chintha, V.; Muppuru, M.K.; Chippada, A.R.; Badri, K.R.; Meriga, B. Bauhiniastatin-1 alleviates diet induced obesity and lipid accumulation through modulating PPAR-Ƴ/AMPK expressions: In-vitro, in-vivo and in-silico studies. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Takeda, Y.; Matoba, K.; Sekiguchi, K.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Endothelial dysfunction in diabetes. Biomedicines 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Antoniades, C.; Nenna, A.; Chung, C.; Will, R.; Chello, M.; Gaudino, M.F.L. Preventing treatment failures in coronary artery disease: What can we learn from the biology of in-stent restenosis, vein graft failure, and internal thoracic arteries? Cardiovasc. Res. 2020, 116, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M.; Hashiguchi, T. Endothelial Cell Aging: How miRNAs Contribute? J. Clin. Med. 2018, 7, 170. [Google Scholar] [CrossRef]
- Rad, R.S. Effect of Exercise and Non-exercise Interventions on Cardiac Angiogenesis in Diabetes Mellitus Patients: A Review. Int. J. Diabetes Endocrinol. 2022, 7, 1. [Google Scholar]
- Gbr, A.A.; Abdel Baky, N.A.; Mohamed, E.A.; Zaky, H.S. Cardioprotective effect of Pioglitazone and curcumin against diabetic cardiomyopathy in type 1 diabetes mellitus: Impact on CaMKII/NF-κB/TGF-β1 and PPAR-Ƴ signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.M.; Abdelgawad, M.A.; Shalaby, K.; Ghoneim, M.M.; AboulMagd, A.M.; Abdelwahab, N.S.; Hassan, H.M.; Othman, A.M. Pioglitazone Synthetic Analogue Ameliorates Streptozotocin-Induced Diabetes Mellitus through Modulation of ACE 2/Angiotensin 1–7 via PI3K/AKT/mTOR Signaling Pathway. Pharmaceuticals 2022, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Molavi, B.; Chen, J.; Mehta, J.L. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H687–H693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Megharbel, S.M.; Al-Thubaiti, E.H.; Qahl, S.H.; Al-Eisa, R.A.; Hamza, R.Z. Synthesis and Spectroscopic Characterization of Dapagliflozin/Zn (II), Cr (III) and Se (IV) Novel Complexes That Ameliorate Hepatic Damage, Hyperglycemia and Oxidative Injury Induced by Streptozotocin-Induced Diabetic Male Rats and Their Antibacterial Activity. Crystals 2022, 12, 304. [Google Scholar]
- Kalai, F.Z.; Boulaaba, M.; Ferdousi, F.; Isoda, H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int. J. Mol. Sci. 2022, 23, 704. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.F.; Alzamil, N.M.; Hewett, P.W.; Momenah, M.A.; Dallak, M.; Kamar, S.S.; Kader, D.H.A.; Yassin, H.; Haidara, M.A.; Maarouf, A.; et al. Metformin Protects against Diabetic Cardiomyopathy: An Association between Desmin–Sarcomere Injury and the iNOS/mTOR/TIMP-1 Fibrosis Axis. Biomedicines 2022, 10, 984. [Google Scholar] [CrossRef] [PubMed]
- Giha, H.A.; Sater, M.S.; Alamin, O.A.O. Diabetes mellitus tendino-myopathy: Epidemiology, clinical features, diagnosis and management of an overlooked diabetic complication. Acta Diabetol. 2022, 59, 871–883. [Google Scholar] [CrossRef]
- Baig, M.A.; Panchal, S.S.; Mirza, A.B. Streptozotocin-Induced Diabetes Mellitus in Neonatal Rats: An Insight into its Applications to Induce Diabetic Complications. Curr. Diabetes Rev. 2019, 16, 26–39. [Google Scholar] [CrossRef]
