Exosomes in Parkinson: Revisiting Their Pathologic Role and Potential Applications
Abstract
1. Introduction
2. Overview of Extracellular Vesicles
2.1. EXOs Biogenesis
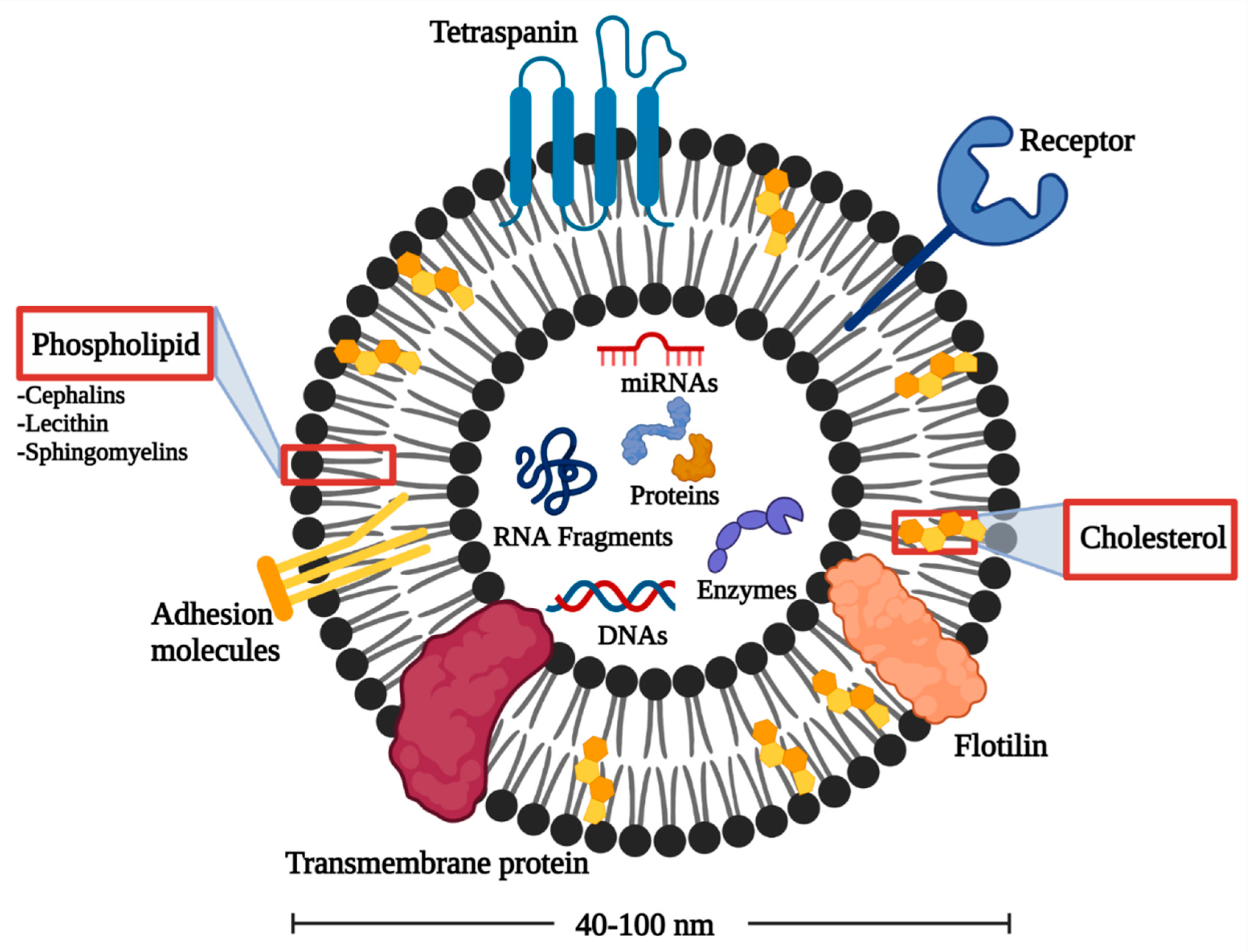
2.2. EXOs Structure
2.3. Isolation of EXOs
3. Pathogenesis of PD
3.1. The α-Syn Role
3.2. The Role of EXOs in Spreading α-Syn
4. EXOs as a Future Approach for PD Diagnosis
| Source | Potential Biomarkers | Findings | p-Value | Patient N | Ref |
|---|---|---|---|---|---|
| Plasma | CNS-derived EXOs α-syn | ↑ | p = 0.004 | 267 PD, 215 controls | [88] |
| miR-331-5p | ↑ | p < 0.05 | 52 PD, 48 controls | [102] | |
| miR-505 | ↓ | ||||
| Serum | Pigmented epithelium-derived factor, Afamin, apolipoprotein D, and J | ↑ | p < 0.05 | 20 PD, 10 controls | [78] |
| Complement C1q | ↓ | ||||
| miR-19b | ↓ | p < 0.05 | 109 PD, 40 controls | [61] | |
| miR-24, miR-195 | ↑ | ||||
| CSF | α-syn | ↓ | p < 0.05 | 76 PD, 58 controls | [103] |
| miR-1 and miR-19b-3p | ↓ | p < 0.05 | 47 PD, 27 controls | [86] | |
| miR-153, miR-409-3p, miR-10a-5p, and let-7g-3p | ↑ | ||||
| Urine | SerP-1292 LRRK2/total LRRK2 ratio | ↑ | p = 0.0014 | 79 PD, 79 controls | [89] |
| Saliva | S100-A16, ARP2/3, and VPS4B | ↓ | p < 0.05 | 24 PD, 15 controls | [89] |
5. Treatment of PD
5.1. Challenges of PD Treatment
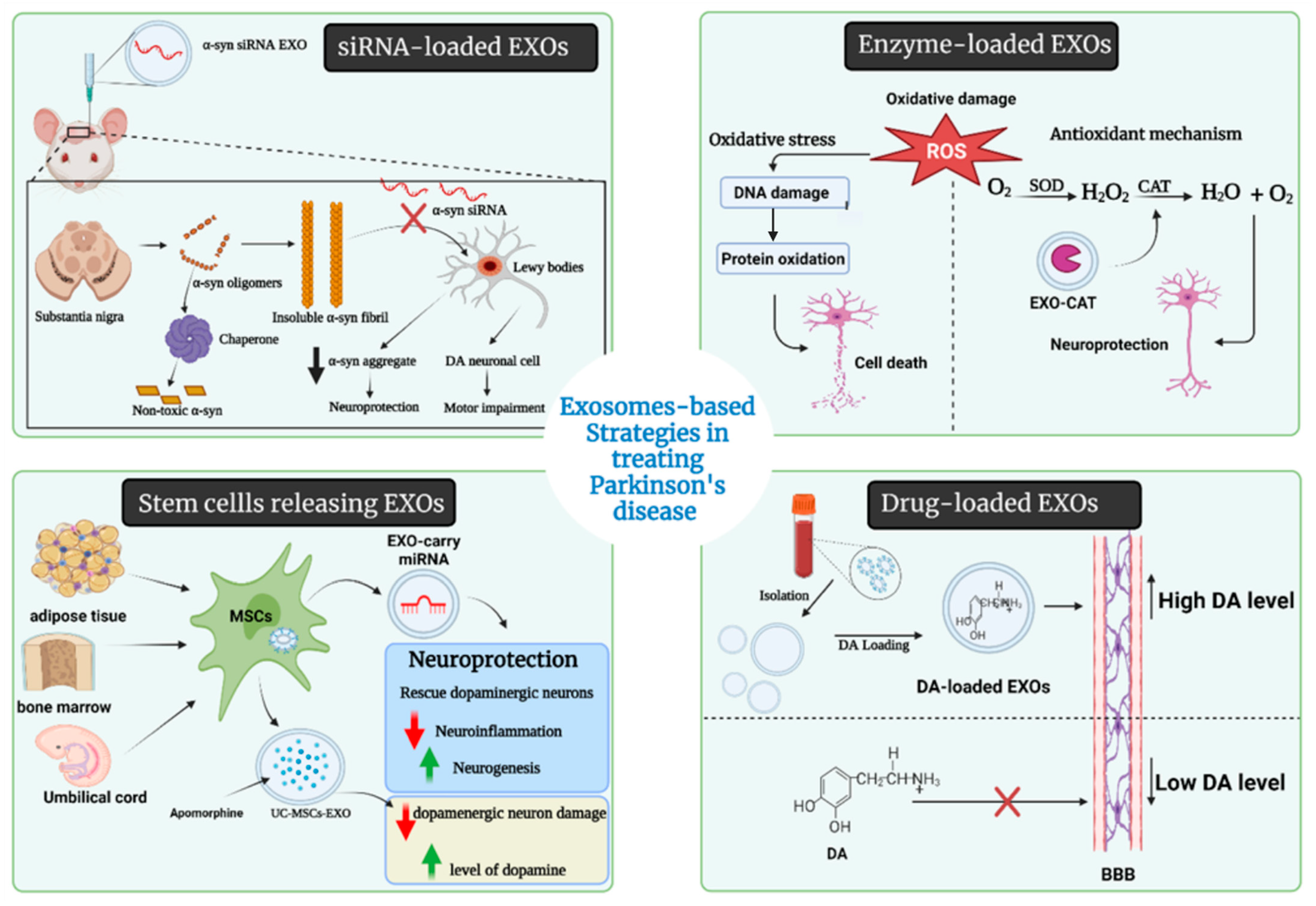
5.2. Therapeutic Aspects of EXOs in PD
5.2.1. Drug-Loaded EXOs
5.2.2. Enzyme-Loaded EXOs
5.2.3. EXOs-Loaded Short Hairpin (sh-), si-, and miRNA Molecules
5.2.4. EXOs as Nanoscavengers
5.3. Stem Cells-Derived EXOs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emard, J.F.; Thouez, J.P.; Gauvreau, D. Neurodegenerative Diseases and Risk Factors: A Literature Review. Soc. Sci. Med. 1995, 40, 847–858. Available online: https://www.sciencedirect.com/science/article/pii/027795369400138J (accessed on 15 July 2021). [CrossRef]
- Ingenta Connect. Current Limitations in the Treatment of Parkinson’s and Alzheimer. Available online: https://www.ingentaconnect.com/content/ben/cmc/2018/00000025/00000041/art00011 (accessed on 15 July 2021).
- Katzenschlager, R.; Lees, A.J. Treatment of Parkinson’s Disease: Levodopa as the First Choice. J. Neurol. 2002, 249 (Suppl. 2), II19–II24. [Google Scholar] [CrossRef]
- Bartel, W.P.; Van Laar, V.S.; Burton, E.A. Chapter 23—Parkinson’s Disease. In Behavioral and Neural Genetics of Zebrafish; Gerlai, R.T., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 377–412. ISBN 978-0-12-817528-6. [Google Scholar]
- Sonne, J.; Reddy, V.; Beato, M.R. Neuroanatomy, Substantia Nigra. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Veljkovic, E.; Xia, W.; Phillips, B.; Wong, E.T.; Ho, J.; Oviedo, A.; Hoeng, J.; Peitsch, M. (Eds.) Chapter 1—Parkinson’s Disease. In Nicotine and Other Tobacco Compounds in Neurodegenerative and Psychiatric Diseases; Academic Press: Cambridge, MA, USA, 2018; pp. 3–12. ISBN 978-0-12-812922-7. [Google Scholar]
- Reisberg, B. Dementia: Overview. In International Encyclopedia of the Social & Behavioral Sciences; Smelser, N.J., Baltes, P.B., Eds.; Pergamon: Oxford, UK, 2001; pp. 3389–3396. ISBN 978-0-08-043076-8. [Google Scholar]
- Gandhi, K.R.; Saadabadi, A. Levodopa (L-Dopa). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Schulz-Schaeffer, W.J. The Synaptic Pathology of α-Synuclein Aggregation in Dementia with Lewy Bodies, Parkinson’s Disease and Parkinson’s Disease Dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef]
- Bahbah, E.I.; Ghozy, S.; Attia, M.S.; Negida, A.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Abdel-Daim, M.M.; Uddin, M.; Simal-Gandara, J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar. Drugs 2021, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Kumari, U.; Tan, E.K. LRRK2 in Parkinson’s Disease: Genetic and Clinical Studies from Patients. FEBS J. 2009, 276, 6455–6463. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Q.; Tan, L.; Yu, J.-T. The Role of the LRRK2 Gene in Parkinsonism. Mol. Neurodegener. 2014, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.S.; Trindade, D.; Vaz, M.; Campelo, I.; Almeida, M.; Trigo, G.; da Cruz e Silva, O.A.B.; Henriques, A.G. Diagnostic and Therapeutic Potential of Exosomes in Alzheimer’s Disease. J. Neurochem. 2021, 156, 162–181. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E. Extracellular Vesicles as Mediators of Neuron-Glia Communication. Front. Cell. Neurosci. 2013, 7, 182. Available online: https://www.frontiersin.org/articles/10.3389/fncel.2013.00182/full?utm_source=newsletter&utm_medium=web&utm_campaign=Neuroscience-w46-2013 (accessed on 15 July 2021). [CrossRef] [PubMed]
