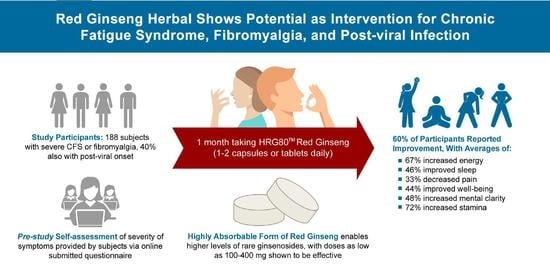
An Open-Label, Pilot Trial of HRG80™ Red Ginseng in Chronic Fatigue Syndrome, Fibromyalgia, and Post-Viral Fatigue
Abstract
:1. Introduction
- A major loss of micronutrients in the processed Western diet, including as much as an 85% loss of the key energy nutrient magnesium [1].
- Insufficient sleep [2].
- The increased speed and stress of modern life.
- Infections triggering chronic fatigue syndrome and fibromyalgia (CFS/FMS).
2. Results
2.1. Generalizability
2.2. Dropouts and Side Effects
3. Discussion
4. Materials and Methods
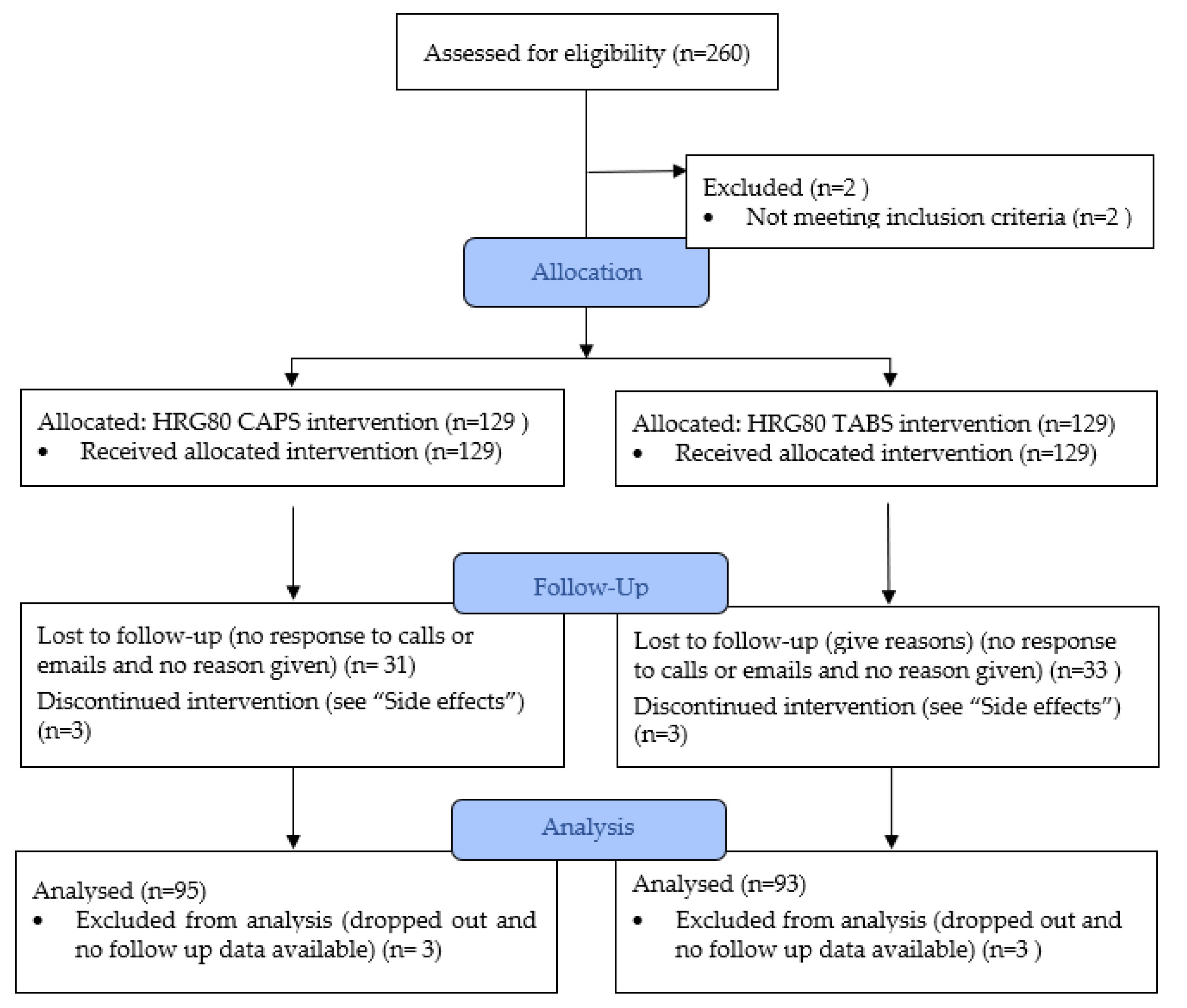
4.1. Patient Enrollment
4.2. Inclusion Criteria
- Subjects needed to rate their overall well-being as 5 or less on the Visual Analogue Scale (VAS).
