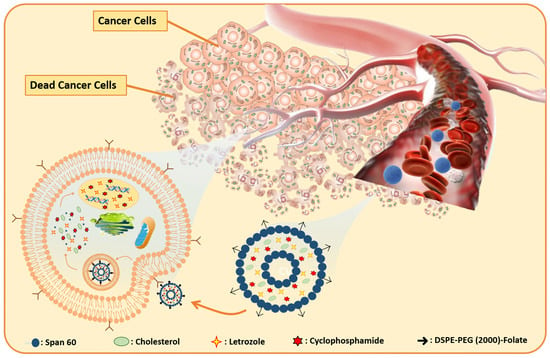
Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression
Abstract
1. Introduction
2. Results and Discussion
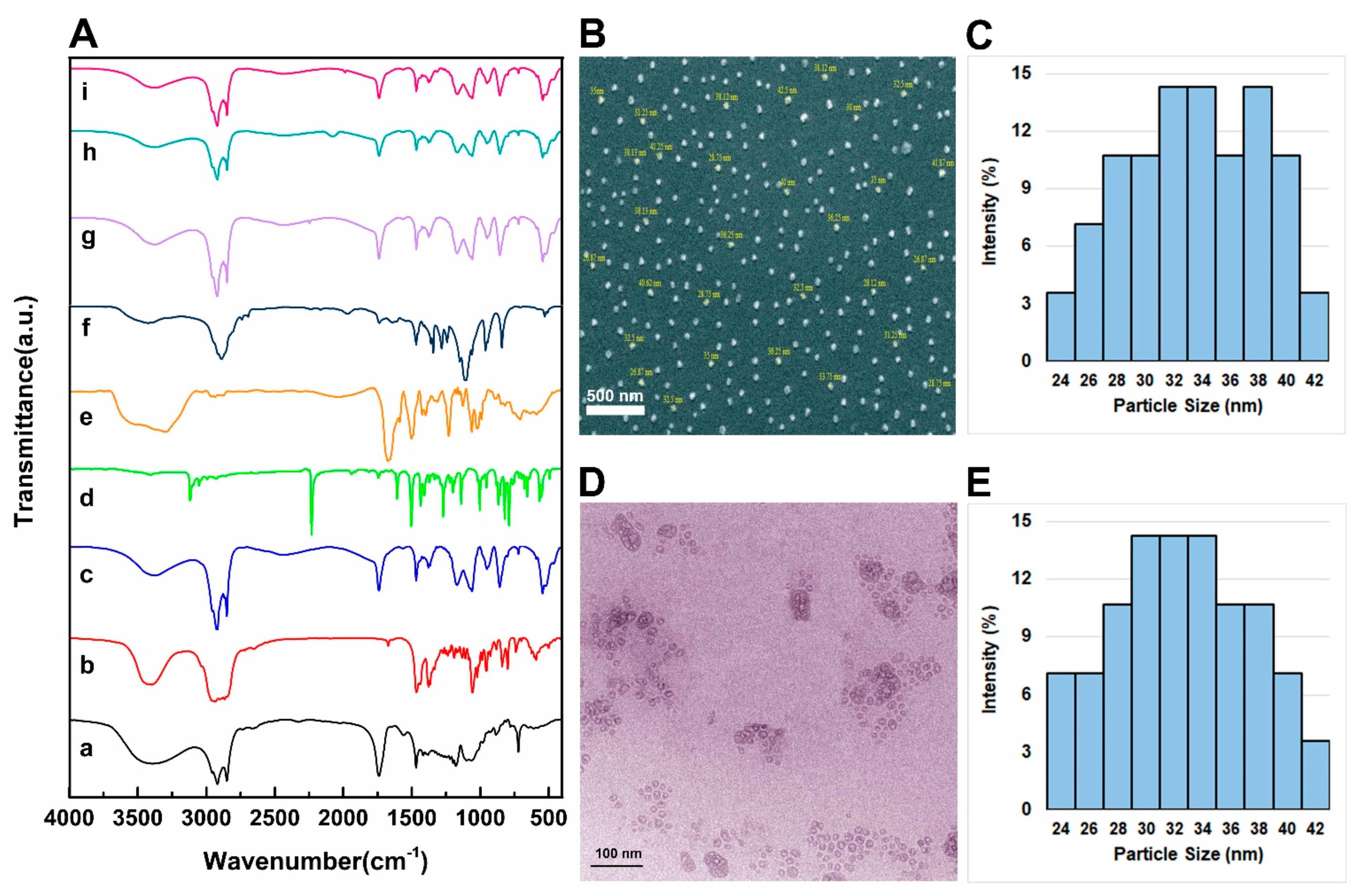
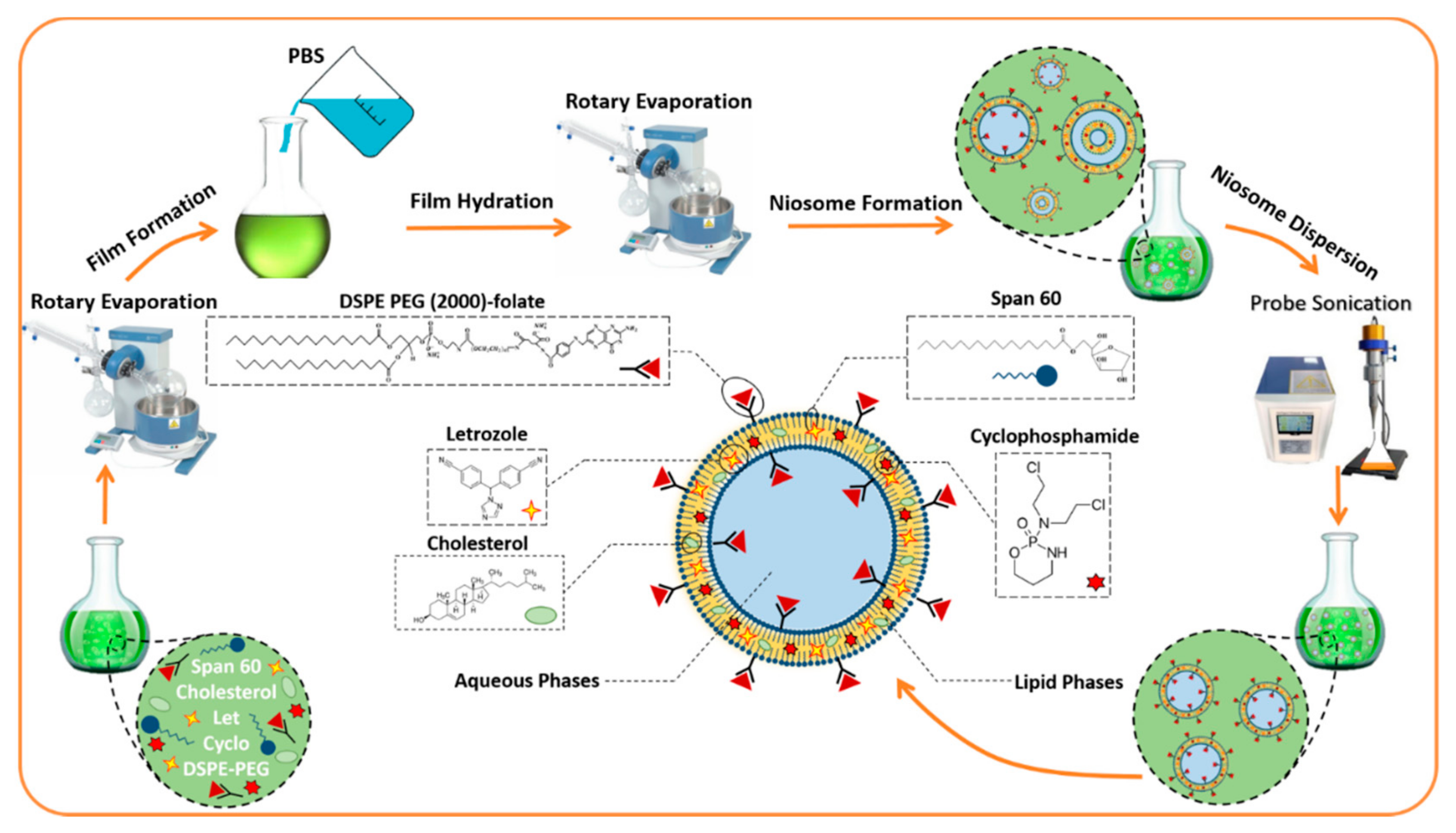
2.1. Noisome Formulations Properties
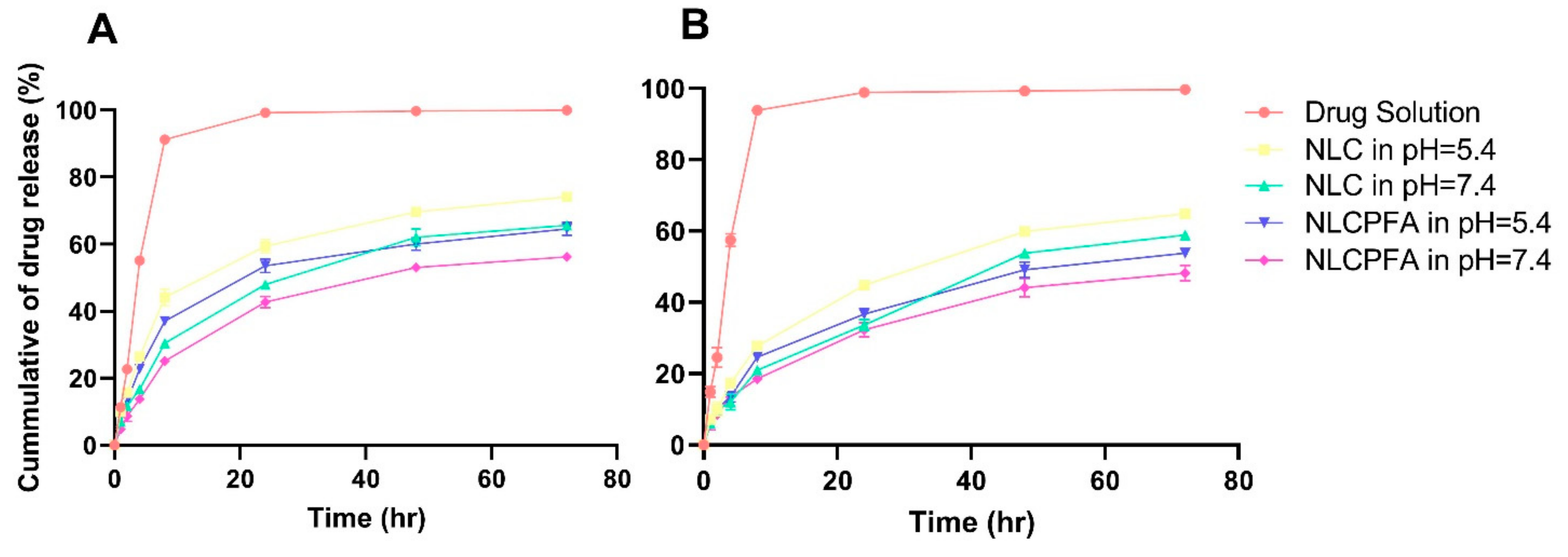
2.2. Release Study
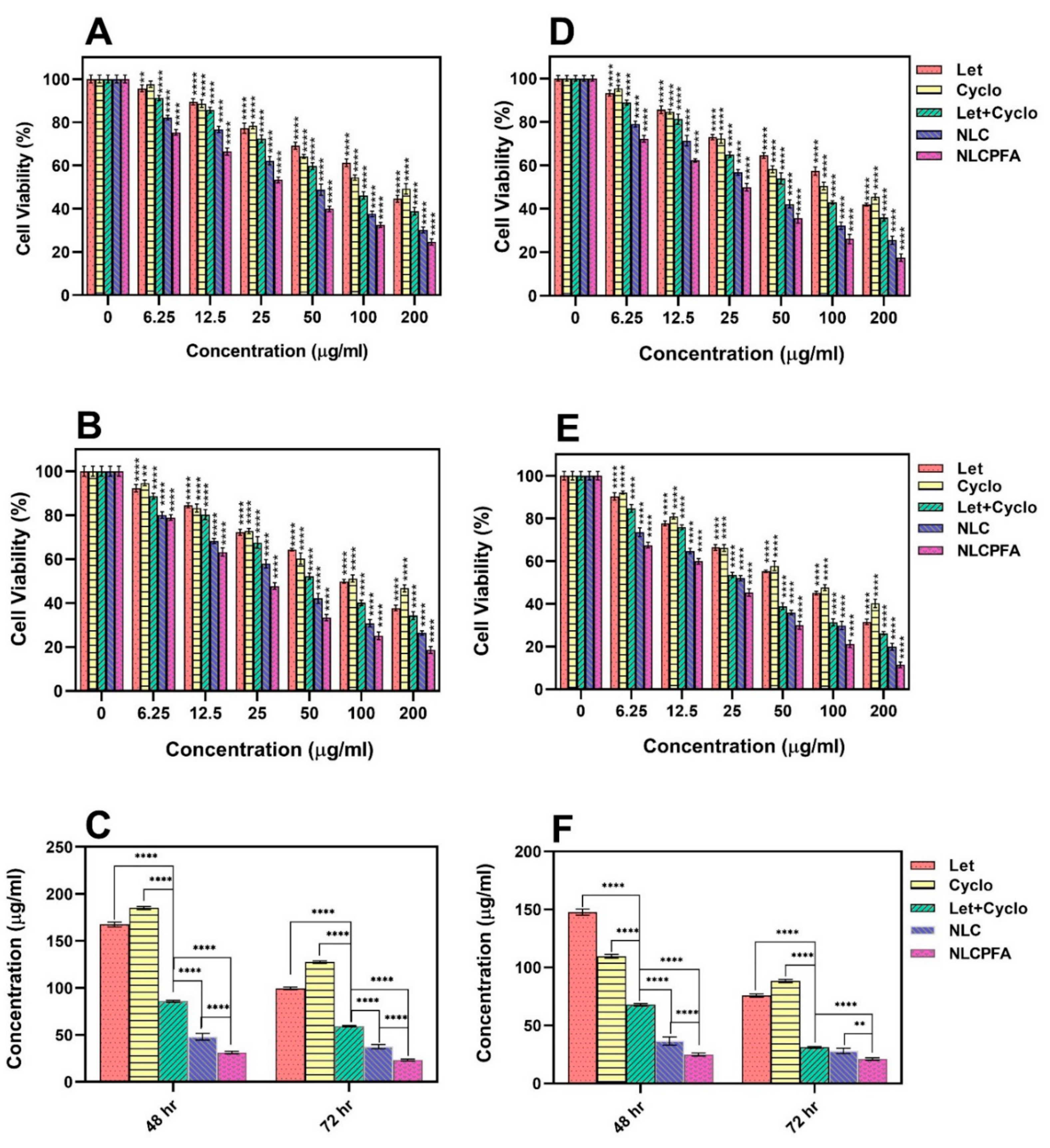
2.3. Cytotoxicity Assay
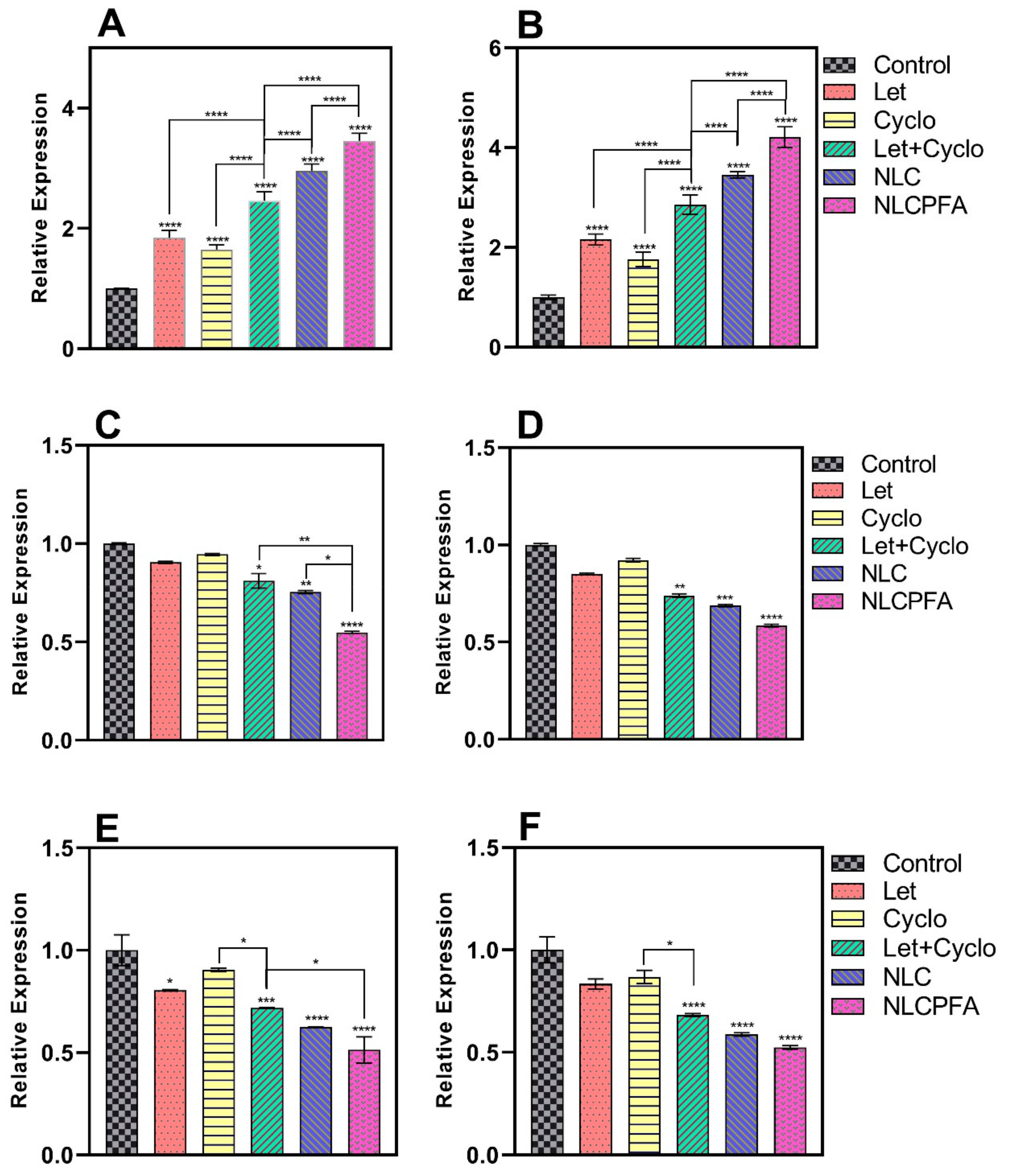
2.4. Gene Expression Analysis
2.5. Apoptosis Analysis
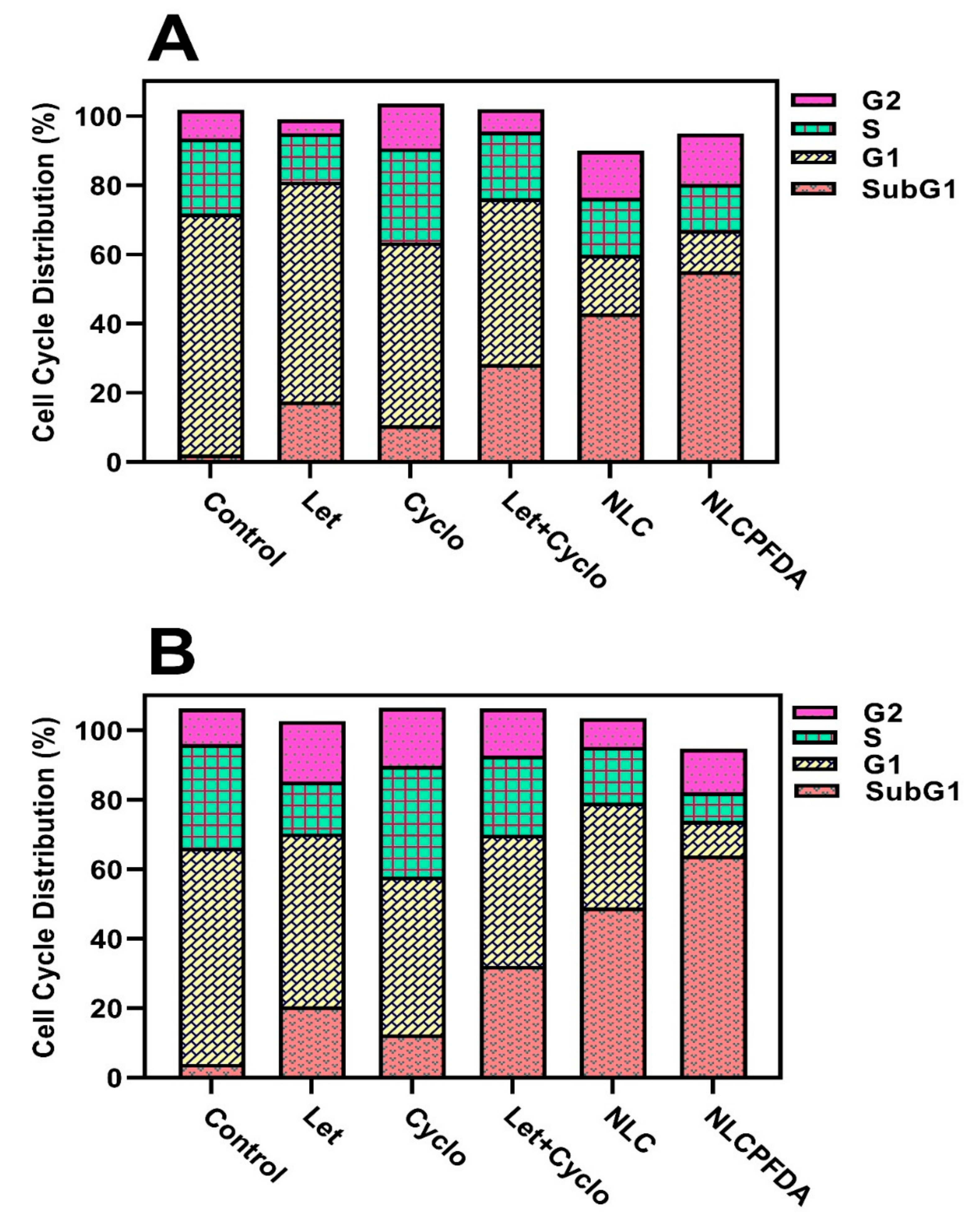
2.6. Cell Cycle Analysis
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Nanoniosomes
3.3. Entrapment Efficiency
3.4. Chemical Structure Analysis
3.5. Size, Polydispersity, and Morphological Investigations
3.6. Drug Release Study
3.7. Cell Culture