- Krämer-Albers, E.-M.; Bretz, N.; Tenzer, S.; Winterstein, C.; Möbius, W.; Berger, H.; Nave, K.-A.; Schild, H.; Trotter, J. Oligodendrocytes Secrete Exosomes Containing Major Myelin and Stress-Protective Proteins: Trophic Support for Axons? Proteom. Clin. Appl. 2007, 1, 1446–1461. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of Exosomal Proteins in Cancer Diagnosis. Mol. Cancer 2017, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-S.; Yang, X.-L.; Xin, R.; Liu, J.-B.; Fu, D. Power and Promise of Exosomes as Clinical Biomarkers and Therapeutic Vectors for Liquid Biopsy and Cancer Control. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188497. [Google Scholar] [CrossRef]
- Si, X.-L.; Fang, Y.-J.; Li, L.-F.; Gu, L.-Y.; Yin, X.-Z.; Tian, J.; Yan, Y.-P.; Pu, J.-L.; Zhang, B.-R. From Inflammasome to Parkinson’s Disease: Does the NLRP3 Inflammasome Facilitate Exosome Secretion and Exosomal Alpha-Synuclein Transmission in Parkinson’s Disease? Exp. Neurol. 2021, 336, 113525. [Google Scholar] [CrossRef]
- Maguire, G. Chapter 7—Exosomes: Smart Nanospheres for Drug Delivery Naturally Produced by Stem Cells. In Fabrication and Self-Assembly of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 179–209. ISBN 978-0-323-41533-0. [Google Scholar]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Exosomes as Drug Delivery Systems: A Brief Overview and Progress Update. Eur. J. Pharm. Biopharm. 2020, 154, 259–269. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ferdous, K.S.; Ahmed, M. Emerging Promise of Nanoparticle-Based Treatment for Parkinson’s Disease. Biointerface Res. Appl. Chem. 2020, 10, 7135–7151. [Google Scholar] [CrossRef]
- Parnham, M.J.; Wetzig, H. Toxicity Screening of Liposomes. Chem. Phys. Lipids 1993, 64, 263–274. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, M.; Xu, J.; Geng, S.; Zhang, Y.; Ye, X.; Gou, J.; Yin, T.; He, H.; Tang, X. Asialoglycoprotein Receptor-Targeted Liposomes Loaded with a Norcantharimide Derivative for Hepatocyte-Selective Targeting. Int. J. Pharm. 2017, 520, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 1–18. Available online: https://cellandbioscience.biomedcentral.com/articles/10.1186/s13578-019-0282-2 (accessed on 25 November 2021). [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as Therapeutic Drug Carriers and Delivery Vehicles across Biological Membranes: Current Perspectives and Future Challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging Roles of Exosomes in Normal and Pathological Conditions: New Insights for Diagnosis and Therapeutic Applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular Vesicles: Lipids as Key Components of Their Biogenesis and Functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of Human Plasma-Derived Exosomal RNAs by Deep Sequencing. BMC Genom. 2013, 14, 1–14. Available online: https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-14-319 (accessed on 25 November 2021). [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional Transfer of MicroRNA-Loaded Exosomes from T Cells to Antigen-Presenting Cells. Nat. Commun. 2011, 2, 1–10. Available online: https://www.nature.com/articles/ncomms1285 (accessed on 25 November 2021). [CrossRef]
- Hewson, C.; Capraro, D.; Burdach, J.; Whitaker, N.; Morris, K.V. Extracellular Vesicle Associated Long Non-Coding RNAs Functionally Enhance Cell Viability. Noncoding RNA Res. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and Properties of a Novel Potential Biomarker for Cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-Speed Centrifugation Induces Aggregation of Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Exosomes in Parkinson Disease—Journal of Neurochemistry—Wiley Online Library. 2021. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/jnc.15288 (accessed on 4 September 2021).
