Influence of Cold-TRP Receptors on Cold-Influenced Behaviour
Abstract
1. Introduction
2. Results
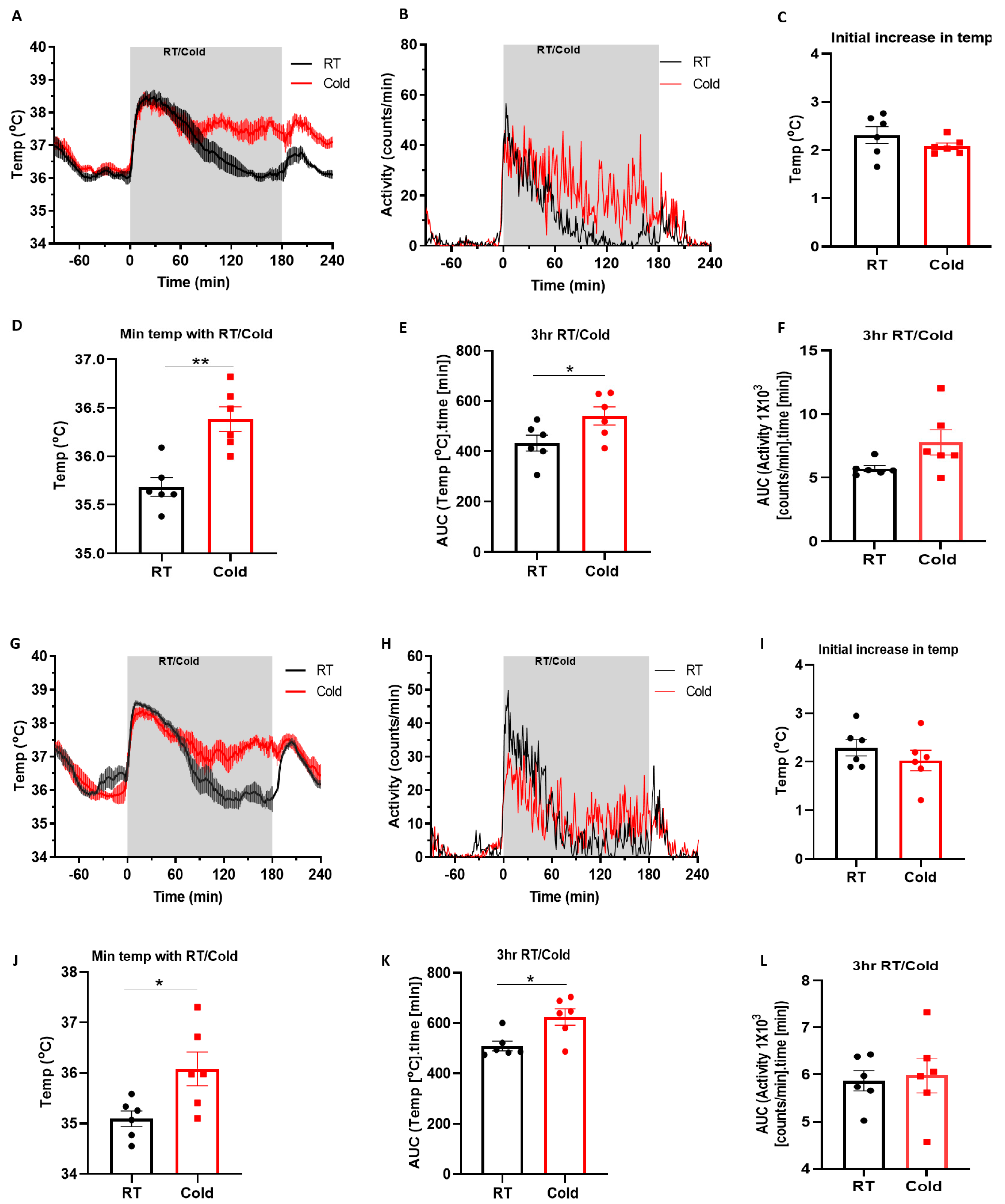
2.1. Cold Induces Increased Core Body Temperature
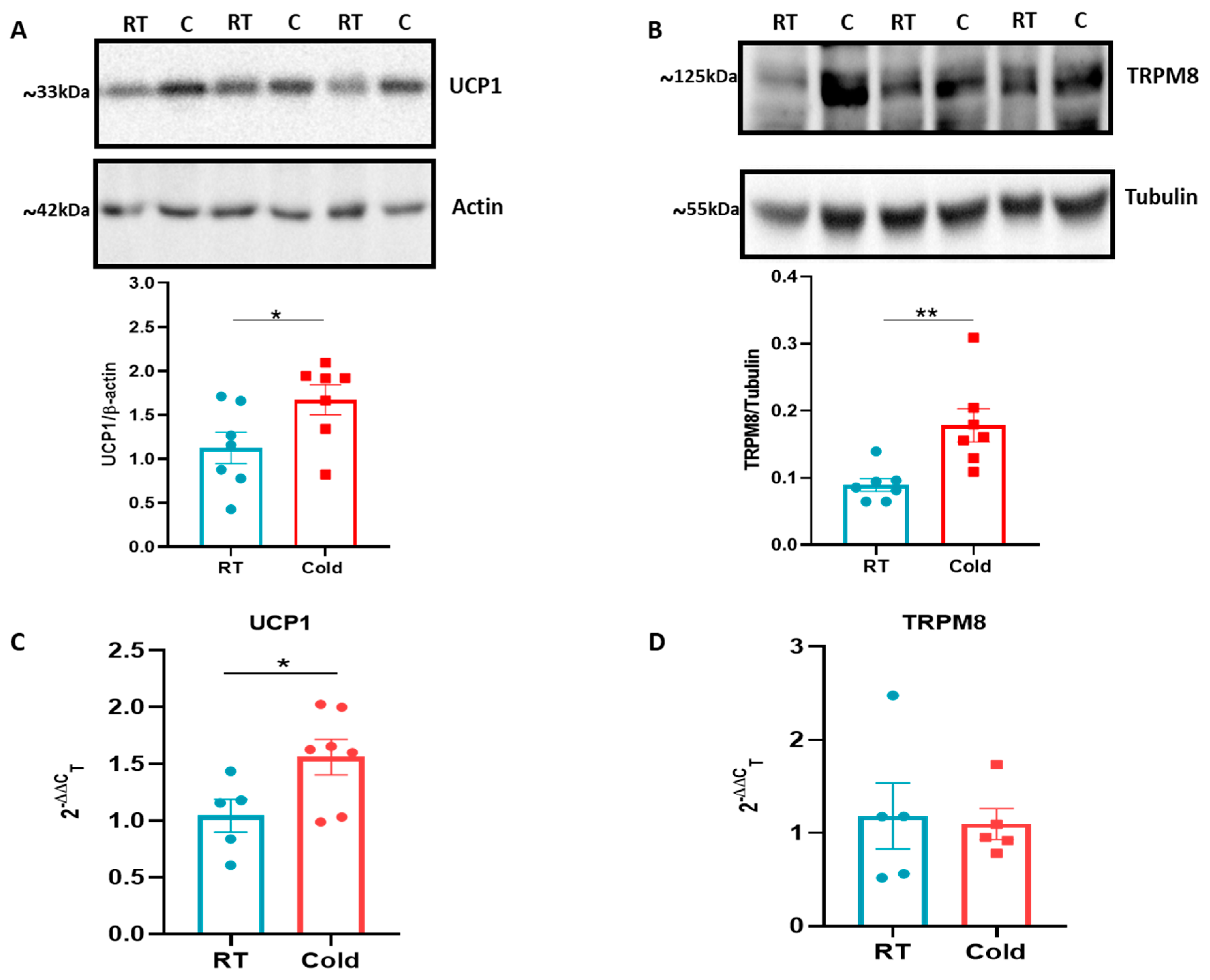
2.2. Cold Upregulates UCP-1 and TRPM8
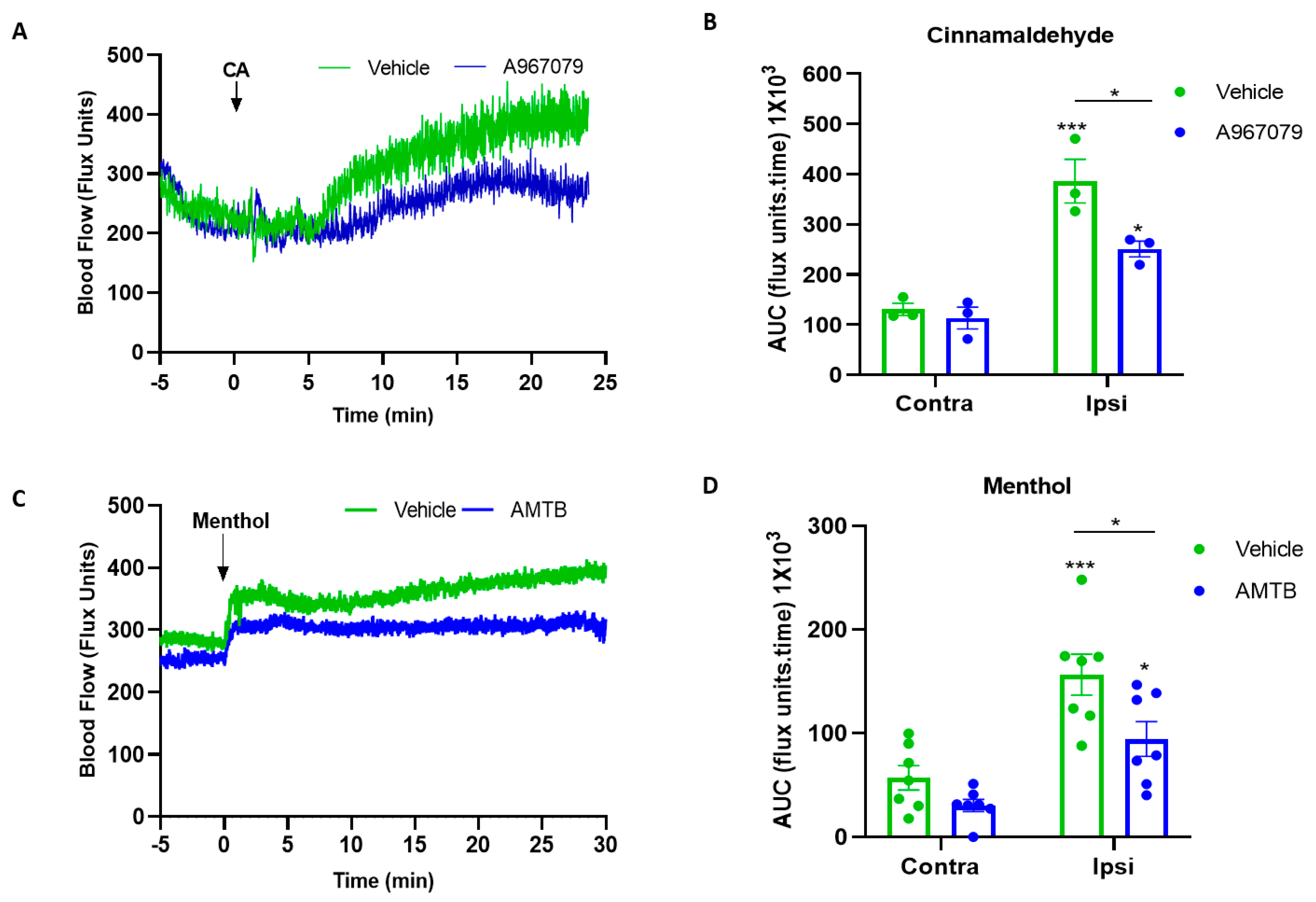
2.3. A967079 and AMTB Inhibits TRPA1 and TRPM8 Response
2.4. TRPM8 but Not TRPA1 Influences Core Body Temperature
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Full-Field Laser Perfusion Imager (FLPI) of Cutaneous Blood Flow in the Ear
4.3. Radiotelemetry and Whole Body Cold Exposure
4.4. Western Blotting
4.5. Polymerase Chain Reaction
4.6. Drugs and Reagents
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef]
- Thompson, C.S.; Holowatz, L.A.; Kenney, W.L. Attenuated noradrenergic sensitivity during local cooling in aged human skin. J. Physiol. 2005, 564, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis Is Driven by β2-AR Stimulation. Cell Metab. 2020, 32, 287–300. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. Brown adipose tissue as a heat-producing thermoeffector. Handb. Clin. Neurol. 2018, 156, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- De Oliveira, C.; Garami, A.; Lehto, S.G.; Pakai, E.; Tekus, V.; Pohoczky, K.; Youngblood, B.D.; Wang, W.; Kort, M.E.; Kym, P.R.; et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J. Neurosci. 2014, 34, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef] [PubMed]
- Tsavaler, L.; Shapero, M.H.; Morkowski, S.; Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001, 61, 3760–3769. [Google Scholar] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel that Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated Cold Sensitivity in TRPM8 Null Mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Steiner, A.A.; Branco, L.G.S.; Romanovsky, A.A. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur. J. Neurosci. 2006, 23, 3359–3367. [Google Scholar] [CrossRef]