3.8. Cytotoxicity Assay
3.9. Gene Expression Analysis
3.10. Flow Cytometry
3.11. Cell Cycle Analysis
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Molani, S.; Madadi, M.; Williams, D. Investigating the effectiveness of breast cancer supplemental screening considering radiologists’ bias. MedRxiv 2020. [Google Scholar] [CrossRef]
- Shirzad, M.; Jamehbozorgi, S.; Akbarzadeh, I.; Aghabozorg, H.R.; Amini, A. The role of polyethylene glycol size in chemical spectra, cytotoxicity, and release of PEGylated nanoliposomal cisplatin. Assay Drug Dev. Technol. 2019, 17, 231–239. [Google Scholar] [CrossRef]
- Boran, G.; Tavakoli, S.; Dierking, I.; Kamali, A.R.; Ege, D. Synergistic effect of graphene oxide and zoledronic acid for osteoporosis and cancer treatment. Sci. Rep. 2020, 10, 7827. [Google Scholar] [CrossRef]
- Targhi, A.A.; Moammeri, A.; Jamshidifar, E.; Abbaspour, K.; Sadeghi, S.; Lamakani, L.; Akbarzadeh, I. Synergistic effect of curcumin-Cu and curcumin-Ag nanoparticle loaded niosome: Enhanced antibacterial and anti-biofilm activities. Bioorganic Chem. 2021, 115, 105–116. [Google Scholar] [CrossRef]
- Khan, R.; Irchhaiya, R. An Overview on Niosomes as Efficient—Google Scholar, (n.d.). Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Khan+R%2C+Irchhaiya+R.+An+overview+on+niosomes+as+efficient+drug+carriers.+Int+J+Pharm+Biosci.+2017%3B8%3A106–116&btnG= (accessed on 8 December 2020).
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Mazzotta, E. Do niosomes have a place in the field of drug delivery? Expert Opin. Drug Deliv. 