Recent Advances in Antiviral Activities of Triterpenoids
Abstract
1. Introduction
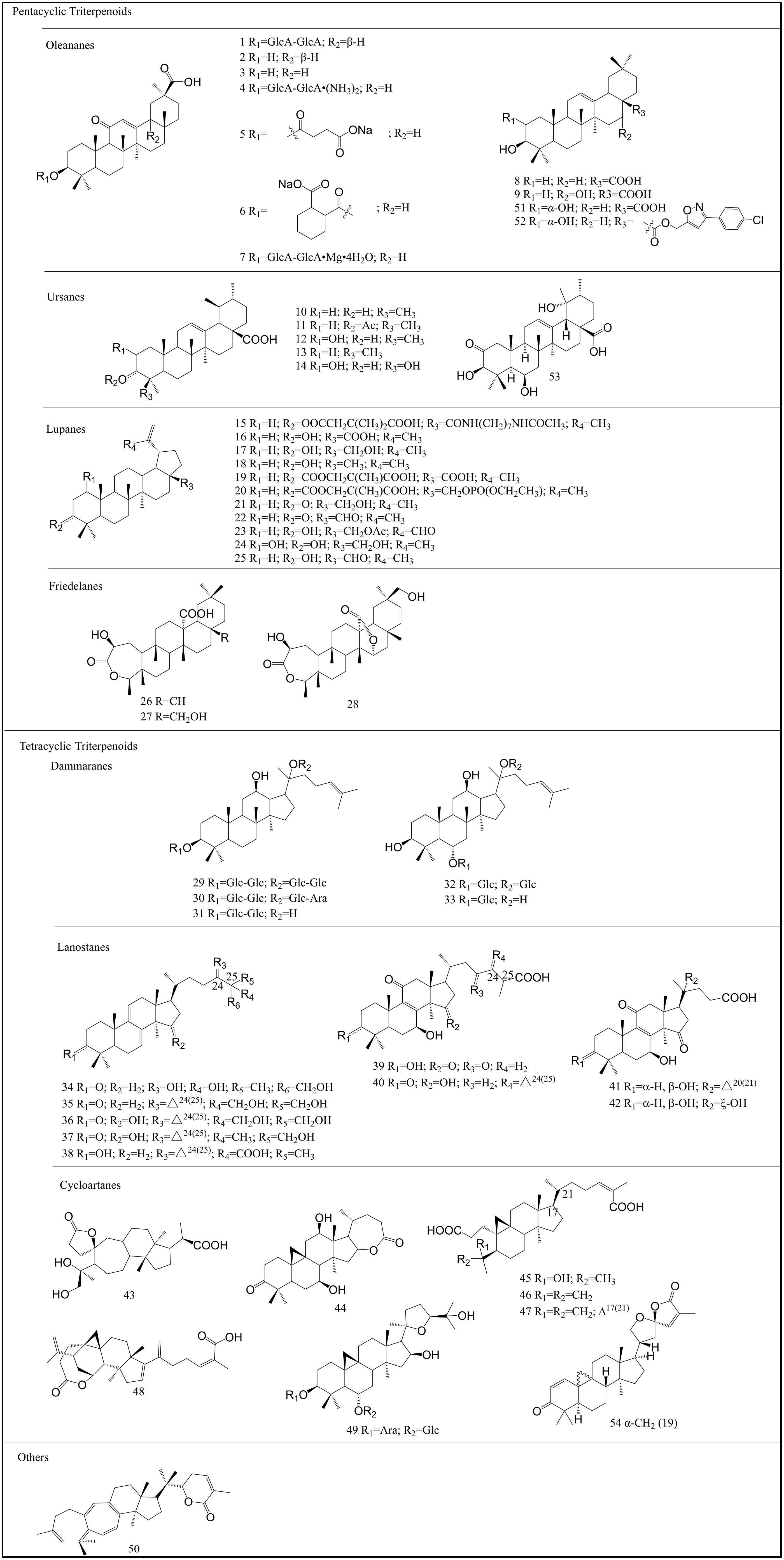
2. Structure and Classification of Triterpenoids
3. Antiviral Activities and Mechanism
3.1. Anti-HIV
| Compounds | IC50 | EC50 | Targets | References |
|---|---|---|---|---|
| 1 | - | - | Entry inhibitors | [16] |
| 8 | 57.7 μM 30.3 μM | - | Protease inhibitors Integrase inhibitors | [46] [45] |
| 10 | 8 μM | - | Protease inhibitors | [28] |
| 35 μM | - | Integrase inhibitors | [45] | |
| 11 | 13 μM | - | Protease inhibitors | [28] |
| 13 | - | - | Protease inhibitors | [30] |
| 16 | 24.8 μM | - | Integrase inhibitors | [45] |
| 17 | 17.7 μM | - | Integrase inhibitors | [45] |
| 18 | 11.6 μM | - | Reverse transcriptase inhibitor | [36] |
| 19 | 0.03 μM | - | Maturation inhibitors | [44] |
| 20 | 0.02 μM | - | - | [44] |
| 21 | 4.08 μM | - | Replication inhibition | [52] |
| 22 | 4.18 μM | - | Replication inhibitors | [52] |
| 23 | 1.70 μM | - | Replication inhibitors | [52] |
| 34 | >1.0 mM | - | Protease inhibitors | [47] |
| 35 | 7.8 μg/mL | - | Protease inhibitors | [48] |
| 40 | 30 μM | - | Protease inhibitors | [48] |
| 41 | 48 μM | - | Protease inhibitors | [48] |
| 42 | 25 μM | - | Protease inhibitors | [48] |
| 43 | - | 20.69 μg/mL | - | [32] |
| 44 | - | 16.6 μg/mL | - | [32] |
| 45 | - | 16.2 μg/mL | - | [32] |
| 46 | - | 10.3 μg/mL | - | [32] |
| 15.79 μM | - | Protease inhibitors | [35] | |
| 47 | 20.44 μM | - | Protease inhibitors | [35] |
| 48 | - | 6.1 μg/mL | - | [33] |
| 50 | - | 1.4 μg/mL | - | [34] |
| 53 | - | 1.24 μM | - | [53] |
| 54 | - | - | - | [54] |
3.2. Anti-Influenza Virus
3.3. Anti-CoV
| Compounds | IC50 | Targets | Virus | References |
|---|---|---|---|---|
| 1 | Inhibits SARS-CoV replication, adsorption, and penetration; | SARS-CoV | [68] | |
| Protease inhibitor | SARS-CoV-2 | [73] | ||
| 7 | - | SARS-CoV-2 | [74] | |
| 8 | 3CL hydrolase inhibitor | SARS-CoV-2 | [75] | |
| 10 | 12.57 | SARS-CoV-2 Mpro inhibitor | SARS-CoV-2 | [76] |
| 16 | 14.55 | SARS-CoV-2 Mpro inhibitor | SARS-CoV-2 | [76] |
| 17 | 89.67 | SARS-CoV-2 Mpro inhibitor | SARS-CoV-2 | [76] |
| 51 | 3.22 | SARS-CoV-2 Mpro inhibitor | SARS-CoV-2 | [76] |
| 52 | 4.12 | Inhibits SARS-CoV replication and adsorption | SARS-CoV | [77] |
| 6.25 | - | MERS-CoV | [77] |
3.4. Anti-Hepatitis Virus
3.4.1. Anti-Hepatitis A Virus (HAV)
3.4.2. Anti-HBV
| Compounds | IC50 | Targets | Cells | References |
|---|---|---|---|---|
| 1 | - | Replication inhibitors | - | [81] |
| 26; 27; 28 | HBsAg: 26.2, 33.7, and 104.0 μM HBeAg: 8.0, 15.2, and 21.6 μM | Inhibits the secretion of HBsAg and HBeAg | HepG2.2.15 cells | [82] |
| 31 | - | Replication inhibitors | - | [85] |
| 32 | - | Immunomodulator | - | [86] |
| 49 | HBsAg: 28.15 μM HBeAg: 33.38 μM | Replication inhibitors | HepG2.2.15 cells | [84] |
3.4.3. Anti-HCV
| Compounds | IC50 | Targets | Cells | References |
|---|---|---|---|---|
| 8 | 1.8 μM | Entry inhibitors Protease inhibitors and polymerase inhibitors | Hep G2-5B cells | [87,89] |
| 9 | - | Entry inhibitors | - | [24] |
| 10 | 6.8 μM | Polymerase inhibitors | Hep G2-5B cells | [89] |
| 31 | - | Infection inhibitors | Human hepatoma Huh7 and Huh7.5.1 cells | [90] |
3.5. Anti-Rotavirus (RV)
3.6. Anti-Herpesvirus
3.7. Anti-DENV
| Compounds | IC50 | Targets | References |
|---|---|---|---|
| 1 | 8.1 μM | Inhibited DENV2-induced CPE and reduced DENV-2 infectivity | [13] |
| 5 | - | Virucidal effect | [109] |
| 16 | 4.3 ± 3.1 μM | Replication inhibitors | [110] |
| 17 | 4.1 ± 0.4 μM | Replication inhibitors | [110] |
| 25 | 7.5 ± 1.1 μM | Replication inhibitors | [110] |
| 34 | - | Protease inhibitors | [111] |
3.8. Anti-Norovirus (NoV)
3.9. Anti-Coxsackievirus (CV)
3.10. Anti-Adenovirus (AdV)
3.11. Anti-Enterovirus (EV71)
3.12. Anti-Porcine Reproductive and Respiratory Syndrome Virus (PRRSV)