- Those with post-viral fatigue were also allowed to participate.
- Subjects needed to live in the United States, be over 18 years of age, and nonpregnant.
4.3. Exclusion Criteria
- Subjects could not be taking the blood thinner Coumadin (generic name: warfarin).
- Subjects could not be pregnant.
4.4. Objectives and Randomization
4.5. Sample Size
4.6. Interventions
4.7. Outcome Measures
- (A)
- How is your energy?1 2 3 4 5 6 7 8 9 101 = “near dead” and 10 = excellent
- (B)
- How is your overall sense of well-being?1 2 3 4 5 6 7 8 9 101 = “near dead” and 10 = excellent
- (C)
- How is your mental clarity?1 2 3 4 5 6 7 8 9 101 = “brain dead” and 10 = good clarity
- (D)
- How is your sleep?1 2 3 4 5 6 7 8 9 101 = no sleep and 10 = 8 h of sleep a night without waking
- (E)
- How severe is your achiness/pain (1 is worst possible pain)?1 2 3 4 5 6 7 8 9 101 = very severe pain and 10 = pain free
- (F)
- How is your stamina?1 = no stamina and 10 = healthy stamina
4.8. Study Design
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Chaput, J.P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping hours: What is the ideal number and how does age impact this? Nat. Sci. Sleep 2018, 10, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, J.A. The Legacy of Ginseng: Ginseng; A Concise Handbook; Reference Publications, Inc.: Algonac, MI, USA, 1989; p. 274. ISBN 0-917256-32-8. [Google Scholar]
- Lu, G.; Liu, Z.; Wang, X.; Wang, C. Recent Advances in Panax ginseng C.A. Meyer as a Herb for Anti-Fatigue: An Effects and Mechanisms Review. Foods 2021, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xue, B.; Yang, C.; Miao, L.; Zhou, L.; Chen, Q.; Cai, Q.; Liu, Y.; Liu, D.; He, H.; et al. Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia 2018, 129, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Dharmananda, S. The Nature of Ginseng: Traditional Use, Modern Research, and the Question of Dosage. HerbalGram 2002, 54, 34–51. Available online: http://herbalgram.org/resources/herbalgram/issues/54/table-of-contents/article2168/ (accessed on 17 November 2021).