2019, 16, 1145–1147. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Tavakkoli Yaraki, M.; Bourbour, M.; Noorbazargan, H.; Lajevardi, A.; Sadat Shilsar, S.M.; Heidari, F.; Mousavian, S.M. Optimized doxycycline-loaded niosomal formulation for treatment of infection-associated prostate cancer: An in-vitro investigation. J. Drug Deliv. Sci. Technol. 2020, 57, 101715. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Fatemizadeh, M.; Heidari, F.; Mousavi-Niri, N. Niosomal formulation for co-administration of hydrophobic anticancer drugs into MCF-7 cancer cells. Arch. Adv. Biosci. 2020, 11. [Google Scholar] [CrossRef]
- Mirzaei-Parsa, M.J.; Najafabadi, M.R.H.; Haeri, A.; Zahmatkeshan, M.; Ebrahimi, S.A.; Pazoki-Toroudi, H.; Adel, M. Preparation, characterization, and evaluation of the anticancer activity of artemether-loaded nano-niosomes against breast cancer. Breast Cancer 2020, 27, 243–251. [Google Scholar] [CrossRef]
- Wu, W.; Deng, H.; Rao, N.; You, N.; Yang, Y.; Cao, M.; Liu, J. Neoadjuvant everolimus plus letrozole versus fluorouracil, epirubicin and cyclophosphamide for ER-positive, HER2-negative breast cancer: Study protocol for a randomized pilot trial. Trials 2017, 18, 497. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Zhao, Z.; Yang, Z.; Xu, F.; Lu, H.L.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Journal of Biological Sciences, 2017. Risk Factors and Preventions of Breast Cancer, Ncbi.Nlm.Nih.Gov. (n.d.). Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmc5715522/ (accessed on 8 December 2020).