- Alvarez-Erviti, L.; Seow, Y.; Schapira, A.H.; Gardiner, C.; Sargent, I.L.; Wood, M.J.A.; Cooper, J.M. Lysosomal Dysfunction Increases Exosome-Mediated Alpha-Synuclein Release and Transmission. Neurobiol. Dis. 2011, 42, 360–367. [Google Scholar] [CrossRef]
- Theillet, F.-X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural Disorder of Monomeric α-Synuclein Persists in Mammalian Cells. Nature 2016, 530, 45–50. Available online: https://www.nature.com/articles/nature16531 (accessed on 25 November 2021). [CrossRef]
- Kumari, P.; Ghosh, D.; Vanas, A.; Fleischmann, Y.; Wiegand, T.; Jeschke, G.; Riek, R.; Eichmann, C. Structural Insights into α-Synuclein Monomer–Fibril Interactions. Proc. Natl. Acad. Sci. USA 2021, 118, e2012171118. [Google Scholar] [CrossRef]
- Vandendriessche, C.; Bruggeman, A.; Van Cauwenberghe, C.; Vandenbroucke, R.E. Extracellular Vesicles in Alzheimer’s and Parkinson’s Disease: Small Entities with Large Consequences. Cells 2020, 9, 2485. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial Exosomes Facilitate α-Synuclein Transmission in Parkinson’s Disease. Brain 2020, 143, 1476–1497. Available online: https://academic.oup.com/brain/article/143/5/1476/5827587?login=true (accessed on 4 September 2021). [CrossRef]
- Fan, R.Z.; Guo, M.; Luo, S.; Cui, M.; Tieu, K. Exosome Release and Neuropathology Induced by α-Synuclein: New Insights into Protective Mechanisms of Drp1 Inhibition. Acta Neuropathol. Commun. 2019, 7, 184. [Google Scholar] [CrossRef]
- Desplats, P.; Lee, H.-J.; Bae, E.-J.; Patrick, C.; Rockenstein, E.; Crews, L.; Spencer, B.; Masliah, E.; Lee, S.-J. Inclusion Formation and Neuronal Cell Death through Neuron-to-Neuron Transmission of α-Synuclein. Proc. Natl. Acad. Sci. USA 2009, 106, 13010–13015. [Google Scholar] [CrossRef]
- Biological Properties of Extracellular Vesicles and Their Physiological Functions. Available online: https://www.tandfonline.com/doi/full/10.3402/jev.v4.27066 (accessed on 4 September 2021).
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Lang, H.; Geng, N.; Wang, J.; Li, N.; Wang, X. Exosomes of BV-2 Cells Induced by Alpha-Synuclein: Important Mediator of Neurodegeneration in PD. Neurosci. Lett. 2013, 548, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, G.; Han, C.; Ma, K.; Guo, X.; Wan, F.; Kou, L.; Yin, S.; Liu, L.; Huang, J.; et al. Microglia as Modulators of Exosomal Alpha-Synuclein Transmission. Cell Death Dis. 2019, 10, 174. [Google Scholar] [CrossRef]
- Grey, M.; Dunning, C.J.; Gaspar, R.; Grey, C.; Brundin, P.; Sparr, E.; Linse, S. Acceleration of α-Synuclein Aggregation by Exosomes. J. Biol. Chem. 2015, 290, 2969–2982. [Google Scholar] [CrossRef] [PubMed]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-Produced α-Synuclein Is Secreted in a Calcium-Dependent Manner by Exosomes and Impacts Neuronal Survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Poehler, A.-M.; Xiang, W.; Spitzer, P.; May, V.E.L.; Meixner, H.; Rockenstein, E.; Chutna, O.; Outeiro, T.F.; Winkler, J.; Masliah, E.; et al. Autophagy Modulates SNCA/α-Synuclein Release, Thereby Generating a Hostile Microenvironment. Autophagy 2014, 10, 2171–2192. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Cho, E.-D.; Lee, K.W.; Kim, J.-H.; Cho, S.-G.; Lee, S.-J. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 2013, 45, e22. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s Disease: A Target for Neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Brück, D.; Wenning, G.K.; Stefanova, N.; Fellner, L. Glia and Alpha-Synuclein in Neurodegeneration: A Complex Interaction. Neurobiol. Dis. 2016, 85, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Dammer, E.B.; Malovic, E.; Olsen, A.L.; Raza, S.A.; Gao, T.; Xiao, H.; Oliver, D.L.; Duong, D.; Joers, V.; et al. Molecular Signatures of Neuroinflammation Induced by ASynuclein Aggregates in Microglial Cells. Front. Immunol. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.E.; Moore, A.P. Parkinson’s Disease. Am. Fam. Phys. 2007, 75, 1045. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Y.; Lu, J.-M.; Zhao, Z.-Q.; Li, M.-C.; Lu, T.; An, X.-S.; Xue, L.-J. MicroRNA Biomarkers of Parkinson’s Disease in Serum Exosome-like Microvesicles. Neurosci. Lett. 2017, 644, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Parkinson’s Disease—Clinical Evidence Handbook—American Family Physician. Available online: https://www.aafp.org/afp/2007/0401/p1045.html (accessed on 4 August 2021).
- Biomarkers in Parkinson’s Disease: An Update. Current Opinion in Neurology. Available online: https://journals.lww.com/co-neurology/Abstract/2012/08000/Biomarkers_in_Parkinson_s_disease__an_update.12.aspx (accessed on 4 August 2021).