- Tajino, K.; Hosokawa, H.; Maegawa, S.; Matsumura, K.; Dhaka, A.; Kobayashi, S. Cooling-sensitive TRPM8 is thermostat of skin temperature against cooling. PLoS ONE 2011, 6, e17504. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.S.; Golden, J.P.; Vogt, S.K.; Dhaka, A.; Story, G.M.; Gereau, R.W. A dynamic set point for thermal adaptation requires phospholipase C-mediated regulation of TRPM8 in vivo. Pain 2014, 155, 2124–2133. [Google Scholar] [CrossRef]
- Yang, S.; Lu, X.; Wang, Y.; Xu, L.; Chen, X.; Yang, F.; Lai, R. A paradigm of thermal adaptation in penguins and elephants by tuning cold activation in TRPM8. Proc. Natl. Acad. Sci. USA 2020, 117, 8633–8638. [Google Scholar] [CrossRef]
- Ma, S.; Yu, H.; Zhao, Z.; Luo, Z.; Chen, J.; Ni, Y.; Jin, R.; Ma, L.; Wang, P.; Zhu, Z.; et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell Biol. 2012, 4, 88–96. [Google Scholar] [CrossRef]
- Almeida, M.C.; Hew-Butler, T.; Soriano, R.N.; Rao, S.; Wang, W.; Wang, J.; Tamayo, N.; Oliveira, D.L.; Nucci, T.B.; Aryal, P.; et al. Pharmacological Blockade of the Cold Receptor TRPM8 Attenuates Autonomic and Behavioral Cold Defenses and Decreases Deep Body Temperature. J. Neurosci. 2012, 32, 2086–2099. [Google Scholar] [CrossRef]
- Moraes, M.N.; de Assis, L.V.M.; Henriques, F.D.S.; Batista, M.L.; Güler, A.D.; Castrucci, A.M.D.L. Cold-sensing TRPM8 channel participates in circadian control of the brown adipose tissue. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2415–2427. [Google Scholar] [CrossRef]