3.13. Anti-Human Respiratory Syncytial Virus (HRSV)
4. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sander, W.J.; O’Neill, H.G.; Pohl, C.H. Prostaglandin E(2) As a Modulator of Viral Infections. Front. Physiol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Gao, Y.; Wen, Y.; Ke, X.; Ou, Z.; Li, Y.; He, H.; Chen, Q. Detection of Virus-Related Sequences Associated With Potential Etiologies of Hepatitis in Liver Tissue Samples From Rats, Mice, Shrews, and Bats. Front. Microbiol. 2021, 12, 653873. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W. Antiviral effect of glycyrrhizic acid. Mod. Chin. Med. 2020, 22, 533–541. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Cao, Y. Host–Virus Interaction: How Host Cells Defend against Influenza A Virus Infection. Viruses 2020, 12, 376. [Google Scholar] [CrossRef]
- Yang, J.; Yue, L.; Yang, Z.; Miao, Y.; Ouyang, R.; Hu, Y. Metal-Based Nanomaterials: Work as Drugs and Carriers against Viral Infections. Nanomaterials 2021, 11, 2129. [Google Scholar] [CrossRef]
- Finsterer, J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol. Scand. 2022, 145, 5–9. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef]
- Pu, J.Y.; He, L.; Wu, S.Y.; Zhang, P.; Huang, X. Anti-virus research of triterpenoids in licorice. Chin. J. Virol. 2013, 29, 673–679. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.; Pu, Y.; Tao, J.; Zhang, T. Techniques for the analysis of pentacyclic triterpenoids in medicinal plants. J. Sep. Sci. 2018, 41, 6–19. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Xiao, S.; Zhang, L.; Zhou, D. Triterpenoid-Mediated Inhibition of Virus–Host Interaction: Is Now the Time for Discovering Viral Entry/Release Inhibitors from Nature? J. Med. Chem. 2020, 63, 15371–15388. [Google Scholar] [CrossRef]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962–998. [Google Scholar] [CrossRef]
- Baltina, L.A.; Tasi, Y.T.; Huang, S.H.; Lai, H.C.; Baltina, L.A.; Petrova, S.F.; Yunusov, M.S.; Lin, C.W. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 126645. [Google Scholar] [CrossRef]
- Hattori, T.; Ikematsu, S.; Koito, A.; Matsushita, S.; Maeda, Y.; Hada, M.; Fujimaki, M.; Takatsuki, K. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antivir. Res. 1989, 11, 255–261. [Google Scholar] [CrossRef]
- Mori, K.; Sakai, H.; Suzuki, S.; Sugai, K.; Akutsu, Y.; Ishikawa, M.; Seino, Y.; Ishida, N.; Uchida, T.; Kariyone, S.; et al. Effects of glycyrrhizin (SNMC: Stronger Neo-Minophagen C) in hemophilia patients with HIV infection. Tohoku J. Exp. Med. 1989, 158, 25–35. [Google Scholar] [CrossRef]
- Ito, M.; Sato, A.; Hirabayashi, K.; Tanabe, F.; Shigeta, S.; Baba, M.; De Clercq, E.; Nakashima, H.; Yamamoto, N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV). Antivir. Res. 1988, 10, 289–298. [Google Scholar] [CrossRef]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology 2002, 70, 229–236. [Google Scholar] [CrossRef]
- Venuti, A.; Pastori, C.; Lopalco, L. The Role of Natural Antibodies to CC Chemokine Receptor 5 in HIV Infection. Front. Immunol. 2017, 8, 1358. [Google Scholar] [CrossRef]
- Yoshida, T.; Kobayashi, M.; Li, X.D.; Pollard, R.B.; Suzuki, F. Inhibitory effect of glycyrrhizin on the neutrophil-dependent increase of R5 HIV replication in cultures of macrophages. Immunol. Cell Biol. 2009, 87, 554–558. [Google Scholar] [CrossRef]
- Takei, M.; Kobayashi, M.; Li, X.D.; Pollard, R.B.; Suzuki, F. Glycyrrhizin inhibits R5 HIV replication in peripheral blood monocytes treated with 1-methyladenosine. Pathobiology 2005, 72, 117–123. [Google Scholar] [CrossRef]
- Jeong, H.G.; Kim, J.Y. Induction of inducible nitric oxide synthase expression by 18β-glycyrrhetinic acid in macrophages. FEBS Lett. 2002, 513, 208–212. [Google Scholar] [CrossRef]