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-Derived Extracellular Vesicles: Neuroreparative Properties and Role in the Pathogenesis of Neurodegenerative Disorders. J. Control. Release 2020, 323, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhang, Z. Activated Microglia Facilitate the Transmission of α-Synuclein in Parkinson’s Disease. Neurochem. Int. 2021, 148, 105094. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Lo Monaco, M.R.; Bentivoglio, A.R.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial-Derived Vesicles as Candidate Biomarkers in Parkinson’s Disease: Rationale, Design and Methods of the EXosomes in PArkiNson Disease (EXPAND) Study. Int. J. Mol. Sci. 2019, 20, 2373. [Google Scholar] [CrossRef] [PubMed]
- Damme, M.; Suntio, T.; Saftig, P.; Eskelinen, E.L. Autophagy in Neuronal Cells: General Principles and Physiological and Pathological Functions. Acta Neuropathol. 2015, 129, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Fader, C.M.; Sánchez, D.; Furlán, M.; Colombo, M.I. Induction of Autophagy Promotes Fusion of Multivesicular Bodies with Autophagic Vacuoles in K562 Cells. Traffic 2008, 9, 230–250. [Google Scholar] [CrossRef]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and Autophagy: Coordinated Mechanisms for the Maintenance of Cellular Fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic Systemic Pesticide Exposure Reproduces Features of Parkinson’s Disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Wu, F.; Xu, H.D.; Guan, J.J.; Hou, Y.S.; Gu, J.H.; Zhen, X.C.; Qin, Z.H. Rotenone Impairs Autophagic Flux and Lysosomal Functions in Parkinson’s Disease. Neuroscience 2015, 284, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.; Marsico, G.; Verbavatz, J.M.; Rodrigo-Angulo, M.; et al. Environmental Toxins Trigger PD-like Progression via Increased Alpha-Synuclein Release from Enteric Neurons in Mice. Sci. Rep. 2012, 2, 898. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on Extracellular Vesicles: Exosomes and Their Role in Protein Trafficking and Biomarker Potential in Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 173. Available online: https://www.mdpi.com/1422-0067/17/2/173/htm (accessed on 16 November 2021). [CrossRef] [PubMed]
- Kim, J.; Inoue, K.; Ishii, J.; Vanti, W.B.; Voronov, S.V.; Murchison, E.; Hannon, G.; Abeliovich, A. A MicroRNA Feedback Circuit in Midbrain Dopamine Neurons. Science 2007, 317, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA Delivery through Nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Akbar, I.; Kumari, B.; Vrati, S.; Basu, A.; Banerjee, A. Japanese Encephalitis Virus Infected Microglial Cells Secrete Exosomes Containing Let-7a/b That Facilitate Neuronal Damage via Caspase Activation. J Neurochem. 2018, 149, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased MiR-124-3p in Microglial Exosomes Following Traumatic Brain Injury Inhibits Neuronal Inflammation and Contributes to Neurite Outgrowth via Their Transfer into Neurons. FASEB J. 2018, 32, 512–528. [Google Scholar] [CrossRef]
- Jiang, R.; Rong, C.; Ke, R.; Meng, S.; Yan, X.; Ke, H.; Wu, S.; Azim, A. Differential Proteomic Analysis of Serum Exosomes Reveals Alterations in Progression of Parkinson Disease. Medicine 2019, 98, e17478. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Vallelunga, A.; Ragusa, M.; Di Mauro, S.; Iannitti, T.; Pilleri, M.; Biundo, R.; Weis, L.; Di Pietro, C.; De Iuliis, A.; Nicoletti, A.; et al. Identification of Circulating MicroRNAs for the Differential Diagnosis of Parkinson’s Disease and Multiple System Atrophy. Front. Cell. Neurosci. 2014, 8, 156. [Google Scholar] [CrossRef]
- Botta-Orfila, T.; Morató, X.; Compta, Y.; Lozano, J.J.; Falgàs, N.; Valldeoriola, F.; Pont-Sunyer, C.; Vilas, D.; Mengual, L.; Fernández, M.; et al. Identification of Blood Serum Micro-RNAs Associated with Idiopathic and LRRK2 Parkinson’s Disease. J. Neurosci. Res. 2014, 92, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Huang, Z.; Chen, M.; Wang, C.; Chen, X.; Chen, J.; Zhang, J. Identification of a Panel of Five Serum MiRNAs as a Biomarker for Parkinson’s Disease. Parkinsonism Relat. Disord. 2016, 22, 68–73. [Google Scholar] [CrossRef]
- Khoo, S.K.; Petillo, D.; Kang, U.J.; Resau, J.H.; Berryhill, B.; Linder, J.; Forsgren, L.; Neuman, L.A.; Tan, A.C. Plasma-Based Circulating MicroRNA Biomarkers for Parkinson’s Disease. J. Parkinson’s Dis. 2012, 2, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of Extracellular MiRNA in Cerebrospinal Fluid and Serum from Patients with Alzheimer’s and Parkinson’s Diseases Correlate with Disease Status and Features of Pathology. PLoS ONE 2014, 9, e94839. [Google Scholar] [CrossRef]
- Cardo, L.F.; Coto, E.; de Mena, L.; Ribacoba, R.; Moris, G.; Menéndez, M.; Alvarez, V. Profile of MicroRNAs in the Plasma of Parkinson’s Disease Patients and Healthy Controls. J. Neurol. 2013, 260, 1420–1422. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered MicroRNA Profiles in Cerebrospinal Fluid Exosome in Parkinson Disease and Alzheimer Disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef]
- Andersen, H.H.; Duroux, M.; Gazerani, P. MicroRNAs as Modulators and Biomarkers of Inflammatory and Neuropathic Pain Conditions. Neurobiol. Dis. 2014, 71, 159–168. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma Exosomal α-Synuclein Is Likely CNS-Derived and Increased in Parkinson’s Disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; West, A.B. Ser(P)-1292 LRRK2 in Urinary Exosomes Is Elevated in Idiopathic Parkinson’s Disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Figura, M.; Sitkiewicz, E.; Świderska, B.; Milanowski, Ł.; Szlufik, S.; Koziorowski, D.; Friedman, A. Proteomic Profile of Saliva in Parkinson’s Disease Patients: A Proof of Concept Study. Brain Sci. 2021, 11, 661. [Google Scholar] [CrossRef]
- Sturchler, E.; Cox, J.A.; Durussel, I.; Weibel, M.; Heizmann, C.W. S100A16, a Novel Calcium-Binding Protein of the EF-Hand Superfamily. J. Biol. Chem. 2006, 281, 38905–38917. [Google Scholar] [CrossRef] [PubMed]
- A Comprehensive Characterisation of the Salivary Proteome of Patients with Parkinson’s Disease—MDS Abstracts. Available online: https://www.mdsabstracts.org/abstract/a-comprehensive-characterisation-of-the-salivary-proteome-of-patients-with-parkinsons-disease/ (accessed on 25 November 2021).