- Ugusman, A.; Kumar, J.; Aminuddin, A. Endothelial function and dysfunction: Impact of sodium-glucose cotransporter 2 inhibitors. Pharmacol. Ther. 2021, 224, 107832. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.L.; Han, L.L.; Qian, J.H.; Wang, H.Z. Molecular Mechanism of Puerarin Against Diabetes and its Complications. Front. Pharmacol. 2021, 12, 780419. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Magro, D.O.; Evangelista-Poderoso, R.; Saad, M.J.A. Diabetes, obesity, and insulin resistance in COVID-19: Molecular interrelationship and therapeutic implications. Diabetol. Metab. Syndr. 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.M.; Messiha, B.A.S.; El-Daly, M.E.S.; Abo-Saif, A.A. Effects of ticagrelor, empagliflozin and tamoxifen against experimentally-induced vascular reactivity defects in rats in vivo and in vitro. Pharmacol. Rep. 2019, 71, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Van Raemdonck, K.; Umar, S.; Szekanecz, Z.; Zomorrodi, R.K.; Shahrara, S. Impact of obesity on autoimmune arthritis and its cardiovascular complications. Autoimmun. Rev. 2018, 17, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Silva, H. Tobacco Use and Periodontal Disease—The Role of Microvascular Dysfunction. Biology 2021, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, H.S.; Ye, C.; Hanley, A.J.; Sermer, M.; Zinman, B.; Retnakaran, R. Biomarkers of vascular injury and endothelial dysfunction after recent glucose intolerance in pregnancy. Diabetes Vasc. Dis. Res. 2018, 15, 449–457. [Google Scholar] [CrossRef]
- Liang, S.; Desai, A.A.; Black, S.M.; Tang, H. Cytokines, Chemokines, and Inflammation in Pulmonary Arterial Hypertension. In Lung Inflammation in Health and Disease; Springer: Cham, Switzerland, 2021; Volume 1, pp. 275–303. [Google Scholar]
- Zou, L.; Xiong, L.; Wu, T.; Wei, T.; Liu, N.; Bai, C.; Huang, X.; Hu, Y.; Xue, Y.; Zhang, T.; et al. NADPH oxidases regulate endothelial inflammatory injury induced by PM2. 5 via AKT/eNOS/NO axis. J. Appl. Toxicol. 2022, 42, 738–749. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Outzen, E.M.; Zaki, M.; Mehryar, R.; Abdolalizadeh, B.; Sajid, W.; Boonen, H.C.; Sams, A.; Sheykhzade, M. Lipopolysaccharides, but not Angiotensin ll, Induces Direct Pro-Inflammatory Effects in Cultured Mouse Arteries and Human Endothelial and Vascular Smooth Muscle Cells. Basic Clin. Pharmacol. Toxicol. 2017, 120, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, P.; Sadeghalvad, M.; Rezaei, N. An inflammatory triangle in Sarcoidosis: PPAR-Ƴ, immune microenvironment, and inflammation. Expert Opin. Biol. Ther. 2021, 21, 1451–1459. [Google Scholar] [CrossRef]
- Jung, C.-Y.; Yoo, T.-H. Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease. Diabetes Metab. J. 2022, 46, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.-C.; Chao, C.-T.; Lin, S.-H. The Dynamics and Plasticity of Epigenetics in Diabetic Kidney Disease: Therapeutic Applications Vis-à-Vis. Int. J. Mol. Sci. 2022, 23, 843. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Kandaraki, E.A.; Poulia, K.A.; Piperi, C.; Papadimitriou, E.; Papaioannou, T.G. Assessment of Early Markers of Cardiovascular Risk in Polycystic Ovary Syndrome. Touch Rev. Endocrinol. 2021, 17, 37. [Google Scholar]
- Manners, N.; Priya, V.; Mehata, A.K.; Rawat, M.; Mohan, S.; Makeen, H.A.; Albratty, M.; Albarrati, A.; Meraya, A.M.; Muthu, M.S. Theranostic Nanomedicines for the Treatment of Cardiovascular and Related Diseases: Current Strategies and Future Perspectives. Pharmaceuticals 2022, 15, 441. [Google Scholar] [CrossRef]