- Uekama, K.; Otagiri, M. Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carrier. Syst. 1987, 3, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Lemerond, T.; Panossian, A.G. Panax ginseng Meyer Herbal Preparation HRG80 for Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects. J. Altern. Complement Integr. Med. 2020, 6, 100. [Google Scholar]
- Kroenke, K.; Arrington, M.E.; Mangelsdorff, A.D. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch. Intern. Med. 1990, 150, 1685–1689. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med. 2021, 7, 1132. [Google Scholar] [CrossRef]
- Maloney, E.M.; Boneva, R.; Nater, U.M.; Reeves, W.C. Chronic fatigue syndrome and high allostatic load: Results from a population-based case-control study in Georgia. Psychosom. Med. 2009, 71, 549–556. [Google Scholar] [CrossRef]
- Leombruni, P.; Zizzi, F.; Pavan, S.; Fusaro, E.; Miniotti, M. Allostatic Overload in Patients with Fibromyalgia: Preliminary Findings. Psychother. Psychosom. 2019, 88, 180–181. [Google Scholar] [CrossRef]
- Tanriverdi, F.; Karaca, Z.; Unluhizarci, K.; Kelestimur, F. The hypothalamo-pituitary-adrenal axis in chronic fatigue syndrome and fibromyalgia syndrome. Stress 2007, 10, 13–25. [Google Scholar] [CrossRef]
- Mariage, P.A.; Hovhannisyan, A.; Panossian, A.G. Efficacy of Panax ginseng Meyer herbal preparation HRG80 in preventing and mitigating stress-induced failure of cognitive functions in healthy subjects: A pilot, randomized, double-blind, placebo-controlled crossover trial. Pharmaceuticals 2020, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.Y.; Woo, T.S.; Yoon, S.Y. Red ginseng supplementation more effectively alleviates psychological than physical fatigue. J. Ginseng Res. 2011, 35, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Rhee, D.K. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J. Ginseng Res. 2017, 41, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Demitrack, M.A.; Crofford, L.J. Evidence for and pathophysiologic implications of hypothalamic-pituitary-adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. Ann. N. Y. Acad. Sci. 1998, 840, 684–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network Pharmacology of Ginseng (Part II): The Differential Effects of Red Ginseng and Ginsenoside Rg5 in Cancer and Heart Diseases as Determined by Transcriptomics. Pharmaceuticals 2021, 14, 1010. [Google Scholar] [CrossRef]