- Serrano, A.; Apolloni, S.; Rossi, S.; Lattante, S.; Sabatelli, M.; Peric, M.; Andjus, P.; Michetti, F.; Carrì, M.T.; Cozzolino, M.; et al. The S100A4 Transcriptional Inhibitor Niclosamide Reduces Pro-Inflammatory and Migratory Phenotypes of Microglia: Implications for Amyotrophic Lateral Sclerosis. Cells 2019, 8, 1261. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Ping, S.; Kyle, M.; Longo, J.; Chin, L.; Zhao, L.-R. S100 Calcium-Binding Protein A9 Knockout Contributes to Neuroprotection and Functional Improvement after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 950–965. [Google Scholar] [CrossRef]
- Kaca-Oryńska, M.; Tomasiuk, R.; Friedman, A. Neuron-Specific Enolase and S 100B Protein as Predictors of Outcome in Ischaemic Stroke. Neurol. Neurochir. Pol. 2010, 44, 459–463. [Google Scholar] [CrossRef]
- Schrank, B.R.; Aparicio, T.; Li, Y.; Chang, W.; Chait, B.T.; Gundersen, G.G.; Gottesman, M.E.; Gautier, J. Nuclear Arp2/3 Drives DNA Break Clustering for Homology-Directed Repair. Nature 2018, 559, 61–66. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-Syntenin-ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Galindez, J.M.; Juwara, L.; Cressatti, M.; Gornitsky, M.; Velly, A.M.; Schipper, H.M. Salivary Heme Oxygenase-1: A Potential Biomarker for Central Neurodegeneration. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029114. [Google Scholar] [CrossRef] [PubMed]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The Enzymatic Conversion of Heme to Bilirubin by Microsomal Heme Oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef]
- Ayuso, P.; Martínez, C.; Pastor, P.; Lorenzo-Betancor, O.; Luengo, A.; Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; Agúndez, J.A.G.; García-Martín, E. An Association Study between Heme Oxygenase-1 Genetic Variants and Parkinson’s Disease. Front. Cell. Neurosci. 2014, 8, 298. [Google Scholar] [CrossRef]
- Cressatti, M.; Galindez, J.M.; Juwara, L.; Orlovetskie, N.; Velly, A.M.; Eintracht, S.; Liberman, A.; Gornitsky, M.; Schipper, H.M. Characterization and Heme Oxygenase-1 Content of Extracellular Vesicles in Human Biofluids. J. Neurochem. 2021, 157, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.F.; Qu, M.W.; Li, G.C.; Zhang, F.B.; Rui, H.C. Circulating exosomal miRNAs as diagnostic biomarkers in Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5278–5283. [Google Scholar]
- Stuendl, A.; Kunadt, M.; Kruse, N.; Bartels, C.; Moebius, W.; Danzer, K.M.; Mollenhauer, B.; Schneide, A. Induction of α-Synuclein Aggregate Formation by CSF Exosomes from Patients with Parkinson’s Disease and Dementia with Lewy Bodies. Brain 2016, 139, 481–494. Available online: https://academic.oup.com/brain/article/139/2/481/1753771?login=true (accessed on 10 October 2021). [CrossRef] [PubMed]
- Olanow, C.W.; Agid, Y.; Mizuno, Y.; Albanese, A.; Bonuccelli, U.; Bonucelli, U.; Damier, P.; De Yebenes, J.; Gershanik, O.; Guttman, M.; et al. Levodopa in the Treatment of Parkinson’s Disease: Current Controversies. Mov. Disord. 2004, 19, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.G.; Shoulson, I.; Fahn, S.; Kieburtz, K.; Lang, A.; Marek, K.; McDermott, M.; Seibyl, J.; Weiner, W.; Musch, B.; et al. Pramipexole vs Levodopa as Initial Treatment for Parkinson Disease: A 4-Year Randomized Controlled Trial. Arch. Neurol. 2004, 61, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- PubMed. A Five-Year Study of the Incidence of Dyskinesia in Patients with Early Parkinson’s Disease Who Were Treated with Ropinirole or Levodopa. Available online: https://pubmed.ncbi.nlm.nih.gov/10816186/ (accessed on 25 November 2021).
- Rinne, U.K.; Bracco, F.; Chouza, C.; Dupont, E.; Gershanik, O.; Marti Masso, J.F.; Montastruc, J.L.; Marsden, C.D. Early Treatment of Parkinson’s Disease with Cabergoline Delays the Onset of Motor Complications. Results of a Double-Blind Levodopa Controlled Trial. The PKDS009 Study Group. Drugs 1998, 55 (Suppl. 1), 23–30. [Google Scholar] [CrossRef]
- Oertel, W.H.; Wolters, E.; Sampaio, C.; Gimenez-Roldan, S.; Bergamasco, B.; Dujardin, M.; Grosset, D.G.; Arnold, G.; Leenders, K.L.; Hundemer, H.-P.; et al. Pergolide versus Levodopa Monotherapy in Early Parkinson’s Disease Patients: The PELMOPET Study. Mov. Disord. 2006, 21, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Wajsbort, J. Post-Menopausal Bleeding after L-DOPA. N. Engl. J. Med. 1972, 286, 784. [Google Scholar] [PubMed]
- Calm, D.; Reid, J. Antiparkinsonian Drugs: Pharmacological and Therapeutic Aspects. Drugs 1972, 4, 49–74. [Google Scholar]
- Calne, D.; Petrie, A.; Rao, S.; Reid, J.; Vakil, S. Action of l-α-Methyldopa-Hydrazine on the Blood Pressure of Patients Receiving Levodopa. Br. J. Pharmacol. 1972, 44, 162. [Google Scholar] [CrossRef]
- Riddoch, D. Gastritis and L-Dopa. Br. Med. J. 1972, 1, 53. [Google Scholar] [CrossRef][Green Version]
- Lieberman, A.; Goodgold, A.; Jonas, S.; Leibowitz, M. Comparison of Dopa Decarboxylase Inhibitor (Carbidopa) Combined with Levodopa and Levodopa Alone in Parkinson’s Disease. Neurology 1975, 25, 911. [Google Scholar] [CrossRef]
- Cedarbaum, J.M. Clinical Pharmacokinetics of Anti-Parkinsonian Drugs. Clin. Pharmacokinet. 1987, 13, 141–178. [Google Scholar] [CrossRef]
- Sasahara, K.; Habara, T.; Morioka, T.; Nakajima, E. Bioavailability of Marketed Levodopa Preparation in Dogs and Parkinsonian Patients. J. Pharm. Sci. 1980, 69, 261–265. [Google Scholar] [CrossRef]
- Yeh, K.; August, T.; Bush, D.; Lasseter, K.; Musson, D.; Schwartz, S.; Smith, M.; Titus, D. Pharmacokinetics and Bioavailability of Sinemet CR: A Summary of Human Studies. Neurology 1989, 39, 25–38. [Google Scholar] [PubMed]
- Lieberman, A.; Estey, E.; Gopinathan, G.; Ohashi, T.; Sauter, A.; Goldstein, M. Comparative Effectiveness of Two Extracerebral DOPA Decarboxylase Inhibitors in Parkinson Disease. Neurology 1978, 28, 964. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.E.; Sisson, T.L.; Ichhpurani, A.K.; Peters, G.R. Steady-state Pharmacokinetic Properties of Pramipexole in Healthy Volunteers. J. Clin. Pharmacol. 1997, 37, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Gill, S.; Samii, A. Comparison of the Risk of Adverse Events with Pramipexole and Ropinirole in Patients with Parkinson’s Disease. Drug Saf. 2003, 26, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kaye, C.M.; Nicholls, B. Clinical Pharmacokinetics of Ropinirole. Clin. Pharmacokinet. 2000, 39, 243–254. [Google Scholar] [CrossRef]
- Titlic, M.; Tonkic, A.; Jukic, I.; Lusic, I.; Dikanovic, M. Side Effects of Ropinirole in Patients with Idiopathic Parkinson’s Disease. Bratisl. Lek. Listy 2008, 109, 273–275. [Google Scholar]
- Batla, A.; Stamelou, M.; Mencacci, N.; Schapira, A.H.; Bhatia, K.P. Ropinirole Monotherapy Induced Severe Reversible Dyskinesias in Parkinson’s Disease. Mov. Disord. 2013, 28, 1159. [Google Scholar] [CrossRef] [PubMed]