- Jain, K.K. Neuroprotection in Alzheimer Disease. In The Handbook of Neuroprotection; Humana: New York, NY, USA, 2019; pp. 465–585. [Google Scholar]
- Abdelsamia, E.M.; Khaleel, S.A.; Balah, A.; Baky, N.A.A. Curcumin augments the cardioprotective effect of metformin in an experimental model of type I diabetes mellitus; Impact of Nrf2/HO-1 and JAK/STAT pathways. Biomed. Pharmacother. 2019, 109, 2136–2144. [Google Scholar] [CrossRef]
- Matsumoto, T.; Noguchi, E.; Kobayashi, T.; Kamata, K. Mechanisms underlying the chronic Pioglitazone treatment-induced improvement in the impaired endothelium-dependent relaxation seen in aortas from diabetic rats. Free. Radic. Biol. Med. 2007, 42, 993–1007. [Google Scholar] [CrossRef]
- Aliperti, L.A.; Lasker, G.F.; Hagan, S.S.; Hellstrom, J.A.; Gokce, A.; Trost, L.W.; Kadowitz, P.J.; Sikka, S.C.; Hellstrom, W.J. Efficacy of Pioglitazone on Erectile Function Recovery in a Rat Model of Cavernous Nerve Injury. Urology 2014, 84, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Giubilato, S.; Zaccardi, F.; Di Stasio, E.; Buffon, A.; Biasucci, L.M.; Liuzzo, G.; Crea, F.; Ghirlanda, G. Pioglitazone reduces monocyte activation in type 2 diabetes. Acta Diabetol. 2008, 46, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.; Tripodi, G.L.; Ferrer, R.; Boscá, L.; Pitta, M.G.; Pitta, I.R.; Abdalla, D.S. New thiazolidinediones affect endothelial cell activation and angiogenesis. Eur. J. Pharmacol. 2016, 782, 98–106. [Google Scholar] [CrossRef]
- Suresh, V.; Reddy, A. Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impediment. J. Diabetes Metab. Disord. 2021, 20, 989–1002. [Google Scholar] [CrossRef]
- Akhmedov, A.; Sawamura, T.; Chen, C.-H.; Kraler, S.; Vdovenko, D.; Lüscher, T.F. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): A crucial driver of atherosclerotic cardiovascular disease. Eur. Heart J. 2020, 42, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chang, G.; Cao, L.; Ding, G. Dysregulation of serum miR-361-5p serves as a biomarker to predict disease onset and short-term prognosis in acute coronary syndrome patients. BMC Cardiovasc. Disord. 2021, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Mohammadi, N.; Haghi-Aminjan, H.; Farhood, B.; Negahdari, B.; Sahebkar, A. Regulation of tumor angiogenesis by microRNAs: State of the art. J. Cell. Physiol. 2019, 234, 1099–1110. [Google Scholar] [CrossRef]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Zhou, K.; Kao, G.; Xiao, J. MicroRNA-126 and VEGF enhance the function of endothelial progenitor cells in acute myocardial infarction. Exp. Ther. Med. 2022, 23, 142. [Google Scholar] [CrossRef]
- Wu, Q.; Qi, B.; Duan, X.; Ming, X.; Yan, F.; He, Y.; Bu, X.; Sun, S.; Zhu, H. MicroRNA-126 enhances the biological function of endothelial progenitor cells under oxidative stress via PI3K/Akt/GSK3β and ERK1/2 signaling pathways. Bosn. J. Basic Med. Sci. 2021, 21, 71. [Google Scholar]