- Gavva, N.R.; Davis, C.; Lehto, S.G.; Rao, S.; Wang, W.; Zhu, D.X. Transient Receptor Potential Melastatin 8 (TRPM8) Channels are Involved in Body Temperature Regulation. Mol. Pain 2012, 8, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Feketa, V.V.; Balasubramanian, A.; Flores, C.M.; Player, M.R.; Marrelli, S.P. Shivering and tachycardic responses to external cooling in mice are substantially suppressed by TRPV1 activation but not by TRPM8 inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 1040. [Google Scholar] [CrossRef][Green Version]
- Feketa, V.V.; Zhang, Y.; Cao, Z.; Balasubramanian, A.; Flores, C.M.; Player, M.R.; Marrelli, S.P. Transient receptor potential melastatin 8 channel inhibition potentiates the hypothermic response to transient receptor potential vanilloid 1 activation in the conscious mouse. Crit. Care Med. 2014, 42, 355. [Google Scholar] [CrossRef]
- Reimúndez, A.; Fernández-Peña, C.; García, G.; Fernández, R.; Ordás, P.; Gallego, R.; Pardo-Vazquez, J.L.; Arce, V.; Viana, F.; Señarís, R. Deletion of the Cold Thermoreceptor TRPM8 Increases Heat Loss and Food Intake Leading to Reduced Body Temperature and Obesity in Mice. J. Neurosci. 2018, 38, 3643–3656. [Google Scholar] [CrossRef]
- Gordon, C.J. Temperature Regulation in Laboratory Rodents. Camb. Core 1993, 186, 228. [Google Scholar] [CrossRef]
- Paricio-Montesinos, R.; Schwaller, F.; Udhayachandran, A.; Rau, F.; Walcher, J.; Evangelista, R.; Vriens, J.; Voets, T.; Poulet, J.F.A.; Lewin, G.R. The Sensory Coding of Warm Perception. Neuron 2020, 106, 830–841.e3. [Google Scholar] [CrossRef]
- Melanaphy, D.; Johnson, C.D.; Kustov, M.V.; Watson, C.A.; Borysova, L.; Burdyga, T.V.; Zholos, A.V. Ion channel mechanisms of rat tail artery contraction-relaxation by menthol involving, respectively, TRPM8 activation and L-type Ca2+ channel inhibition. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1416–H1430. [Google Scholar] [CrossRef]