- Sanford, M.; Scott, L.J. Rotigotine Transdermal Patch. CNS Drugs 2011, 25, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Ly, A.-V. Rasagiline: A Second-Generation Monoamine Oxidase Type-B Inhibitor for the Treatment of Parkinson’s Disease. Am. J. Health-Syst. Pharm. 2006, 63, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Swope, D.M. Clinical Pharmacology of Rasagiline: A Novel, Second-generation Propargylamine for the Treatment of Parkinson Disease. J. Clin. Pharmacol. 2005, 45, 878–894. [Google Scholar] [CrossRef] [PubMed]
- Azzaro, A.J.; Ziemniak, J.; Kemper, E.; Campbell, B.J.; VanDenBerg, C. Pharmacokinetics and Absolute Bioavailability of Selegiline Following Treatment of Healthy Subjects with the Selegiline Transdermal System (6 Mg/24 h): A Comparison with Oral Selegiline Capsules. J. Clin. Pharmacol. 2007, 47, 1256–1267. [Google Scholar] [CrossRef]
- Heinonen, E.; Myllylä, V. Safety of Selegiline (Deprenyl) in the Treatment of Parkinson’s Disease. Drug Saf. 1998, 19, 11–22. [Google Scholar] [CrossRef]
- Blair, H.A.; Dhillon, S. Safinamide: A Review in Parkinson’s Disease. CNS Drugs 2017, 31, 169–176. [Google Scholar] [CrossRef]
- Heikkinen, H.; Saraheimo, M.; Antila, S.; Ottoila, P.; Pentikäinen, P.J. Pharmacokinetics of Entacapone, a Peripherally Acting Catechol-O-Methyltransferase Inhibitor, in Man. Eur. J. Clin. Pharmacol. 2001, 56, 821–826. [Google Scholar] [CrossRef]
- Brooks, D.J. Safety and Tolerability of COMT Inhibitors. Neurology 2004, 62, S39–S46. [Google Scholar] [CrossRef]
- Assal, F.; Spahr, L.; Hadengue, A.; Rubbici-Brandt, L.; Burkhard, P.R. Tolcapone and Fulminant Hepatitis. Lancet 1998, 352, 958. [Google Scholar] [CrossRef]
- Borges, N. Tolcapone-Related Liver Dysfunction. Drug Saf. 2003, 26, 743–747. [Google Scholar] [CrossRef]
- Dingemanse, J.; Jorga, K.M.; Schmitt, M.; Gieschke, R.; Fotteler, B.; Zürcher, G.; Prada, M.; van Brummelen, P. Integrated Pharmacokinetics and Pharmacodynamics of the Novel Catechol-O-methyltransferase Inhibitor Tolcapone during First Administration to Humans. Clin. Pharmacol. Ther. 1995, 57, 508–517. [Google Scholar] [CrossRef]
- Scott, L.J. Opicapone: A Review in Parkinson’s Disease. Drugs 2016, 76, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Rocha, J.F.; Falcão, A.; Palma, P.N.; Loureiro, A.I.; Pinto, R.; Bonifácio, M.J.; Wright, L.C.; Nunes, T.; Soares-da-Silva, P. Pharmacokinetics, Pharmacodynamics and Tolerability of Opicapone, a Novel Catechol-O-Methyltransferase Inhibitor, in Healthy Subjects. Clin. Pharmacokinet. 2013, 52, 139–151. [Google Scholar] [CrossRef]
- Aoki, F.Y.; Sitar, D.S. Clinical Pharmacokinetics of Amantadine Hydrochloride. Clin. Pharmacokinet. 1988, 14, 35–51. [Google Scholar] [CrossRef]
- Rees, W.; Harkins, J.; Woods, W.; Blown, R.; Lu, M.; Fenger, C.; Holland, R.; Chambers, T.; Tobin, T. Amantadine and Equine Influenza: Pharmacology, Pharmacokinetics and Neurological Effects in the Horse. Equine Vet. J. 1997, 29, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.Y.; Sitar, D.S.; Ogilvie, R.I. Amantadine Kinetics in Healthy Young Subjects after Long-term Dosing. Clin. Pharmacol. Ther. 1979, 26, 729–736. [Google Scholar] [CrossRef]
- Dragašević-Mišković, N.; Petrović, I.; Stanković, I.; Kostić, V.S. Chemical Management of Levodopa-Induced Dyskinesia in Parkinson’s Disease Patients. Expert Opin. Pharmacother. 2019, 20, 219–230. [Google Scholar] [CrossRef]
- Pessoa, R.R.; Moro, A.; Munhoz, R.P.; Teive, H.A.; Lees, A.J. Apomorphine in the Treatment of Parkinson’s Disease: A Review. Arq. Neuro-Psiquiatr. 2018, 76, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, P.; Fowle, A.; Hamilton, M.; Peck, A.; Bye, A.; Dean, K.; Webster, A. Pharmacokinetics and Pharmacodynamics of Procyclidine in Man. Eur. J. Clin. Pharmacol. 1985, 28, 73–78. [Google Scholar] [CrossRef]
- Wisher, D. Martindale: The Complete Drug Reference. J. Med. Libr. Assoc. 2012, 100, 815. [Google Scholar] [CrossRef]
- Begbie, F.; Walker, G.; Kubba, H.; Sabharwal, A. Acute Colonic Pseudo-Obstruction in a Child Taking Trihexyphenidyl for Drooling: Prescribers Beware. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group. Pramipexole vs Levodopa as Initial Treatment for Parkinson Disease: A Randomized Controlled Trial. Parkinson Study Group. JAMA 2000, 284, 1931–1938. [Google Scholar] [CrossRef]
- PubMed. Toxicity of 6-Hydroxydopamine and Dopamine for Dopaminergic Neurons in Culture. Available online: https://pubmed.ncbi.nlm.nih.gov/1977925/ (accessed on 25 November 2021).
- Spencer, J.P.; Jenner, A.; Aruoma, O.I.; Evans, P.J.; Kaur, H.; Dexter, D.T.; Jenner, P.; Lees, A.J.; Marsden, D.C.; Halliwell, B. Intense Oxidative DNA Damage Promoted by L-Dopa and Its Metabolites. Implications for Neurodegenerative Disease. FEBS Lett. 1994, 353, 246–250. [Google Scholar] [CrossRef]
- Mena, M.A.; Pardo, B.; Casarejos, M.J.; Fahn, S.; García de Yébenes, J. Neurotoxicity of Levodopa on Catecholamine-Rich Neurons. Mov. Disord. 1992, 7, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. Is Levodopa Toxic? Neurology 1996, 47, S184–S195. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.H. Levodopa Prolongs Life Expectancy and Is Non-Toxic to Substantia Nigra. Parkinsonism Relat. Disord. 2001, 8, 95–100. [Google Scholar] [CrossRef]
- Hoehn, M.M. The Natural History of Parkinson’s Disease in the Pre-Levodopa and Post-Levodopa Eras. Neurol. Clin. 1992, 10, 331–339. [Google Scholar] [CrossRef]
- Hagenah, J.; Klein, C.; Sieberer, M.; Vieregge, P. Exogenous Levodopa Is Not Toxic to Elderly Subjects with Non-Parkinsonian Movement Disorders: Further Clinical Evidence. J. Neural Transm. 1999, 106, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Urso, D.; Chaudhuri, K.R.; Qamar, M.A.; Jenner, P. Improving the Delivery of Levodopa in Parkinson’s Disease: A Review of Approved and Emerging Therapies. CNS Drugs 2020, 34, 1149–1163. [Google Scholar] [CrossRef]
- Poewe, W.; Antonini, A.; Zijlmans, J.C.; Burkhard, P.R.; Vingerhoets, F. Levodopa in the Treatment of Parkinson’s Disease: An Old Drug Still Going Strong. Clin. Interv. Aging 2010, 5, 229–238. [Google Scholar]
- Titova, N.; Qamar, M.A.; Chaudhuri, K.R. The Nonmotor Features of Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 132, 33–54. [Google Scholar] [CrossRef]
- Non-Oral Dopaminergic Therapies for Parkinson’s Disease: Current Treatments and the Future. NPJ Parkinson’s Dis. 2016, 2, 1–7. Available online: https://www.nature.com/articles/npjparkd201623 (accessed on 25 November 2021).
- Gut Dysfunction in Parkinson’s Disease. Available online: https://www.wjgnet.com/1007-9327/full/v22/i25/5742.htm (accessed on 25 November 2021).
- Chaudhuri, K.R.; Qamar, M.A.; Rajah, T.; Loehrer, P.; Sauerbier, A.; Odin, P.; Jenner, P. Pharmacokinetics of Levodopa: Clinical Neuropharmacology. Available online: https://journals.lww.com/clinicalneuropharm/Citation/1984/03000/Pharmacokinetics_of_Levodopa.2.aspx (accessed on 25 November 2021).