- Baltina, L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003, 10, 155–171. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.H. Molecular targets of anti-HIV-1 triterpenes. Curr. Drug Targets Infect. Disord. 2002, 2, 33–36. [Google Scholar] [CrossRef]
- Fan, B.Z.; Wang, Y.X.; Lian, X.T.; Xie, W.S.; Yu, Y.; Liang, J.H. Structure-activity relationships and mechanisms of triterpenoids against virus. CIESC J. 2020, 71, 4071–4101. [Google Scholar] [CrossRef]
- Harada, S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem. J. 2005, 392 Pt 1, 191–199. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Quéré, L.; Wenger, T.; Schramm, H.J. Triterpenes as potential dimerization inhibitors of HIV-1 protease. Biochem. Biophys. Res. Commun. 1996, 227, 484–488. [Google Scholar] [CrossRef]
- Yang, X.W.; Zhao, J.; Ma, C.M.; Hattori, M. Inhibition of HIV-1 protease in vitro with escins and ursolic acid derivatives. Chin. J. New Drugs 2007, 16, 366–369. [Google Scholar] [CrossRef]
- Ohigashi, H.; Takamura, H.; Koshimizu, K.; Tokuda, H.; Ito, Y. Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation; ursolic acid and oleanolic acid from an anti-inflammatory Chinese medicinal plant, Glechoma hederaceae L. Cancer Lett. 1986, 30, 143–151. [Google Scholar] [CrossRef]
- Yanuar, A.; Suhartanto, H.; Munim, A.; Anugraha, B.H.; Syahdi, R.R. Virtual Screening of Indonesian Herbal Database as HIV-1 Protease Inhibitor. Bioinformation 2014, 10, 52–55. [Google Scholar] [CrossRef]
- Xiao, W.L.; Li, R.T.; Li, S.H.; Li, X.L.; Sun, H.D.; Zheng, Y.T.; Wang, R.R.; Lu, Y.; Wang, C.; Zheng, Q.T. Lancifodilactone F: A novel nortriterpenoid possessing a unique skeleton from Schisandra lancifolia and its anti-HIV activity. Org. Lett. 2005, 7, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.L.; Tian, R.R.; Pu, J.X.; Li, X.; Wu, L.; Lu, Y.; Li, S.H.; Li, R.T.; Zheng, Y.T.; Zheng, Q.T.; et al. Triterpenoids from Schisandra lancifolia with anti-HIV-1 activity. J. Nat. Prod. 2006, 69, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Song, H.C.; Wang, C.R.; Shen, K.Z.; Xu, Y.B.; Gao, Y.X.; Chen, Y.G.; Dong, J.Y. Compounds from Kadsura angustifolia with anti-HIV activity. Bioorg. Med. Chem. Lett. 2011, 21, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Zhang, S.X.; Wang, H.K.; Zhang, S.Y.; Sun, Q.Z.; Cosentino, L.M.; Lee, K.H. Novel anti-HIV lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999, 62, 94–97. [Google Scholar] [CrossRef]
- Xu, L.J.; Peng, Z.G.; Chen, H.S.; Wang, J.; Xiao, P.G. Bioactive triterpenoids from Kadsura heteroclita. Chem. Biodivers. 2010, 7, 2289–2295. [Google Scholar] [CrossRef]
- Esposito, F.; Mandrone, M.; Del Vecchio, C.; Carli, I.; Distinto, S.; Corona, A.; Lianza, M.; Piano, D.; Tacchini, M.; Maccioni, E.; et al. Multi-target activity of Hemidesmus indicus decoction against innovative HIV-1 drug targets and characterization of Lupeol mode of action. Pathog. Dis. 2017, 75, ftx065. [Google Scholar] [CrossRef]
- Huang, Q.X.; Chen, H.F.; Luo, X.R.; Zhang, Y.X.; Yao, X.; Zheng, X. Structure and Anti-HIV Activity of Betulinic Acid Analogues. Curr. Med. Sci. 2018, 38, 387–397. [Google Scholar] [CrossRef]
- Wu, H.F.; Morris-Natschke, S.L.; Xu, X.D.; Yang, M.H.; Cheng, Y.Y.; Yu, S.S.; Lee, K.H. Recent advances in natural anti-HIV triterpenoids and analogs. Med. Res. Rev. 2020, 40, 2339–2385. [Google Scholar] [CrossRef]
- Huang, L.; Ho, P.; Lee, K.H.; Chen, C.H. Synthesis and anti-HIV activity of bi-functional betulinic acid derivatives. Bioorg. Med. Chem. 2006, 14, 2279–2289. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Chrobak, E.; Marciniec, K.; Dąbrowska, A.; Pęcak, P.; Bębenek, E.; Kadela-Tomanek, M.; Bak, A.; Jastrzębska, M.; Boryczka, S. New Phosphorus Analogs of Bevirimat: Synthesis, Evaluation of Anti-HIV-1 Activity and Molecular Docking Study. Int. J. Mol. Sci. 2019, 20, 5209. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Hashimoto, F.; Cosentino, L.M.; Chen, C.H.; Garrett, P.E.; Lee, K.H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J. Med. Chem. 1996, 39, 1016–1017. [Google Scholar] [CrossRef]