- Gauchan, P.; Andoh, T.; Kato, A.; Kuraishi, Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 2009, 458, 93–95. [Google Scholar] [CrossRef]
- Wilde, E.; Aubdool, A.A.; Thakore, P.; Baldissera, L.; Alawi, K.M.; Keeble, J.; Nandi, M.; Brain, S.D. Tail-Cuff Technique and Its Influence on Central Blood Pressure in the Mouse. J. Am. Heart Assoc. 2017, 6, e005204. [Google Scholar] [CrossRef]
- Wang, X.P.; Yu, X.; Yan, X.J.; Lei, F.; Chai, Y.S.; Jiang, J.F.; Yuan, Z.Y.; Xing, D.M.; Du, L.J. TRPM8 in the negative regulation of TNFα expression during cold stress. Sci. Rep. 2017, 7, 45155. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Thapa, D.; Baldissera, L.; Argunhan, F.; Aubdool, A.A.; Brain, S.D. Relevance of TRPA1 and TRPM8 channels as vascular sensors of cold in the cutaneous microvasculature. Pflugers Arch. 2018, 470, 779–786. [Google Scholar] [CrossRef]
- Thapa, D.; de Sousa Valente, J.; Barrett, B.; Smith, M.J.; Argunhan, F.; Sheng, L.Y.; Nikitochkina, S.; Kodji, X.; Brain, S.D. Dysfunctional TRPM8 signalling in the vascular response to environmental cold in ageing. elife 2021, 10, e70153. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.; Enerbäck, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am. J. Physiol. Endocrinol. Metab. 2006, 291, 350. [Google Scholar] [CrossRef]
- Rossato, M.; Granzotto, M.; Macchi, V.; Porzionato, A.; Petrelli, L.; Calcagno, A.; Vencato, J.; De Stefani, D.; Silvestrin, V.; Rizzuto, R.; et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol. Cell. Endocrinol. 