- Eradication of Helicobacter pylori Infection Improves Levodopa Action, Clinical Symptoms and Quality of Life in Patients with Parkinson’s Disease. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0112330 (accessed on 25 November 2021).
- Dingemanse, J. Issues Important for Rational COMT Inhibition. Neurology 2000, 55, S24–S27. [Google Scholar]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood–Brain Barrier Structure and Function and the Challenges for CNS Drug Delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Huang, Y.; Zhang, H.; Lu, H.; Zheng, J.C. Exosomal MiRNAs in Central Nervous System Diseases: Biomarkers, Pathological Mediators, Protective Factors and Therapeutic Agents. Prog. Neurobiol. 2019, 183, 101694. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Peng, H.; Liu, R.; Ji, W.; Shi, Z.; Shen, J.; Ma, G.; Zhang, X. Targeted Exosome Coating Gene-Chem Nanocomplex as “Nanoscavenger” for Clearing α-Synuclein and Immune Activation of Parkinson’s Disease. Sci. Adv. 2020, 6, eaba3967. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes Derived from MiR-188-3p-Modified Adipose-Derived Mesenchymal Stem Cells Protect Parkinson’s Disease. Mol. Ther. Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic Exosomal SiRNA Delivery Reduced Alpha-Synuclein Aggregates in Brains of Transgenic Mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of ShRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-Loaded Blood Exosomes Targeted to Brain for Better Treatment of Parkinson’s Disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, Y.; Xue, F.; Zheng, Y.; Huang, H.; Wang, W.; Chang, Y.; Yang, H.; Zhang, J. Exosomal DNA Aptamer Targeting α-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol. Ther. Nucleic Acids 2019, 17, 726–740. [Google Scholar] [CrossRef]
- Zhao, Y.; Haney, M.J.; Gupta, R.; Bohnsack, J.P.; He, Z.; Kabanov, A.V.; Batrakova, E.V. GDNF-Transfected Macrophages Produce Potent Neuroprotective Effects in Parkinson’s Disease Mouse Model. PLoS ONE 2014, 9, e106867. [Google Scholar] [CrossRef]
- Lotvall, J.; Valadi, H. Cell to Cell Signalling via Exosomes Through EsRNA. Cell Adhes. Migr. 2007, 1, 156–158. [Google Scholar] [CrossRef]
- Jarmalavičiūtė, A.; Pivoriūnas, A. Exosomes as a Potential Novel Therapeutic Tools against Neurodegenerative Diseases. Pharmacol. Res. 2016, 113, 816–822. [Google Scholar] [CrossRef]
- Gangadaran, P.; Ahn, B.-C. Extracellular Vesicle- and Extracellular Vesicle Mimetics-Based Drug Delivery Systems: New Perspectives, Challenges, and Clinical Developments. Pharmaceutics 2020, 12, 442. [Google Scholar] [CrossRef]
- Luo, S.; Du, L.; Cui, Y. Potential Therapeutic Applications and Developments of Exosomes in Parkinson’s Disease. Mol. Pharm. 2020, 17, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Haddad, F.; Sawalha, M.; Khawaja, Y.; Najjar, A.; Karaman, R. Dopamine and Levodopa Prodrugs for the Treatment of Parkinson’s Disease. Molecules 2017, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Colamartino, M.; Padua, L.; Meneghini, C.; Leone, S.; Cornetta, T.; Testa, A.; Cozzi, R. Protective Effects of L-Dopa and Carbidopa Combined Treatments on Human Catecholaminergic Cells. DNA Cell Biol. 2012, 31, 1572–1579. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Shen, S.; Pang, Z.; Jiang, X. Ligand Modified Nanoparticles Increases Cell Uptake, Alters Endocytosis and Elevates Glioma Distribution and Internalization. Sci. Rep. 2013, 3, 2534. [Google Scholar] [CrossRef]
- Kanazawa, T.; Taki, H.; Okada, H. Nose-to-Brain Drug Delivery System with Ligand/Cell-Penetrating Peptide-Modified Polymeric Nano-Micelles for Intracerebral Gliomas. Eur. J. Pharm. Biopharm. 2020, 152, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.S.; Hassaballah, M.Y.; Abdelqawy, M.A.; Mohamed, M.E.; Farag, A.K.; Negida, A.; Ghaith, H.; Emam, S.E. An Updated Review of Mesoporous Carbon as Novel Drug Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Silindir Gunay, M.; Yekta Ozer, A.; Chalon, S. Drug Delivery Systems for Imaging and Therapy of Parkinson’s Disease. Curr. Neuropharmacol. 2016, 14, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Subra, C.; Silvente-Poirot, S.; Poirot, M. Exosomes as Intercellular Signalosomes and Pharmacological Effectors. Biochem. Pharmacol. 2011, 81, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted MiR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting Transferrin Receptors at the Blood-Brain Barrier Improves the Uptake of Immunoliposomes and Subsequent Cargo Transport into the Brain Parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Dunnett, S.B. The Amphetamine Induced Rotation Test: A Re-Assessment of Its Use as a Tool to Monitor Motor Impairment and Functional Recovery in Rodent Models of Parkinson’s Disease. J. Parkinson’s Dis. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Hadinezhad, T.; Fallah, M.; Shahmirzadi, A.R.; Taghizadeh, M.; Behnam, M.; Asemi, Z. The Therapeutic Potential of Quercetin in Parkinson’s Disease: Insights into Its Molecular and Cellular Regulation. Curr. Drug Targets 2020, 21, 509–518. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain Delivery of Quercetin-Loaded Exosomes Improved Cognitive Function in AD Mice by Inhibiting Phosphorylated Tau-Mediated Neurofibrillary Tangles. Drug Deliv. 2020, 27, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of SiRNA. Int. J. Biomed. Sci. IJBS 2017, 13, 48–57. [Google Scholar]
- Lewis, J.; Melrose, H.; Bumcrot, D.; Hope, A.; Zehr, C.; Lincoln, S.; Braithwaite, A.; He, Z.; Ogholikhan, S.; Hinkle, K.; et al. In Vivo Silencing of Alpha-Synuclein Using Naked SiRNA. Mol. Neurodegener. 2008, 3, 19. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Curcumin Affords Neuroprotection and Inhibits α-Synuclein Aggregation in Lipopolysaccharide-Induced Parkinson’s Disease Model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Zharikov, A.D.; Cannon, J.R.; Tapias, V.; Bai, Q.; Horowitz, M.P.; Shah, V.; El Ayadi, A.; Hastings, T.G.; Greenamyre, J.T.; Burton, E.A. ShRNA Targeting α-Synuclein Prevents Neurodegeneration in a Parkinson’s Disease Model. J. Clin. Investig. 2015, 125, 2721–2735. [Google Scholar] [CrossRef] [PubMed]