- Curigliano, G.; Gómez Pardo, P.; Meric-Bernstam, F.; Conte, P.; Lolkema, M.P.; Beck, J.T.; Bardia, A.; Martínez García, M.; Penault-Llorca, F.; Dhuria, S.; et al. Hurvitz, ribociclib plus letrozole in early breast cancer: A presurgical, window-of-opportunity study. Breast 2016, 28, 191–198. [Google Scholar] [CrossRef]
- Ueno, T.; Masuda, N.; Kamigaki, S.; Morimoto, T.; Akiyama, F.; Kurosumi, M.; Tsuda, H.; Mikami, Y.; Tanaka, S.; Morita, S.; et al. A multicenter phase II trial of neoadjuvant letrozole plus low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer (JBCRG-07): Therapeutic efficacy and clinical implications of circulating endothelial cells. Cancer Med. 2018, 7, 2442–2451. [Google Scholar] [CrossRef]
- Pineda-Moncusí, M.; Garcia-Giralt, N.; Diez-Perez, A.; Servitja, S.; Tusquets, I.; Prieto-Alhambra, D.; Nogués, X. Increased fracture risk in women treated with aromatase inhibitors versus tamoxifen: Beneficial effect of bisphosphonates. J. Bone Miner. Res. 2020, 35, 291–297. [Google Scholar] [CrossRef]
- Fantacuzzi, M.; de Filippis, B.; Gallorini, M.; Ammazzalorso, A.; Giampietro, L.; Maccallini, C.; Aturki, Z.; Donati, E.; Ibrahim, R.S.; Shawky, E.; et al. Synthesis, biological evaluation, and docking study of indole aryl sulfonamides as aromatase inhibitors. Eur. J. Med. Chem. 2020, 185, 111815. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xiao, H.; Niu, C.; Wu, H.; Jin, H.; Yao, C.; He, H.; Tian, H.; Han, F.; et al. Adjuvant treatment combining cellular immunotherapy with chemotherapy improves the clinical outcome of patients with stage II/III gastric cancer. Cancer Med. 2017, 6, 45–53. [Google Scholar] [CrossRef]
- Mei, L.; Liu, Y.; Rao, J.; Tang, X.; Li, M.; Zhang, Z.; He, Q. Enhanced tumor retention effect by click chemistry for improved cancer immunochemotherapy. ACS Appl. Mater. Interfaces. 2018, 10, 17582–17593. [Google Scholar] [CrossRef]
- Yang, X.; Hu, C.; Tong, F.; Liu, R.; Zhou, Y.; Qin, L.; Ouyang, L.; Gao, H. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv. Funct. Mater. 2019, 29, 1901896. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Scurr, M.; Pembroke, T.; Bloom, A.; Roberts, D.; Thomson, A.; Smart, K.; Bridgeman, H.; Adams, R.; Brewster, A.; Jones, R.; et al. Godkin, Low-dose cyclophosphamide induces antitumor T-cell responses, which associate with survival in metastatic colorectal cancer. Clin. Cancer Res. 2017, 23, 6771–6780. [Google Scholar] [CrossRef]
- Roghanian, A.; Hu, G.; Fraser, C.; Singh, M.; Foxall, R.B.; Meyer, M.J.; Lees, E.; Huet, H.; Glennie, M.J.; Beers, S.A.; et al. Cyclophosphamide enhances cancer antibody immunotherapy in the resistant bone marrow niche by modulating macrophage FcγR expression. Cancer Immunol. Res. 2019, 7, 1876–1890. [Google Scholar] [CrossRef]
- Oral Metronomic Cyclophosphamide with and without Methotrexate as Palliative Treatment for Patients with Metastatic Breast Carcinoma, (n.d.). Available online: http://ar.iiarjournals.org/content/32/2/529.short (accessed on 9 December 2020).