- Li, F.; Goila-Gaur, R.; Salzwedel, K.; Kilgore, N.R.; Reddick, M.; Matallana, C.; Castillo, A.; Zoumplis, D.; Martin, D.E.; Orenstein, J.M.; et al. PA-457: A potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc. Natl. Acad. Sci. USA 2003, 100, 13555–13560. [Google Scholar] [CrossRef]
- Marciniec, K.; Chrobak, E.; Dąbrowska, A.; Bębenek, E.; Kadela-Tomanek, M.; Pęcak, P.; Boryczka, S. Phosphate Derivatives of 3-Carboxyacylbetulin: SynThesis, In vitro Anti-HIV and Molecular Docking Study. Biomolecules 2020, 10, 1148. [Google Scholar] [CrossRef]
- Chaniad, P.; Sudsai, T.; Septama, A.W.; Chukaew, A.; Tewtrakul, S. Evaluation of Anti-HIV-1 Integrase and Anti-Inflammatory Activities of Compounds from Betula alnoides Buch-Ham. Adv. Pharmacol. Sci. 2019, 2019, 2573965. [Google Scholar] [CrossRef]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Cano-Muñoz, M.; Martinez, A.; Lupiañez, J.A.; Parra, A. Oleanolic Acid Derivatives as Potential Inhibitors of HIV-1 Protease. J. Nat. Prod. 2019, 82, 2886–2896. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- Sato, N.; Zhang, Q.; Ma, C.M.; Hattori, M. Anti-human immunodeficiency virus-1 protease activity of new lanostane-type triterpenoids from Ganoderma sinense. Chem. Pharm. Bull. 2009, 57, 1076–1080. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, X.Y.; Zhu, P.C.; Tong, Q.; Zheng, G.Q.; Wang, Y. Ginsenoside Rb1 for Myocardial Ischemia/Reperfusion Injury: Preclinical Evidence and Possible Mechanisms. Oxid. Med. Cell. Longev. 2017, 2017, 6313625. [Google Scholar] [CrossRef]
- Jeong, J.J.; Kim, B.; Kim, D.H. Ginsenoside Rb1 eliminates HIV-1 (D3)-transduced cytoprotective human macrophages by inhibiting the AKT pathway. J. Med. Food 2014, 17, 849–854. [Google Scholar] [CrossRef]
- Jeong, J.J.; Kim, B.; Kim, D.H. Ginsenoside Rh1 eliminates the cytoprotective phenotype of human immunodeficiency virus type 1-transduced human macrophages by inhibiting the phosphorylation of pyruvate dehydrogenase lipoamide kinase isozyme 1. Biol. Pharm. Bull. 2013, 36, 1088–1094. [Google Scholar] [CrossRef]
- Callies, O.; Bedoya, L.M.; Beltrán, M.; Muñoz, A.; Calderón, P.O.; Osorio, A.A.; Jiménez, I.A.; Alcamí, J.; Bazzocchi, I.L. Isolation, Structural Modification, and HIV Inhibition of Pentacyclic Lupane-Type Triterpenoids from Cassine xylocarpa and Maytenus cuzcoina. J. Nat. Prod. 2015, 78, 1045–1055. [Google Scholar] [CrossRef]
- Cui, J.J.; Han, Y.S.; Zhou, B.; Yue, J.M. Ursane and 24-Noroleanane-Type Triterpenoids with Anti-HIV Activity from the Twigs and Leaves of Antirhea chinensis. Chem. Biodivers. 2022, e202200716. [Google Scholar] [CrossRef]
- Rahim, A.; Saito, Y.; Miyake, K.; Goto, M.; Chen, C.H.; Alam, G.; Morris-Natschke, S.; Lee, K.H.; Nakagawa-Goto, K. Kleinhospitine E and Cycloartane Triterpenoids from Kleinhovia hospita. J. Nat. Prod. 2018, 81, 1619–1627. [Google Scholar] [CrossRef]
- Sakai-Sugino, K.; Uematsu, J.; Kamada, M.; Taniguchi, H.; Suzuki, S.; Yoshimi, Y.; Kihira, S.; Yamamoto, H.; Kawano, M.; Tsurudome, M.; et al. Glycyrrhizin inhibits human parainfluenza virus type 2 replication by the inhibition of genome RNA, mRNA and protein syntheses. Drug Discov. Ther. 2017, 11, 246–252. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhou, H.X.; Qi, W.B.; Ning, Z.Y.; Ma, Y.J.; Li, Y.L.; Wang, G.C.; Chen, J.X. Antiviral effects of the combination of glycyrrhizin and ribavirin against influenza A H1N1 virus infection in vivo. Acta Pharm. Sin. 2015, 50, 966–972. [Google Scholar]
- Liang, S.; Li, M.; Yu, X.; Jin, H.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019, 166, 328–338. [Google Scholar] [CrossRef]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef]