2014, 383, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, C.; Jall, S.; Kleinert, M.; Quarta, C.; Gruber, T.; Reber, J.; Sachs, S.; Fischer, K.; Feuchtinger, A.; Karlas, A.; et al. Coordinated targeting of cold and nicotinic receptors synergistically improves obesity and type 2 diabetes. Nat. Commun. 2018, 9, 4304. [Google Scholar] [CrossRef]
- Vinuela-Fernandez, I.; Sun, L.; Jerina, H.; Curtis, J.; Allchorne, A.; Gooding, H.; Rosie, R.; Holland, P.; Tas, B.; Mitchell, R.; et al. The TRPM8 channel forms a complex with the 5-HT(1B) receptor and phospholipase D that amplifies its reversal of pain hypersensitivity. Neuropharmacology 2014, 79, 136–151. [Google Scholar] [CrossRef]
- Goralczyk, A.; van Vijven, M.; Koch, M.; Badowski, C.; Yassin, M.S.; Toh, S.; Shabbir, A.; Franco-Obregón, A.; Raghunath, M. TRP channels in brown and white adipogenesis from human progenitors: New therapeutic targets and the caveats associated with the common antibiotic, streptomycin. FASEB J. 2017, 31, 3251–3266. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, D.; Barrett, B.; Argunhan, F.; Brain, S.D. Influence of Cold-TRP Receptors on Cold-Influenced Behaviour. Pharmaceuticals 2022, 15, 42. https://doi.org/10.3390/ph15010042
Thapa D, Barrett B, Argunhan F, Brain SD. Influence of Cold-TRP Receptors on Cold-Influenced Behaviour. Pharmaceuticals. 2022; 15(1):42. https://doi.org/10.3390/ph15010042
Chicago/Turabian StyleThapa, Dibesh, Brentton Barrett, Fulye Argunhan, and Susan D. Brain. 2022. "Influence of Cold-TRP Receptors on Cold-Influenced Behaviour" Pharmaceuticals 15, no. 1: 42. https://doi.org/10.3390/ph15010042
APA StyleThapa, D., Barrett, B., Argunhan, F., & Brain, S. D. (2022). Influence of Cold-TRP Receptors on Cold-Influenced Behaviour. Pharmaceuticals, 15(1), 42. https://doi.org/10.3390/ph15010042