- Titze-de-Almeida, R.; Titze-de-Almeida, S.S. MiR-7 Replacement Therapy in Parkinson’s Disease. Curr. Gene Ther. 2018, 18, 143–153. [Google Scholar] [CrossRef]
- Meng, X.; Zhong, J.; Zeng, C.; Yung, K.K.L.; Zhang, X.; Wu, X.; Qu, S. MiR-30a-5p Regulates GLT-1 Function via a PKCα-Mediated Ubiquitin Degradation Pathway in a Mouse Model of Parkinson’s Disease. ACS Chem. Neurosci. 2021, 12, 1578–1592. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barceló, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential Disease Biomarkers and New Therapeutic Targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef]
- Soliman, H.M.; Ghonaim, G.A.; Gharib, S.M.; Chopra, H.; Farag, A.K.; Hassanin, M.H.; Nagah, A.; Emad-Eldin, M.; Hashem, N.E.; Yahya, G.; et al. Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics. Int. J. Mol. Sci. 2021, 22, 10794. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Fujimiya, M. Potential Effects of Mesenchymal Stem Cell Derived Extracellular Vesicles and Exosomal MiRNAs in Neurological Disorders. Neural Regen. Res. 2021, 16, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Benmelouka, A.Y.; Munir, M.; Sayed, A.; Attia, M.S.; Ali, M.M.; Negida, A.; Alghamdi, B.S.; Kamal, M.A.; Barreto, G.E.; Ashraf, G.M. Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2258. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, T.; An, J.; Wen, L.; Liu, F.; Bu, Z.; Cui, Y.; Feng, J. Potential Roles of Exosomes in Parkinson’s Disease: From Pathogenesis, Diagnosis, and Treatment to Prognosis. Front. Cell Dev. Biol. 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, P.R.; Zheng, Y.; Fischer, R.; Heidasch, R.; Gardiner, C.; Evetts, S.; Hu, M.; Wade-Martins, R.; Turner, M.R.; Morris, J.; et al. Identification of Distinct Circulating Exosomes in Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2015, 2, 353–361. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lin, M.-C.; Tsai, J.-S.; He, P.-L.; Luo, W.-T.; Chiu, I.-M.; Herschman, H.R.; Li, H.-J. Exosomal 2′,3′-CNP from Mesenchymal Stem Cells Promotes Hippocampus CA1 Neurogenesis/Neuritogenesis and Contributes to Rescue of Cognition/Learning Deficiencies of Damaged Brain. Stem Cells Transl. Med. 2020, 9, 499–517. [Google Scholar] [CrossRef]


| Name/Chemical Structure | Class | Pharmacokinetic Features | Peripheral Side Effects | Ref |
|---|---|---|---|---|
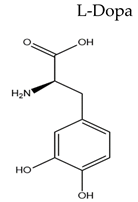 | Dopamine Precursor |
|
| [109,110,111,112,113,114,115] |
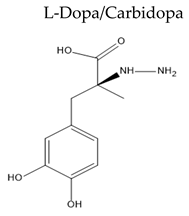 | Carbidopa is a peripheral DOPA decarboxylase inhibitor |
|
| [116,117] |
 | Non-ergoline dopamine agonists |
|
| [118,119] |
 |
|
| [120,121,122] | |
 | Non-selective non-ergoline dopamine agonist |
|
| [123] |
 | MAO B inhibitors |
|
| [124,125] |
 |
|
| [126,127] | |
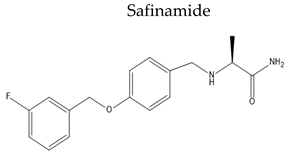 |
|
| [128] | |
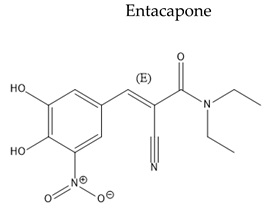 | COMT inhibitors |
|
| [129,130] |
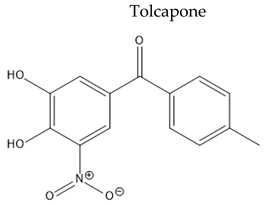 |
|
| [131,132,133] | |
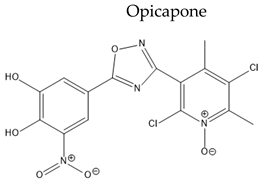 |
|
| [134,135] | |
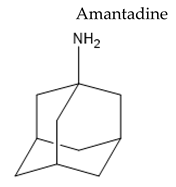 | Weak dopamine agonist with some antimuscarinic activity and N-methyl-D-aspartate antagonist |
|
| [136,137,138,139] |
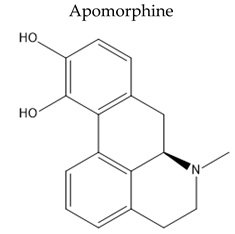 | non-ergoline dopamine D1 and D2 agonist |
|
| [140] |
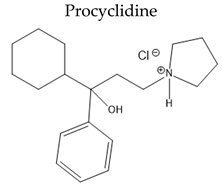 | Antimuscarinic anticholinergic drugs |
|
| [141,142] |
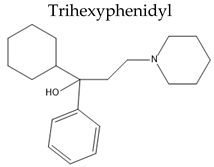 |
|
| [114,143] |
| Cargo | Vesicle Size (nm) | Source | Isolation Method | Loading Method | Therapeutic Efficacy | Ref |
|---|---|---|---|---|---|---|
| CAT | 100–200 | Mouse macrophage | Differential centrifugation followed by filtration | Incubation with or without saponin, freeze-thaw cycle, sonication, or extrusion | Enhanced CAT bioavailability in neuronal cells, therefore, increased therapeutic efficacy and decreased ROS level in the brain | [163] |
| CUR and siRNA molecules | 70 | imDC | Differential centrifugation followed by ultrafiltration and passed through a size exclusion chromatography | Sonication | Observed slowness in movement speed, an improvement in the time to tip of the rod and an immune suppressive effect, an increase in Fox p3 in CD4+ T cells and a decrease in the IL-22 and IL-17 cytokines | [165] |
| miR-188-3p | - | ADSC | Differential centrifugation | Culturing cells with miR-188-3p-overexpressed EXOs | Alleviated the damaged substantia nigra and suppressed the levels of CDK5 and NLRP3 in the PD mice model | [166] |
| siRNA | - | BMDCs | Electroporation | A significant decrease in total α-syn mRNA and protein level | [167] | |
| shRNA-MCs | - | DCs transfected with RVG-Lamp2b. | Reduction in the α-syn aggregation and loss of dopaminergic neurons | [168] | ||
| L-Dopa | 40–200 | Blood of Kunming mice | Incubation | Boosting the brain delivery of DA | [169] | |
| DNA aptamers | 100 | myc-RVG-lamp2b | PFF-induced insoluble α-syn aggregates were reduced, therefore reducing PD progression | [170] | ||
| GDNF | 96.0 ± 9.1 | Macrophages | Enabled GDNF to reach CNS and consequently induced a neuroprotective effect, and reduced inflammation and levels of activated microglia in the targeted regions | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouerdane, Y.; Hassaballah, M.Y.; Nagah, A.; Ibrahim, T.M.; Mohamed, H.A.H.; El-Baz, A.; Attia, M.S. Exosomes in Parkinson: Revisiting Their Pathologic Role and Potential Applications. Pharmaceuticals 2022, 15, 76. https://doi.org/10.3390/ph15010076
Ouerdane Y, Hassaballah MY, Nagah A, Ibrahim TM, Mohamed HAH, El-Baz A, Attia MS. Exosomes in Parkinson: Revisiting Their Pathologic Role and Potential Applications. Pharmaceuticals. 2022; 15(1):76. https://doi.org/10.3390/ph15010076
Chicago/Turabian StyleOuerdane, Yassamine, Mohamed Y. Hassaballah, Abdalrazeq Nagah, Tarek M. Ibrahim, Hosny A. H. Mohamed, Areej El-Baz, and Mohamed S. Attia. 2022. "Exosomes in Parkinson: Revisiting Their Pathologic Role and Potential Applications" Pharmaceuticals 15, no. 1: 76. https://doi.org/10.3390/ph15010076
APA StyleOuerdane, Y., Hassaballah, M. Y., Nagah, A., Ibrahim, T. M., Mohamed, H. A. H., El-Baz, A., & Attia, M. S. (2022). Exosomes in Parkinson: Revisiting Their Pathologic Role and Potential Applications. Pharmaceuticals, 15(1), 76. https://doi.org/10.3390/ph15010076