- Song, G.; Shen, X.; Li, Y.; Zheng, Y.; Xiong, P.; Liu, S. 3-O-β-chacotriosyl benzyl ursolate inhibits entry of H5N1 influenza virus into target cells. J. South Med. Univ. 2015, 35, 789–794. [Google Scholar] [CrossRef]
- Fu, X.L.; Fan, D.; Liu, Y.X.; Fang, B.; Liu, W.J.; Tian, Y.B.; Huang, Y.M. Anti-influenza virus effects and mechanise of glycryrrhizin. Chin. J. Vet. Sci. 2020, 40, 330–335. [Google Scholar] [CrossRef]
- Dong, W.; Farooqui, A.; Leon, A.J.; Kelvin, D.J. Inhibition of influenza A virus infection by ginsenosides. PLoS ONE 2017, 12, e0171936. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Zhu, Y.; Si, L.; Zhang, B.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis of a Hexavalent Betulinic Acid Derivative as a Hemagglutinin-Targeted Influenza Virus Entry Inhibitor. Mol. Pharm. 2020, 17, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.K.; Li, P.L.; Qiao, D.; Zhang, X.W.; Chu, M.J.; Qin, G.F.; Tang, X.L.; Li, G.Q. Cytotoxic and Antiviral Triterpenoids from the Mangrove Plant Sonneratia paracaseolaris. Molecules 2017, 22, 1319. [Google Scholar] [CrossRef] [PubMed]
- Iannarella, R.; Lattanzi, C.; Cannata, G.; Argentiero, A.; Neglia, C.; Fainardi, V.; Pisi, G.; Esposito, S. Coronavirus infections in children: From SARS and MERS to COVID-19, a narrative review of epidemiological and clinical features. Acta Bio Med. Atenei Parm. 2020, 91, e2020032. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Veesler, D. Structural insights into coronavirus entry. Adv. Virus Res. 2019, 105, 93–116. [Google Scholar] [CrossRef]
- Jacobs, J.J.L. Persistent SARS-2 infections contribute to long COVID-19. Med. Hypotheses 2021, 149, 110538. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Cinatl, J., Jr.; Michaelis, M.; Hoever, G.; Preiser, W.; Doerr, H.W. Development of antiviral therapy for severe acute respiratory syndrome. Antivir. Res. 2005, 66, 81–97. [Google Scholar] [CrossRef]
- Chrzanowski, J.; Chrzanowska, A.; Graboń, W. Glycyrrhizin: An old weapon against a novel coronavirus. Phytother. Res. 2021, 35, 629–636. [Google Scholar] [CrossRef]
- Ahmad, S.; Waheed, Y.; Abro, A.; Abbasi, S.W.; Ismail, S. Molecular screening of glycyrrhizin-based inhibitors against ACE2 host receptor of SARS-CoV-2. J. Mol. Model. 2021, 27, 206. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2021, 85, 153364. [Google Scholar] [CrossRef]
- Srivastava, V.; Yadav, A.; Sarkar, P. Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS-CoV2. Mater. Today Proc. 2022, 49, 2999–3007. [Google Scholar] [CrossRef]
- Tang, C.; Ding, H.; Sun, Y.; Han, Z.; Kong, L. A narrative review of COVID-19: Magnesium isoglycyrrhizinate as a potential adjuvant treatment. Ann. Palliat. Med. 2021, 10, 4777–4798. [Google Scholar] [CrossRef]
- Li, J.L.; Yang, L.J.; Zhou, H.L.; Lin, K.Y.; Liang, Q.T.; He, W.; Zhao, Z.M.; Pan, H.F. Mechanism of sovereign medicines in Sanren Decoction on COVID-19 based on network pharmacology and molecular docking. Chin. Tradit. Herb. Drugs 2020, 51, 2345–2353. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Sharif, A.M.; Azhar, E.I.; Rateb, M.E. Olive-Derived Triterpenes Suppress SARS COV-2 Main Protease: A Promising Scaffold for Future Therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef]
- Soltane, R.; Chrouda, A.; Mostafa, A.; Al-Karmalawy, A.A.; Chouaïb, K.; Dhahri, A.; Pashameah, R.A.; Alasiri, A.; Kutkat, O.; Shehata, M.; et al. Strong Inhibitory Activity and Action Modes of Synthetic Maslinic Acid Derivative on Highly Pathogenic Coronaviruses: COVID-19 Drug Candidate. Pathogens 2021, 10, 623. [Google Scholar] [CrossRef]
- Crance, J.M.; Lévêque, F.; Biziagos, E.; van Cuyck-Gandré, H.; Jouan, A.; Deloince, R. Studies on mechanism of action of glycyrrhizin against hepatitis A virus replication in vitro. Antivir. Res. 1994, 23, 63–76. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, B.H.; Lee, S.; Choi, C. Reduction of hepatitis A virus on FRhK-4 cells treated with Korean red ginseng extract and ginsenosides. J. Food Sci. 2013, 78, M1412–M1415. [Google Scholar] [CrossRef]