- Jamshidifar, E.; Eshrati Yeganeh, F.; Shayan, M.; Tavakkoli Yaraki, M.; Bourbour, M.; Moammeri, A.; Akbarzadeh, I.; Noorbazargan, H.; Hossein-Khannazer, N. Super magnetic niosomal nanocarrier as a new approach for treatment of breast cancer: A case study on SK-BR-3 and MDA-MB-231 cell lines. Int. J. Mol. Sci. 2021, 22, 7948. [Google Scholar] [CrossRef]
- Kulkarni, P.; Rawtani, D. Application of Box-Behnken Design in the Preparation, Optimization, and In Vitro Evaluation of Self-assembly–based Tamoxifen-and Doxorubicin-loaded and Dual Drug–loaded Niosomes for Combinatorial Breast Cancer Treatment. J. Pharm. Sci. 2019, 108, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Raei, M.; Hushmandi, K.; Zarrabi, A.; Voelcker, N.H.; Aref, A.R.; et al. Hyaluronic acid-based nanoplatforms for Doxorubicin: A review of stimuli-responsive carriers, co-delivery and resistance suppression. Carbohydr. Polym. 2021, 272, 118491. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V.P. Polymeric co-delivery systems in cancer treatment: An overview on component drugs’ dosage ratio effect. Molecules 2019, 24, 1035. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Akbarzadeh, I.; Tavakkoli Yaraki, M.; Lajevardi, A.; Fatemizadeh, M.; Heidarpoor Saremi, L. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int. J. Pharm. 2019, 569, 118580. [Google Scholar] [CrossRef]
- Azandaryani, A.H.; Kashanian, S.; Shahlaei, M.; Derakhshandeh, M.; Motiei, M.; Moradi, S. A comprehensive physicochemical, in vitro and molecular characterization of letrozole incorporated chitosan-lipid nanocomplex. Pharm. Res. 2019, 36, 1–11. [Google Scholar] [CrossRef]
- Reis-Mendes, A.; Carvalho, F.; Remião, F.; Sousa, E.; de Lourdes Bastos, M.; Costa, V.M. The main metabolites of fluorouracil + adriamycin + cyclophosphamide (FAC) are not major contributors to FAC toxicity in H9c2 cardiac differentiated cells. Biomolecules 2019, 9, 98. [Google Scholar] [CrossRef]
- Knyazev, E.N.; Nikulin, S.V.; Khristichenko, A.Y.; Gerasimenko, T.N.; Kindeeva, O.V.; Petrov, V.A.; Belyakova, G.A.; Maltseva, D.V. Transport and toxicity of 5-fluorouracil, doxorubicin, and cyclophosphamide in in vitro placental barrier model based on BeWo b30 cells. Russ. Chem. Bull. 2019, 68, 2344–2349. [Google Scholar] [CrossRef]
- Mirzaie, A.; Peirovi, N.; Akbarzadeh, I.; Moghtaderi, M.; Heidari, F.; Yeganeh, F.E.; Noorbazargan, H.; Mirzazadeh, S.; Bakhtiari, R. Preparation and optimization of ciprofloxacin encapsulated niosomes: A new approach for enhanced antibacterial activity, biofilm inhibition and reduced antibiotic resistance in ciprofloxacin-resistant methicillin-resistance Staphylococcus aureus. Bioorg. Chem. 2020, 103, 104231. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids. 2018, 133, 239–252. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Mostafa, S.K.; Helmy, M.W.; El Demellawy, M.A.; Sheweita, S.A. Multi-reservoir phospholipid shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapy. Pharm. Res. 2017, 34, 1956–1969. [Google Scholar] [CrossRef]
- Siddiqa, A.J.; Shrivastava, N.K.; Ali Mohsin, M.E.; Abidi, M.H.; Sharaf, M.A.F.; Shaikh, T.A. In vitro release and degradation study of letrozole-loaded poly(lactic-co-glycolic acid) microparticles. JOM 2021, 73, 450–459. [Google Scholar] [CrossRef]
- Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Dorkoosh, F.A.; Handali, S. Folic acid-modified liposomal drug delivery strategy for tumor targeting of 5-fluorouracil. Eur. J. Pharm. Sci. 2018, 114, 166–174. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhao, J.; Li, C.; Zhou, Y.; Du, J.; Wang, Y. In vitro and in vivo evaluation of targeting tumor with folate-based amphiphilic multifunctional stabilizer for resveratrol nanosuspensions. Colloids Surf. B Biointerfaces 2017, 160, 462–472. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Mirzaie, A.; Zabet, N.; Moammeri, A.; Mansoori-Kermani, A.; Akbarzadeh, I.; Ren, Q. Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation. Nanomaterials 2021, 11, 1573. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Shayan, M.; Bourbour, M.; Moghtaderi, M.; Noorbazargan, H.; Eshrati Yeganeh, F.; Saffar, S.; Tahriri, M. Preparation, optimization and in-vitro evaluation of curcumin-loaded niosome@calcium alginate nanocarrier as a new approach for breast cancer treatment. Biology 2021, 10, 173. [Google Scholar] [CrossRef]
- Rinaldi, F.; del Favero, E.; Rondelli, V.; Pieretti, S.; Bogni, A.; Ponti, J.; Rossi, F.; di Marzio, L.; Paolino, D.; Marianecci, C.; et al. Ph-sensitive niosomes: Effects on cytotoxicity and on inflammation and pain in murine models. J. Enzyme Inhib. Med. Chem. 2017, 32, 538–546. [Google Scholar] [CrossRef]
- pH-Sensitive, Polymer Modified, Plasma Stable Niosomes: Promising Carriers for Anti-Cancer Drugs, Ncbi.Nlm.Nih.Gov. (n.d.). Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4553888/ (accessed on 17 March 2021).
- You, L.; Liu, X.; Fang, Z.; Xu, Q.; Zhang, Q. Synthesis of multifunctional Fe3O4@PLGA-PEG nano-niosomes as a targeting carrier for treatment of cervical cancer. Mater. Sci. Eng. C 2019, 94, 291–302. [Google Scholar] [CrossRef]
- 5-Mathematical Models of Drug Release, Google Scholar, (n.d.). Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=5+-+Mathematical+models+of+drug+release%2C+in%3A+M.L.+Bruschi+%28Ed.%29%2C+Strategies+to+Modify+the+Drug+Release+from+Pharmaceutical+Systems%2C+Woodhead+Publishing%2C+2015%2C+pp.+63–86&btnG= (accessed on 17 March 2021).