- Pérez-Cano, H.J.; Moreno-Murguía, M.B.; Morales-López, O.; Crow-Buchanan, O.; English, J.A.; Lozano-Alcázar, J.; Somilleda-Ventura, S.A. Anxiety, depression, and stress in response to the coronavirus disease-19 pandemic. Cir. Cir. 2020, 88, 562–568. (In English) [Google Scholar] [CrossRef]
- Official Press Conference: COVID-19 Conference Highlights. YouTube Video, 00:48:32, Posted by “International AIDS Conference”; (34 min in). 9 July 2020. Available online: https://www.youtube.com/watch?v=UMmT48IC0us&t=1999s (accessed on 17 November 2021).
- Teitelbaum, J.E.; Bird, B.; Greenfield, R.M.; Weiss, A.; Muenz, L.; Gould, L. Effective Treatment of CFS and FMS: A Randomized, Double-Blind Placebo Controlled Study. J. Chronic Fatigue Syndr. 2001, 8, 3–15. Available online: https://www.tandfonline.com/doi/abs/10.1300/J092v08n02_02 (accessed on 17 November 2021). [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, J.E.; Bird, B. Effective Treatment of Severe Chronic Fatigue: A Report of a Series of 64 Patients. J. Musculoskelet. Pain 1995, 3, 91–110. Available online: http://informahealthcare.com/doi/abs/10.1300/J094v03n04_11 (accessed on 15 June 2018). [CrossRef]
- Fielding, S.; Maclennan, G.; Cook, J.A.; Ramsay, C.R. A review of RCTs in four medical journals to assess the use of imputation to overcome missing data in quality of life outcomes. Trials 2008, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The Chronic Fatigue Syndrome: A Comprehensive Approach to its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Age in years, mean (SD) | 58.6 (11.6) |
| Gender, female % | 89.4 (168) |
| Duration of illness in years, mean (SD) | 18.9 (10.6) |
| Onset with viral illness, % (n) | 40.4 (76) |
| Diagnosis of CFS, % (n) | 96.8 (182) |
| Diagnosis of FMS, % (n) | 94.1 (177) |
| Self-reported feeling post-treatment, % (n) | |
| Much worse Worse Same Better Much better | 2.7 (5) 3.2 (6) 34.0 (64) 46.8 (88) 13.3 (25) |
| Variable | Pre-tx Mean (SD) | Post-tx Mean (SD) | t Statistic (p-Value) | Effect Size (d) | Percent Improvement |
|---|---|---|---|---|---|
| VAS Composite * | 12.2 (3.5) | 17.1 (4.8) | −14.31 (<0.001) | −1.04 | 40.2 |
| VAS 1. Energy | 3.6 (1.3) | 5.4 (1.8) | −13.30 (<0.001) | −0.97 | 50.0 |
| VAS 2. Sleep | 4.5 (1.7) | 6.0 (2.0) | −10.46 (<0.001) | −0.76 | 33.3 |
| VAS 3. Pain | 4.6 (1.8) | 5.5 (1.8) | −5.84 (<0.001) | −0.43 | 19.6 |
| VAS 4. Well-Being | 3.9 (1.4) | 5.7 (1.9) | −12.89 (<0.001) | −0.94 | 46.2 |