- Jiang, K.; Qu, S.J.; Tan, C.H. Research progress in natural inhibitors against hepatitis B and C viruses. Prog. Pharm. Sci. 2016, 40, 30–41. [Google Scholar]
- Matsuo, K.; Takenaka, K.; Shimomura, H.; Fujii, N.; Shinagawa, K.; Kiura, K.; Harada, M. Lamivudine and glycyrrhizin for treatment of chemotherapy-induced hepatitis B virus (HBV) hepatitis in a chronic HBV carrier with non-Hodgkin lymphoma. Leuk. Lymphoma 2001, 41, 191–195. [Google Scholar] [CrossRef]
- Dai, J.J.; Tao, H.M.; Min, Q.X.; Zhu, Q.H. Anti-hepatitis B virus activities of friedelolactones from Viola diffusa Ging. Phytomedicine 2015, 22, 724–729. [Google Scholar] [CrossRef]
- Li, Z.J. The Synthesis of Seco-A-Pentacyclic Triterpene Derivatives and Their Anti-HBV Activities In Vitro; Southern Medical University: Guangzhou, China, 2018. [Google Scholar]
- Zhang, J.; Chen, J.Z.; Zhang, J.P.; Jia, X.; Jiang, W.F. Inhibitory effect of astragaloside IV on human hepatitis B virus replication in vitro. Negative 2007, 24, 2291–2293. [Google Scholar] [CrossRef]
- Kang, L.J.; Choi, Y.J.; Lee, S.G. Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1 signalling by ginsenoside Rg3 attenuates hepatitis B virus replication. Int. J. Biochem. Cell Biol. 2013, 45, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Yuan, Q.; Cui, Q.; Liu, C.; Zhou, Z.; Zhao, H.; Dun, Y.; Wang, T.; Zhang, C. Vaccine adjuvant ginsenoside Rg1 enhances immune responses against hepatitis B surface antigen in mice. Can. J. Physiol. Pharmacol. 2016, 94, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Q.; Zhang, Z.; Peng, Y.; Qiu, Y.; Shi, Y.; Zheng, Y.; Xiao, S.; Wang, H.; Huang, X.; et al. Development of oleanane-type triterpenes as a new class of HCV entry inhibitors. J. Med. Chem. 2013, 56, 4300–4319. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, Q.; Si, L.; Shi, Y.; Wang, H.; Yu, F.; Zhang, Y.; Li, Y.; Zheng, Y.; Zhang, C.; et al. Synthesis and anti-HCV entry activity studies of β-cyclodextrin-pentacyclic triterpene conjugates. ChemMedChem 2014, 9, 1060–1070. [Google Scholar] [CrossRef]
- Sun, R.N.; Zhang, Y.N.; Wang, J.; Liu, H.J.; Kong, L.B. Active components of Ligustrum lucidum inhibiting hepatitis C virus replicase activity. Acta Pharm. Sin. 2013, 48, 1390–1396. [Google Scholar] [CrossRef]
- Kim, S.J.; Jang, J.Y.; Kim, E.J.; Cho, E.K.; Ahn, D.G.; Kim, C.; Park, H.S.; Jeong, S.W.; Lee, S.H.; Kim, S.G.; et al. Ginsenoside Rg3 restores hepatitis C virus-induced aberrant mitochondrial dynamics and inhibits virus propagation. Hepatology 2017, 66, 758–771. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chen, B.T.; Zhu, Q.H.; Chen, G.B.; Wei, L.B.; Xiong, B. In vitro antirotaviral effects of quercetin and 2α-hydroxyursolic acid extracted from Psidium guajava leaves. Chin. Tradit. Herb. Drugs 2005, 36, 76–79. [Google Scholar] [CrossRef]
- Tohmé, M.J.; Giménez, M.C.; Peralta, A.; Colombo, M.I.; Delgui, L.R. Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents 2019, 54, 601–609. [Google Scholar] [CrossRef]
- Yang, H.; Oh, K.H.; Kim, H.J.; Cho, Y.H.; Yoo, Y.C. Ginsenoside-Rb2 and 20(S)-Ginsenoside-Rg3 from Korean Red Ginseng Prevent Rotavirus Infection in Newborn Mice. J. Microbiol. Biotechnol. 2018, 28, 391–396. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, D.W.; Li, R. Conserved Herpesvirus Protein Kinases Target SAMHD1 to Facilitate Virus Replication. Cell Rep. 2019, 28, 449–459.e445. [Google Scholar] [CrossRef]
- Dargan, D.J.; Subak-Sharpe, J.H. The antiviral activity against herpes simplex virus of the triterpenoid compounds carbenoxolone sodium and cicloxolone sodium. J. Antimicrob. Chemother. 1986, 18 (Suppl. B), 185–200. [Google Scholar] [CrossRef]
