Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Results
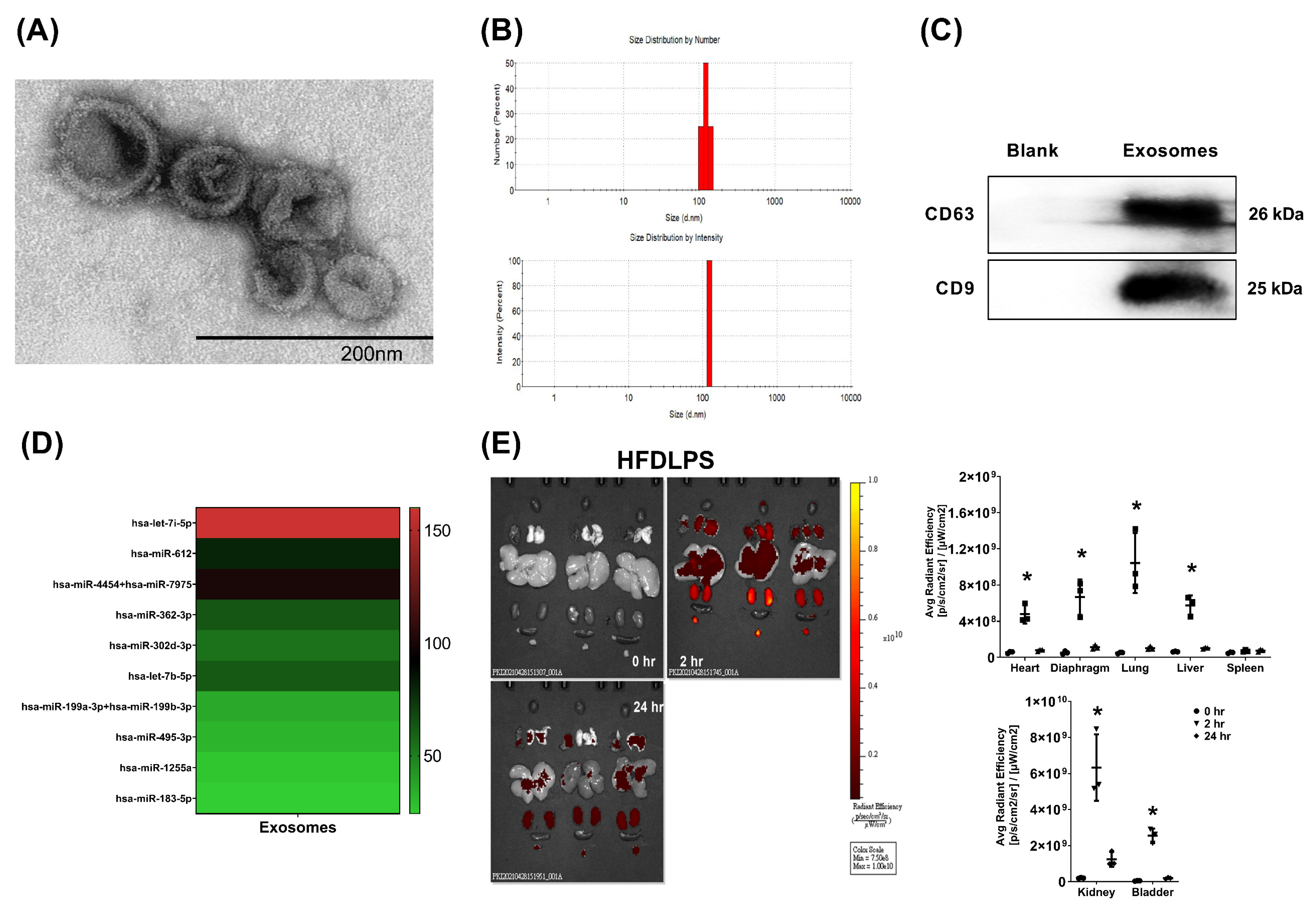
2.1. Confirmation, miRNA Identification, and Biodistribution of Exosomes
2.2. Survivorship, Metabolic Profiling, and Systemic Inflammation
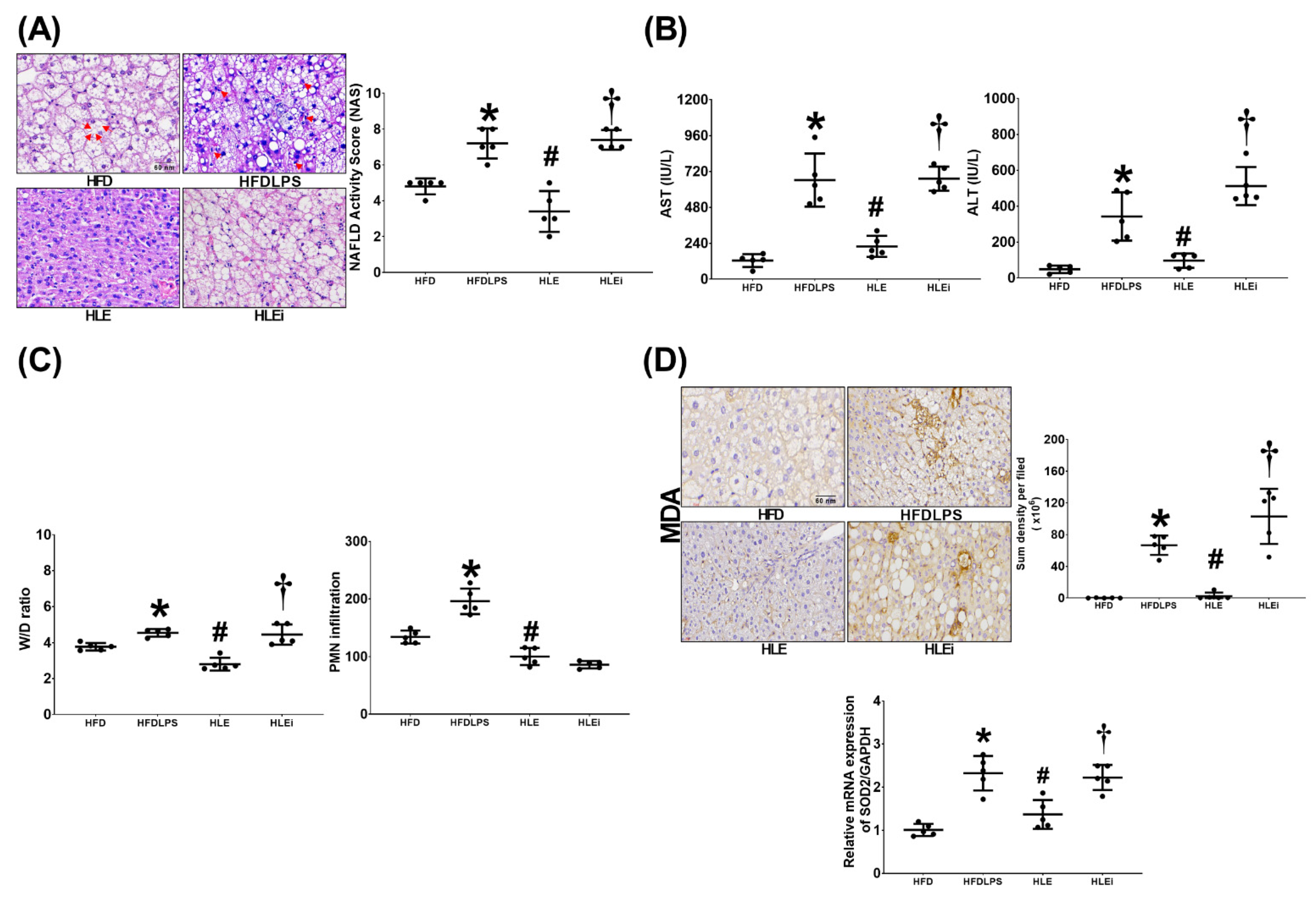
2.3. Liver Injury and Oxidation
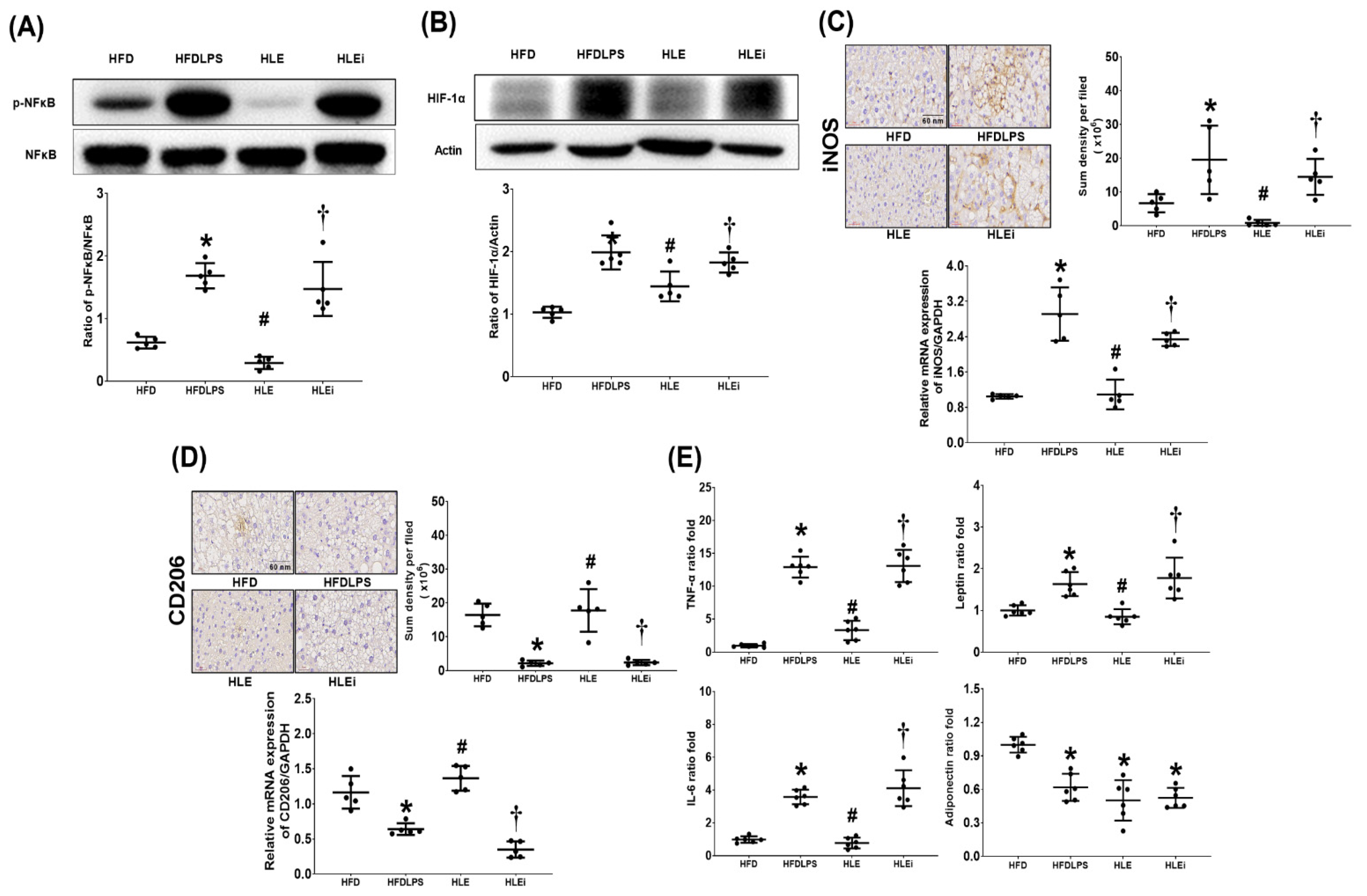
2.4. Liver Inflammation
2.5. Mitochondrial Injury and Dysfunction
2.6. Apoptosis in the Liver
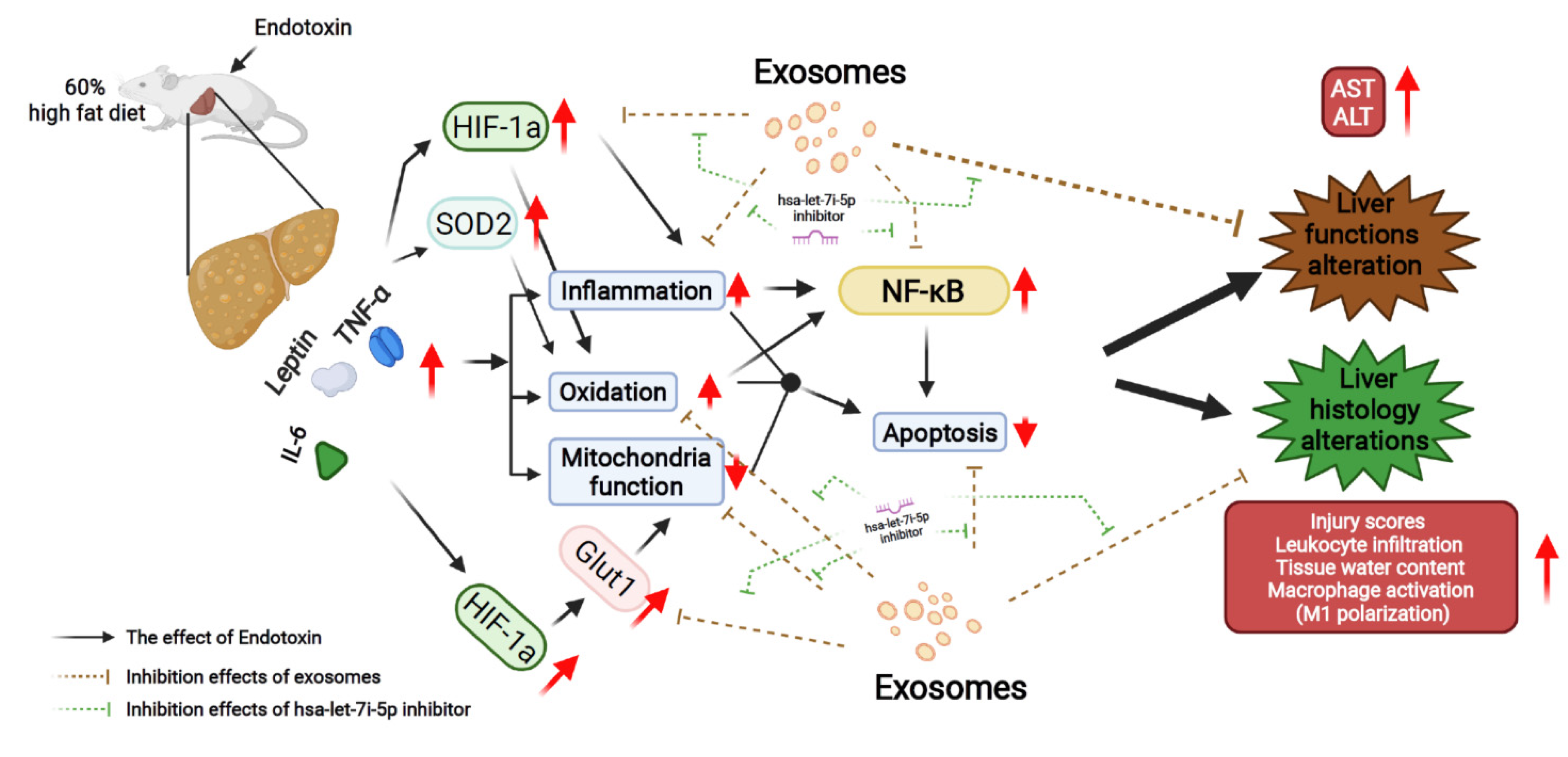
3. Discussion
4. Materials and Methods
4.1. Isolation, Purification, and Characterization of Exosomes
4.2. Analysis of the miRNA in Exosomes
4.3. Electroporation of the miRNA Inhibitor into Exosomes
4.4. High-Fat Diet-Induced Obesity and Endotoxin-Induced Monomicrobial Sepsis Models
4.5. Biodistribution of Exosomes Using an Ex Vivo Bioluminescence Imaging Assay
4.6. Survivorship, Blood Sampling, Liver Harvesting, and Wet/Dry Weight Ratio
4.7. Liver Enzyme and Metabolic Profile
4.8. Histological Analysis of the Liver
4.9. Enzyme-Linked Immunosorbent Assay
4.10. Immunohistochemistry Staining
4.11. Immunoblotting Assay
4.12. Quantitative Real-Time Polymerase Chain Reaction
4.13. Hepatocyte Isolation, Treatment, and the Mitochondrial Function Assay
4.14. TEM Analysis
4.15. TUNEL Assay
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Richter, T.; Mühlhauser, I. The morbidity and mortality associated with overweight and obesity in adulthood: A systematic review. Dtsch. Arztebl. Int. 2009, 106, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisaka, S.; Usui, I.; Ikutani, M.; Aminuddin, A.; Takikawa, A.; Tsuneyama, K.; Mahmood, A.; Goda, N.; Nagai, Y.; Takatsu, K.; et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 2013, 56, 1403–1412. [Google Scholar] [CrossRef] [Green Version]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, v192, 26–32. [Google Scholar] [CrossRef]