- Shen, Y.; Du, Y.; Zhang, Y.; Pan, Y. Synergistic effects of combined treatment with simvastatin and exemestane on MCF-7 human breast cancer cells. Mol. Med. Rep. 2015, 12, 456–462. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Aparajay, P.; Dev, A. Functionalized niosomes as a smart delivery device in cancer and fungal infection. Eur. J. Pharm. Sci. 2022, 168, 106052. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Hu, Q.; Bai, X.C.; Huang, W.; Scheres, S.H.W.; Shi, Y. Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme. Proc. Natl. Acad. Sci. USA 2017, 114, 1542–1547. [Google Scholar] [CrossRef]
- Expression of NPAT, a Novel Substrate of Cyclin E–CDK2, Promotes S-Phase Entry, (n.d.). Available online: http://genesdev.cshlp.org/content/12/4/456.short (accessed on 12 May 2021).
- Ries, C.; Pitsch, T.; Mentele, R.; Zahler, S.; Egea, V.; Nagase, H.; Jochum, M. 2007. Identification of a Novel 82 kDa proMMP-9 Species Associated with the Surface of Leukaemic Cells: (Auto-) Catalytic Activation and Resistance to Inhibition by TIMP-1, Portlandpress.Com (n.d.). Available online: https://portlandpress.com/biochemj/article-abstract/405/3/547/42383 (accessed on 14 December 2021).
- Sun, Y.S.; Thakur, K.; Hu, F.; Zhang, J.G.; Wei, Z.J. Icariside II inhibits tumorigenesis via inhibiting AKT/Cyclin E/CDK 2 pathway and activating mitochondria-dependent pathway. Pharmacol. Res. 2020, 152, 104616. [Google Scholar] [CrossRef]
- Fard, S.; Tafvizi, F.; Torbati, M.B. Nanobiotechnology, 2018, Silver Nanoparticles Biosynthesised Using Centella asiatica Leaf Extract: Apoptosis Induction in MCF-7 Breast Cancer Cell Line Ieeexplore.Ieee.Org. (n.d.). Available online: https://ieeexplore.ieee.org/document/8471679/ (accessed on 14 December 2021).


| Sample | Surfactant: Cholesterol (Molar Ratio) | Lipid: Drug (Molar Ratio) | Let (mg) | Cyclo (mg) | Size (nm) | PDI | EE (%) | |
|---|---|---|---|---|---|---|---|---|
| Cyclo | Let | |||||||
| NLC | 1:1 | 10:1 | 10 | 10 | 224.7 ± 6.4 | 0.224 ± 0.009 | 89.60 ± 0.86 | 91.58 ± 0.96 |
| NLC | 2:1 | 10:1 | 10 | 10 | 231.3 ± 7.2 | 0.257 ± 0.011 | 92.75 ± 1.06 | 93.50 ± 1.12 |
| NLC | 1:1 | 20:1 | 10 | 10 | 251.7 ± 5.3 | 0.249 ± 0.010 | 91.39 ± 0.98 | 94.85 ± 1.23 |
| NLC | 2:1 | 20:1 | 10 | 10 | 240.9 ± 4.4 | 0.198 ± 0.008 | 93.95 ± 1.36 | 96.22 ± 1.42 |
| NLCPFA | 2:1 | 20:1 | 10 | 10 | 213.9 ± 3.2 | 0.143 ± 0.007 | 94.10 ± 1.85 | 98.50 ± 1.88 |
| NL | 2:1 | 20:1 | - | - | 186.1 ± 3.1 | 0.158 ± 0.009 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahrayi, H.; Hosseini, E.; Karimifard, S.; Khayam, N.; Meybodi, S.M.; Amiri, S.; Bourbour, M.; Farasati Far, B.; Akbarzadeh, I.; Bhia, M.; et al. Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression. Pharmaceuticals 2022, 15, 6. https://doi.org/10.3390/ph15010006
Sahrayi H, Hosseini E, Karimifard S, Khayam N, Meybodi SM, Amiri S, Bourbour M, Farasati Far B, Akbarzadeh I, Bhia M, et al. Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression. Pharmaceuticals. 2022; 15(1):6. https://doi.org/10.3390/ph15010006
Chicago/Turabian StyleSahrayi, Hamidreza, Elham Hosseini, Sara Karimifard, Nazanin Khayam, Seyed Mohammadmahdi Meybodi, Sahar Amiri, Mahsa Bourbour, Bahareh Farasati Far, Iman Akbarzadeh, Mohammed Bhia, and et al. 2022. "Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression" Pharmaceuticals 15, no. 1: 6. https://doi.org/10.3390/ph15010006
APA StyleSahrayi, H., Hosseini, E., Karimifard, S., Khayam, N., Meybodi, S. M., Amiri, S., Bourbour, M., Farasati Far, B., Akbarzadeh, I., Bhia, M., Hoskins, C., & Chaiyasut, C. (2022). Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression. Pharmaceuticals, 15(1), 6. https://doi.org/10.3390/ph15010006