- Huang, W.; Chen, X.; Li, Q.; Li, P.; Zhao, G.; Xu, M.; Xie, P. Inhibition of intercellular adhesion in herpex simplex virus infection by glycyrrhizin. Cell Biochem. Biophys. 2012, 62, 137–140. [Google Scholar] [CrossRef]
- Lin, J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antivir. Res. 2003, 59, 41–47. [Google Scholar] [CrossRef]
- Laconi, S.; Madeddu, M.A.; Pompei, R. Autophagy activation and antiviral activity by a licorice triterpene. Phytother. Res. 2014, 28, 1890–1892. [Google Scholar] [CrossRef]
- Lampi, G.; Deidda, D.; Pinza, M.; Pompei, R. Enhancement of anti-herpetic activity of glycyrrhizic acid by physiological proteins. Antivir. Chem. Chemother. 2001, 12, 125–131. [Google Scholar] [CrossRef]
- Curreli, F.; Friedman-Kien, A.E.; Flore, O. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J. Clin. Investig. 2005, 115, 642–652. [Google Scholar] [CrossRef]
- Cohen, J.I. Licking latency with licorice. J. Clin. Investig. 2005, 115, 591–593. [Google Scholar] [CrossRef]
- Wright, S.; Altman, E. Inhibition of Herpes Simplex Viruses, Types 1 and 2, by Ginsenoside 20(S)-Rg3. J. Microbiol. Biotechnol. 2020, 30, 101–108. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, J.; Xu, X.G.; Su, H.L.; Xing, W.M.; Zhang, Z.S.; Jin, W.H.; Dai, J.H.; Wang, Y.Z.; He, X.Y.; et al. Inhibitory effects of piceatannol on human cytomegalovirus (hCMV) in vitro. J. Microbiol. 2020, 58, 716–723. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Liu, T.; Fang, J.; Wan, J.; Zhao, J.; Li, W.; Liu, J.; Zhao, X.; Chen, S. Anti-viral effects of urosolic acid on guinea pig cytomegalovirus in vitro. J. Huazhong Univ. Sci. Technol. 2012, 32, 883–887. [Google Scholar] [CrossRef]
- Zhao, J.J.; Chen, S.H.; Fang, J.G.; Wan, J.; Liu, T.; Li, W.; Chen, J.J.; Zhao, J.H.; Liu, J. Antiviral effects of oleanolic acid on guniea cytomegalovirus in vitro. Lishizhen Med. Mater. Med. Res. 2011, 22, 2862–2864. [Google Scholar] [CrossRef]
- Heinemann, M.; Bigdon, E.; Veletzky, L.; Jordan, S.; Jochum, J.; Knospe, V.; Schmiedel, S.; Ramharter, M. Case Report: Acute Vision Loss in a Young Returning Traveler with Dengue Fever. Am. J. Trop. Med. Hyg. 2020, 103, 2026–2028. [Google Scholar] [CrossRef]
- Loe, M.W.C.; Hao, E.; Chen, M.; Li, C.; Lee, R.C.H.; Zhu, I.X.Y.; Teo, Z.Y.; Chin, W.X.; Hou, X.; Deng, J.; et al. Betulinic acid exhibits antiviral effects against dengue virus infection. Antivir. Res. 2020, 184, 104954. [Google Scholar] [CrossRef]
- Ji, X.M.; Liang, Y.H.; He, Z.M.; Zhou, Y.; Xiao, W.; Xu, Q.H.; Xu, Y.; Lai, W.H.; Zhang, J.K.; Zhang, L.; et al. The antiviral effect of Qingkailing injection against Dengue virus type 1 in vitro. China Trop. Med. 2021, 21, 107–111. [Google Scholar] [CrossRef]
- Pu, J.; He, L.; Xie, H.; Wu, S.; Li, Y.; Zhang, P.; Yang, Z.; Huang, X. Antiviral activity of Carbenoxolone disodium against dengue virus infection. J. Med. Virol. 2017, 89, 571–581. [Google Scholar] [CrossRef]
- Peyrat, L.A.; Eparvier, V.; Eydoux, C.; Guillemot, J.C.; Litaudon, M.; Stien, D. Betulinic Acid, The First Lupane-Type Triterpenoid Isolated from Both a Phomopsis sp. and Its Host Plant Diospyros carbonaria Benoist. Chem. Biodivers. 2017, 14, e1600171. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Lee, K.E.; Dwivedi, V.D.; Yadava, U.; Panwar, A.; Lucas, S.J.; Pandey, A.; Kang, S.G. Discovery of Ganoderma lucidum triterpenoids as potential inhibitors against Dengue virus NS2B-NS3 protease. Sci. Rep. 2019, 9, 19059. [Google Scholar] [CrossRef]
- Maimaitiming, A.; Wu, Y.Z.; Xu, W.; Hu, C.; Chen, J.; Zhou, D.H.; Xiao, K.; Dong, X.P.; Shi, Q. The therapeutic role of Mitochondria-targeted antioxidants Mitoquinone and asiatic acid in scrapie infected cell line SMB-S15. Chin. J. Virol. 2019, 35, 247–254. [Google Scholar] [CrossRef]