- Li, Z.L.; Woollard, J.R.; Ebrahimi, B.; Crane, J.A.; Jordan, K.L.; Lerman, A.; Wang, S.M.; Lerman, L.O. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.E.; Griffin, R.; Judd, S.; Shapiro, N.I.; Safford, M.M. Obesity and risk of sepsis: A population-based cohort study. Obesity 2013, 21, E762–E769. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L. Clinical sepsis and septic shock–definition, diagnosis and management principles. Langenbecks Arch. Surg. 2008, 393, 817–824. [Google Scholar] [CrossRef]
- Yao, Y.M.; Luan, Y.Y.; Zhang, Q.H.; Sheng, Z.Y. Pathophysiological aspects of sepsis: An overview. Methods Mol. Biol. 2015, 1237, 5–15. [Google Scholar]
- Papadimitriou-Olivgeris, M.; Aretha, D.; Zotou, A.; Koutsileou, K.; Zbouki, A.; Lefkaditi, A.; Sklavou, C.; Marangos, M.; Fligou, F. The Role of Obesity in Sepsis Outcome among Critically Ill Patients: A Retrospective Cohort Analysis. Biomed Res. Int. 2016, 2016, 5941279. [Google Scholar] [CrossRef] [Green Version]
- Utzolino, S.; Ditzel, C.M.; Baier, P.K.; Hopt, U.T.; Kaffarnik, M.F. The obesity paradox in surgical intensive care patients with peritonitis. J. Crit. Care 2014, 29, 887.e1–887.e5. [Google Scholar] [CrossRef]
- Paulsen, J.; Askim, Å.; Mohus, R.M.; Mehl, A.; Dewan, A.; Solligård, E.; Damås, J.K.; Åsvold, B.O. Associations of obesity and lifestyle with the risk and mortality of bloodstream infection in a general population: A 15-year follow-up of 64 027 individuals in the HUNT Study. Int. J. Epidemiol. 