- Ye, P.; Liu, J.; He, F.; Xu, W.; Yao, K. Hypoxia-Induced Deregulation of miR-126 and Its Regulative Effect on VEGF and MMP-9 Expression. Int. J. Med. Sci. 2014, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Gong, L.; Tan, F.; Liu, Y.; Li, S.; Shen, H.; Zhu, M.; Cai, W.; Xu, F.; Hou, B.; et al. Vaccarin ameliorates insulin resistance and steatosis by activating the AMPK signaling pathway. Eur. J. Pharmacol. 2019, 851, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Wei, K. miRNA in cardiac development and regeneration. Cell Regen. 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, Actions, and Therapeutic Potential of Specialized Pro-resolving Lipid Mediators for the Treatment of Inflammation in Cystic Fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Guzmán, R.R.; Centofanti, F.; Doldo, E.; Miranda, E.M.C.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Ngcobo, S.R.; Nkambule, B.B.; Nyambuya, T.M.; Mokgalaboni, K.; Ntsethe, A.; Mxinwa, V.; Ziqubu, K.; Ntamo, Y.; Nyawo, T.A.; Dludla, P.V. Activated monocytes as a therapeutic target to attenuate vascular inflammation and lower cardiovascular disease-risk in patients with type 2 diabetes: A systematic review of preclinical and clinical studies. Biomed. Pharmacother. 2022, 146, 112579. [Google Scholar] [CrossRef]
- Pereira, C.A.; Carneiro, F.S.; Matsumoto, T.; Tostes, R.C. Bonus Effects of Antidiabetic Drugs: Possible Beneficial Effects on Endothelial Dysfunction, Vascular Inflammation and Atherosclerosis. Basic Clin. Pharmacol. Toxicol. 2018, 123, 523–538. [Google Scholar] [CrossRef]
- Bancroft, G.D.; Steven, A. Theory and Practice of Histological Technique; Churchil Livingstone Publications: London, UK, 1983. [Google Scholar]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef]
- Lequin, R.M. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef]
- Burnette, W.N. “Western Blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Merz, H.; Malisius, R.; Mannweiler, S.; Zhou, R.; Hartmann, W.; Orscheschek, K.; Moubayed, P.; Feller, A.C. ImmunoMax. A maximized immunohistochemical method for the retrieval and enhancement of hidden antigens. Lab. Investig. A J. Tech. Methods Pathol. 1995, 73, 149–156. [Google Scholar]
| Groups | Fasting Blood Glucose mg/dL |
|---|---|
| Normal control | 101.66 ± 4.73 |
| Positive control | 289.33 ± 12.90 a |
| PIO | 129.66 ± 8.08 b |
| P-PPARƳ synthetic derivative | 96.33 ± 8.14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, Y.M.; Orfali, R.; Abdelwahab, N.S.; Hassan, H.M.; Rateb, M.E.; AboulMagd, A.M. Partial Synthetic PPARƳ Derivative Ameliorates Aorta Injury in Experimental Diabetic Rats Mediated by Activation of miR-126-5p Pi3k/AKT/PDK 1/mTOR Expression. Pharmaceuticals 2022, 15, 1175. https://doi.org/10.3390/ph15101175
Ahmed YM, Orfali R, Abdelwahab NS, Hassan HM, Rateb ME, AboulMagd AM. Partial Synthetic PPARƳ Derivative Ameliorates Aorta Injury in Experimental Diabetic Rats Mediated by Activation of miR-126-5p Pi3k/AKT/PDK 1/mTOR Expression. Pharmaceuticals. 2022; 15(10):1175. https://doi.org/10.3390/ph15101175
Chicago/Turabian StyleAhmed, Yasmin M., Raha Orfali, Nada S. Abdelwahab, Hossam M. Hassan, Mostafa E. Rateb, and Asmaa M. AboulMagd. 2022. "Partial Synthetic PPARƳ Derivative Ameliorates Aorta Injury in Experimental Diabetic Rats Mediated by Activation of miR-126-5p Pi3k/AKT/PDK 1/mTOR Expression" Pharmaceuticals 15, no. 10: 1175. https://doi.org/10.3390/ph15101175
APA StyleAhmed, Y. M., Orfali, R., Abdelwahab, N. S., Hassan, H. M., Rateb, M. E., & AboulMagd, A. M. (2022). Partial Synthetic PPARƳ Derivative Ameliorates Aorta Injury in Experimental Diabetic Rats Mediated by Activation of miR-126-5p Pi3k/AKT/PDK 1/mTOR Expression. Pharmaceuticals, 15(10), 1175. https://doi.org/10.3390/ph15101175