- Dong, Y.; Zhan, P.; Liu, X.Y. New progress in anti-norovirus drugs and vaccines. Acta Pharm. Sin. 2020, 55, 640–651. [Google Scholar] [CrossRef]
- Hua, Y.W.; Zhu, L.Y.; Lin, J.R. Effects of four different Chinese medicine monomers on the levels of TNF-α, IL-1 β, IL-6 and PKR / PKR in norovirus infected enteritis mice. J. Liaoning Univ. Tradit. Chin. Med. 2021, 23, 24–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Huang, C.; Cui, X.; Gao, Y.; Huang, Y.; Gong, W.; Zhao, Y.; Guo, S. Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-gamma. J. Cardiovasc. Pharmacol. 2006, 47, 190–195. [Google Scholar] [CrossRef]
- Shang, L.; Qu, Z.Y.; Ning, L.L.; Wang, Y.C.; Wei, F.X.; Gao, H.; Lu, W.J. The anti-adenovirus effect of astragaloside IV in vitro. Chin. Pharmacol. Bul. 2013, 29, 854–858. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Wang, L.H.; Lian, G.W.; Lin, Z.F.; Li, Y.H.; Guo, M.; Chen, Y.; Liu, X.M.; Zhu, B. Characterization of lymphocyte subsets in peripheral blood cells of children with EV71 infection. J. Microbiol. Immunol. Infect. 2020, 53, 705–714. [Google Scholar] [CrossRef]
- Kang, N.; Gao, H.; He, L.; Liu, Y.; Fan, H.; Xu, Q.; Yang, S. Ginsenoside Rb1 is an immune-stimulatory agent with antiviral activity against enterovirus 71. J. Ethnopharmacol. 2021, 266, 113401. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, L.; Ma, S.; Liu, Y. Anti-Enterovirus 71 Agents of Natural Products. Molecules 2015, 20, 16320–16333. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, J.; Yang, X.; Yang, Z.; Zhang, L.; Liu, H.; Wu, K.; Wu, J. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem. Biophys. Res. Commun. 2014, 449, 307–312. [Google Scholar] [CrossRef]
- Duan, E.; Wang, D.; Fang, L.; Ma, J.; Luo, J.; Chen, H.; Li, K.; Xiao, S. Suppression of porcine reproductive and respiratory syndrome virus proliferation by glycyrrhizin. Antivir. Res. 2015, 120, 122–125. [Google Scholar] [CrossRef]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 2020, 16, e1906206. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Yi, H.Y.; Ma, J.; Wei, Y.F.; Cai, M.K.; Li, Q.; Qin, C.X.; Chen, Y.J.; Han, X.L.; Zhong, R.T.; et al. Ginsenoside Rg1 Suppresses Type 2 PRRSV Infection via NF-κB Signaling Pathway In vitro, and Provides Partial Protection against HP-PRRSV in Piglet. Viruses 2019, 11, 1045. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Wu, L.; Zhang, M.; Gao, Y.; Wang, H.; Xu, D.; Chen, W.; Song, G.; Chen, J. Ursolic acid derivatives are potent inhibitors against porcine reproductive and respiratory syndrome virus. RSC Adv. 2020, 10, 22783–22796. [Google Scholar] [CrossRef]
- Liu, J.D.; Zhang, J.F.; Liu, J.K.; Zheng, H.F. Progress in research on human respiratory syncytial virus vaccine. Chin. J. Biol. 2021, 34, 244–248. [Google Scholar] [CrossRef]
- Yeh, C.F.; Wang, K.C.; Chiang, L.C.; Shieh, D.E.; Yen, M.H.; Chang, J.S. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 148, 466–473. [Google Scholar] [CrossRef]




| Compounds | Targets | Cells | References |
|---|---|---|---|
| 1 | Replication inhibitors | PLC/PRF/5 cells | [78] |
| 29; 32 | - | Fetal rhesus monkey kidney (FRhK-4) cells | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, L.; Wang, H.; Xiong, Y. Recent Advances in Antiviral Activities of Triterpenoids. Pharmaceuticals 2022, 15, 1169. https://doi.org/10.3390/ph15101169
Liu Y, Yang L, Wang H, Xiong Y. Recent Advances in Antiviral Activities of Triterpenoids. Pharmaceuticals. 2022; 15(10):1169. https://doi.org/10.3390/ph15101169
Chicago/Turabian StyleLiu, Yue, Liangyu Yang, Hong Wang, and Yongai Xiong. 2022. "Recent Advances in Antiviral Activities of Triterpenoids" Pharmaceuticals 15, no. 10: 1169. https://doi.org/10.3390/ph15101169
APA StyleLiu, Y., Yang, L., Wang, H., & Xiong, Y. (2022). Recent Advances in Antiviral Activities of Triterpenoids. Pharmaceuticals, 15(10), 1169. https://doi.org/10.3390/ph15101169