2017, 46, 1573–1581. [Google Scholar] [CrossRef] [Green Version]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Giebel, B.; Kordelas, L.; Börger, V. Clinical potential of mesenchymal stem/stromal cell derived extracellular vesicles. Stem Cell Investig. 2017, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Pini, M.; Castellanos, K.J.; Rhodes, D.H.; Fantuzzi, G. Obesity and IL-6 interact in modulating the response to endotoxemia in mice. Cytokine 2013, 61, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Haljamäe, H. Metabolic effects of high dose corticosteroids. Acta Chir. Scand Suppl. 1985, 526, 27–36. [Google Scholar]
- Waage, A.; Brandtzaeg, P.; Halstensen, A.; Kierulf, P.; Espevik, T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 1989, 169, 333–338. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, C.K.; Yu, J.Q.; Tan, R.; Yang, P.L. Hypoglycemia and mortality in sepsis patients: A systematic review and meta-analysis. Heart Lung 2021, 50, 933–940. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y.; et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2018, 12, 7613–7628. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Wu, L.; Liu, M.; Zhu, L.; Chen, Y.; Zhou, H.; Shi, X.; Wei, J.; Zheng, L.; Hu, X.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate LPS-Induced ARDS by Modulating Macrophage Polarization Through Inhibiting Glycolysis in Macrophages. Shock 2020, 54, 828–843. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquinelli, A.E.; Bettinger, J.C.; Rougvie, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nat. Cell Biol. 2000, 403, 901–906. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, X.; Liu, F. Silencing TTTY15 mitigates hypoxia-induced mitochondrial energy metabolism dysfunction and cardiomyocytes apoptosis via TTTY15/let-7i-5p and TLR3/NF-κB pathways. Cell Signal 2020, 76, 109779. [Google Scholar] [CrossRef]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, H.J.; Foo, J.; Shih, H.J.; Huang, C.J. Peptide-Based TNF-α-Binding Decoy Therapy Mitigates Lipopolysaccharide-Induced Liver Injury in Mice. Pharmaceuticals 2020, 13, 280. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Awe, O.; Rao, R.C.; Kirby, P.A.; Hitchon, P.W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J. Neurosurg. Spine 2014, 21, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials-an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Sjaastad, F.V.; Jensen, I.J.; Berton, R.R.; Badovinac, V.P.; Griffith, T.S. Inducing Experimental Polymicrobial Sepsis by Cecal Ligation and Puncture. Curr. Protoc. Immunol. 2020, 13, e110. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y.; Shibata, R.; Ouchi, N.; Kambara, T.; Ohashi, K.; Jie, L.; Inoue, Y.; Murohara, T.; Komori, K. Adiponectin ameliorates endotoxin-induced acute cardiac injury. BioMed Res. Int. 2014, 2014, 382035. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Wu, M.S.; Hui, Y.Y.; Chang, B.M.; Pan, L.; Hsu, P.C.; Chen, Y.T.; Ho, H.N.; Huang, Y.H.; Ling, T.Y.; et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci. Rep. 2017, 7, 45607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; A B da Cruz E Silva, O.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Pérez Lanzón, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, Z.; Ma, T.; Yang, Z.; Zhang, J.; Liu, X.; Lu, D.; Shen, Z.; Yang, J.; Meng, Q. Endothelial progenitor cell-derived exosomes, loaded with miR-126, promoted deep vein thrombosis resolution and recanalization. Stem Cell Res. Ther. 2018, 9, 223. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Nadeem Aslam, M.; Bassis, C.M.; Zhang, L.; Zaidi, S.; Varani, J.; Bergin, I.L. Calcium Reduces Liver Injury in Mice on a High-Fat Diet: Alterations in Microbial and Bile Acid Profiles. PLoS ONE 2016, 11, e0166178. [Google Scholar] [CrossRef]
- Shih, H.J.; Chang, C.Y.; Chiang, M.; Le, V.L.; Hsu, H.J.; Huang, C.J. Simultaneous Inhibition of Three Major Cytokines and Its Therapeutic Effects: A Peptide-Based Novel Therapy against Endotoxemia in Mice. J. Pers. Med. 2021, 11, 436. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Ye, J.; Liu, J.; Xu, Y.; Wang, Z.; Ye, D.; Zhao, M.; Wan, J. The Anti-inflammatory Mediator Resolvin E1 Protects Mice Against Lipopolysaccharide-Induced Heart Injury. Front. Pharmacol. 2020, 11, 203. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Xing, Z.; Tan, M.; Wu, Y.; Zhang, C. Propofol regulates activated macrophages metabolism through inhibition of ROS-mediated GLUT1 expression. Inflamm. Res. 2021, 70, 473–481. [Google Scholar] [CrossRef]
- Oliveira, L.S.; de Queiroz, N.M.; Veloso, L.V.; Moreira, T.G.; Oliveira, F.S.; Carneiro, M.B.; Faria, A.M.; Vieira, L.Q.; Oliveira, S.C.; Horta, M.F. A defective TLR4 signaling for IFN-β expression is responsible for the innately lower ability of BALB/c macrophages to produce NO in response to LPS as compared to C57BL/6. PLoS ONE 2014, 9, e98913. [Google Scholar] [CrossRef] [Green Version]
- Li, W.C.; Ralphs, K.L.; Tosh, D. Isolation and culture of adult mouse hepatocytes. Methods Mol. Biol. 2010, 633, 185–196. [Google Scholar] [CrossRef]