| VAS 5. Mental Clarity | 4.7 (1.6) | 6.1 (1.8) | −10.18 (<0.001) | −0.74 | 29.8 |
| VAS 6. Stamina | 3.3 (1.5) | 4.8 (2.1) | −10.04 (<0.001) | −0.73 | 45.5 |
| Variable | Pre-tx Mean (SD) | Post-tx Mean (SD) | t Statistic (p-Value) | Effect Size (d) | Percent Improvement |
|---|---|---|---|---|---|
| VAS Composite * | 11.9 (3.2) | 18.8 (4.3) | −17.71 (<0.001) | −1.67 | 58.0 |
| VAS 1. Energy | 3.6 (1.4) | 6.0 (1.7) | −14.86 (<0.001) | −1.40 | 66.7 |
| VAS 2. Sleep | 4.4 (1.7) | 6.4 (1.8) | −11.95 (<0.001) | −1.36 | 45.5 |
| VAS 3. Pain | 4.5 (1.9) | 6.0 (1.7) | −7.36 (<0.001) | −0.90 | 33.3 |
| VAS 4. Well-Being | 3.8 (1.2) | 6.3 (1.6) | −15.63 (<0.001) | −1.74 | 43.6 |
| VAS 5. Mental Clarity | 4.4 (1.3) | 6.5 (1.7) | −12.80 (<0.001) | −1.45 | 47.7 |
| VAS 6. Stamina | 3.2 (1.4) | 5.5 (2.0) | −12.48 (<0.001) | −1.41 | 71.9 |
| Outcome Variable | F-Statistic (p-Value) |
|---|---|
| VAS Composite | |
| Treatment Treatment * Group | 204.57 (<0.001) 1.57 (0.212) |
| VAS 1. Energy | |
| Treatment Treatment * Group | 172.22 (<0.001) 0.26 (0.614) |
| VAS 2. Sleep | |
| Treatment Treatment * Group | 113.03 (<0.001) 2.77 (0.098) |
| VAS 3. Pain | |
| Treatment Treatment * Group | 31.47 (<0.001) 0.33 (0.567) |
| VAS 4. Well-Being | |
| Treatment Treatment * Group | 168.03 (<0.001) 2.03 (0.156) |
| VAS 5. Mental Clarity | |
| Treatment Treatment * Group | 104.74 (<0.001) 1.49 (0.224) |
| VAS 6. Stamina | |
| Treatment Treatment * Group | 103.65 (<0.001) 2.37 (0.125) |
| Outcome Variable | F-Statistic (p-Value) |
|---|---|
| VAS Composite | |
| Treatment Treatment * Group | 203.65 (<0.001) 0.04 (0.844) |
| VAS 1. Energy | |
| Treatment Treatment * Group | 220.65 (<0.001) 2.44 (0.120) |
| VAS 2. Sleep | |
| Treatment Treatment * Group | 109.70 (<0.001) 1.19 (0.277) |
| VAS 3. Pain | |
| Treatment Treatment * Group | 33.91 (<0.001) 0.58 (0.447) |
| VAS 4. Well-Being | |
| Treatment Treatment * Group | 165.39 (<0.001) 0.02 (0.891) |
| VAS 5. Mental Clarity | |
| Treatment Treatment * Group | 103.00 (<0.001) 0.18 (0.676) |
| VAS 6. Stamina | |
| Treatment Treatment * Group | 100.85 (<0.001) 1.82 (0.179) |
| Outcome Variable (% Improvement in Improvers) | One Tablet (n = 25) | One Capsule (n = 16) | Tablet vs. Capsule t Statistic (p-Value) | Effect Size (d) | Two Tablets (n = 24) | Two Capsules (n = 39) |
|---|---|---|---|---|---|---|
| VAS Composite * | 60.6 | 69.0 | 0.56 (0.581) | 0.18 | 70.7 | 68.3 |