- Chen, C.P.; Tsai, P.S.; Huang, C.J. Antiinflammation effect of human placental multipotent mesenchymal stromal cells is mediated by prostaglandin E2 via a myeloid differentiation primary response gene 88-dependent pathway. Anesthesiology 2012, 117, 568–579. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.J.; Chang, C.Y.; Huang, I.T.; Tsai, P.S.; Han, C.L.; Huang, C.J. Testicular torsion-detorsion causes dysfunction of mitochondrial oxidative phosphorylation. Andrology 2021, 9, 1902–1910. [Google Scholar] [CrossRef]
- Dlasková, A.; Špaček, T.; Engstová, H.; Špačková, J.; Schröfel, A.; Holendová, B.; Smolková, K.; Plecitá-Hlavatá, L.; Ježek, P. Mitochondrial cristae narrowing upon higher 2-oxoglutarate load. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 659–678. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Chen, K.-Y.; Shih, H.-J.; Chiang, M.; Huang, I.-T.; Huang, Y.-H.; Huang, C.-J. Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice. Pharmaceuticals 2022, 15, 36. https://doi.org/10.3390/ph15010036
Chang C-Y, Chen K-Y, Shih H-J, Chiang M, Huang I-T, Huang Y-H, Huang C-J. Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice. Pharmaceuticals. 2022; 15(1):36. https://doi.org/10.3390/ph15010036
Chicago/Turabian StyleChang, Chao-Yuan, Kung-Yen Chen, Hung-Jen Shih, Milton Chiang, I-Tao Huang, Yen-Hua Huang, and Chun-Jen Huang. 2022. "Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice" Pharmaceuticals 15, no. 1: 36. https://doi.org/10.3390/ph15010036
APA StyleChang, C.-Y., Chen, K.-Y., Shih, H.-J., Chiang, M., Huang, I.-T., Huang, Y.-H., & Huang, C.-J. (2022). Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice. Pharmaceuticals, 15(1), 36. https://doi.org/10.3390/ph15010036