| VAS 1. Energy | 70.9 | 78.4 | 0.31 (0.757) | 0.11 | 92.1 | 96.5 |
| VAS 2. Sleep | 62.5 | 78.8 | 0.64 (0.531) | 0.23 | 59.4 | 60.9 |
| VAS 3. Pain | 45.5 | 67.0 | 0.90 (0.375) | 0.29 | 36.2 | 67.2 |
| VAS 4. Well-Being | 68.5 | 80.6 | 0.61 (0.547) | 0.20 | 79.6 | 80.1 |
| VAS 5. Mental Clarity | 56.4 | 67.9 | 0.70 (0.490) | 0.22 | 55.0 | 58.7 |
| VAS 6. Stamina | 74.7 | 123.7 | 1.41 (0.168) | 0.45 | 87.8 | 105.1 |
| Full Sample (n = 188) | Subgroup: Capsule (n = 95) | Subgroup: Tablet (n = 93) | |
|---|---|---|---|
| Daily dose, % (n) | |||
| One per day Two per day Other | 30.3 (57) 52.1 (98) 17.6 (33) | 23.2 (22) 62.1 (59) 14.7 (14) | 37.6 (35) 41.9 (39) 20.4 (19) |
| Daily frequency, % (n) | |||
| Once per day Twice per day Other | 70.2 (132) 17.6 (33) 12.2 (23) | 74.7 (71) 16.8 (16) 8.4 (8) | 65.6 (61) 18.3 (17) 16.1 (15) |
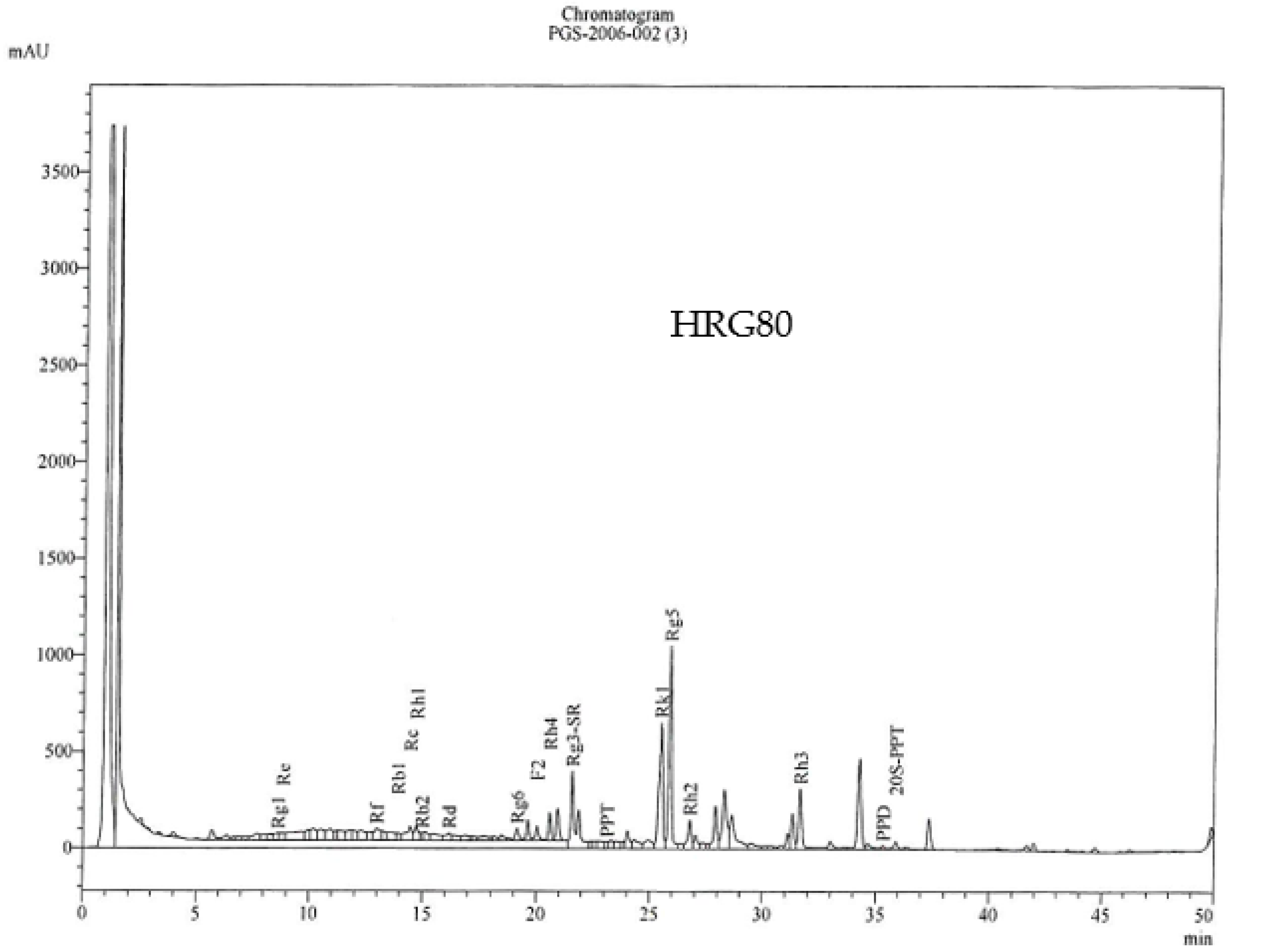
| Batch | Total Ginsenosides (%) * | Rare Ginsenosides (%) ** |
|---|---|---|
| 7 September 2021 | 10.6% | 10.1% |
| 9 July 2021 | 12.6% | 11.7% |
| 26 May 2021 | 12.5% | 10.6% |
| Ginsenosides | Content (%) in HRG80 TM, |
|---|---|
| Rg1 | 0.023 |
| Re | 0.114 |
| Rf | 0.081 |
| Rb1 | 0.046 |
| Rg2 | 0.00 |
| Rc | 0.473 |
| Rh1 | 0.071 |
| Rb2 | 0.130 |
| F1 | 0.005 |
| Rd | 0.477 |
| Rg6 | 0.218 |
| F2 | 0.131 |
| Rh4 | 1.092 |
| Rg3-(S-R) | 1.080 |
| PPT (20-R) | 0.022 |
| Rk1 | 1.009 |
| C(k) | 0.00 |
| Rg5 | 1.888 |
| Rh2 | 0.232 |
| Rh3 | 0.455 |
| 20S-PPT | 0.015 |
| PPD | 0.038 |
| Total | 7.599 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teitelbaum, J.; Goudie, S. An Open-Label, Pilot Trial of HRG80™ Red Ginseng in Chronic Fatigue Syndrome, Fibromyalgia, and Post-Viral Fatigue. Pharmaceuticals 2022, 15, 43. https://doi.org/10.3390/ph15010043
Teitelbaum J, Goudie S. An Open-Label, Pilot Trial of HRG80™ Red Ginseng in Chronic Fatigue Syndrome, Fibromyalgia, and Post-Viral Fatigue. Pharmaceuticals. 2022; 15(1):43. https://doi.org/10.3390/ph15010043
Chicago/Turabian StyleTeitelbaum, Jacob, and Sarah Goudie. 2022. "An Open-Label, Pilot Trial of HRG80™ Red Ginseng in Chronic Fatigue Syndrome, Fibromyalgia, and Post-Viral Fatigue" Pharmaceuticals 15, no. 1: 43. https://doi.org/10.3390/ph15010043
APA StyleTeitelbaum, J., & Goudie, S. (2022). An Open-Label, Pilot Trial of HRG80™ Red Ginseng in Chronic Fatigue Syndrome, Fibromyalgia, and Post-Viral Fatigue. Pharmaceuticals, 15(1), 43. https://doi.org/10.3390/ph15010043